Abstract
Background
Hormonal changes during menstrual cycling may affect susceptibility to HIV.
Methods
We determined the SHIV acquisition time point in 43 cycling pigtail macaques infected by repeated vaginal virus exposures initiated randomly in the cycle.
Results
SHIV infection was first detected in the follicular phase in 38 macaques (88%), and in the luteal phase in 5 macaques (12%), indicating a statistically significant timing difference. Assuming a 7-day eclipse phase, most infections occurred during or following a high-progesterone period associated with menstruation, vaginal epithelium thinning and suppressed mucosal immunity.
Conclusions
This raises questions whether other high progesterone conditions (pregnancy, hormonal contraception) similarly affect HIV risk.
Keywords: HIV risk, HIV acquisition, menstrual cycle, reproductive hormone, hormonal contraception, mucosal immunity
Introduction
Susceptibility to sexual HIV infection is variable in women (reviewed in [5]). High viral load in the transmitter, co-infections or genetic determinants (also reviewed in [5]) are known risk factors. Identification of additional susceptibility factors could prove beneficial for HIV prevention efforts. In 2008, Wira and Fahey formulated the hypothesis that there is a 7–10 day window of vulnerability to HIV infection during the menstrual cycle [18]. This was postulated as a result of an observed, temporary suppression of mucosal immunity during the cycle, likely to facilitate immune tolerance of paternal antigens during reproduction. During the first (follicular) phase of the cycle, the ovum has not yet matured and there is no potential for pregnancy. Immune defenses are high, resulting in low vulnerability to HIV. In contrast, in the later, luteal, progesterone-dominated phase of the cycle initiated by ovulation, immunity is down-regulated to allow tolerance of potential paternal antigens. Changes in mucosal barriers, e.g., in epithelial cell layer thickness and in properties of mucus, also alter susceptibility to pathogens throughout the menstrual cycle [2]. Wira and Fahey hypothesized that this results in highest susceptibility to HIV infection late in the cycle. Experimental proof for the hypothesis has been elusive, likely due to difficulties in timing both HIV acquisition and menstrual cycle accurately and retrospectively in HIV-infected women.
We previously reported a window of high susceptibility to vaginal SHIV infection late in the menstrual cycle of 19 pigtail macaques (macaca nemestrina) [16]. Contrary to rhesus macaques (macaca mulatta), another macaque species commonly used for HIV research, pigtail macaques have median 32-day menstrual cycles year-round, and no progestin treatments are needed prior to vaginal S(H)IV infection in the repeat low-dose (RLD) virus exposure model [9]. We used existing longitudinal specimens of opportunity to retrospectively analyze the timing of infection relative to the menstrual cycle in control animals from prevention evaluation trials [3–7]. Our analysis focused on the time point of first virus detection following transmission, using a sensitive RT-PCR assay for early detection of infection. Infection timing was found not to be random over the menstrual cycle, but rather occurred in the follicular phase of the cycle for 18 of the 19 macaques [16]. Accounting for an eclipse period before infection was detected, we concluded that transmission likely occurred at the end of the menstrual cycle, thus supporting Wira and Fahey’s hypothesis. This also confirmed the conclusions of a small study in rhesus macaques that did not reach statistical significance [15].
In this study, we further analyze susceptibility fluctuations by examining a larger number of animals that totaled 46 with the addition of 27 newly analyzed macaques. Included in the present analysis are three uninfected macaques, and a subset with twice-weekly rather than once-weekly virus exposures and specimen collections. We now more extensively divide the median 32-day cycle of pigtail macaques into four 8-day periods, as opposed to previous analysis of only two, follicular and luteal, phases of the ovarian cycle. The 8-day periods correspond approximately to commonly described phases of the ovarian and uterine cycle in women [13], and permit analysis of periods with equal average virus exposures. The follicular phase was further divided into menstrual and proliferative phases, during which menstruation and then proliferation of the lining of the uterus occur, respectively. Within the luteal phase, we analyzed secretory and pre-menstrual phases, representing progesterone secretion by the corpus luteum and preparation for menstruation, respectively. Our results confirm the existence of periods of increased vulnerability to SHIV infection in this species, occurring during two 8-day pre-menstrual and menstrual phases.
Materials and Methods
Humane Care Guidelines, Macaques, SHIV infections, Hormone analyses
Adult female pigtail macaques were purchased and housed at CDC according to humane animal experimentation guidelines put forth by the National Research Council [8]. All procedures were approved by CDC’s Institutional Animal Care and Use Committee. Macaque infections were described [4, 9, 12, 14, 16]. Briefly, we obtained SHIVSF162P3 [3] from the NIH AIDS Research and Reference Reagent Program, and expanded the stock on pigtail peripheral blood mononuclear cells in 2002 and 2008 (“old and new stock”, respectively). Each stock was titrated in vivo; and used at 10 or 50 TCID-50, causing infection after a median of four to five vaginal challenges (data not shown). Virus exposures and blood collection were once or twice per week, as indicated in Table 1, and were given regardless of whether macaques were menstruating or not. The timing of infection was when plasma viral load first reached 50 copies/mL, determined with an in-house RT-PCR assay [9, 16]. Infection data were retrospectively compared to menstrual cycle data (Table 1 and Figure 1). Progesterone in blood plasma was analyzed at the University of Wisconsin National Primate Center as described [16], from all available blood specimens. Day one of the menstrual cycle was defined as the time point after steepest progesterone decline, as previously described [16], cycles lasted until the following progesterone drop. We previously found cycles of median 32 day length in our facility [16]. Macaque data were not used when infections occurred before we could determine day one of the cycle. For analysis purposes, we used the following phase definitions (in days): follicular: 1–16, luteal: 17–32, and for sub-phases: menstrual: 1–8, proliferative 9–16, secretory: 17–24, pre-menstrual 25 – 32.
Table 1. Macaque infections.
| Macaque # | Macaque ID | Menstrual cycle day at 1st SHIV RNA+ | Graph of menstrual cycle data, reference | RLD virus challenges/week | Progesterone data/week | RLD virus challenges to infection | Virus stock, dose |
|---|---|---|---|---|---|---|---|
| 1 | POh2 | 1 | Fig. 1 | 1 | 2 | 6 | N-50 |
| 2 | 96PO25 | 26 | 1 | 1 | 7 | N-50 | |
| 3 | A1 | 15 | 2 | 2 | 7 | N-50 | |
| 4 | A2 | 15 | 2 | 2 | 9 | N-50 | |
| 5 | A3 | 12 | 2 | 2 | 11 | N-50 | |
| 6 | A4 | 29 | 2 | 2 | 20 | N-50 | |
| 7 | PVC2 | 4 | 2 | 1 | 7 | N-50 | |
| 8 | PZA2 | NI | 2 | 1 | >14 | N-50 | |
| 9 | PSd2 | 4 | 2 | 1 | 11 | N-50 | |
| 10 | PEG2 | 4 | 2 | 1 | 10 | N-50 | |
| 11 | PUG2 | NI | 2 | 1 | >14 | N-50 | |
| 12 | PZG2 | 4 | 2 | 1 | 6 | N-50 | |
| 13 | P8P | 7 | [16] | 1 | 1 | 4 | O-10 |
| 14 | Pct-1 | 18 | 2 | 2 | 9 | O-10 | |
| 15 | 96Po17 | 1 | 2 | 2 | 1 | N-50 | |
| 16 | Pra-2 | 4 | 2 | 2 | 3 | O-10 | |
| 17 | 96Po26 | 9 | 2 | 2 | 5 | N-50 | |
| 18 | TD6 | 1 | 2 | 1 | 6 | N-10 | |
| 19 | PGB2 | 1 | 2 | 2 | 2 | O-10 | |
| 20 | Ptd2 | 15 | 1 | 1 | 4 | O-10 | |
| 21 | FH3 | 10 | 2 | 2 | 2 | N-50 | |
| 22 | 96Po78 | 4 | 2 | 2 | 6 | N-50 | |
| 23 | Pde-2 | 11 | 2 | 2 | 3 | O-10 | |
| 24 | PMc-2 | 8 | 2 | 2 | 10 | O-10 | |
| 25 | PsC-2 | 8 | 2 | 2 | 2 | O-10 | |
| 26 | Pvr-1 | 15 | 2 | 2 | 4 | O-10 | |
| 27 | P8X | 1 | 1 | 1 | 2 | O-10 | |
| 28 | Pya-2 | 4 | 2 | 2 | 2 | O-10 | |
| 29 | Pzu-1 | 8 | 2 | 2 | 5 | O-10 | |
| 30 | 303 | 1 | 1 | 1 | 14 | O-10 | |
| 31 | FF2 | 8 | 1 | 1 | 3 | O-10 | |
| 32 | PQB1 | 1 | [12] | 1 | 1 | 1 | N-50 |
| 33 | PRW | 15 | 1 | 1 | 2 | N-50 | |
| 34 | FJ24 | 15 | 1 | 1 | 5 | N-50 | |
| 35 | FJ25 | 15 | 1 | 1 | 4 | N-50 | |
| 36 | DL82 | 29 | 1 | 1 | 3 | N-50 | |
| 37 | Ppi | 8 | 1 | 1 | 10 | N-50 | |
| 38 | BB336 | NI | [14] | 1 | 1 | >14 | N-50 |
| 39 | BB401 | 8 | 1 | 1 | 1 | N-50 | |
| 40 | BB966 | 14 | 1 | 1 | 11 | N-50 | |
| 41 | BB925 | 21 | 1 | 1 | 7 | N-50 | |
| 42 | BB588 | 1 | 1 | 1 | 3 | N-50 | |
| 43 | BB432 | 1 | 1 | 1 | 4 | N-50 | |
| 44 | PRG2 | 1 | [4] | 2 | 2 | 4 | N-10 |
| 45 | CN19 | 10 | 2 | 2 | 8 | N-10 | |
| 46 | PYH2 | 8 | 2 | 2 | 9 | N-10 |
The table lists time point of first detected plasma viremia in the menstrual cycle of 46 female pigtail macaques, exposed to SHIVSF162P3 by vaginal route using the repeat-low dose (RLD) challenge approach. They received one or two challenges/week, for up to 14 or 20 challenges, using a dose of 10 – 50 TCID-50. The number of virus challenges required for infection is listed, using an eclipse phase of 7 days to determine the infecting challenge. Abbreviation and symbols used: NI=not infected, >14= maximal number of 14 challenges was administered, but the animal remained uninfected; O-10=old virus stock, given at 10 Tissue culture infectious doses (TCID-50); N=new virus stock; ID=identification; RNA+ = above 49 viral RNA copies/ml of plasma.
Figure 1. Progesterone levels and SHIV infection.
A–L: Each graph depicts plasma progesterone levels for one macaque, used to identify day one of the menstrual cycle (defined at steepest drop in progesterone). Cycles lasted until the next progesterone drop occurred. Negative days indicate that day one of the cycle could not be determined because no initial drop in progesterone was recorded. The first arrow (and accompanying asterisk at menstrual cycle day) indicates initiation of virus exposures, the second arrow (and asterisk) refers to time point of initial viremia > 49 copies/ml, except for in 1H and 1K, where it indicates the last virus challenge. Only data are shown that have not been reported elsewhere.
Statistical Analysis
A two-sided one sample test for proportion was conducted using SAS 9.2 (SAS institute, Cary, NC) to examine if initial viremia detection in the luteal phase was different from that in the follicular phase.
Results
Vaginal virus infection occurs mostly before and after menstruation, and infrequently in mid-cycle
SHIV was first detected in the follicular phase in 38 of 43 (88%) macaques, compared to five macaques (12%) in the luteal phase (Fig. 2A). The median menstrual cycle day of detection was day nine. Three additional macaques remained uninfected despite documented cycling (Table 1, Fig. 1H, 1K). The proportion of infections detected in the follicular phase was 7.6- times as great as in the luteal phase. The difference was statistically significant (P<0.0001, two-sided one sample test for proportion).
Figure 2. SHIV infection in relation to the menstrual cycle.
A. The figure shows the number of macaques with detected infections during each day of the menstrual cycle. Three uninfected animals are not shown. B. Time of infection was calculated by subtracting a seven-day viral eclipse phase from the data shown in A. C. Schematic depiction of reproductive events during the cycle, and select physiologic processes with suspected effect on HIV susceptibility.
To further define when virus infection occurred rather than when infections were detected, we subtracted an eclipse period from time point of initial viremia. Macaques 96PO17, PQB1, and BB401 had initial viremia detected seven days after their first virus exposure (Table 1, [12, 16]). We therefore subtracted an eclipse phase of seven days from the time point of virus detection, and analyzed likely virus transmissions in eight-day sub-phases (Fig. 2B). Most virus infections from vaginal exposures occurred during the pre-menstrual (n=17; 40%) and the subsequent menstrual (n=21; 49%) phases and few during the proliferative (n=2; 5%) and secretory phases (n=3; 7%) (Fig. 2B).
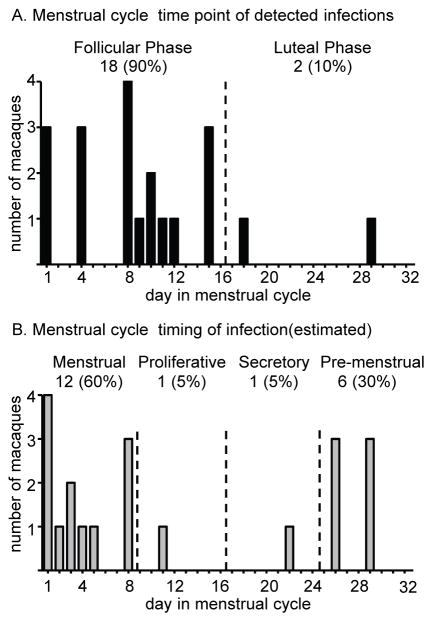
To allow for greater accuracy in assessing menstrual cycle and time point of infection, we analyzed a sub-group of 20 infected macaques with virus exposures and sample collection twice, rather than once, per week. Eighteen macaques (90%) had first viremia detection in follicular, and two in luteal phase (10%, Fig. 3A, P<0.0001, two-sided one sample test for proportion). Again, assuming an eclipse period of seven days, most infections occurred during the pre-menstrual (n=6; 30%) and the subsequent menstrual (n=12; 60%) phases and few during the proliferative (n=1; 5%) and secretory phases (n=1; 5%; Fig. 3B).
Figure 3. SHIV infection in relation to the menstrual cycle in a subset of macaques with twice weekly virus exposure and progesterone analysis.
A. The figure shows data from 20 macaques with virus exposures and sample collection performed twice per week (as opposed to once per week). The number of macaques is given with detected infections during each day of the menstrual cycle. B. Time of infection was calculated by subtracting a seven-day viral eclipse phase from the data shown in A.
Discussion
We report that susceptibility to vaginal SHIV infection is greater in the week before and after menstruation than it is in the two mid-menstrual cycle weeks in pigtail macaques. The study analyzed by the same methods a large number of cycling pigtail macaques (n=43 infected macaques). Our data confirm and expand key observations of our previous work examining 19 macaques [16]. We now more precisely define a window of increased virus transmission in the pre- and menstrual phases of the cycle. Fig. 2C schematically depicts major reproductive events associated with these phases, as well as variations in suspected HIV or SHIV susceptibility factors such as mucosal immunity and vaginal epithelial thickness.
High susceptibility to infection was not only seen during the late luteal phase, as postulated by Wira and Fahey, but also during menstruation, when progesterone levels have just declined. This may be explained by a lingering effect of preceding high progesterone, as it likely takes time to re-build the differentiated vaginal epithelial layers lost in the late luteal phase, and to restore immune factors. It is also feasible that vascular permeability and shedding of endometrial layers in the uterus during menstruation constitutes a breakdown of a barrier against virus entry, although precise anatomical location of virus acquisition is unknown in the macaque model and in women [2]. Intercourse during menses is practiced by 3–30% of sexually active women according to some studies (reviewed in [7]). Our model suggests an increased risk of HIV acquisition during that period.
Timing of infection in our model depends largely on accurately defining the eclipse period for SHIV infection. While it is easy to determine eclipse periods that follow conventional single high-dose challenges, RLD models do not readily allow such measurements because infection rarely occurs after the first challenge. However, we note that of 43 infected macaques, three had detectable viremia seven days after first virus exposure and thus had a measured seven-day eclipse period, supporting use of this window in this study. We recognize that animals may infrequently show longer eclipse phases and have indeed identified one macaque for which infection was detected 14 days after last challenge (Table 1, macaque with ID 303). Eclipse length after vaginal low-dose S(H)IV challenge is not as well studied as for intra-rectal exposures [6] and, therefore, could not be supported by literature data. Nevertheless, to better assess the impact of a potentially longer eclipse period, we reanalyzed our data of Fig. 2A based on a 10-day eclipse phase. With this window, 28% and 42% of infections had calculated transmission times during the menstrual and pre-menstrual period, respectively, and the remaining 30% in mid-cycle (not shown). Thus, the late luteal phase maintains the highest SHIV susceptibility, further supporting our conclusions. A prospective study with virus exposures only in the luteal or follicular phase could further corroborate infection risk during the menstrual cycle.
We also note that we only used progesterone measurements to define the beginning of each cycle, constituting a potential study limitation. Considering other markers of ovulation, corpus luteum formation and menstruation possibly could have allowed greater accuracy in linking SHIV infection with reproductive events. The time segments correspond roughly, but not entirely, to physiologic events. Pigtails have longer cycles than women (average 32 compared to 28 days, respectively [1, 13]), and sub-phases are not well described, complicating a more in-depth evaluation of physiologic sub-phases.
We recently found that genital co-infections can alter vaginal SHIV susceptibility patterns [4]. Subclinical infections, inflammation, or associated changes in the vaginal microbiome and pH, may have contributed to the altered infection pattern of the five macaques in this study with estimated transmission in mid cycle. Similarly, the three cycling but uninfected animals may have host resistance factors that negated hormonal susceptibility periods. For example, we have previously defined immune parameters such as systemic RANTES levels associated with resistance to infection in this model [11]; other factors may be able to override menstrual cycle-dependent susceptibility issues.
HIV infection risk during the menstrual cycle of women has not been sufficiently analyzed, and it remains unknown whether similar windows of increased HIV vulnerability exist. However, as summarized by Wira and Fahey [18], there is ample evidence of broad regulation of host defenses during the human menstrual cycle, i.e., concerning innate, cell-mediated, and humoral immunity, thickness of the vaginal epithelial layers, pH, amount and permeability of cervical mucus, and composition of vaginal microflora, among others (reviewed in [5, 18]). Research on the contribution of each of these factors and other factors not yet identified may provide new targets for intervention. Furthermore, concerns remain regarding HIV risk and hormonal contraception with progestins [10, 17] which prevent pregnancy by mimicking the luteal phase of the cycle and pregnancy. We propose that pigtail macaques are an invaluable model for sexual transmission studies, due to similarities to human FRT physiology, and due to their demonstrated differential SHIV susceptibility during the cycle.
Acknowledgments
We gratefully acknowledge progesterone measurements by Dr. Toni Ziegler and Dan Wittwer, and CDC’s animal care staff who performed the studies from which archived specimens were obtained. We thank Dr. M. Hendry for helpful discussions. Funded in part by an interagency agreement (Y1-A1-0681-02) between CDC and NIH.
Footnotes
Disclaimer: The findings and conclusions in this presentation are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. SHIVSF162P3 was obtained through the NIH AIDS Reagent Program, NIAID, NIH from Drs. Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal and the DAIDS, NIAID.
References
- 1.Blakley GB, Beamer TW, Dukelow WR. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Laboratory animals. 1981;15:351–353. doi: 10.1258/002367781780953059. [DOI] [PubMed] [Google Scholar]
- 2.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. Journal of virology. 2013;87:11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science (New York, NY. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 4.Henning T, Butler K, Hanson D, Sturdevant G, Ellis S, Sweeney EM, Mitchell J, Deyounks F, Phillips C, Farshy C, Fakile Y, Papp J, Secor WE, Caldwell H, Patton D, McNicholl J, Kersh EN. Increased Susceptibility to Vaginal SHIV Transmission in Pigtail Macaques Coinfected with Chlamydia trachomatis and Trichomonas vaginalis. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nature reviews. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. Journal of virology. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lurie S. Does intercourse during menses increase the risk for sexually transmitted disease? Arch Gynecol Obstet. 2010;282:627–630. doi: 10.1007/s00404-010-1564-4. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals. Washington, D.C: National Academies Press; 2011. [Google Scholar]
- 9.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. The Journal of infectious diseases. 2005;191:164–173. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 10.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 11.Promadej-Lanier N, Hanson DL, Srinivasan P, Luo W, Adams DR, Guenthner PC, Butera S, Otten RA, Kersh EN. Resistance to Simian HIV infection is associated with high plasma interleukin-8, RANTES and Eotaxin in a macaque model of repeated virus challenges. Journal of acquired immune deficiency syndromes (1999) 2010;53:574–581. doi: 10.1097/QAI.0b013e3181d3521f. [DOI] [PubMed] [Google Scholar]
- 12.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, Bachman S, Pau CP, Heneine W, Garcia-Lerma JG. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One. 2012;7:e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverthorn DU. Human Physiology: An Integrated Approach. Pearson Education, Inc; 2013. [Google Scholar]
- 14.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, McNicholl JM, Hendry RM, Dinh CT, Martin A, Herold BC, Kiser PF. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16145–50. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS research and human retroviruses. 1998;14 (Suppl 1):S119–123. [PubMed] [Google Scholar]
- 16.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. Journal of acquired immune deficiency syndromes (1999) 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Hormonal Contraception and HIV: Technical Statement. 2012. [PubMed] [Google Scholar]
- 18.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS (London, England) 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]