Supplemental Digital Content is Available in the Text.
Key Words: HIV infections, neuropsychological tests, cognition disorders, cross-sectional studies, men who have sex with men
Abstract
Background:
To determine the prevalence of neurocognitive impairment (NCI) in UK HIV-positive and HIV-negative men who have sex with men (MSM).
Methods:
HIV-positive and HIV-negative participants were recruited to a cross-sectional study from 2 London clinics and completed computer-assisted neuropsychological tests and questionnaires of depression, anxiety, and activities of daily living. Published definitions of HIV-associated neurocognitive disorders (HAND) and global deficit scores were used. Age- and education-adjusted neuropsychological test scores were directly compared with reference population data.
Results:
A total of 248 HIV-positive and 45 HIV-negative MSM participated. In the HIV-positive group, median time since diagnosis was 9.4 years, median CD4+ count was 550 cells per cubic millimeter, and 88% were on antiretroviral therapy. Prevalence of HAND was 21.0% in HIV-positive MSM (13.7% asymptomatic neurocognitive impairment, 6.5% mild neurocognitive disorder, and 0.8% HIV-associated dementia). Using a global deficit score threshold of 0.5, the prevalence of NCI was 31.5% (when averaged over 5 neuropsychological domains) and 40.3% (over 10 neuropsychological test scores). These results were not significantly different from the HIV-negative study sample. No consistent pattern of impairment was seen in HIV-positive patients relative to general male population data (n = 380).
Conclusions:
We found a prevalence of HAND and degree of impairment on neuropsychological testing of HIV-positive MSM that could represent a normal population distribution. These findings suggest that NCI may be overestimated in HIV-positive MSM, and that the attribution of NCI to HIV infection implied by the term HAND requires revision.
INTRODUCTION
The prevalence and implications of HIV-related neurocognitive impairment (NCI) are important but disputed issues in research and clinical practice. The incidence of HIV-associated dementia (HAD) has declined dramatically and is now rarely seen in patients receiving effective antiretroviral therapy (ART).1–3 However, the prevalence of NCI has been estimated in some studies of HIV-positive people both on and off ART at around or above 50%.4–8 In contrast, using broadly the same definition but different neuropsychological (NP) testing methods, only 19% of people in a UK cohort were found to have NCI.9 The same prevalence (19%) was found using another definition of NCI, the global deficit score (GDS), in a cohort of HIV-positive US military veterans receiving ART,10 and there was no impairment in group mean performance relative to HIV-negative controls.
Updated criteria for HIV-associated neurocognitive disorders (HAND), known as the Frascati criteria, include 3 categories: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HAD.11 Studies reporting lower prevalence of NCI in HIV lend weight to concerns regarding potential overclassification when using current definitions of HAND and raise the possibility that the category of ANI does not reflect HIV-related factors but is instead part of a normal population distribution, insomuch as performance needs to fall only 1 SD below normal means in 2 of 5 NP domains to meet the criteria.12 This level of impairment can result in a classification of MND or ANI, depending on whether everyday function is impaired or not, provided HIV seropositivity is confirmed and major confounders such as delirium, intoxication, and severe depression are excluded. However, determining everyday function may be problematic because in most cases it is based on patients' self-report, and depressive symptoms themselves have been shown to predict self-reported functional difficulties.13 Given the high rates of depression associated with HIV infection, this presents a potentially serious confounder.
The wide variation in prevalence estimates and the difficulties in applying current criteria present significant challenges to the interpretation of available data on NCI in HIV-positive individuals. Our aim was to use a computerized test battery (CogState) supplemented by standard paper-and-pencil NP tests to determine the prevalence of NCI in HIV-positive men who have sex with men (MSM), the largest demographic group affected by incident and prevalent HIV in Europe,14 defined by both the Frascati HAND criteria11 and a GDS threshold,15,16 and to compare the NP scores to general population norms and to a study control group of HIV-negative MSM.
METHODS
Study Population
The Cognitive Impairment in People with HIV in the European Region (CIPHER) study is a cross-sectional study of NCI in HIV outpatients in 4 countries. HIV-positive patients were consecutively invited to enroll in the Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) study,17 a questionnaire survey of sexual behavior, in 7 outpatient clinics in the United Kingdom in 2011–2012. Those enrolled at the Mortimer Market Centre (MMC) and the Royal Free Hospital (RFH) (both in London) who consented to have routine clinical information linked to study data were invited to participate in CIPHER. Exclusion criteria were age <18 years, being unable to understand the questionnaire component of the study because of language difficulties, and being too ill or distressed to complete the assessments. This article refers to the analysis only of CIPHER participants who were MSM and enrolled in the UK.
Additionally, HIV-negative MSM attending a sexual health clinic at MMC were recruited in 2011–2012 through clinician referral, leaflets, and advertisement in clinic waiting rooms. HIV status was confirmed serologically within 1 month before the time of assessment.
Ethical Approval
Ethical approval for the study was obtained from the National Research Ethics Service (London—Hampstead committee), reference 11/LO/0077. All study participants gave written informed consent before commencing the study.
Neuropsychological Testing
CogState is a computerized battery of NP assessments that has been used in a range of clinical settings.18–23 The CogState battery was chosen because of its ease of administration, low testing burden to participants and speed; these are important factors, given our intention to conduct longitudinal assessments at a later date. The software has a track record of use in HIV-positive patients and has been compared with paper-and-pencil NP tests in this group.9,19,24,25 It performs well in participants across a range of cultural, economic, linguistic, and social backgrounds. It is robust against practice effects with performance considered to become stable after 1 full practice session. We devised a battery to detect patterns of impairment expected in HIV, with particular focus on attention, psychomotor speed, and executive function (see text in Supplemental Digital Content, http://links.lww.com/QAI/A548, which describes the battery in more detail). The CogState battery comprised detection (reaction time), identification (choice reaction time), international shopping list, Groton Maze learning, and one-back tasks. In addition, participants completed 2 tests of verbal fluency and executive function: the controlled oral word asssociation test (COWAT), using the letters C, F, and L, and the category fluency test (CFT). All NP tasks generated a single performance score except for the one-back task, which defined performance using both accuracy and speed scores. Performance measures from the NP tasks were organized into cognitive domains by combining pairs of test scores as follows: psychomotor speed (detection and identification), verbal memory (shopping list learning and recall), executive function (Groton Maze learning and recall), working memory maintenance (one-back speed and accuracy), and verbal fluency (COWAT and CFT).
Functional impairment due to cognitive difficulties was measured using a self-report questionnaire based on an older tool for measuring function in the elderly26 that has been used in other observational studies of HIV-related cognitive impairment5,27,28 (see text in Supplemental Digital Content, http://links.lww.com/QAI/A548, which describes the questionnaire in more detail). Declining function was registered if a participant recorded their current level of functioning as lower than their best ever in 2 or more of 12 activities of daily living (ADL) and attributed this decline to cognitive rather than physical causes. Declining function was graded as mild (decline in 2–3 ADL) or significant (4 or more ADL).
Social, Clinical, and Other Health Data
Participants completed a self-completed confidential questionnaire developed for the ASTRA study,17 which collected information on age, ethnicity, education, smoking, alcohol (using the CAGE questionnaire),29 recreational drug use, and sexual behavior. The questionnaire included validated depression and anxiety symptom inventories (the PHQ-930 and GAD-7,31 respectively). Viral load, CD4 count, ART regimen, hepatitis C virus (HCV) status, mental health, current medication, and other health data were also collected. Data were corroborated by merging with routinely collected clinical information. Case notes were reviewed by clinicians to identify participants who met criteria for serious confounding comorbidities as listed in the Frascati criteria.11
General Population Data
Data were provided by CogState with scores generated by the computerized tasks from 380 healthy men. These individuals had been recruited to clinical trials through employment services, community advertisements, and word of mouth. Studies providing data for normative databases had been conducted in Western and Eastern Europe, United States of America, Southeast Asia, Australia, and New Zealand. All assessments had been conducted in individual's first language. Exclusion criteria for individuals contributing data to the normative database for the CogState tasks included evidence or history of clinically significant neurological, psychiatric, hematological, renal, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, immunological or allergic disease, and routine use of sedative medication, analgesic, or other central nervous system (CNS)-active medications. All data were drawn from individuals with normal or corrected to normal visual and auditory acuity. These normative data had been used previously to classify cognitive function in HIV-positive adults18 (also unpublished data presented by K. Robertson, PhD, et al. at the Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2013). Data were sampled to provide a similar distribution within strata to the HIV-positive MSM study sample. The general male population CogState data were grouped by age (18–34, 35–50, 51–59 years) and years of education (up to 11, 12–14, 15–18, ≥19). The resulting 12 groups were used to determine normative mean and SD values. Published age-, sex-, and education-stratified norms were used for the COWAT and CFT,32,33 and CogState's preexisting norms were used for age ≥60.
Case Definitions
Raw NP test scores were log-transformed or arcsine root–transformed where necessary and converted into age- and education-adjusted Z scores using normative means and SD (as above). A single Z score was calculated for each of the 5 cognitive domains by averaging pairs of Z scores (see Neuropsychological Testing).
For each patient, the Z scores were then used to determine they met 3 separate definitions. The first was the Frascati criteria,11 in which HAND was defined by combining functional assessments with NP scores and other clinical information. Participants with a Z score of −1 SD or less in 2 or more domains were classified as ANI if there was no functional decline attributed to cognitive difficulties, or MND if there was mild decline. Those scoring −2 SD or less in 2 or more domains were classified as ANI if there was no functional decline, MND if there was mild decline, or HAD if there was a significant decline.
Two GDS were calculated from the average deficit across all 10 NP test scores and the average deficit across all 5 domains. Deficit was defined for each test by assigning a score of 0 to Z scores lying above −1.0 and assigning 1 point for each 0.5 decrement in Z score below −1.0. By assigning normal and above-average Z scores a deficit score of 0, this system places less weight on tests in the normal or superior range. In keeping with previous work,15,16 NCI was defined as GDS ≥0.5. These case definitions did not incorporate functional status, depression, or other factors.
Statistical Analyses
Overall group characteristics, and the prevalence of NCI according to the 3 definitions, were compared between HIV-positive and HIV-negative study groups, using χ2 tests to compare categorical variables and the t test to compare age. Tests for trend were used to compare ordered categorical variables (grade of HAND, functional impairment, anxiety, and depression) between the 2 groups. In sensitivity analyses, comparisons of the prevalence and grade of NCI were repeated after exclusion of participants with severe symptoms of depression (defined as scoring ≥20 on the PHQ-9), psychosis, delirium, intoxication, or a previous major CNS condition.
The χ2 and Wilcoxon rank-sum tests were used to assess associations between NCI and covariates including demographic (age, education, and ethnicity), behavioral (smoking, alcohol, and drug use), psychological (depression and anxiety symptoms), and disease-related factors (viral load, CD4, time since HIV diagnosis, ART status, time on ART, and HCV coinfection). Multivariable logistic regression was used to assess the independent effects of these factors found to have P < 0.15 in univariate analyses. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated.
Additionally, group mean test scores were compared between HIV-positive MSM and both the general male population data supplied by CogState (N = 380) and the group of HIV-negative MSM recruited to the study, using analysis of covariance (ANCOVA) with adjustment for age and education.
All reported P values are 2-sided, and a P value of <0.05 was considered statistically significant. Data were analyzed using Stata 13 (StataCorp, College Station, TX).
RESULTS
Participant Characteristics
A total of 263 HIV-positive and 49 HIV-negative MSM were enrolled to CIPHER at 2 UK sites, of whom 251 HIV-positive and 45 HIV-negative men completed full practice run and baseline CogState tests. Of these, 3 HIV-positive MSM had other missing COWAT and CFT scores, leaving 248 HIV-positive and 45 HIV-negative MSM suitable for analysis. Participant characteristics are summarized in Table 1. The HIV-positive participants were older than the HIV-negative group [mean (SD) age of 45.9 (9.1) years compared with 33.4 (7.8); P < 0.001]. Over 80% of both groups were of white ethnicity and around 60% were university educated. HIV-positive participants had been diagnosed with HIV for a median of 9.4 years previously, and around 80% were virologically suppressed on ART and/or had a CD4 count >350 cells per cubic millimeter.
TABLE 1.
Characteristics of the Study Sample, Comparing HIV-Positive and HIV-Negative Men
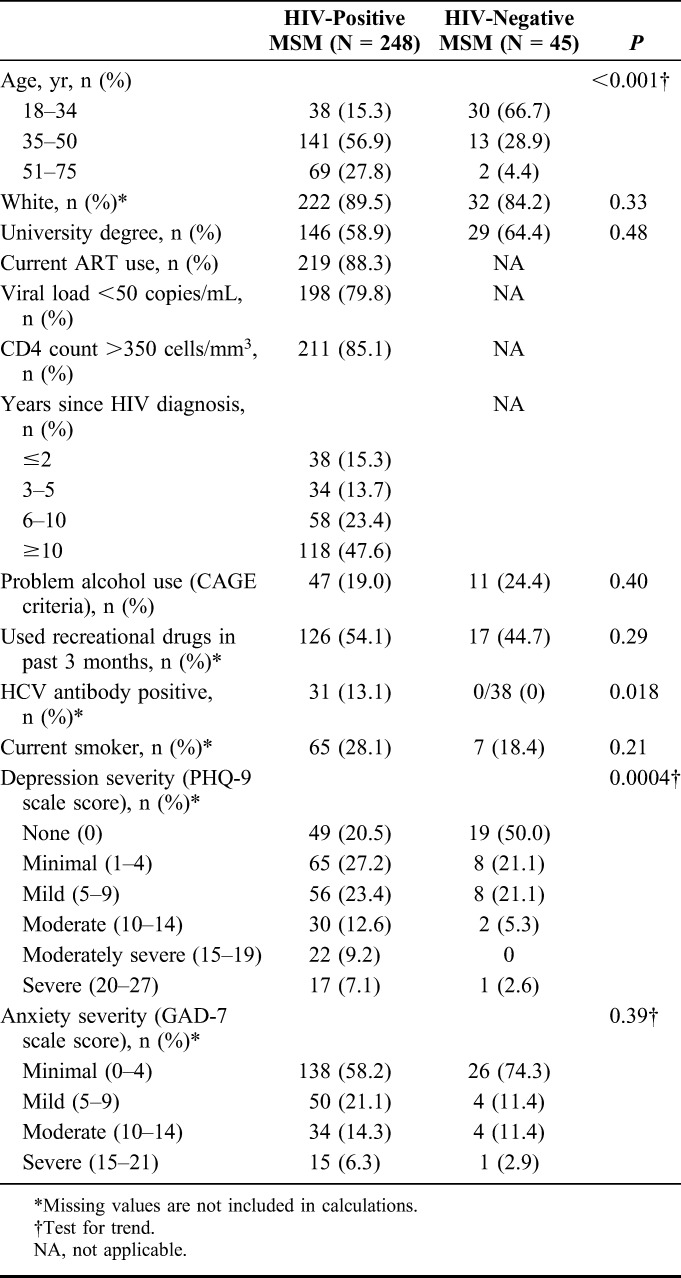
Problem alcohol use (CAGE score of 2 or more) was apparent in 47 (19.0%) of the HIV-positive and 11 (24.4%) of the HIV-negative MSM. Recreational drug use in the past 3 months was reported by 126 (54.1%) HIV-positive and 17 (44.7%) HIV-negative participants. Recreational substances most commonly used (>20% of respondents) were amyl nitrite (poppers) (29.0%), cannabis (24.0%), and phosphodiesterase inhibitors (23.3%), followed by cocaine (19.4%), 3,4-methylenedioxy-N-methylamphetamine (ecstasy) (14.7%), ketamine (11.5%), and γ-hydroxybutyric acid (11.1%). Grade of depressive symptoms was higher in HIV-positive respondents than in HIV-negative respondents (χ2 for trend = 0.0004), and the proportion graded as moderate depression or above (≥10 on PHQ-9) was 28.9% in HIV-positive compared with 7.9% in HIV-negative respondents. Moderate-to-severe anxiety symptoms were reported by a similar proportion of each group (20.7% compared with 14.3%; P = 0.38).
Neurocognitive Impairment According to Standard Criteria
In the HIV-positive sample, the prevalence of HAND defined by the Frascati criteria was 21.0% (n = 52, 95% CI: 16.1% to 26.6%), graded as ANI in 13.7% (n = 34, 95% CI: 9.7% to 18.6%), MND in 6.5% (n = 16, 95% CI: 3.7% to 10.3%), and HAD in 0.8% (n = 2, 95% CI: 0.1% to 2.9%) (Table 2). Although the Frascati criteria in the strictest sense do not apply to HIV-negative individuals, applying the other elements of the criteria to the HIV-negative sample gave a prevalence estimate of 28.9% (n = 13, 95% CI: 16.4% to 44.3%), graded as ANI in 24.4% (n = 11, 95% CI: 12.9% to 39.5%), MND in 4.4% (n = 2, 95% CI: 0.5% to 15.1%), and HAD in none. The difference between the HIV-positive and HIV-negative groups was not statistically significant (P = 0.32, test for trend). Self-reported functional impairment resulting from cognitive difficulties, the sole discriminator between ANI and MND, was higher in HIV-positive (29.0%) than in HIV-negative participants (11.1%) (P = 0.007, test for trend), although this did not translate into a statistically significant difference in the proportion of those meeting HAND criteria who were classified as symptomatic (MND or HAD) (P = 0.31).
TABLE 2.
Estimates of Prevalence of Neurocognitive Impairment in HIV-Positive and HIV-Negative Men
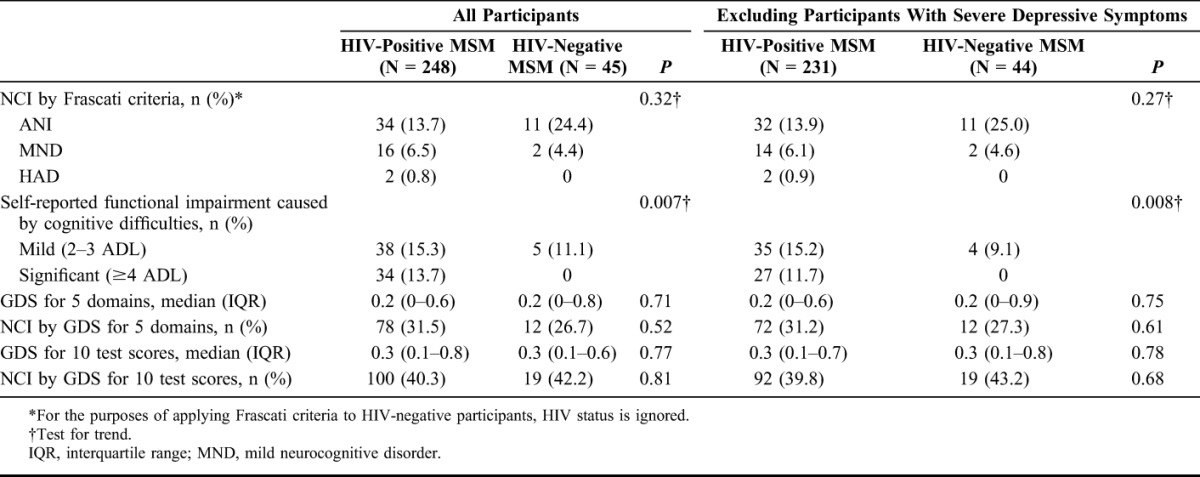
In a sensitivity analysis, removing those with severe depressive symptoms reported on the PHQ-9 (n = 17 HIV positive and n = 2 HIV negative) had little effect on the prevalence of HAND in either group (Table 2). There were 6 HIV-positive participants with previous AIDS-defining conditions of the CNS that may also be considered confounders in the causation of NCI: 3 with toxoplasmosis (of whom 1 had MND) and 3 with cryptococcosis (of whom 1 had ANI and 1 had HAD). Removal of these participants and those with severe depressive symptoms resulted in there being 31 HIV-positive patients (13.7%) with ANI, 13 (5.8%) with MND, and 1 (0.4%) with HAD.
Using the GDS algorithm to average 5 cognitive domains gave a prevalence of NCI of 31.5% (n = 78, 95% CI: 25.7% to 37.6%) in HIV-positive and 26.7% (n = 12, 95% CI: 14.6% to 41.9%) in HIV-negative participants; comparison between these 2 proportions was not statistically significant (P = 0.52). Averaging all 10 test scores gave higher estimates of 40.3% (n = 100, 95% CI: 34.2% to 46.7%) in HIV-positive and 42.2% (n = 19, 95% CI: 27.7% to 57.8%) in HIV-negative participants (again not statistically significant, P = 0.81). There was little difference to the results after removal of participants with severe depression symptoms.
Assessment of agreement between the 2 scoring systems in the HIV-positive sample demonstrated that the GDS algorithm (averaged over 5 domains) was more sensitive and/or less specific than the Frascati criteria: 28 of the 248 (11.3%) participants were classified as having NCI by the GDS algorithm but normal by the Frascati criteria, and 2 of the 248 (0.8%) participants were differentially classified in the opposite direction.
Comparison of Neuropsychological Test Scores Between Groups
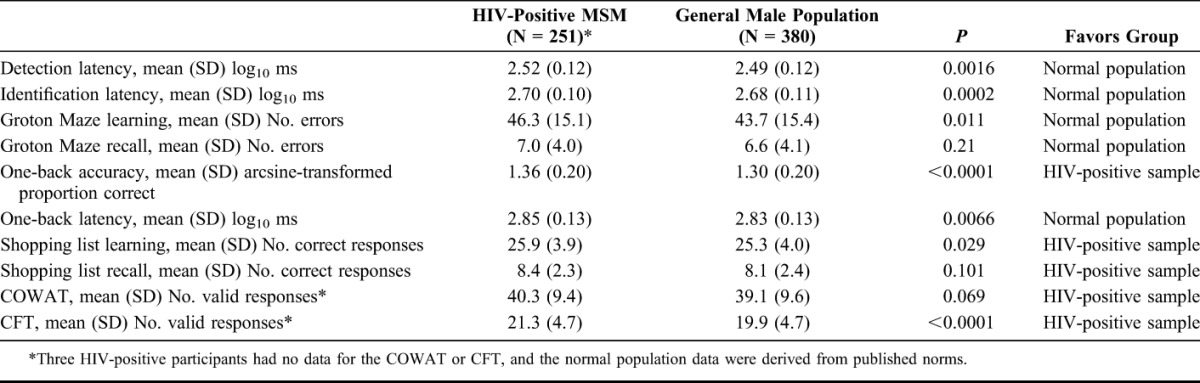
Complete CogState test scores were available from 251 HIV-positive participants. To explore which specific tasks showed better or worse performance in these patients, individual test scores were compared with the general male population data provided by CogState (N = 380) using ANCOVA, adjusted for age and education (Table 3). Statistically significant differences were found between HIV-positive MSM and general male population in 8 of the 10 scores, although the absolute differences between the mean scores were small. The direction of difference favored the HIV-positive sample in 4 tasks (mainly involving verbal abilities) and favored the general male population in 4 tasks (mainly involving speed and attention).
TABLE 3.
Age- and Education-Adjusted Neuropsychological Test Scores in HIV-Positive Male Participants and General Male Population Data Supplied by CogState
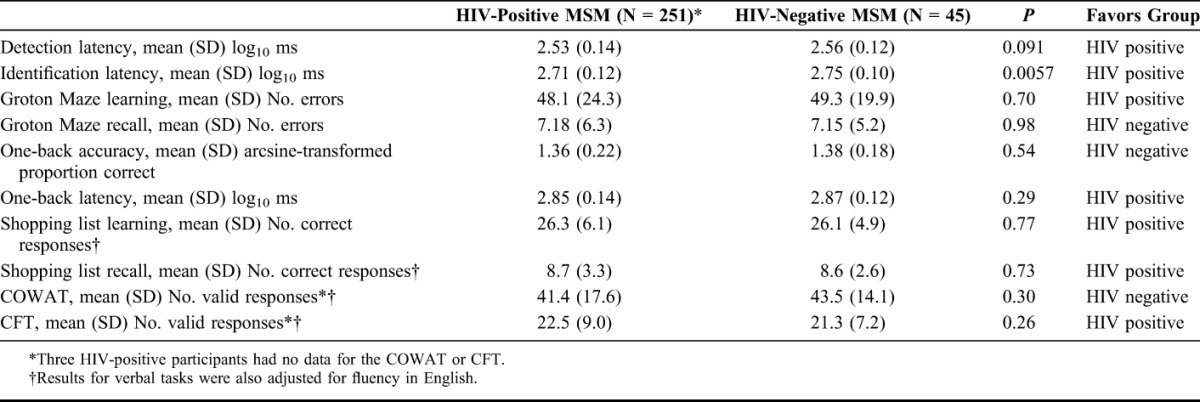
Individual test scores were also compared between HIV-positive and HIV-negative (N = 45) study samples, again adjusting for age and education using ANCOVA, with additional adjustment for fluency in English for the verbal memory and verbal fluency tasks (Table 4). Only 1 test had a statistically significant difference between the 2 groups, the identification task (choice reaction time), which favored HIV-positive participants (P = 0.0057). These comparisons should be interpreted with caution as the group sizes were small (particularly in HIV-negative MSM group), they relied heavily on statistical adjustment, and information was limited on the composition of the general male population sample (N = 380).
TABLE 4.
Age- and Education-Adjusted Neuropsychological Test Scores in HIV-Positive and HIV-Negative Participants
Factors Associated With Neurocognitive Impairment in HIV-Positive Participants
In the HIV-positive group only, factors associated with any grade of HAND (by Frascati criteria) in the final model were of lower educational attainment (OR = 3.41 vs. university or higher degree, 95% CI: 1.73 to 6.70, P < 0.001) and increasing age (OR = 1.05 per +1 year, 95% CI: 1.01 to 1.08, P = 0.017). Other social and demographic factors, ART status, viral load, HCV status, and current and nadir CD4 count were not significantly associated with HAND. There were no significant associations between self-reported risky sexual behavior and any of the executive function–based tasks or global cognitive function. In the HIV-negative group, no factors were identified as significantly associated with NCI, but statistical power was limited for these analyses.
DISCUSSION
In this study, we found prevalence of NCI in HIV-positive participants, all of whom were MSM attending clinics in London, United Kingdom, of 21% using the Frascati criteria for HAND, 31% using GDS across 5 cognitive domains, and 40% using GDS across 10 neuropsychological test scores. Prevalence estimates were very similar for HIV-negative participants, and direct group comparison with general population data found little overall difference in neuropsychological performance. Results were not materially altered after exclusion of those with severe self-reported symptoms of depression. This contrasts with studies from the United States, Switzerland, and France,4–6 which found rates of HAND in HIV-positive subjects exceeding 50% using similar criteria. The low prevalence of symptomatic HAND (MND or HAD) at around 6% after exclusion of those with significant confounding conditions similarly contrasts with previous estimates ranging from 14% to 34%.4–6 Our findings are more in keeping with a study of US veterans in which HIV-positive participants had the same or better cognitive function compared with HIV-negative controls.10 Variation between studies may reflect differences in population characteristics, the testing methodology, and/or the scores used as normative data. Even within our study sample, the choice of case definition had a substantial effect on the estimated prevalence of abnormality.
The term “HIV-associated neurocognitive disorder” contains an implicit attribution of mild neurological impairment in HIV-positive people to HIV itself. However, around one-fifth of the general population would achieve scores more than 1 SD below the mean on 2 of 5 NP tests, rising to half on 2 of 10 tests.12,34 ANI in particular may be a misclassification of those individuals who merely represent 1 end of the normal population distribution. A recent analysis of data from a general population sample in Kenya has estimated that the Frascati criteria may result in a false-positive HAND classification of up to 74% of HIV-positive participants.35
Regarding our neuropsychological testing methodology, the sensitivity of CogState for detecting impairment across the full range of abilities and HAND classifications in HIV-positive patients is not known. These may be limitations to our methods, but one should note that CogState is based on traditional neuropsychological testing methods and has been used to assess cognitive function across many different conditions in clinical and nonclinical settings.18–25 Our conclusions warrant replication using other tests of the same cognitive domains in other samples. We used 2 approaches to measuring executive function: semantic fluency and a visual maze–based learning task. Iudicello et al36 have shown that HIV predominantly affects the executive components of semantic fluency tasks, that is, the cognitive operations related to switching sets, rather than any impairment in semantic memory stores. As with all neuropsychological testing, poor performance observed in some individuals may reflect impairment in cognitive domains other than that for which the test was intended.
Much of the prevalent NCI in HIV-positive populations could be caused by factors other than HIV. For example, drug and alcohol use were high in both HIV-positive and HIV-negative MSM who took part in this study, and such findings are not atypical of HIV-positive populations in many regions.37–39 Mental health conditions, poor premorbid educational attainment, suboptimal effort by participants, and culturally inappropriate tests or norms may all lead to underestimates of NP function. This raises some important clinical and ethical concerns. First, HIV management decisions, such as altering an ART regimen on the basis of a diagnosis of HAND, are questionable, and other factors should be considered and addressed. Second, the diagnosis of ANI could be potentially distressing for HIV-positive individuals, and neuropsychological labels in general may have negative effects and should be applied with caution especially when the prognosis and impact are uncertain.
We found higher rates of self-reported attribution of functional impairment to cognitive difficulties in the HIV-positive group compared with the HIV-negative group (18.8% vs. 6.6%). This suggests that HIV-positive patients are already more vigilant to possible NCI symptoms, perhaps resulting from knowledge of high published prevalence rates. Another possible explanation may be that the study's opportunistic sampling strategy led to over-recruitment of patients with particular concerns about their neuropsychological health.
Estimates of NCI prevalence in HIV-positive samples also depend on the characteristics of the reference population from whom norms are derived. Choice of reference data can significantly affect overall rates of neuropsychological abnormality, in some cases quadrupling it.40 It is important to use a suitable control population, and lack of appropriate methods to control for education, lifestyle, and behavioral characteristics is a limitation of previous studies.41 Our control group was small, which limits our ability to compare neurocognitive function between the 2 groups. We were able to adjust our analyses for age and education, the most important confounders. Substance misuse and mental health problems were common in both HIV-positive and negative groups, but there may have been other unrecognized confounders in our sample.
Education is an important protective factor for dementia in the general population. It is theorized that “cognitive reserve”42 may compensate for neurodegeneration and aging and thus afford preservation of neuropsychological function in the face of these effects.43 Both groups of MSM in our study had relatively high levels of education and may have had greater cognitive reserve, which is also potentially protective against the neurological effects of HIV.44
In summary, these findings have implications for classifying NCI, show that levels of NCI in HIV-positive MSM in the United Kingdom could have been overestimated, and suggest that diagnosed deficits may often not be related to HIV. We propose a re-evaluation of current diagnostic criteria for NCI in HIV, for example, by increasing the level of deficit required to meet the criteria, improving the validity of assessments of everyday function, standardizing NP assessment procedures or weighting particular cognitive domains, including biomarkers, or incorporating repeat assessments. We also propose that the cognitive health of HIV-positive patients should be protected by addressing factors associated with cognitive dysfunction in the general population, for example, by promoting regular exercise and smoking cessation, reducing alcohol and recreational drug use, increasing fruit and vegetable consumption, and treating diabetes and depression.43 When interpreting estimates of HAND prevalence, it is important to recognize our inability to specifically attribute NCI to HIV infection, the inadequacy of imaging and biochemical markers except in severe cases,45,46 issues with the neuropsychological tests themselves, and the confounding effects of age, education, and culture. Only through correct classification we can maximize the efficacy of recommended interventions in those with true HIV-related cognitive impairment.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all study participants for undergoing testing. They also thank Christina Broussard and Robert Pralat for coordinating and administering assessments, Adrian Schembri from CogState for supplying general population data and contributing to the manuscript, and Pamela Muniina and Colette Smith for retrieval of clinical data. The CIPHER study group manages the project across 4 European countries and includes, in addition to the authors, Andrzej Horban, Jens Lundgren, Igor Karpov, and Graham Hart (see http://www.astra-study.org/cipher/c_mgmt).
Footnotes
Supported by the European AIDS Treatment Network and the National Institute for Health Research (NIHR) under its Program Grants for Applied Research funding scheme (RP-PG-0608-10142).
All authors contributed to the conception, conduct and analysis of the study, and have reviewed the final manuscript. S.W., J.M, and colleagues conducted neuropsychological assessments. Data were analyzed by L.H., J.M., M.D., F.L., and AR. The final manuscript was written by L.H., J.M., M.D., F.L., P.M., R.G., L.S., and A.R.
P.M. is one of the founders of CogState and employed by the company as Chief Scientific Officer. A.S. is Director of Science at CogState. The remaining authors have no conflicts of interest to disclose.
Presented at the 20th Conference on Retroviruses and Opportunistic Infections, March 3–6, 2013, Atlanta, CA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.d'Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–328 [DOI] [PubMed] [Google Scholar]
- 2.Garvey LJ, Winston A, Walsh J, et al. HIV-associated central nervous system diseases in the recent combination antiretroviral therapy era. Eur J Neurol. 2011;18:527–534 [DOI] [PubMed] [Google Scholar]
- 3.Bhaskaran K, Mussini C, Antinori A, et al. ; CASCADE Collaboration. Changes in the incidence and predictors of human immunodeficiency virus associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221 [DOI] [PubMed] [Google Scholar]
- 4.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250 [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Clifford DB, Franklin DRJ, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet F, Amieva H, Marquant F, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS. 2013;27:391–400 [DOI] [PubMed] [Google Scholar]
- 7.Tozzi V, Balestra P, Serraino D, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005;21:706–713 [DOI] [PubMed] [Google Scholar]
- 8.Bragança M, Palha A. Depression and neurocognitive performance in Portuguese patients infected with HIV. AIDS Behav. 2011;15:1879–1887 [DOI] [PubMed] [Google Scholar]
- 9.Garvey LJ, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12:333–338 [DOI] [PubMed] [Google Scholar]
- 10.Crum-Cianflone NF, Moore DJ, Letendre S, et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology. 2013;80:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisslen M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis. 2011;11:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadek JR, Vigil O, Grant I, et al. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Exp Neuropsychol. 2007;29:266–276 [DOI] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2012. Stockholm: European Centre for Disease Prevention and Control; 2013 [Google Scholar]
- 15.Blackstone K, Moore DJ, Franklin DRJ, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26:894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of Global Deficit Scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319 [DOI] [PubMed] [Google Scholar]
- 17.Speakman A, Rodger AJ, Phillips AN, et al. The “Antiretrovirals, sexual transmission risk and attitudes” (ASTRA) study. Design, methods and participant characteristics. PLoS One. 2013;8:e77230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruff P, Thomas E, Cysique LA, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178 [DOI] [PubMed] [Google Scholar]
- 19.Cysique LAJ, Maruff P, Darby D, et al. The assessment of cognitive function in advanced HIV-1 infection and AIDS dementia complex using a new computerised cognitive test battery. Arch Clin Neuropsychol. 2006;21:185–194 [DOI] [PubMed] [Google Scholar]
- 20.Makdissi M, Collie A, Maruff P, et al. Computerised assessment of concussed Australian Rules footballers. Br J Sports Med. 2001;35:354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver Cargin J, Maruff P, Collie A, et al. Mild memory impairment in healthy older adults is distinct from normal aging. Brain Cogn. 2006;60:146–155 [DOI] [PubMed] [Google Scholar]
- 22.Harrison J, Maruff P. Measuring the mind: assessing cognitive change in clinical drug trials. Expert Rev Clin Pharmacol. 2008;1:471–473 [DOI] [PubMed] [Google Scholar]
- 23.Lim YY, Ellis KA, Harrington K, et al. Use of the CogState Brief Battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34:345–358 [DOI] [PubMed] [Google Scholar]
- 24.Garvey LJ, Yerrakalva D, Winston A. Correlations between computerized battery testing and a memory questionnaire for identification of neurocognitive impairment in HIV type 1-infected subjects on stable antiretroviral therapy. AIDS Res Hum Retroviruses. 2009;25:765–769 [DOI] [PubMed] [Google Scholar]
- 25.Overton ET, Kauwe JS, Paul R, et al. Performances on the CogState and standard neuropsychological batteries among HIV patients without dementia. AIDS Behav. 2011;15:1902–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186 [PubMed] [Google Scholar]
- 27.Gandhi NS, Skolasky RL, Peters KB, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011;17:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331 [DOI] [PubMed] [Google Scholar]
- 29.Mayfield D, McLoed G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–1123 [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097 [DOI] [PubMed] [Google Scholar]
- 32.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177 [PubMed] [Google Scholar]
- 33.Ruff RM, Light RH, Parker SB, et al. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338 [PubMed] [Google Scholar]
- 34.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10:120–124 [Google Scholar]
- 35.Meyer AC, Boscardin WJ, Kwasa JK, et al. Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-Associated Neurocognitive Disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology. 2013;41:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iudicello JE, Woods SP, Deutsch R, et al. Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. J Clin Exp Neuropsychol. 2012;34:476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker BW, Thames AD, Woo E, et al. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav. 2011;15:1888–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothlind JC, Greenfield TM, Bruce AV, et al. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 2004;18:S19–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winston A, Arenas-Pinto A, Stoehr W, et al. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8:e61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M, Cooper V. HIV and ageing: premature ageing or premature conclusions? Curr Opin Infect Dis. 2012;25:1–3 [DOI] [PubMed] [Google Scholar]
- 42.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460 [PubMed] [Google Scholar]
- 43.Ritchie K, Carrière I, Ritchie CW, et al. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341:c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foley JM, Ettenhofer ML, Kim MS, et al. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Appl Neuropsychol Adult. 2012;19:16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brew BJ, Letendre SL. Biomarkers of HIV related central nervous system disease. Int Rev Psychiatry. 2008;20:73–88 [DOI] [PubMed] [Google Scholar]
- 46.Tucker KA, Robertson KR, Lin W, et al. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol. 2004;157:153–162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


