Abstract
MicroRNAs (miRNAs) constitute a relatively recently-discovered class of small non-coding RNAs (sncRNAs) that are gaining considerable attention in the molecular-genetic regulatory mechanisms that contribute to human health and disease. As highly soluble and mobile entities, emerging evidence indicates that miRNAs posess a highly selected ribonucleotide sequence structure, are part of an evolutionary ancient genetic signaling system, resemble the plant pathogens known as viroids in their structure, mode of generation and function, and are very abundant in the physiological fluids that surround cells and tissues. Persistence and altered abundance of miRNAs in the extracellular fluid (ECF) or cerebrospinal fluid (CSF) may play a role in the intercellular spreading of disease systemically, and throughout functionally-linked cellular and tissue systems such as the central nervous system (CNS). This short communication will review some of the more fascinating features of these highly structured single stranded RNAs (ssRNAs) with emphasis on their presence and function in the human CNS, with particular reference to Alzheimer's disease (AD) wherever possible.
Keywords: Alzheimer's disease, CSF, ECF, Endocrine, Evolution, MicroRNA (miRNA), Neurological disease, Paracrine, Stability, Translocation, Transmissibility, Viroids
1. Introduction
Originally described about 12 years ago across diverse organisms ranging from Caenorhabditis elegans to Mus musculus, microRNAs (miRNAs) constitute a class of ~21 to 24-ribonucleotide small non-coding RNAs (sncRNAs) involved in the post-transcriptional regulation of eukaryotic gene expression (Lagos-Quintana et al., 2001; Pasquinelli and Ruvkun, 2002, Ambros et al., 2003, 2004; Baulcombe, 2005; Neilson and Sharp, 2008). While our perceptions on the neurobiologicalmechanism and relevance of miRNA signaling continues to evolve, it is now widely accepted that the primary mode of miRNA action is to recognize and bind to specific complementary ribonucleotide sequences in the 3′ untranslated region (3′-UTR) of target messenger RNAs (mRNAs), and in doing so, down-regulate their expression (Sempere et al., 2004; Lukiw, 2007, 2008; Mehler and Mattick, 2007; Neilson and Sharp, 2008; Hébert and De Strooper, 2009; Guo et al., 2010; Witkos et al., 2011). Although miRNAs are considered to be critically important epigenetic regulators of gene expression in human development, aging and disease, it is not often appreciated that these single stranded RNAs (ssRNAs): (i) are very highly selected in their ribonucleotide sequence and their tissue abundance and specificity (as further discussed below); (ii) represent a signaling system that is evolutionarily ancient; (iii) are the smallest yet identified ribonucleic acid carriers of genetic regulatory information, possessing viroid-like properties; (iv) are the most abundant nucleic acids contained in extracellular fluid (ECF) and cerebrospinal fluid (CSF) of humans; and (v) as abundant constituents of the ECF, CSF and blood serum may spread both homeostatic and pathological signalings amongst neighboring cells, tissues and perhaps even between individual organisms or species (Arteaga-Vazquez et al., 2006; Mehler and Mattick, 2007; Madathil et al., 2011; Alexandrov et al., 2012; Sarkies and Miska, 2013). This short communication will briefly review some of the more neglected aspects on the structure, function and mechanism of these fascinating ssRNAs with emphasis on human central nervous system (CNS) disease and relevance to Alzheimer's disease (AD) wherever possible.
2. miRNA sequence selectivity, evolution, tissue specificity and stability
Firstly, rudimentary bioinformatics and ribonucleic acid sequence analysis indicates that a ‘typical’ 22 nucleotide single stranded RNA (ssRNA) composed of 4 different ribonucleotides (adenine, guanine, cytosine and uridine; A, G, C, U) could have over 1013 possible sequence combinations. There are typically only about 2 × 103 different miRNAs so far identified, and miRNAs are highly developmental stage-, tissue- and cell-specific, even in adjacent cell types. The fact that there are probably less than 102 abundant miRNAs in any single cell type suggests an extremely high evolutionary selection pressure to utilize only specific ribonucleotide sequences in miRNAs that will yield biologically useful miRNA–mRNA interactions (Ambros et al., 2003, 2004; Arteaga-Vazquez et al., 2006; Neilson and Sharp, 2008; Guo et al., 2010; Lukiw, 2012a, 2013). Further to this idea, direct RNA-sequencing-, RT-PCR-, Northern dot blot and miRNA array-based analyses suggest that abundant miRNAs in the human neocortex number probably less than 40 or 50, and only a smaller fraction of these are misregulated in Alzheimer's disease (AD) and other inflammatory neurodegenerative disorders of the human CNS (Burmistrova et al., 2007; Lukiw and Pogue, 2007; Yuva-Aydemir et al., 2011; Lukiw, 2012b; Alexandrov et al., 2012). The abundance, speciation and complexity of these highly selected miRNAs may vary amongst human populations in health and disease (Lukiw, 2013). Like messenger RNA (mRNA), miRNAs appear to follow the same stability rules involving adenine–uridine (AU) rich elements (AREs) in their linear sequence; a higher content of AREs in miRNAs is generally correlated with a shorter miRNA half-life, and absence of AU or UA dinucleotide elements may confer miRNA stability and lengthen miRNA half-life (Chen and Shyu, 1995; Cui et al., 2005; Sethi and Lukiw, 2009). Interestingly, while mammalian brain and retinal miRNAs in particular may have in general a relatively short half-life, miRNA half-lives may be considerably extended by miRNA-binding proteins, extensive miRNA secondary and tertiary structures, circularization, or by combinations of these (Krol and Krzyzosiak, 2006; Krol et al., 2010; Perkel, 2013). Within miRNA precursors, virtually all of the miRNA sequence base-pairs with complementary sequences in other parts of the same molecule forming RNA double helices and highly structured regions that are somewhat more resistant to degradation than ssRNA alone (Saetrom et al., 2006; Krol et al., 2010; Fig. 1). Interestingly, while different miRNAs seem to be resident of different cells, tissues and species, primary structures are often very highly conserved, for example as demonstrated by a significant presence or absence of certain bases at specific positions in the miRNA precursors and their immediate flanking regions (Na and Kim, 2013; Hill et al., 2014). Indeed, miRNA sequences contain fingerprints for conservation across multiple species, and these fingerprints represent some of the most highly conserved nucleic acid sequences known (Shi et al., 2012; Arteaga-Vazquez et al., 2006; Hill et al., 2014). For example, using novel genome-wide computational approaches to detect miRNAs based on both sequence and structure alignments, the miRNA-854 family has been shown to be expressed in Arabidopsis thaliana, Caenorhabditis elegans, Mus musculus, and Homo sapiens. In these diverse species, miRNA-854 commonly targets an oligouridylate binding protein 1b (UBP1b) mRNA 3′–UTR that normally encodes a member of a heterogeneous nuclear RNA binding protein (hnRNP) family. This suggests a common origin for miRNA-854 as a regulator of basal transcriptional mechanisms in both plants and animals over vast periods of time (A. thaliana–H. sapiens divergence about 1.5 billion years; Wang et al., 1999; Hedges, 2002; Arteaga-Vazquez et al., 2006; Hill et al., 2014). Moreover secondary structures also appear to be conserved between multiple miRNAs and internal stems, loops and mis-paired RNA ‘bulges’ are evident in identical positions in many pre-miRNA sequences (Fig. 1; Saetrom et al., 2006; Arteaga-Vazquez et al., 2006; Lukiw, 2012b, 2013; Hill et al., 2014).
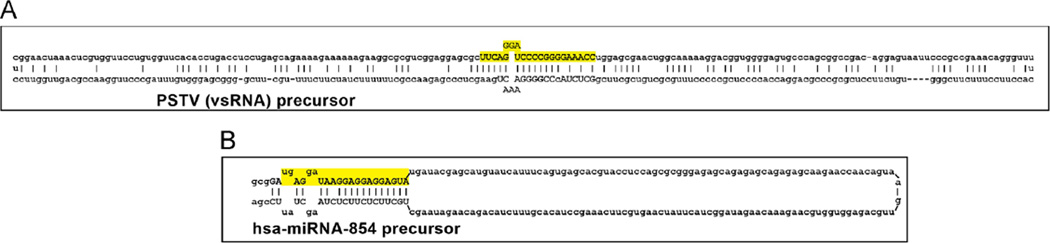
Fig. 1.
This highly schematicized figure shows the predicted structures of (A) the 359 nucleotide (nt) circular potato spindle tuber viroid (PSTV) viroid specific (vsRNA) precursor; and (B) a 214 nt hairpin-shaped precursor to the 21 nt microRNA-854 (hsa-miRNA-854; ultimately processed from the 214 nt precursor sequence and overlaid in yellow); the hsa-miRNA-854 precursor contains a large (162 nt) terminal loop structure; for both PSTV and miRNA-854 other internal free energy bonding schemes and/or secondary or tertiary structures may exist; hsa-miRNA-854 is amongst one of the most evolutionary ancient miRNAs (see text; Arteaga-Vazquez et al., 2006; Krol and Krzyzosiak, 2006; Ritchie et al., 2007; Ding, 2009; Triboulet and Gregory, 2010; unpublished). In both cases these ssRNA precursors are further processed by an RNase III of the family of Dicer-like proteins to generate smaller ssRNA species (mature vsRNA or miRNA sequences highlighted in yellow); nucleotides thought to be critical in the mature PSTV or miRNA-854 ~21–22 nt sequences are in upper case; mature vsRNAs or miRNAs have potential to alter the normal gene expression patterns in the host plant by viroids, or of mRNAs via miRNA–mRNA interactions in many plant and animal species including humans (Krol and Krzyzosiak, 2006; Arteaga-Vazquez et al., 2006; Ritchie et al., 2007; Ding, 2009; Navarro et al., 2012; Hammann and Steger, 2012). While naked ssRNAs such as these depicted have relatively short half-lives in vitro, and retinal and human brain neuronal miRNAs appear to have very limited stabilities (Sethi and Lukiw, 2009; Krol et al., 2010), viroid and miRNA half-lives may be greatly extended by single- or double-stranded RNA-binding proteins, by complex secondary and tertiary structures, by RNA circularization, by storage in vesicles, or by combinations of these and other factors (Chen and Shyu, 1995; Cui et al., 2005; Sethi and Lukiw, 2009; Krol et al., 2010; see text). Interestingly, PSTV is the smallest known self-replicating pathogen of all species; it is noteworthy that both the plant and animal kingdoms have adopted similar ‘minimalistic’ ssRNA strategies to convey highly specific- and selective-genetic regulatory information in the propagation of both homeostatic and potentially pathogenic RNA signals. Other secondary and tertiary structures are possible; PSTV and miRNA-854 ssRNA sequences and/or precursor structures were derived from GenBank accession M36163.1; GI:333356 (http://www.ncbi.nlm.nih.gov/nuccore/M36163.1) or miRBase Accession MI0005412 (http://www.mirbase.org/cgi-bin/mirnaentry.pl?acc=MI0005412); secondary structures were predicted using several freely available online web servers (such as http://mfold.rna.albany.edu/?q=mfold).
3. miRNAs have extensive similarity to viroids
miRNAs are the smallest yet identified carriers of highly selective genetic regulatory information. Their ribonucleotide sequences, some of which are conserved across both plant and animal species, further define and regulate the expression of a relatively discrete subset of cellular mRNAs with which they may interact, thus defining a highly associative gene regulation network (Ambros, 2004; Taganov et al., 2006; Taft et al., 2010; Alexandrov et al., 2012; Na and Kim, 2013). ssRNAs such as miRNA presumably carry genetic signals encoded within the 5′-3′ sequence of their ribonucleotides and these may be transmitted via unique 3-dimensional molecular shape, surface topology and RNA charge density across the entire miRNA sequence, often in cooperation with other miRNAs or miRNA binding proteins to form complex association networks (Na and Kim, 2013; Hill et al., 2014). Interestingly, miRNAs are typically less than about one one-thousandth of the size of a ‘typical virus’; currently, seven orders, 96 families, 22 subfamilies, 420 genera, and 2618 species of viruses have been recently classified by the International Committee on the Taxonomy of Viruses (International Committee on the Taxonomy of Viruses; http://www.ictvonline.org/; Adams and Carstens., 2012), including 5 orders and 47 families of RNA viruses, both double and single stranded (Hammann and Steger, 2012; Adams and Carstens, 2012). Interestingly, there is approximately the same number of species of viruses as there are all currently known mammalian miRNAs (about 2000). The smallest ssRNA viruses in terms of genome size are retroviruses such as Rous Sarcoma virus with a 3.5 × 104 nucleotide genome (Adams and Carstens, 2012; http://www.ictvonline.org/). Smal ler than any known ssRNA viruses are viroids, a small family of about 30 plant pathogens consisting of circular ssRNAs ranging in size from 246 to 401 nucleotides, and of evolu tionary, virological and biological interest since they probably represent living fossils of pre-cellular evolution in a hypothe tical RNA world (Diener, 1991, 2003; Ding, 2009). The first discovered, and one of the smallest viroids is the potato spindle tuber viroid (PSTV), a circular ssRNA which causes infectious disease in potato plants (chiefly Solanum tubero sum), and remains as an important agricultural and economic concern throughout the world (Fig. 1; Ding, 2009; Navarro et al., 2012). Viroids are truly minimalist plant pathogens; it remains unclear whether they are a biological oddity or an evolutionary fossil (Diener, 1991, 2003); they are transcribed by a unidirectional nucleic acid rolling-circle mechanism in the plant host's nuclei (for the genus Pospiviroidae) or chlor oplast (for the genus Avsunviroidae). Recent findings indicate that viroid infection is associated with the appearance of viroid-specific small RNA (vsRNA), ~20 to 24 nucleotides in size, processed by an RNase III of the family of Dicer-like proteins from a pre-viroid precursor (Hammann and Steger, 2012; Hill et al., 2014). These vsRNAs have sizes similar to naturally occurring endogenous small interfering RNAs and miRNAs, and are known to permanently alter the normal cultivar- and viroid-dependent gene expression patterns in the host plant (Diener, 1991, 2003; Ding, 2009; Navarro et al., 2012; Hammann and Steger, 2012). Very much like miRNAs, viroid ssRNAs encode no proteins, have no naturally protec tive protein capsid or coat, do not reverse-transcribe into DNA when they replicate, and are significantly inducible by external stress and environmental factors, including environ mentally abundant neurotoxic metal sulfates (Diener, 1991, 2003; Lukiw and Pogue, 2007; Hammann and Steger, 2012). As naked, infectious ssRNA molecules viroid replication requires endogenous polymerase, initially generated as a highly complementary double-stranded precursor RNA (pre-viroid) struc ture from which a mature ssRNA is excised by RNAse III-type Dicer enzymes (see Fig. 1). As far as is known, miRNAs do not replicate in vivo; in fact they are probably physically too small to do so, and in vitro usually require a linker DNA or RNA to effectively copy them using externally supplied polymerase systems and cofactors. Interestingly, circularization of both pre-viroids, pri-miRNAs or miRNAs and the formation of complex higher order secondary structures may stabilize them from the rapid RNA degradation of these normally labile, single stranded RNA entities (Rocheleau and Pelchat, 2006; Ritchie et al., 2007; Sethi and Lukiw, 2009; Perkel, 2013).
4. miRNAs are abundant in ECF and CSF
Recent studies on human brain extracellular fluid (ECF, a cell-free preparation of the extracellular fluid that surrounds and bathes human brain cells) and cerebrospinal fluid (CSF) indicate that miRNAs are the most abundant nucleic acids contained within the fluids which circulate within the human brain and CNS (Alexandrov et al., 2012). In these studies increases in specific miRNAs were confirmed independently using both miRNA arrays (LC Sciences, Houston, TX) and reconfirmed using a highly sensitive LED-Northern dot-blot assay (Alexandrov et al., 2012). Several of the NF-κB-sensitive miRNAs detected were formerly identified to be up-regulated in brain anatomical regions targeted by AD, and strongly associate with the progressive spreading of inflammatory neurodegeneration and neuroimmune signaling (Li et al., 2011a, 2011b; Cui et al., 2010). The ECF- and CSF-enriched miRNA species included, prominently, miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, miRNA-155 and others; their selective enrichment in circulating CSF and ECF in AD suggests that they may be involved in the modulation or proliferation of miRNA-triggered pathogenic signaling throughout the brain and CNS (Alexandrov et al., 2012; Rao et al., 2013). If abundance is any indication of importance, then these miRNAs may be performing significant pathogenic signaling and disease-transmission functions in these CNS fluids. Recent in vivo studies further indicate the importance of these and other miRNAs in the regulation of human brain endothelial and epithelial cell-barrier functions and their impact on neuroinflammatory and neuroimmune diseases such as multiple sclerosis (Zhou et al., 2009; Reijerkerk et al., 2013). A corollary to this is that over-expressed miRNAs may be involved in AD spreading as they pass so easily out of the cell, into adjacent cells, and throughout biological fluids (Alexandrov et al., 2012; Gallego et al., 2012; Zhou et al., 2009; Machida et al., 2013; Müller et al., 2014; Rao et al., 2013; Reijerkerk et al., 2013). Indeed, many independent research groups have analyzed, detected and characterized highly selected subfamilies of potentially pathogenic miRNAs in human CSF (Cogswell et al., 2008; Alexandrov et al., 2011; Li et al., 2011a, 2011b; Gallego et al., 2012; Machida et al., 2013; Müller et al., 2014; Rao et al., 2013). Interestingly, despite the analysis of AD CSF from many diverse human population samples, increases in the pro-inflammatory miRNA-146a have been reported by multiple independent AD miRNA researchers; miRNA-146a has also been detected to be significantly up-regulated in transgenic AD murine models, in rare human and rodent prion diseases and in other neurological disorders associated with progressive inflammatory neurodegeneration (Taganov et al., 2006; Lukiw et al., 2008, 2011; Li et al., 2011a, 2011b; Aronica et al., 2010; Alexandrov et al., 2011; Saba et al., 2012; Machida et al., 2013; Müller et al., 2014). Cumulatively these data support the hypothesis that paracrine or endocrine effects of miRNAs originating in stressed constituent cells of the human neurovascular unit may contribute to “spreading events” of AD pathology, and these appear to be a unifying characteristic of progressive neurodegenerative disorders (Erickson and Banks, 2013; Sagare et al., 2013). As further discussed below; these data further suggest that (1) circulating miRNAs may have diagnostic value and (2) that peripheral anti-miRNA strategies may be therapeutically useful in containing the spread of neuropathology not only in AD but in other progressive inflammatory and neuroimmune degenerative diseases (Alexandrov et al., 2011; Rao et al., 2013; Reijerkerk et al., 2013).
5. miRNA and the spreading of CNS disease
One of the classical neuropathological features of AD is the progressive and propagating nature of AD inflammatory neuropathology, and the age-related deposition of Aβ42 peptides as insoluble senile plaque deposits. AD appears to originate in the lateral entorhinal cortex of the human brain and subsequently spreads radially from the hippocampal CA1 and superior temporal lobe regions to more distal lobes of the brain, including, eventually, the frontal and parietal poles and primary visual cortex (Cui et al., 2007; Lee et al., 2011; Alexandrov et al., 2011; Jucker and Walker, 2011). Interestingly, amyloid pathology, including progressive Aβ42 peptide deposition, pro-inflammatory signaling and AD-relevant secretase activities may ultimately extend into more distant anatomical regions such as retinal neurons in late-stage AD (Lukiw et al., 2001; Cui et al., 2007; Alexandrov et al., 2011; Busch et al., 2012; Sivak, 2013). Accompanying this spreading mechanism is an up-regulation in AD-specific neuropathology including increases in Aβ42 peptide deposition and senile plaque formation, neurofibrillary tangling and a progressive inflammatory neurodegeneration (Lukiw, 2007; Cui et al., 2007; Lee et al., 2011; Alexandrov et al., 2011; Jucker and Walker, 2011). The spreading mechanism for AD inflammatory signaling throughout these anatomical regions of the human brain involved in cognition and memory is not well understood. The small size of miRNAs, recent identification of miRNA protective proteins, circularization of miRNAs into complex tertiary structures, and miRNA-containing vesicles suggest that miRNAs may be a novel vector for paracrine, endocrine and related forms of intercellular and inter-tissue communication and potential disease spreading amongst the neurons, astroglia and endothelial cells of the neurovascular unit (Lukiw et al., 1998; Arroyo et al., 2011; Wang et al., 2010). Understanding the mechanism for the pathological proliferation of AD may of course expose unique opportunities for the development of novel diagnostic techniques and therapeutic strategies for AD and related neurodegenerative disorders such as Parkinsons' disease, Down's syndrome (trisomy 21), fronto-temporal dementia, multiple sclerosis, prion disease, and other progressive neurological diseases with an inflammatory component.
Six independent lines of evidence currently support the contention that miRNAs may be involved in the pathological spreading of AD including the observations: (i) of their similarity in structure, function and pathological mechanism to viroids, the smallest infectious agent known, which spread degenerative disease in plants (Diener, 2003; Lukiw, 2007; Ding, 2009, Jucker and Walker, 2011; Sarkies and Miska, 2013); (ii) that the growth medium of primary human brain cells stressed with AD-relevant stressors (such as TNFα and Aβ peptides) contains elevated pro-inflammatory miRNA levels (Arroyo et al., 2011; Alexandrov et al., 2012); (iii) that these similar potentially pathogenic miRNAs are abundant in AD ECF and CSF in a complex mixture with other pathogenic molecules (Zhao et al., 2006; Cogswell et al., 2008; Wang et al., 2010; Lee et al., 2011; Alexandrov et al., 2012; Rao et al., 2013); (iv) that the pathogenic miRNAs found to be elevated in AD brain tissues are also abundant in AD ECF and CSF (Alexandrov et al., 2012; Rao et al., 2013); (v) that miRNA-containing conditioned medium (CM) from stressed primary human neuronal-glial cells in co-culture and microglial cells can in vitro down-regulate proinflammatory regulatory glycoproteins and receptors such as complement factor H (CFH) and the triggering receptor in myeloid cells (TREM2; CFH and TREM2 are known to be down-regulated in AD; Cui et al., 2010; Alexandrov et al., 2012; Zhao et al., 2013); and (vi) that anti-miRNA (AM) strategies can quench the phenomenon of miRNA-mediated inflammatory spreading in stressed primary human brain cells (Lukiw et al., 2008; Zhang et al., 2012; Reijerkerk et al., 2013; Sarkies and Miska, 2013). What is even more remarkable is that miRNAs appear to be highly mobile not only between cells and tissues but between species, carrying genetic regulatory information outside of the cell(s) in which they were initially generated (Alexandrov et al., 2012; Rao et al., 2013; Zhang et al., 2012; Sarkies and Miska, 2013). While the results need to be independently replicated, the very recent observation of human microbiome-derived sncRNA and miRNA and plant miRNA translocation across endothelial barriers, between cells and tissues, and even between individual species indicates that human neurobiology may be significantly impacted by the actions of human micobiome-mediated or externally-derived sncRNA or miRNA trafficking, and the integration of a cell, tissue or an entire organism into its local environment (Zhao et al., 2006; Alexandrov et al., 2012;; Zhang et al., 2012; Bhattacharjee and Lukiw, 2013; Sarkies and Miska, 2013; Reijerkerk et al., 2013).
6. Summary
Our ideas on the structure and function miRNA and sncRNA, their regulatory mechanisms in development and aging, and how they fit into the fascinating realm of RNA metabolism in progressive age-related neurological disease continues to evolve. Pathogenically up-regulated miRNAs and sncRNAs can be considered as an ancient, epigenetic signaling system used to down-regulate specific mRNAs and their expression to ultimately shape the transcriptome of cells, and this elaborate regulatory scheme has been significantly conserved across multiple plant and animal species for evolutionary periods of hundreds of millions of years (Wang et al., 1999; Hedges, 2002; Arteaga-Vazquez et al., 2006). The observed upregulation of miRNAs in neurodegenerative disorders such as AD may in part explain the large number of brain gene mRNAs that are found to be consistently down-regulated in anatomical regions of the human brain targeted by the AD process (Lukiw et al., 1992; Loring et al., 2001; Colangelo et al., 2002; Lukiw, 2004; Ginsberg et al., 2012). The use of anti-NF-kB compounds against NF-kB-sensitive miRNAs, and anti-miRNA (AM, antagomir) directed strategies represent an obvious therapeutic choice in the future clinical management of AD and related neurological disorders for which miRNAs are now known to play critical pathological roles.
7. Experimental procedures
Peer-reviewed publications involving recent research into miRNA, AD, viroids, the innate immune response and inflammatory neurodegeneration were searched and acquired using Medline (National Center for Biological Information, National Library of Medicine, National Institutes of Health; http://www.ncbi.nlm.nih.gov). Free energy minimized secondary and tertiary structures involving PSTV and miRNA-854 ssRNA sequences and/or precursor structures were derived from GenBank accession M36163.1; GI:333356 (http://www.ncbi.nlm.nih.gov/nuccore/M36163.1) or miRBase Accession MI0005412 (http://www.mirbase.org/cgi-bin/mirnaentry.pl?acc=MI0005412); secondary structures such as those depicted in Fig. 1 were predicted using several freely available online web algorithms such as http://mfold.rna.albany.edu/?q=mfold.
Acknowledgments
The work in this report was presented in part at the Alzheimer Association International Conference 2013 (AAIC 2013) held in Boston, MA, July 13–18, 2013. Sincere thanks are extended to Drs. L. Carver, E. Head, W. Poon, H. LeBlanc, F. Culicchia, C. Eicken, S. Bhattacharjee and C. Hebel for short post-mortem interval (PMI) human brain tissues or extracts, miRNA array work and initial data interpretation, and to D. Guillot and A.I. Pogue for expert technical assistance. Thanks are also extended to the many physicians and neuropathologists who have provided high quality, short post-mortem interval (PMI) human brain tissues for study; additional human temporal lobe and other control and AD brain tissues were provided by the Memory Impairments and Neurological Disorders (MIND) Institute and the University of California, Irvine Alzheimer's Disease Research Center (UCI-ADRC; NIA P50 AG16573). Research on miRNA in the Lukiw laboratory involving the innate-immune response in AD and in retinal disease, amyloidogenesis and neuroinflamamtion was supported through a COBRE III Pilot Project, Research to Prevent Blindness (RPB); a Translational Research Initiative Grant from LSUHSC, the Louisiana Biotechnology Research Network (LBRN), Alzheimer Association Investigator-Initiated Research Grants IIRG-09-131729, NEI EY006311 and NIA Grants AG18031 and AG038834.
REFERENCES
- Adams MJ, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch. Virol. 2012;157:1411–1422. doi: 10.1007/s00705-012-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov PN, Pogue A, Bhattacharjee S, et al. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer’s disease. NeuroReport. 2011;22:623–627. doi: 10.1097/WNR.0b013e3283497334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov PN, Dua P, Hill JM, et al. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF) Int. J. Biochem. Mol. Biol. 2012;3:365–373. [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Ambros V, Bartel B, Bartel DP, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Fluiter K, Iyer A, et al. Expression pattern of miRNA-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 2010;31:1100–1107. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Vazquez M, Caballero-Pérez J, Vielle-Calzada JP. A family of miRNAs present in plants and animals. Plant Cell. 2006;18:3355–3369. doi: 10.1105/tpc.106.044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. RNA silencing. Trends Biochem. Sci. 2005;30:290–293. doi: 10.1016/j.tibs.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Front Cell Neurosci. 2013;7:153. doi: 10.3389/fncel.2013.00153. http://dx.doi.org/10.3389/fncel.2013.00153 (eCollection 2013. PubMed PMID: 24062644; PubMed Central PMCID: PMC3775450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmistrova OA, Goltsov AY, Abramova, et al. MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11) Biochemistry (Mosc.) 2007;72:578–582. doi: 10.1134/s0006297907050161. [DOI] [PubMed] [Google Scholar]
- Busch S, Wu L, Feng Y, et al. Alzheimer’s disease and retinal neurodegeneration share a consistent stress response of the neurovascular unit. Cell Physiol. Biochem. 2012;30:1436–1443. doi: 10.1159/000343331. [DOI] [PubMed] [Google Scholar]
- Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Cogswell JP, Ward J, Taylor IA, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimer’s Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- Colangelo V, Schurr J, Ball MJ, et al. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1JNeurosci. Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- Cui JG, Zhao Y, Lukiw WJ. Isolation of high spectral quality RNA using run-on gene transcription; application to gene expression profiling of human brain. Cell Mol. Neurobiol. 2005;25:789–794. doi: 10.1007/s10571-005-4035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JG, Hill JM, Zhao Y, et al. Expression of inflammatory genes in the primary visual cortex of late-stage Alzheimer’s disease. NeuroReport. 2007;18:115–119. doi: 10.1097/WNR.0b013e32801198bc. [DOI] [PubMed] [Google Scholar]
- Cui JG, Li YY, Zhao Y, et al. Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK- 2 by miRNA-146a and NF-kB in stressed human astroglial cells and in Alzheimer’s disease. J. Biol. Chem. 2010;285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B. The biology of viroid-host interactions. Ann. Rev. Phytopathol. 2009;47:105–131. doi: 10.1146/annurev-phyto-080508-081927. [DOI] [PubMed] [Google Scholar]
- Diener TO. Subviral pathogens of plants: viroids and viroidlike satellite RNAs. FASEB J. 1991;5:2808–2813. doi: 10.1096/fasebj.5.13.1717335. [DOI] [PubMed] [Google Scholar]
- Diener TO. Discovering viroids – a personal perspective. Nat. Rev. Microbiol. 2003;1:75–80. doi: 10.1038/nrmicro736. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Banks WA. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013;33(10):1500–1513. doi: 10.1038/jcbfm.2013.135. http://dx.doi.org/10.1038/jcbfm.2013.135 Epub 2013 Aug 7. PubMed PMID: 23921899; PubMed Central PMCID: PMC3790938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego JA, Gordon ML, Claycomb K, et al. In vivo microRNA detection and quantitation in cerebrospinal fluid. J. Mol. Neurosci. 2012;47:243–248. doi: 10.1007/s12031-012-9731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Che S. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol. Dis. 2012;45:99–107. doi: 10.1016/j.nbd.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammann C, Steger G. Viroid-specific small RNA in plant disease. RNA Biol. 2012;9:809–819. doi: 10.4161/rna.19810. [DOI] [PubMed] [Google Scholar]
- Hébert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32:199–206. doi: 10.1016/j.tins.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Hedges SB. The origin and evolution of model organisms. Nat. Rev. Genet. 2002;3:838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zhao Y, Bhattacharjee S, et al. miRNAs and viroids utilize common strategies in genetic signal transfer. Front J. Mol. Neurosci. 2014;7:10. doi: 10.3389/fnmol.2014.00010. http://dx.doi.org/10.3389/fnmol.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee on the Taxonomy of Viruses. 〈 http://www.ictvonline.org/〉), [Google Scholar]
- Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Krzyzosiak WJ. Structure analysis of microRNA precursors. Methods Mol. Biol. 2006;342:19–32. doi: 10.1385/1-59745-123-1:19. [DOI] [PubMed] [Google Scholar]
- Krol J, Busskamp V, Markiewicz I, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lim HS, Masliah E, et al. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci. Res. 2011;70:339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Cui JG, Dua P, et al. Differential expression of miRNA-146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neurosci. Lett. 2011a;499:109–113. doi: 10.1016/j.neulet.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Cui JG, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. Increased expression of miRNA-146a in Alzheimer’s disease transgenic mouse models. Neurosci Lett. 2011b;487(1):94–98. doi: 10.1016/j.neulet.2010.09.079. http://dx.doi.org/10.1016/j.neulet.2010.09.079 (Epub 2010 Oct 8. PubMed PMID: 20934487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring JF, Wen X, Lee JM, et al. A gene expression profile of Alzheimer’s disease. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Handley P, Wong L, et al. BC200 and other small RNAs RNA in normal human neocortex, non-Alzheimer dementia (NAD), and senile dementia of the Alzheimer type (AD) Neurochem. Res. 1992;17:591–597. doi: 10.1007/BF00968788. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Gordon WC, Rogaev EI, Thompson H, Bazan NG. Presenilin-2 (PS2) expression up-regulation in a model of retinopathy of prematurity and pathoangiogenesis. Neuroreport. 2001;12(1):53–57. doi: 10.1097/00001756-200101220-00019. (PubMed PMID: 11201091) [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, LeBlanc HJ, Carver LA, McLachlan DR, Bazan NG. Run-on gene transcription in human neocortical nuclei. Inhibition by nanomolar aluminum and implications for neurodegenerative disease. J Mol Neurosci. 1998;11(1):67–78. doi: 10.1385/JMN:11:1:67. (PubMed PMID: 9826787) [DOI] [PubMed] [Google Scholar]
- Lukiw WJ. Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem. Res. 2004;29:1287–1297. doi: 10.1023/b:nere.0000023615.89699.63. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Pogue AI. Induction of specific microRNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J. Inorg. Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. NeuroReport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Zhao Y, Cui JG. An NF-κB-sensitive microRNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J. Biol. Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Dua P, Pogue AI, et al. Upregulation of microRNA-146a (miRNA-146a), a marker for inflammatory neurodegeneration, in sporadic Creutzfeldt–Jakob disease (sCJD) and Gerstmann–Straussler–Scheinker (GSS) syndrome. J. Toxicol. Environ. Health A. 2011;74:1460–1468. doi: 10.1080/15287394.2011.618973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ. NF-κB-regulated microRNAs (miRNAs) in primary human brain cells. Exp. Neurol. 2012a;235:484–490. doi: 10.1016/j.expneurol.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ. Evolution and complexity of microRNA in the human brain. Front. Genet. 2012b;3:166. doi: 10.3389/fgene.2012.00166. http://dx.doi.org/10.3389/fgene.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ. Variability in micro RNA (miRNA) abundance, speciation and complexity amongst different human populations and potential relevance to Alzheimer’s disease (AD) Front. Cell Neurosci. 2013 Aug 27;7:133. doi: 10.3389/fncel.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida A, Ohkubo T, Yokota T. Circulating microRNAs in the cerebrospinal fluid of patients with brain diseases. Methods Mol. Biol. 2013;1024:203–209. doi: 10.1007/978-1-62703-453-1_16. [DOI] [PubMed] [Google Scholar]
- Madathil SK, Nelson PT, Saatman KE, Wilfred BR, et al. MicroRNAs in CNS injury: potential roles and therapeutic implications. Bioessays. 2011;33:21–26. doi: 10.1002/bies.201000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification and neurological disease. Physiol. Rev. 2007;87:799–823. doi: 10.1152/physrev.00036.2006. [DOI] [PubMed] [Google Scholar]
- Müller M, Kuiperij HB, Claassen JA, Küsters B, Verbeek MM. MicroRNAs in Alzheimer’s disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol Aging. 2014;35(1):152–158. doi: 10.1016/j.neurobiolaging.2013.07.005. http://dx.doi.org/10.1016/j.neurobiolaging.2013.07.005 (Epub 2013 Aug 17. PubMed PMID: 23962497) [DOI] [PubMed] [Google Scholar]
- Na YJ, Kim JH. Understanding cooperativity of microRNAs via microRNA association networks. BMC Genomics. 2013;14(Suppl 5):S17. doi: 10.1186/1471-2164-14-S5-S17. http://dx.doi.org/10.1186/1471-2164-14-S5-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Gisel A, Rodio ME, et al. Viroids: how to infect a host and cause disease without encoding proteins. Biochimie. 2012;94:1474–1480. doi: 10.1016/j.biochi.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Neilson JR, Sharp PA. Small RNA regulators of gene expression. Cell. 2008;134:899–902. doi: 10.1016/j.cell.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE, Ruvkun G. Control of developmental timing by microRNAs and their targets. Annu. Rev. Cell. Dev. Biol. 2002;18:495–513. doi: 10.1146/annurev.cellbio.18.012502.105832. [DOI] [PubMed] [Google Scholar]
- Perkel JM. Assume nothing: the tale of circular RNA. Biotechniques. 2013;55:55–57. doi: 10.2144/000114061. [DOI] [PubMed] [Google Scholar]
- Rao P, Benito E, Fischer A. MicroRNAs as biomarkers for CNS disease. Front Mol Neurosci. 2013;6(39) doi: 10.3389/fnmol.2013.00039. (eCollection 2013. PubMed PMID: 24324397; PubMed Central PMCID: PMC3840814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, et al. miRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J. Neurosci. 2013;17:6857–6863. doi: 10.1523/JNEUROSCI.3965-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie W, Legendre M, Gautheret D. RNA stem-loops: to be or not be cleaved by RNAse III. RNA. 2007;13:457–462. doi: 10.1261/rna.366507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau L, Pelchat M. The subviral RNA database: a toolbox for viroids, the hepatitis delta virus and satellite RNAs research. BMC Microbiol. 2006;6:24. doi: 10.1186/1471-2180-6-24. http://dx.doi.org/10.1186/1471-2180-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Gushue S, Huzarewich RL, Manguiat K, Medina S, Robertson C, et al. MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS One. 2012;7:e30832. doi: 10.1371/journal.pone.0030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetrom P, Snøve O, Nedland M, et al. Conserved microRNA characteristics in mammals. Oligonucleotides. 2006;16:115–144. doi: 10.1089/oli.2006.16.115. [DOI] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer’s disease. J. Alzheimer’s Dis. 2013;33:S87–S100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkies P, Miska EA. Molecular biology. Is there social RNA? Science. 2013;341:467–468. doi: 10.1126/science.1243175. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, et al. Expression profiling of mammalian miRNAs uncovers a subset of brain-expressed miRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci. Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- Shi B, Gao W, Wang J. Sequence fingerprints of microRNA conservation. PLoS One. 2012;7(10):e48256. doi: 10.1371/journal.pone.0048256. http://dx.doi.org/10.1371/journal.pone.0048256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak JM. The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest Ophthalmol Vis Sci. 2013;54(1):871–880. doi: 10.1167/iovs.12-10827. http://dx.doi.org/10.1167/iovs.12-10827 (Review. PubMed PMID: 23364356) [DOI] [PubMed] [Google Scholar]
- Taft RJ, Pang KC, Mercer TR, et al. Non-coding RNAs: regulators of disease. J. Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, et al. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triboulet R, Gregory RI. Autoregulatory mechanisms controlling the Microprocessor. Adv Exp Med Biol. 2010;700:56–66. (Review. PubMed PMID: 21627030) [PubMed] [Google Scholar]
- Wang DY, Kumar S, Hedges SB. Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc. Biol. Sci. 1999;266:163–171. doi: 10.1098/rspb.1999.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang S, Weber J, et al. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkos TM, Koscianska E, Krzyzosiak WJ. Practical aspects of miRNA target prediction. Curr. Mol. Med. 2011;11:93–109. doi: 10.2174/156652411794859250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuva-Aydemir Y, Simkin A, Gascon E, et al. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8:557–564. doi: 10.4161/rna.8.4.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hou D, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of crosskingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cui JG, Lukiw WJ. Natural secretory products of human neural and microvessel endothelial cells: implications in pathogenic “spreading” and Alzheimer’s disease. Mol. Neurobiol. 2006;34:181–192. doi: 10.1385/MN:34:3:181. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Bhattacharjee S, Jones BM, et al. Regulation of TREM2 expression by an NF-κB-sensitive miRNA-34a. NeuroReport. 2013;24:318–323. doi: 10.1097/WNR.0b013e32835fb6b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Hu G, Liu J, et al. NF-kB p65-dependent transactivation of miRNA genes following cryptosporidium parvum infection stimulates epithelial cell immune responses. PLoS Pathol. 2009;5:e1000681. doi: 10.1371/journal.ppat.1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]