Abstract
The hindgut, as a part of the tick excretory system, plays an important physiological role in maintaining homeostasis and waste elimination. Immunoreactive projections from the synganglion to the hindgut were found using antibodies against four different neuropeptides: FGLamide related allatostatin (FGLa/AST), myoinhibitory peptide (MIP), SIFamide, and orcokinin. The presence of FGLa/AST, MIP and SIFamide in both synganglia (source) and hindgut (target organ) extracts was confirmed by MALDI-TOF. Tissue-specific PCR revealed the expression of four putative FGLa/AST receptors and an SIFamide receptor (SIFa-R) in the hindgut. An antibody against Ixodes scapularis SIFa-R detected immunoreactive spots in epithelial cells as well as the visceral muscles surrounding the rectal sac, while staining with the antibody against MIP receptor 1 (MIP-R1) revealed that the immunoreactivity was only associated with the visceral muscles. In hindgut motility assays, SIFamide activated hindgut motility in a dose-dependent manner. None of other three neuropeptides (FGLa/AST, MIP and orcokinin) activated hindgut motility when tested alone. MIP antagonized the SIFamide-stimulated hindgut mobility when it was tested in combination with SIFamide.
Keywords: Neuropeptide, G protein-coupled receptor, GPCR, Hindgut, Excretion
1. Introduction
Ticks are obligatory ectoparasites that take up large amounts of blood over several days during their attachment to the skin of a vertebrate host. Homeostasis during feeding requires excretion of excessive ions, water and metabolic waste. A study in an ixodid tick, Demacentor andersoni, found that the majority of potassium ion and nitrogeous waste is removed through the anus, while excessive water and sodium ions are excreted back to the host through salivary secretion (Kaufman and Phillips, 1973). Although very little is known about the mechanisms involved in defecation in ticks, the removal of hematin and undigested hemoglobin during feeding and of nirtrogenous wastes concentrated in guanine crystals after eclosion are likely important biological processes involving neural and hormonal controls.
The excretory organs in ticks are comprised of Malpighian tubules and a rectal sac (RS) connected to a short anal canal (AC, often called the “rectum” in ticks) and the anus. These tick organs are likely structural and functional homologs of the insect Malpighian tubules and hindgut, although it is unknown whether the homologous organs have the same evolutionary origin. Mechanisms controlling diuresis in insects, including mosquitoes, have been extensively studied, and a large number of hormonal controllers of the Malpigian tubule and the hindgut have been described (reviewed in Dow and Davies, 2006; Coast, 2009; Beyenbach et al., 2010; Park, 2012). However, there has been no study on the control of excretion in ticks and other arachnids.
In ticks, guanine-rich excreta deposited from the paired Malphigian tubules and undigested waste from the midgut are further concentrated in the RS and delivered to the anus via a short ectodermal AC (Raikhel, 1983; Sonenshine, 2014). Earlier ultra-structural studies (Raikhel, 1983) and recent descriptions of the presence of a water channel aquaporin in the RS (Campbell et al., 2010) suggest its role in water reabsorption. During tick feeding, the periodic filling of the RS is followed by the opening of the anus by the flap muscles, leading to defecation (Sonenshine, 1991). An ultrastructural study described axon terminals in between the muscle sheaths of the RS (Raikhel, 1983), suggesting neuronal control of hindgut activities. The complex neuropeptidergic innervations of the tick hindgut in Ixodes scapularis were described. We identified the neuropeptides and investigated the activities underlying hindgut motility.
2. Materials and methods
2.1. Tick samples and chemicals
Unfed adult I. scapularis ticks were obtained from a tick-rearing facility at Oklahoma State University, USA. Approximately 30 females were kept in a polypropylene tube (9 × 2.5 cm) with a small piece of filter paper (4 × 1 cm). The tubes were kept in a dark, humid chamber at 4°C. Two to 3 weeks before the physiological experiments, ticks were placed in an incubator at 21°C with a 12 h light/dark cycle. For all experiments, we used approximately 3 - 6 month old unfed I. scapularis females.
Ixodes scapularis neuropeptides SIFamide (AYRKPPFNGSIFamide), myoinhibitory peptide 2 (MIP2, ASNWDRLSGMWamide), FGLamide related allatostatin 1 (FGLa/AST1, RPPAAMYGFGLamide) and orcokinin (NFDEIDRTGFEGFY) were synthetized by Bio Basic Inc. (Canada) at greater than 80% purity. Peptides were dissolved in deionized distilled water (SIFamide, FGLa/AST and orcokinin) or 50% DMSO (for MIP2) to a final concentration of 1 mM and stored at −20°C. Working solut ions were made in a saline solution consisting of 140 mM NaCl, KCl 5 mM, MgCl2 1 mM, CaCl2 5 mM, NaHCO3 4 mM, and 5 mM HEPES (Sigma, USA), pH 7.2.
2.2. Antibodies and immunohistochemistry
Antibodies used in this study are listed in Table 1. All monoclonal antibodies or polyclonal antisera originally raised against insect neuropeptides were previously described in tick or insect immunohistochemistry (Šimo et al., 2009a, b; Yamanaka et al., 2011). Characterization of affinity-purified antisera against Ixodes SIFamide receptor (SIFa-R) and MIP receptor 1 (MIP-R1) were previously described (Šimo et al., 2013). The negative controls for the immunohistochemistry of the RS for neuropeptide receptors were performed with the primary antibodies preadsorbed with 1 μM of the 20 antigenic amino acid peptides for 4 h at room temperature (RT) or incubation of tissue in pre-immune serum. These treatments invariably abolished specific staining. F-actin staining for visceral muscles used Alexa 546 phalloidin (Molecular Probes, Carlsbad, CA, USA).
Table 1.
Antibodies used in this study.
| Antibody name | Host | Dilution | Source/characterization | Immunogen |
|---|---|---|---|---|
| Dippu FGLa/AST (Allatostatin A) | Mouse monoclonal | 1:1000 | Creative diagnostics (CSC-H1911)/Stay et al., 1992 | APSGAQRLYGFGLamide |
| Drome SIFamide | Rabbit polyclonal | 1:1,000 | Terhzaz et al., 2006 | AYRKPPFNGSIFamide |
| Manse Myoinhibitory peptide 1 (MIP1) | Mouse monoclonal | 1:1,000 | Kim et al., 2006 | AWQDLNSAWamide |
| Bommo orcokinin | Mouse polyclonal | 1:2,000 | Yamanaka et al., 2011 | NFDEIDR (orcokinin N-terminal) |
| Ixosc SIFa-R | Chicken polyclonal, affinity purified | 1:1,000 | Šimo et al., 2013 | CTRGLSRYDTQCEYLSTSAV |
| Ixosc MIP-R1 | Chicken polyclonal, affinity purified | 1:1,000 | Šimo et al., 2013 | CSSRYSLVNGPRTVTNETVL |
For whole-mounting of tick synganglia and hindguts, we followed a procedure that was previously established by Šimo et al. (2009a,b). Dilutions for primary antibodies were as follows: FGLa/AST, MIP and SIFamide 1:1000; orcokinin 1:2000; and SIFa-R 1:500. The secondary antibodies used in the study were: Alexa 594 or 488-goat anti-mouse IgG, Alexa 488-goat anti-rabbit IgG, Alexa 488-goat anti-chicken IgG and Alexa 647-goat anti-rabbit (Molecular Probes) at a 1:1000 dilution. Nuclei were stained with 300 nM DAPI (Sigma). Images were acquired using a Zeiss LSM 700 confocal microscope (Zeiss, Germany). Image enhancements (i.e., alterations in contrast and brightness) were performed in Adobe Photoshop 7.0.
2.3. MALDI-TOF analyses of neuropeptides in synganglion and hindgut
Extracts of synganglia and RS were analyzed by MALDI-TOF (Bruker Daltonics, Bremen, Germany). Fifteen synganglia and 12 RS were dissected in 20 μl of ice-cold PBS (137 mM NaCl, 1.45 mM NaH2PO4 and 20.5 mM Na2HPO4; pH 7.2; Fisher Scientific, Fair Lawn, NJ, USA). Each tissue was sonicated for 5 - 7 s in PBS in a glass tube. The supernatant was centrifuged at 12,000 g for 1 min at RT, loaded onto an equilibrated ZipTip C18 column (Millipore, Bedford, MA, USA), and eluted twice (to the same tube) with 4 μl of a solution containing 0.1% trifluoroacetic acid (TFA) and 70% acetonitrile (ACN; Fisher Scientific). The matrix of α-cyano-4-hydroxycinnamic-acid (CHCA; saturated solution in ACN/0.1% TFA acid 1:1) was freshly prepared. A 1 μl CHCA aliquot was spotted on the MALDI target plate (Bruker Daltonics) and immediately mixed with 1 μl of sample. Mass spectra were acquired in the reflector positive ion mode. All acquisitions were made in the manual mode in Flex Control 3.0 software (Bruker Daltonics).
2.4. Genes encoding the neuropeptides and their receptors and tissue-specific PCR
For FGLa/AST receptor(s), BLAST searches were undertaken in Vectorbase using the Drosophila melanogaster sequence (GenBank Accession number AAG22404) as a query (Birgul et al., 1999). The search yielded four different putative FGLa/AST receptors 1 to 4 (FGLa/AST-R1-4). The phylogenetic tree for FGLa/AST-Rs was constructed by a neighbor-joining method using 500 bootstrap replicates in MEGA5 (Kumar et al., 2008) to confirm the orthology with Drosophila FGLa/AST-R. Each FGLa/AST and orcokinin gene in I. scapularis was previously described (Christie, 2008; Donohue et al., 2010; Šimo et al., 2014). Receptors for the MIP and SIFamide, including their ligands, in I. scapularis were described by Šimo et al. (2013) and were further investigated in hindgut tissues in this study.
Tissue-specific expression patterns were examined by reverse transcriptase PCR using dissected tissues in ice-cold sterile PBS. RSs/recta, synganglia and carcasses (without salivary glands) were dissected and immediately frozen in tubes, placed on dry ice and stored at −80°C until use. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) followed by a further clean-up with the RNeasy Plus Micro Kit (Qiagen, Chatsworth, CA, USA) that uses an on-column DNase treatment to obtain DNA-free RNA. Reverse transcription according to the manufacturer's oligo(dT)20-based protocol was followed by a standard 35 cycles of PCR. All primers used in this study are listed in Supplementary Table S1. Due to high nucleotide identity among all four fgla/ast receptors 1 to 4, we designed primers in untranslated regions to achieve specificity. The primers for mip-r1 and sifa-r were previously used in quantitative real-time PCR in Šimo et al. (2013). The 80 bp-long amplicon encoding ribosomal protein S4 (rps4, GenBank Accession No. DQ066214) served as the control gene, as described in previous studies (Koči et al., 2013; Šimo et al., 2013). The specificities of PCR products were verified by electrophoresis on a 1% agarose gel.
2.5. Hindgut motility assay
Intact hindguts were dissected in ice-cold saline (see Section 2.1 for tick samples and chemicals) leaving a portion (~0.7 × ~0.7 mm) of the ventral cuticle attached to the anus. After removing the tissues surrounding the hindgut, such as tracheae, muscles, fatbodies and Malphigian tubules, the preparation was washed three times in saline within 30 s. In each experiment, the tissue was incubated in 50 μL of saline for 10 - 15 min at RT in a plastic petri dish. Hindguts showing continuous twitches during the preparation were rare (<~10%) and were excluded from further experiments. All experiments were performed in a drop of ~20 μL saline on the surface of white colored double-sided foam mounting tape (Scotch, St. Paul, USA). The hindgut movements were recorded by a video camera (DCR-SR100, Sony, Japan) for 15 - 25 min after the treatments. The working concentration of peptide(s) was applied by replacing the saline with 30 μl of saline containing the peptide(s) at the desired concentration. For the assays of the of synergistic or antagonistic activity of the peptides and their effects on the SIFamide-mediated activity, only the hindguts showing a response to 100 nM SIFamide within 20 min (~50% of the hindguts tested), in the initial pre-treatment, were used for the subsequent experiments.
For all experiments, a continuous 1 min video clip with the least noise in the recording between 10 - 15 min post-treatment was selected for the analysis using Ethovision XT 7.0 software (Noldus Information Technology, Waqeningen, Netherland). Off-line filtering was performed by sampling images in 200 ms intervals with a setting of high pixel smoothing for the dark coloration of the posterior part of the midgut as the area for the object. The mean values of the speed (mm/min) representing hindgut motility among different treatments were statistically analyzed by an one-way Student's t-test for a minimum of three biological replicates using Microsoft Excel (2010) software. The median effective concentration (EC50) value was calculated in Origin v.7 (Origin Lab, USA).
3. Results
3.1. Neuropeptide immunohistochemistry in synganglion and hindgut
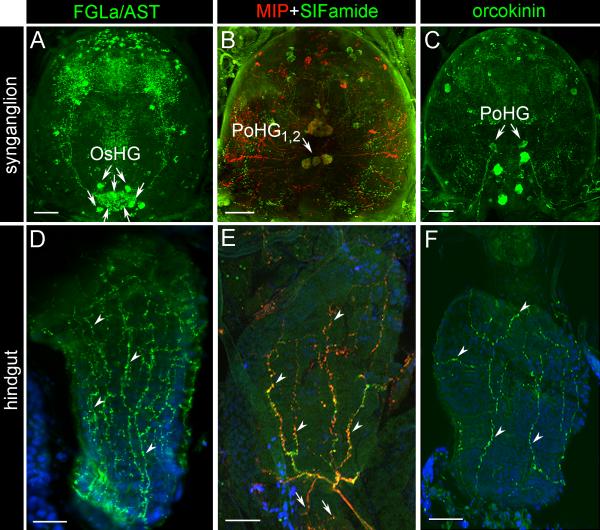
Antibodies against FGLa/AST, MIP, SIFamide and orcokinin revealed a high number of immunoreactive (IR) neurons in various regions of I. scapularis synganglion (Fig. 1A-C). These antibodies commonly recognized IR axonal projections exiting the synganglion via the opistosomal nerves that reached the surface of the hindgut (Fig. 1D-F). Strong FGLa/AST IR was observed in four pairs of dorsal opistosomal neurons although we were unable to observe the clear connections between these neurons and hindgut innervation (Fig. 1A). We conclude that a number of opistosomal FGLa/AST-IR neurons are likely the source (OsHG) of hindgut innervation. On the other hand, two pairs of neuronal cells reacting to both MIP and SIFamide antibodies (post-esophageal hindgut neurons (PoHG1,2), previously named as Pd3DM1,2 in Šimo et al., 2009a, b), and one pair to only the orcokinin antibody (PoHG) were clearly recognized as the sources of the hindgut innervations. The descriptions of all IR neurons in the synganglion for FGLa/ASTIR are in Supplementary Fig. S1 and were previously described for MIP/SIFamide-IR (Šimo et al., 2009b). Paired FGLa/AST-, MIP/SIFamide- and orcokinin-IR axons exit the synganglion via the opistosomal nerves and run postero-ventrally along the midline of the tick body near the midgut diventriculus and continue toward to the AC, where they turn medially and reach the surface region of the RS/AC boundary. From this region, they anteriorly arborize on the surface of the RS up to the posterior midgut boundary (Figs. 1DF, 2). Short MIP/SIFamide axons arborize posteriorly on the anterior part of the AC (Fig. 1E). Double-staining for FGLa/AST, MIP/SIFamide and orcokinin revealed that most of the IR axons run close to each other and contain multiple varicosities (Fig. 3). While most of the FGLa/AST-IR axons run along with MIP/SIFamide axons, some run independently (Fig. 3 A-C). MIP/SIFamide-IR axons (Fig. 3 D-E) run along with orcokinin-IR axons (Fig. 3 G-I), but with clear separation. Although the double staining for FGLa/AST and orcokinin was not possible due to both of the antisera being raised in mouse, the IRs appear to be distinguished by a richer FGLa/AST-IR network compared with the orcokinin-IR, which is running along with the MIP/SIFamide-IR axons.
Fig. 1.
Immunoreactivity (IR) of neuropeptides in unfed Ixodes scapularis female synganglia and hindguts. (A-C) IR of neuropeptides in synganglia. Arrows indicate neuronal cells innervating the hindgut. For FGLamide related allatostatin (FGLa/AST) IR, the specific source of hindgut innervation has not been identified, while four putative pairs of opistosomal hindgut neurons (OsHG) are indicated by arrows. The yellow color in B represents the colocalization of myoinhibitory peptide (MIP, red) and SIFamide (green). (D) FGLa/AST IR innervation of the rectal sac (RS, arrowheads). (E) Colocalization of MIP and SIFamide in the innervation of the RS (arrowheads) and the anal canal (arrows). (F) Orcokinin IR innervation of the RS (arrowheads). PoHG, postesophageal hindgut neurons. For descriptions of all peptidergic neurons reacting with antibodies against FGLa/AST and MIP/SIFamide, see Supplementary Fig. S2 and Šimo et al. (2009b), respectively. Blue color represents DAPI staining for nuclei. Scale bars = 20 μm.
Fig. 2.
Simplified diagrams showing peptidergic innervation of Ixodes scapularis female hindgut. (A) Complex view of synganglion (Syn) neurons innervating the hindgut and salivary glands (SG). (B) Magnified view of dorsal-postesophageal region of the synganglion with a focus on putative peptidergic neurons innervating the hindgut described in this study. Note that immunoreactive (IR) axonal projections from all detected neurons exit the synganglion via the opistosomal nerves (OsN) and reach the surface of the rectal sac and the anal canal as shown in (C). In the case of FGLamide related allatostatin (FGLa/AST) IR neurons (green), four putative pairs of neurons are shown, however the specific source of hindgut innervation has not been identified. (C) IR axonal projections reaching the surface on the rectal sac and the anal canal. Note that all three different IR axonal projections run close to each other and arborize on the surface of the rectal sac. In the case of myoinhibitory peptide (MIP)/SIFamide projections (red), short axon terminals innervating the anal canal were also found. Inset in C is magnified in C’ (see Fig. 3 for more details). Protocerebral salivary gland (PcSG) neurons producing MIP and SIFamide in innervation of the salivary gland were previously described in Šimo et al. (2009b). E, esophagus; MT, Malphigian tubule; OsHG, opistosomal hindgut neurons; PoHG, postesophageal hindgut neurons.
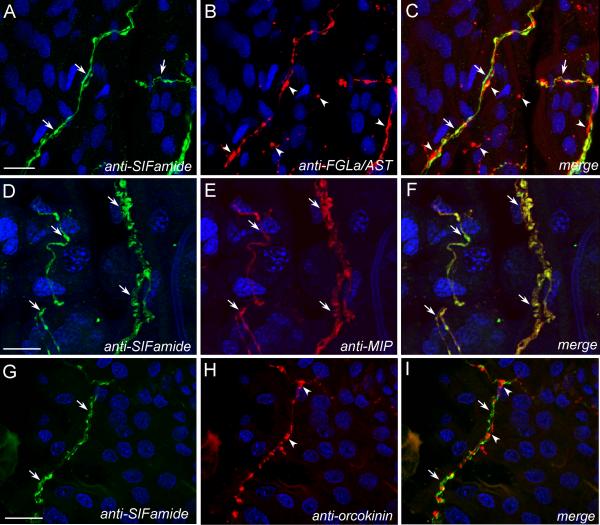
Fig. 3.
Double-stained axonal projections innervating the surface of the rectal sac of Ixodes scapularis. Combinations of SIFamide immunoreactive (IR) (green in A, D and G) with FGLamide related allatostatin (FGLa/AST) (red in B), myoinhibitory peptide (MIP) (E) or orcokinin IR (H) staining and merged images (C, F and E, respectively) are shown. Note that FGLa/AST (arrowheads in B and C) as well as orcokinin (arrowheads in H and I) IR revealed independent axonal projections compared with those reacting with SIFamide (arrows in A and G). MIP (arrows in E) IR axons were colocalized with SIFamide (arrows, D) as shown in the merged image (yellow, arrows in F). For a schematic view, see Fig. 2 C’. Blue color represents DAPI staining for nuclei. Scale bars = 20 μm.
3.2. Mass spectrometry of neuropeptides
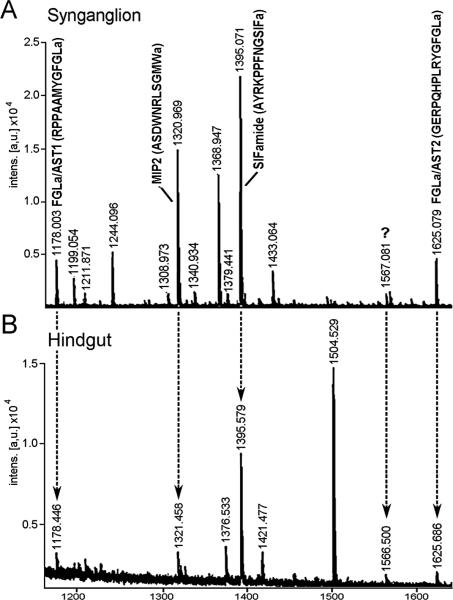
MALDI analyses were performed for each synganglia and hindgut extract based on the prediction that the source and target tissue extracts contain the same neuropeptides. The MALDI peaks revealed that the mass of the peptides were consistent with the results obtained in the immunohistochemistry. The two peaks of [M+H]+ 1178 and 1625 correspond to FGLa/AST1 (RPPAAMYGFLamide) and FGLa/AST2 (GERPQHPLRYGamide), respectively, the [M+H]+ 1321 peak represents MIP2 (ASNWDRLGSMWamide), and the [M+H]+ 1396 peak represents SIFamide (AYRKPPFNGSIFamide) (Fig. 4). Previous tandem mass spectrometry identified the sequences for MIP2 and SIFamide peaks in I. scapularis synganglion (MS/MS, Šimo et al., 2009b). In the current study, we were unable to detect the putative orcokinin peak in the hindgut extracts. The predicted mature peptides in fgla/ast, mip, sifamide and orcokinin genes are noted in Supplementary Fig. S2. We identified a strong peak [M+H]+ 1567 in the hindgut extract (Fig. 4.) that was also present in the synganglia extract, albeit at a smaller size, which still remains to be identified.
Fig. 4.
MALDI spectra of Ixodes scapularis synganglia (A) and hindgut extract (B). The peaks are annotated based on the [M+H]+ with the peptide sequences in parentheses. The “a” notation at C-termini represents putative amidation on the C-terminus of the peptide. Note that five common peaks, [M+H]+ 1178 (FGLamide related allatostatin 1, FGLa/AST1), 1321 (myoinhibitory peptide 2, MIP2), 1395 (SIFamide), 1566 and 1625 (FGLa/AST2) were found in both extracts. The unknown peak at 1566 Da (question mark) still awaits identification. The hindgut extract includes a small portion of posterior midgut, rectal sac, and anal canal.
3.3. Expression of the neuropeptide receptors
BLAST searches of FGLa/AST Rs revealed four putative receptor sequences in the I. scapularis genome. All four FGLa/AST Rs genes were highly similar to each other at both the nucleotide and protein levels and contained typical complete seven transmembrane (TM) domains (Supplementary Fig. S3). Phylogenetic analyses of the FGLa/AST Rs from I. scapularis and from other insect species suggested recent gene expansions for all four I. scapularis FGLa/AST Rs and a clear orthology with other insect FGLa/AST Rs (Supplementary Fig. S4). Receptors for MIP and SIFamide in I. scapularis were previously identified and functionally characterized (Šimo et al., 2013). Orcokinin receptor has not yet been identified in any arthropods.
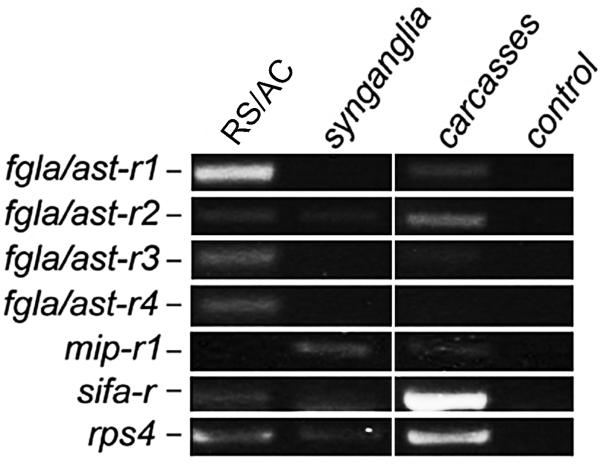
In tissue-specific reverse transcriptase PCR of unfed females, the fgla/ast-r1-4 andsifa-r transcripts were detected in RS/AC, while the mip-r1 transcript was not detected. In synganglia, faint bands for mip-r1, sifa-r and fgla/ast-r2 transcripts were observed. In carcasses, strong bands for sifa-r and fgls/ast-r2 were observed, while mip-r1, fgla/ast-r1 and fgla/ast-r3 showed faint bands (Fig. 5).
Fig. 5.
Tissue-specific reverse transcriptase-PCR for four FGLamide related allatostatin receptors 1 - 4 (fgla/ast-r1 - 4), myoinhibitory peptide receptor 1 (mip-r1) and SIFamide receptor (sifa-r) in an Ixodes scapularis female. rps4, ribosomal protein s4; control, no template added; RS, rectal sac; AC, anal canal. For primers used in this experiment, see Supplementary Table S1.
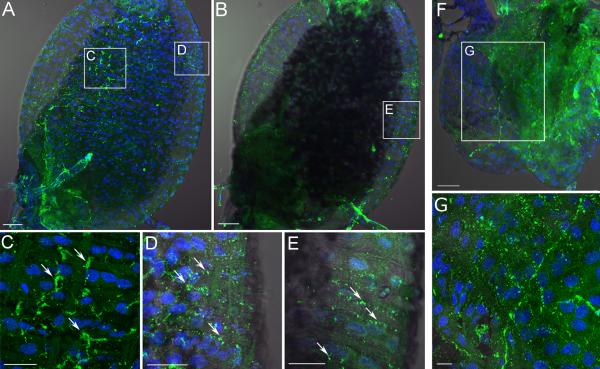
Immunohistochemistry of the RS using the affinity purified antibody against SIFa-R revealed high numbers of IR spots on the surface of the hindgut. The IR spots clustered along the visceral muscles, while some IR spots were also found on epithelial cells and were devoid of muscle (Fig. 6A-E). Anti-MIP-R1 also showed IR spots, mainly lining the RS visceral muscles (Fig. 6F, G). The negative controls, SIFa-R and MIP-R1 preimmune sera and preadsorptions, showed no specific patterns of IR in either case.
Fig. 6.
Immunohistochemistry of the SIFamide receptor (SIFa-R; A - E) and the myoinhibitory peptide receptor (MIP-R; F and G) in Ixodes scapularis unfed female rectal sac (RS). (A) Immunoreactivity (IR) for the optical sections focused on the dorsal region. (B) IR for the optical sections focused on the lateral regions of the RS. Insets in A and B are magnified in C, D and E. (F) MIP-R1 immunohistochemistry. Inset in F is magnified in G. Note that for both receptors the IR was likely found on the visceral muscles on the surface of the RS with some IR associated with the epithelial cells in the case of SIFa IR (C - E). Blue color represents DAPI staining for nuclei. The scale bar in A, B, F = 50 μm and in C, D, E, G = 20 μm.
3.4. Bioactivity of neuropeptides on hindgut motility
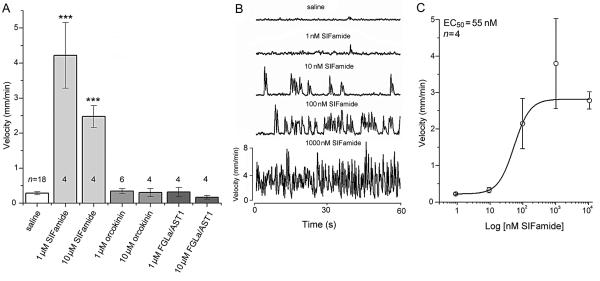
The workflow of the hindgut mobility bioassay is illustrated in Fig. 7. Among the four different peptides tested, only SIFamide showed activity in stimulating hindgut motility (Fig. 8, Supplementary Movie S1) and for the potentiation of the spontaneous movements of the hindgut (Supplementary Fig. S5). This movement was the consequence of muscle contractions on the AC resulting in the movements of the entire hindgut (Fig. 7D). All other peptides tested showed no stimulatory effects when applied at 1 and 10 μM concentrations (Fig. 8A). The most effective dose of SIFamide was 1 μM, and the lowest concentration providing a detectable effect was 10 nM. Based on a dose-responsescurve, the estimated EC50 value was 55 nM (Fig. 8B,C).
Fig. 7.

Experimental flow of hindgut physiology experiments. (A) Dissection and preparation of hindgut for in vitro assay. (B) The microscopic view of the hindgut. (C) The experimental setup for peptide treatments on the hindgut and the recording of treatment results. (D) An exemplary view of the hindgut movement. (E) An example of the data acquired and analyzed in EthoVision. Note that the origin of the recorded movement is in the posterior hindgut region called the anal canal.
Fig. 8.
Effects of selected neuropeptides on isolated hindgut of Ixodes scapularis. (A) Only SIFamide stimulated hindgut movement among the three different neuropeptides tested (SIFamide, orcokinin, FGLamide related allatostatin 1 (FGLa/AST1); each tested in 1 and 10 μM concentrations). (B) Representative hindgut responses and the effect of SIFamide (1, 10, 100 and 1000 nM) on hindgut movement recorded for 1 min. (C) Dose-dependent responses of hindgut motility by SIFamide. In A and C, the velocities are shown by the mean ± S.E. with the numbers (n). *** P ≤ 0.001 compared with saline treatment using an one-way Student's t-test.
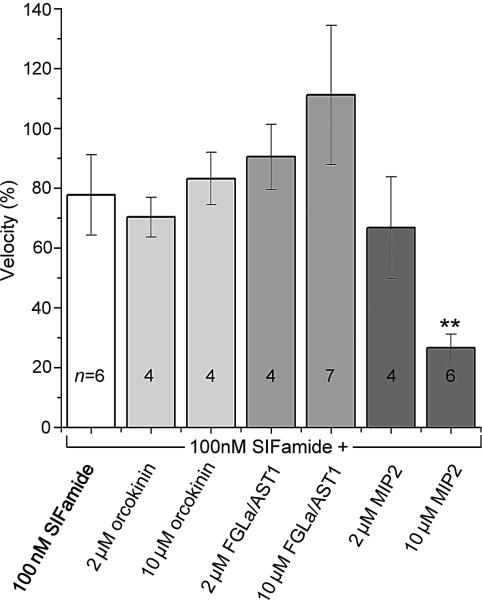
The inhibitory activity of the peptides on SIFamide-stimulated hindgut movement was tested. For this purpose, we applied a mixture of 100 nM SIFamide with the subject peptides in a ratio of 1:20 or 1:100 (2 or 10 μM of test peptides, respectively), on the hindgut that was pre-stimulated by 100 nM SIFamide. Among the three different peptides tested (orcokinin, FGLa/AST1 and MIP2), only MIP2 (10 μM) showed a statistically significant reduction (~65%) of the SIFamide-mediated motility (Fig. 9). SIFamide and MIP2 activities were consistently observed among different individual guts, although the sizes of the RS and the presence of iridescent guanine crystals in the RS largely varied among individuals in the unfed females. During our experiments, we rarely observed autonomous massive peristaltic movements of the RS without neuropeptide treatment (~5% of RS examined in this study).
Fig. 9.
Combinatory actions of neuropeptides on the SIFamide pre-stimulated hindgut motility (100 nM). Note that among the three neuropeptides tested (orcokinin, FGLamide related allatostatin 1 (FGLa/AST1), myoinhibitory peptide 2 (MIP2); each in 2 and 10 μM concentrations) only 10 μM MIP showed statistically significant reductions (~65%) in SIFamide-stimulated hindgut motility. The normalized velocity data were obtained by using the velocity of the initial treatment with SIFamide alone, assigned a value of 100 in each experiment. For the activity in subsequent individual peptide treatments, including repeated SIFamide treatment used as a control, the velocity percentages are shown as the mean ± S.E. with the numbers (n). **P ≤ 0.01 compared with SIFamide alone using an one-way Student's t-test.
4. Discussion
Malpighian tubules and the rectum are the major excretory organs in different phyla of arthropods. Although the anatomy and the function of excretory systems of ticks and insects are strikingly similar, this similarity appears to be a consequence of convergent evolution having different developmental origins. These organs in arachnids are known to be endodermal in development, while those in insects are ectodermal (Hazelton et al., 2002). However, the set of neuropeptides found in this study for control of the tick hindgut has not been previously described for excretory functions in insect species.
In this study, we found three peptidergic neuronal projections reaching the hindgut surface: FGLa/AST-IR, SIFa/MIP-IR and orcokinin-IR neurons. The immunoreactivity was further supported by tissue-specific MALDI showing MS for SIFamide, MIP2, FGLa/AST1 and FGLa/AST2. Among those neuropeptides, SIFamide showed activity in stimulating hindgut motility that was antagonized by MIP2, while the others did directly act on hindgut motility in this assay.
We identified SIFa_R IR on the RS, both on the visceral muscle and on the epithelial cells (Fig. 6A-E). The result indicates that SIFamide is the direct activator of the muscle, while it may also act on the epithelial cells for ion and water transport (resorption). MIP colocalized with SIFamide in the axonal projections and the varicosities showed an antagonistic effect on SIFamide-mediated hindgut motility, however, with much lower potency. We found MIP-R IR on the visceral muscles of the RS (Fig. 6F,G), although we were unable to detect the transcript for mip-r in the hindgut (Fig. 5).
Antagonistic activity in the hindgut between SIFamide and MIP are not surprising because the myostimulatory activity of SIFamide and the inhibitory activity of MIP are well known in other invertebrate species. SIFamide stimulates the oviduct in Locusta migratoria (Janssen et al., 1996) or the pyloric valve between foregut and midgut of the lobster Homarus americanus (Christie et al., 2006). MIPs were commonly identified as peptides, inhibiting muscle activity in many different species of invertebrates (Schoofs et al., 1991; Lorenz et al., 1995; Davis et al., 2003; Kim et al., 2006).
In a previous study, MIP and SIFamide were co-expressed in the protocerebral salivary gland neurons (PcSG) that control the salivary glands and were proposed to be associated with a regulatory mechanism by temporal differences in the expression patterns of the two antagonistic peptides (Šimo et al., 2009b). The presence of the same set of antagonistic neuropeptides in different pairs of neurons (PoHG1,2) controlling another excretory organ hindgut is an interesting coincidence. It may be explained by concurring temporal needs of excretory functions in both salivary glands and the hindgut in the feeding stages.
We found that SIFamide stimulated robust movement of the entire hindgut (Fig. 8A-C), which occurs by the contractions of AC muscles located close to the junction between the RS and the AC. The robust free hindgut movement in our in vitro experiments may be due to the preparation of the samples by removing the tissues surrounding the hindgut (i.e., tracheae, fatbody and possibly muscles). Balashov (1972) described smooth muscles attached from the anal valve to the posterior part of the AC. In our experiments, the anterior region of the AC, which was identified as the origin of movement, is likely different from the anal valve muscles described in the earlier study (Balashov, 1972). We suggest that the posteriorly running MIP/SIFamide short axon terminals on the surface of the AC (Figs. 1E, 2C) may be the release sites for neuropeptides controlling the anterior AC muscles. Despite the rich peptidergic networks arborizing on the surface of the RS, we were unable to detect contractions of the visceral muscles in the RS.
The presence of FGLa/AST in tick hindgut innervation was first reported in the brown ear tick, Rhipicephalus appendiculatus (Šimo et al., 2014). Although the involvement of FGLa/AST in hindgut control in this study is well supported by immunohistochemistry, MALDI and expression FGLa/AST receptors in this tissue, the specific role of this neuropeptide in the tick hindgut remains enigmatic. In general, myoinhibitory activity of FGLa/AST has been reported in many insects: inhibition of the peristaltic movements of the ileum in the blowfly Calliphora vomitoria (Duve and Thorpe, 1994) and inhibition of proctolin-stimulated hindgut contractions in L. migratoria, Diploptera punctata or Schistocerca gregaria (Lange et al., 1993; Veelaert et al., 1996; Robertson et al., 2012). Therefore, the inhibitory functions of FGLa/AST in insects may be expandable to the tick hindgut, although the activity was not found in our experimental setup. Alternatively, FGLa/AST and other neuropeptides may have additional functions as neurohormones, using the hindgut surface as the haemocoel release site. The identity of the orcokinin IR needs additional verification using independent techniques.
Neural and hormonal control of excretory organs is important for maintaining homeostasis in fluctuating environments and during the biological demands of hematophagous arthropods. In this study, we provide evidence supporting the involvement of two antagonistic neuropeptides, SIFamide and MIP, and of FGLa/AST in tick hindgut control.
Supplementary Material
Highlights.
Complex neuropeptidergic innervations of the tick hindgut were identified by immunostaining
Peptidomics supports the presence of SIFamide, MIP, and FGLa/AST in the hindgut
Expressions of the genes encoding receptors for each neuropeptide were confirmed
Hindgut motility assays revealed antagonistic actions between SIFamide and MIP
Acknowledgements
We are grateful to Dr. Akira Mizoguchi, Nagoya University, Japan, for providing the MIP and orcokinin antibodies and to Dr. Jan Veenstra, Université de Bordeaux, France, for providing SIFamide antibody. This paper is contribution no. 14-387-J from the Kansas Agricultural Experiment Station, USA. The project was supported by National Institutes of Health, USA, Grant Number R01AI090062.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balashov YS. Bloodsucking ticks (Ixodoidea)—vectors of disease of man and animals (English translation). Misc. Pub. Entomol. Soc. Am. 1972;8:163–376. [Google Scholar]
- Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Burdin M, Hoppler S, Bowman AS. Role of an aquaporin in the sheep tick Ixodes ricinus: assessment as a potential control target. Int J Parasitol. 2010;40:15–23. doi: 10.1016/j.ijpara.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Christie AE. Neuropeptide discovery in Ixodoidea: an in silico investigation using publicly accessible expressed sequence tags. Gen Comp Endocrinol. 2008;157:174–185. doi: 10.1016/j.ygcen.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stemmler EA, Peguero B, Messinger DI, Provencher HL, Scheerlinck P, Hsu YW, Guiney ME, de la Iglesia HO, Dickinson PS. Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val1)-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus. J Comp Neurol. 2006;496:406–421. doi: 10.1002/cne.20932. [DOI] [PubMed] [Google Scholar]
- Coast GM. Neuroendocrine control of ionic homeostasis in blood-sucking insects. J Exp Biol. 2009;212:378–386. doi: 10.1242/jeb.024109. [DOI] [PubMed] [Google Scholar]
- Davis NT, Blackburn MB, Golubeva EG, Hildebrand JG. Localization of myoinhibitory peptide immunoreactivity in Manduca sexta and Bombyx mori, with indications that the peptide has a role in molting and ecdysis. J Exp Biol. 2003;206:1449–1460. doi: 10.1242/jeb.00234. [DOI] [PubMed] [Google Scholar]
- Donohue KV, Khalil SM, Ross E, Grozinger CM, Sonenshine DE, Michael Roe R. Neuropeptide signaling sequences identified by pyrosequencing of the American dog tick synganglion transcriptome during blood feeding and reproduction. Insect Biochem Mol Biol. 2010;40:79–90. doi: 10.1016/j.ibmb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Dow JA, Davies SA. The Malpighian tubule: rapid insights from post-genomic biology. J Insect Physiol. 2006;52:365–378. doi: 10.1016/j.jinsphys.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Duve H, Thorpe A. Distribution and functional significance of Leu-callatostatins in the blowfly Calliphora vomitoria. Cell Tissue Res. 1994;276:367–379. doi: 10.1007/BF00306122. [DOI] [PubMed] [Google Scholar]
- Hazelton SR, Townsend VR, Jr., Richter C, Ritter ME, Felgenhauer BE, Spring JH. Morphology and ultrastructure of the Malpighian tubules of the Chilean common tarantula (Araneae: Theraphosidae). J Morphol. 2002;251:73–82. doi: 10.1002/jmor.1074. [DOI] [PubMed] [Google Scholar]
- Janssen I, Schoofs L, Spittaels K, Neven H, Vanden Broeck J, Devreese B, Van Beeumen J, Shabanowitz J, Hunt DF, De Loof A. Isolation of NEB-LFamide, a novel myotropic neuropeptide from the grey fleshfly. Mol Cell Endocrinol. 1996;117:157–165. doi: 10.1016/0303-7207(95)03746-2. [DOI] [PubMed] [Google Scholar]
- Kaufman WR, Phillips JE. Ion and water balance in the Ixodid tick Dermacentor andersoni. I. Routes of ion and water excretion. J Exp Biol. 1973;58:523–536. [Google Scholar]
- Kim YJ, Žitňan D, Cho KH, Schooley DA, Mizoguchi A, Adams ME. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci U S A. 2006;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koči J, Šimo L, Park Y. Validation of internal reference genes for real-time quantitative polymerase chain reaction studies in the tick, Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2013;50:79–84. doi: 10.1603/me12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AB, Chan KK, Stay B. Effect of allatostatin and proctolin on antennal pulsatile organ and hindgut muscle in the cockroach, Diploptera punctata. Arch Insect Biochem Physiol. 1993;24:79–92. doi: 10.1002/arch.940240203. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Kellner R, Hoffmann KH. Identification of two allatostatins from the cricket, Gryllus bimaculatus de Geer (Ensifera, Gryllidae): additional members of a family of neuropeptides inhibiting juvenile hormone biosynthesis. Regul Pept. 1995;57:227–236. doi: 10.1016/0167-0115(95)00036-B. [DOI] [PubMed] [Google Scholar]
- Park Y. Endocrine regulation of insect diuresis in the early postgenomic era. Can J Zoology. 2012;90:507–520. [Google Scholar]
- Raikhel AS. The excretory system. In: Balashov Yu.S., editor. An Atlas of Ixodid Tick Ultrastructure [English translation] Entomological Society of America; Lanham, MD: 1983. pp. 129–147. [Google Scholar]
- Robertson L, Rodriguez EP, Lange AB. The neural and peptidergic control of gut contraction in Locusta migratoria: the effect of an FGLa/AST. J Exp Biol. 2012;215:3394–3402. doi: 10.1242/jeb.073189. [DOI] [PubMed] [Google Scholar]
- Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A. Isolation, identification and synthesis of locustamyoinhibiting peptide (LOM-MIP), a novel biologically active neuropeptide from Locusta migratoria. Regul Pept. 1991;36:111–119. doi: 10.1016/0167-0115(91)90199-q. [DOI] [PubMed] [Google Scholar]
- Šimo L, Koči J, Park Y. Receptors for the neuropeptides, myoinhibitory peptide and SIFamide, in control of the salivary glands of the blacklegged tick Ixodes scapularis. Insect Biochem Mol Biol. 2013;43:376–387. doi: 10.1016/j.ibmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Slovak M, Park Y, Žitňan D. Identification of a complex peptidergic neuroendocrine network in the hard tick, Rhipicephalus appendiculatus. Cell Tissue Res. 2009a;335:639–655. doi: 10.1007/s00441-008-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Sonenshine DE, Park Y, Žitňan D. Nervous and sensory systems: structure, function, genomics and proteomics. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. 2nd ed. Oxford University Press; New York City, USA: 2014. p. 58. [Google Scholar]
- Šimo L, Žitňan D, Park Y. Two novel neuropeptides in innervation of the salivary glands of the black-legged tick, Ixodes scapularis: myoinhibitory peptide and SIFamide. J Comp Neurol. 2009b;517:551–563. doi: 10.1002/cne.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. Vol. 1. Oxford University Press; New York: 1991. [Google Scholar]
- Sonenshine DE. Excretion and Water Balance: Hindgut, Malpighian Tubules, and Coxal Glands. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. 2nd ed. Oxford University Press; New York City, USA: 2014. p. 14. [Google Scholar]
- Stay B, Chan KK, Woodhead AP. Allatostatin-immunoreactive neurons projecting to the corpora allata of adult Diploptera punctata. Cell Tissue Res. 1992;270:15–23. doi: 10.1007/BF00381875. [DOI] [PubMed] [Google Scholar]
- Terhzaz S, Rosay P, Goodwin SF, Veenstra JA. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem Biophys Res Commun. 2007;352:305–310. doi: 10.1016/j.bbrc.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Veelaert D, Devreese B, Schoofs L, Van Beeumen J, Vanden Broeck J, Tobe SS, De Loof A. Isolation and characterization of eight myoinhibiting peptides from the desert locust, Schistocerca gregaria: new members of the cockroach allatostatin family. Mol Cell Endocrinol. 1996;122:183–190. doi: 10.1016/0303-7207(96)03889-0. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Roller L, Žitňan D, Satake H, Mizoguchi A, Kataoka H, Tanaka Y. Bombyx orcokinins are brain-gut peptides involved in the neuronal regulation of ecdysteroidogenesis. J Comp Neurol. 2011;519:238–246. doi: 10.1002/cne.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.