Supplemental digital content is available in the text.
Keywords: ASKP1240, Costimulation blockade, Kidney transplantation, Nonhuman primate
Abstract
Background
Blocking the CD40-CD154 signal pathway has previously shown promise as a strategy to prevent allograft rejection. In this study, the efficacy of a novel fully human anti-CD40 monoclonal antibody—ASKP1240, administered as a monotherapy or combination therapy (subtherapeutic dose of tacrolimus or mycophenolate mofetil), on the prevention of renal allograft rejection was evaluated in Cynomolgus monkeys.
Methods
Heterotopic kidney transplants were performed in ABO-compatible, stimulation index 2.5 or higher in the two-way mixed lymphocyte reaction monkey pairs. Animals were divided into 12 groups and observed for a maximum of 180 days. Histopathologic, hematology, and biochemistry analyses were conducted in all groups. Cytokine release (interleukin [IL]-2, IL-4, IL-5, IL-6, tumor necrosis factor, and interferon-γ) was investigated in several groups.
Results
ASKP1240 prolonged renal allograft survival in a dose-dependent manner in monotherapy. Low-dose (2 mg/kg) or high-dose (5 mg/kg) ASKP1240, in combination with mycophenolate mofetil (15 mg/kg) or tacrolimus (1 mg/kg), showed a significantly longer allograft survival time compared with monotherapy groups. No obvious side effects including drug-related thromboembolic complications were found. Cytokine release was not induced by ASKP1240 administration.
Conclusion
The present study indicates that ASKP1240, alone or in combination with other immunosuppressive drugs, could be a promising antirejection agent in organ transplantation.
Costimulatory signals play crucial roles in fully activating T cells that are involved in allograft rejection (1–3). The CD40-CD154 costimulatory pathway has previously been shown to be an important interaction that mediates both the humoral and cellular immune responses (4–7). It has been demonstrated that blocking CD40-CD154 signaling pathway is a successful strategy to reduce allograft rejection (8–10). CD154 was initially chosen as primary therapeutic target, but further studies encountered setbacks because of the unexpected thromboembolic complications associated with the administration of anti-CD154 antibodies (11–14). Targeting CD40 as an alternate therapeutic strategy was developed when it was recognized that these thrombotic complications were CD40-independent (15, 16). To date, several anti-CD40 antibodies that are engineered as immunoglobulin G (IgG)1 or IgG4 chimeric isotypes or fully human IgG4 have been shown to prevent allograft rejection in experimental studies (17–21).
ASKP1240 is an anti-CD40 monoclonal antibody (mAb) consisting of fully human IgG4. It interrupts the CD40-CD154 pathway by preventing the interaction between CD40 and CD154. In addition, ASKP1240 does not cause antibody-dependent cell-mediated cytotoxicity or complement dependent cytotoxicity (19, 22). ASKP1240 monotherapy has been demonstrated to delay renal, islet, and hepatic allograft rejection in nonhuman primate (NHP) models (19, 23–25).
This study evaluated the effects of ASKP1240 when administered as a monotherapy or in combination with a subtherapeutic dose of tacrolimus or mycophenolate mofetil (MMF) on renal allograft survival in NHPs. We also assessed the pharmacokinetic (PK) profile of ASKP1240 and its effect on cytokine release in transplanted monkeys. We found that ASKP1240 significantly prolonged allograft survival as a monotherapy or in combination with tacrolimus or MMF without causing obvious side effects.
RESULTS
Renal Allograft Survival
Renal allograft survival day was recorded according to the following definitions: (1) The final day that the serum creatinine (sCr) level did not exceed a value of 10 mg/dL. (2) The day when significant asthenia had been observed. (3) The day before the finding of death. (4) Animal that survived to the end of study was described as longer than 180 days. As shown in Table 1, the median survival time (MST) of groups 1 to 12 was 7, 10, 52, 100.5, 53, 57, 29, 121, longer than 180, 28.5, longer than 180, and longer than 180 days, respectively. Compared with group 1, MST was significantly prolonged in groups 3 to 12 (P=0.005, 0.012, 0.005, 0.001, 0.002, 0.005, 0.005, 0.002, 0.001, 0.001, respectively). ASKP1240 combined with MMF showed longer MST than that of MMF monotherapy (groups 8 and 9 vs. group 7; P=0.001 and 0.017, respectively) (Fig. 1E and F). ASKP1240 combined with a low dose of tacrolimus (0.5 mg/kg) did not show a prolongation in the MST compared with ASKP1240 monotherapy (Fig. 1A and B). However, ASKP1240, in low dose or high dose, combined with a subtherapeutic dose of tacrolimus (1.0 mg/kg), showed significantly longer MST compared with tacrolimus monotherapy groups (groups 11 and 12 vs. group 10; P=0.006 and 0.025, respectively) or the ASKP1240 monotherapy group (group 11 vs. group 3; P=0.011; group 12 vs. group 4; P=0.329) (Fig. 1C and D). In all 12 groups, 19 animals survived to the end of the study. Except for one animal in the tacrolimus 1.0 mg/kg monotherapy group, the remaining animals were treated with low dose or high dose of ASKP1240 as a monotherapy or in combination with tacrolimus or MMF.
TABLE 1.
Renal allograft survival and histopathologic evaluation
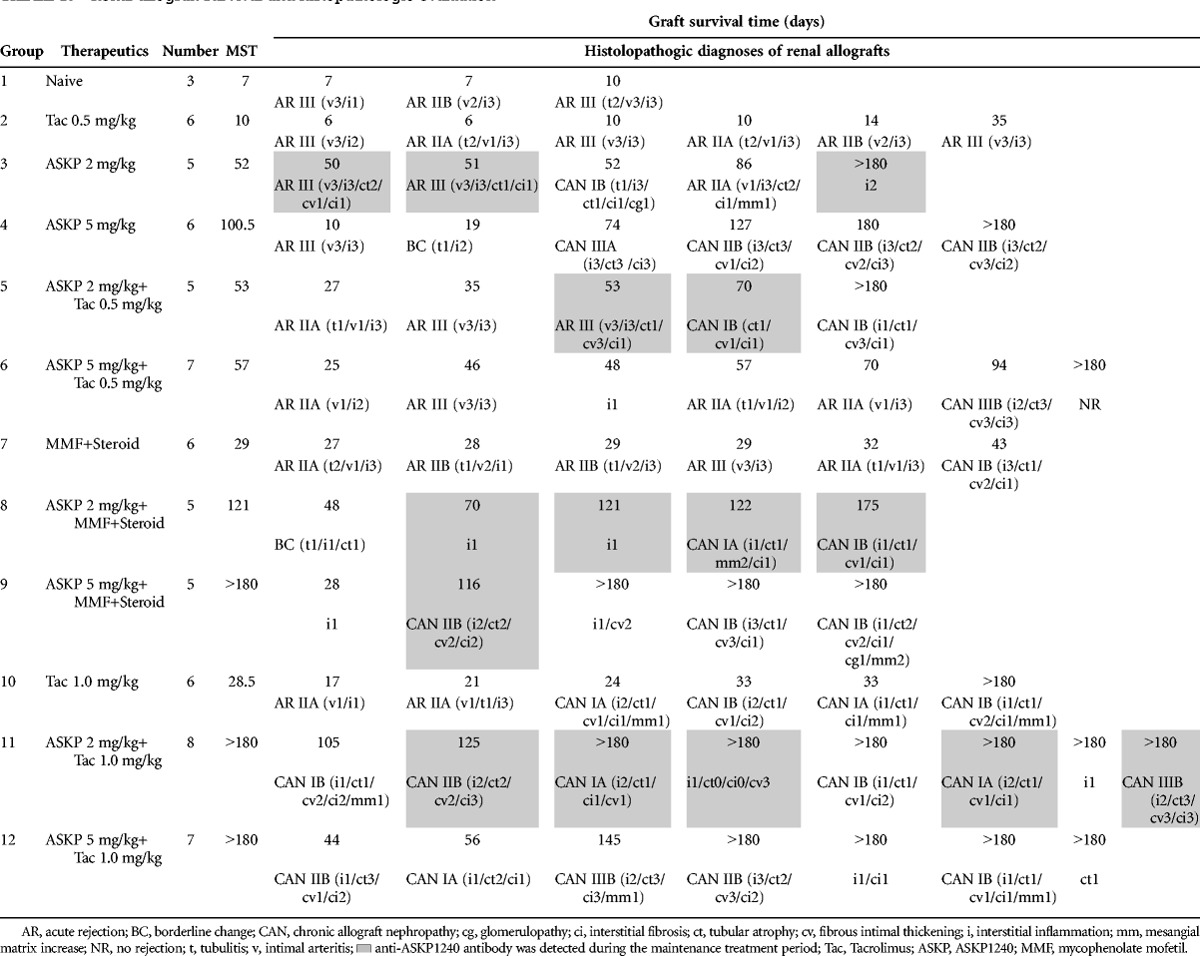
FIGURE 1.
Renal allograft survival in each group. For comparison, each ASKP1240 combination therapy group and the relevant control groups were replicated in panels A to F. The curves represent renal graft survival in naive control (gray solid line), tacrolimus or MMF monotherapy (black dot dash line), ASKP1240 monotherapy (gray dotted line), and combination therapy (black dashed line) group. (Tac 0.5=tacrolimus 0.5 mg/kg, Tac 1.0=tacrolimus 1.0 mg/kg, ASKP 2.0=ASKP1240 2.0 mg/kg, ASKP 5.0=ASKP1240 5.0 mg/kg, MMF=15 mg/kg; *P<0.05 ASKP1240 combination group vs. Tacrolimus or MMF monotherapy group, **P<0.01 ASKP1240 combination group versus tacrolimus or MMF monotherapy group, #P<0.05 ASKP1240 combination group vs. ASKP1240 monotherapy group). 127×152 mm (600×600 DPI). MMF, mycophenolate mofetil.
Renal Graft Function
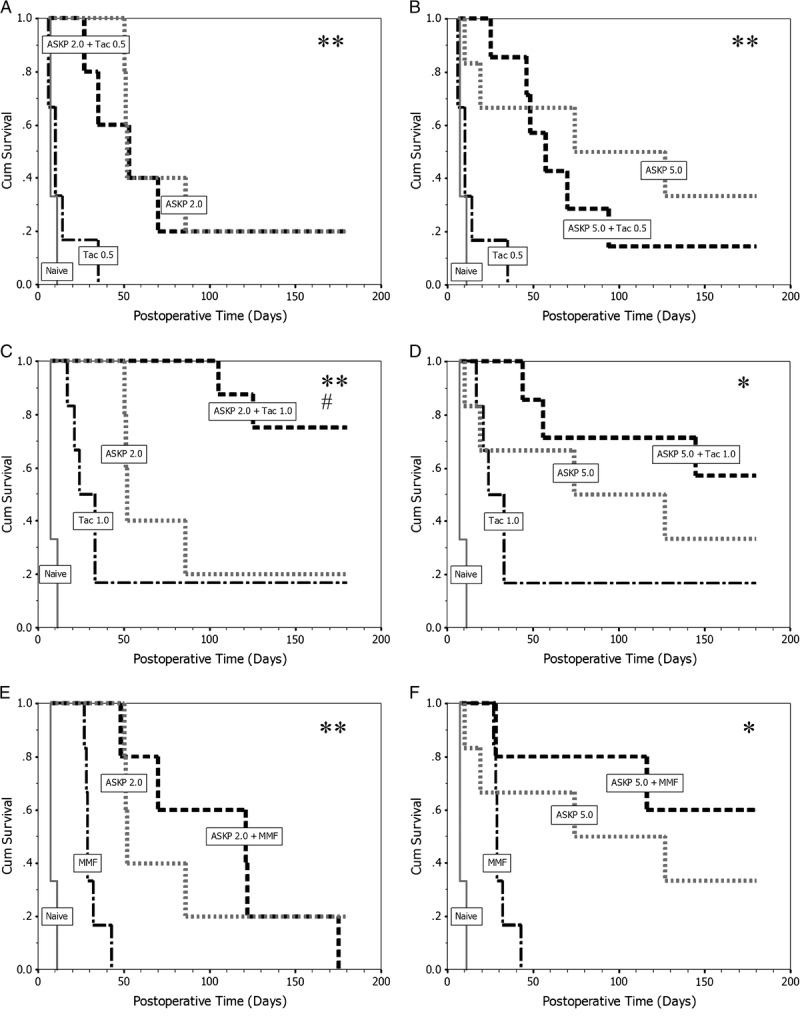
In general, when acute allograft rejection occurred, the sCr and blood urea nitrogen levels of recipient monkeys were rapidly elevated. The renal function parameters were relatively stable in long-term survival monkeys (Fig. 2A and B).
FIGURE 2.
Renal graft function. A, Mean (±SD) serum creatinine level in each group. The elevation of sCr level was consistent with the occurrence of acute allograft rejection. B, Mean (±SD) BUN level in all groups. Changes of mean BUN levels were similar to sCr. 93×89 mm (600×600 DPI). BUN, blood urea nitrogen, SD, standard deviation; sCr, serum creatinine.
Biochemistry
Serum liver enzymes, creatinine kinase, electrolyte levels, and all other biochemical parameters were unaffected by the administration of ASKP1240 (Table S1–S8, SDC, http://links.lww.com/TP/B4).
Hematologic Determinations
The red blood cell, hemoglobin, and hematocrit decreased on week 1 in groups 1, 11, and 12. These parameters were stable in other groups. This phenomenon can be explained based on the blood loss that may occur during the transplantation surgery. There were no consistent changes in white blood cell or platelets counts. The number of CD3+ T cells or CD20+ B cells in peripheral blood mononuclear cells fluctuated during the study period in all groups. However, no clear relationship between CD3+ and CD20+ cell counts and the administration of ASKP1240 was observed.
Body Weight and Clinic Symptoms
Most of the recipient monkey weights remained stable during the study. After recovery from surgery, all recipient monkeys were active with good appetite. However, when acute rejection occurred, animal became lethargic, lost appetite and weight, and developed oliguria which was accompanied by an increase of sCr.
PK Evaluation
Serum ASKP1240 Concentrations
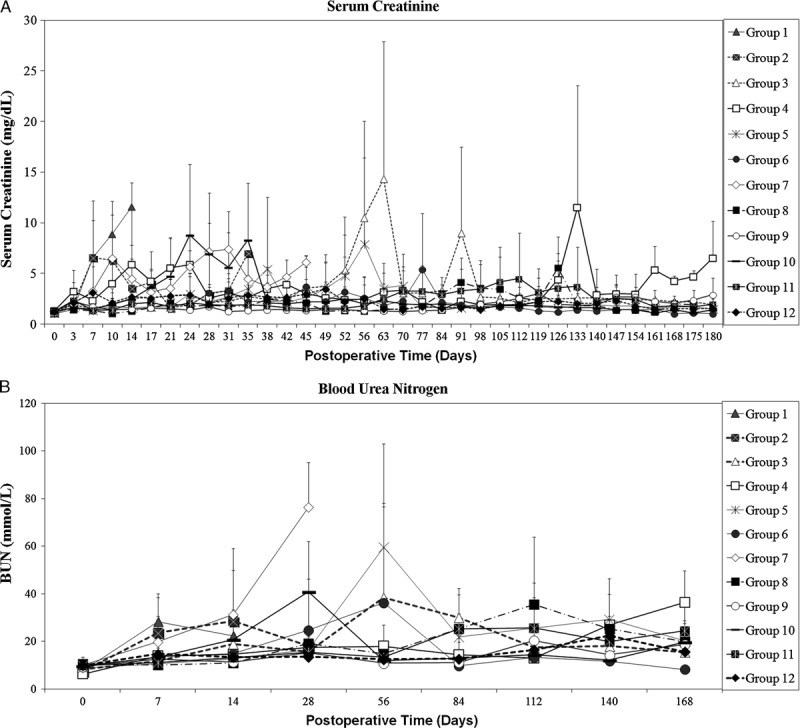
The ASKP1240 concentration-time data are depicted in Figure 3(A). In general, serum concentrations of ASKP1240 increased in a dose-dependent manner. The highest mean values of ASKP1240 trough level in most group appeared on day 14. Thereafter, these values decreased to relative lower levels that were consistent with dose reduction in maintenance phase. From day 56, serum ASKP1240 trough level in most animals dropped to a low level. That was different with what was observed in normal monkeys in another study (26).
FIGURE 3.
Pharmacokinetic evaluation. A, The mean (±SD) serum trough levels of ASKP1240 in groups 3 to 6, 8, and 9, as well as mean (±SD) serum concentration transitions of ASKP1240 in groups 11 and 12. Serum concentrations of ASKP1240 increased in a dose-dependent manner and decreased to relative lower levels 2 weeks after drug administration in maintenance phases. (56BA=before ASKP1240 administration on day 56, 56AA=1 hr after ASKP1240 administration on day 56, day 59=3 days after ASKP1240 administration, day 63=7 days after ASKP1240 administration). B, The mean (±SD) whole blood tacrolimus trough levels increased in a dose-related manner. C, Mean (±SD) plasma trough levels of mycophenolic acid. In general, in the induction phrase, plasma trough levels of mycophenolic acid revealed relatively higher level that was associated with the frequency of MMF administration. The plasma trough levels of mycophenolic acid were stable in maintenance phases. Tac 0.5, tacrolimus 0.5 mg/kg; Tac 1.0, tacrolimus 1.0 mg/kg; ASKP 2.0, ASKP1240 2.0 mg/kg; ASKP 5.0, ASKP1240 5.0 mg/kg; MMF, mycophenolate mofetil 15 mg/kg; SD, standard deviation. 118×157 mm (600×600 DPI).
Blood Concentration of Tacrolimus
The profiles of mean blood trough level of tacrolimus are illustrated in Figure 3(B). The blood trough level of tacrolimus ranged from 0.31 to 85.47 ng/mL. Blood tacrolimus trough level increased in a dose-related manner.
Plasma Concentration of Mycophenolic Acid
The mean plasma trough levels of mycophenolic acid (MPA) are shown in Figure 3(C). Mycophenolic acid was detected in all submitted samples with the minimum value 0.126 μg/mL and the maximum value 10.3 μg/mL from day 7 to day 168. The range of mean trough level of MPA in each group was 1.45 to 3.70 μg/mL for group 7, 0.437 to 3.22 μg/mL for group 8, and 0.411 to 1.47 μg/mL for group 9.
Anti-ASKP1240 Antibody Assay
Anti-ASKP1240 antibodies in serum were detected in 15 of 48 animals during the maintenance treatment period (Table 1). They were mainly found in ASKP1240 2 mg/kg monotherapy or combination therapy groups (14/23), and only one was found in ASKP1240 5 mg/kg treated groups (1/25).
Cytokine Assay
Serum interleukin (IL)-2, IL-4, IL-5, IL-6, tumor necrosis factor (TNF), and interferon-gamma (IFN-γ) were measured in groups 10, 11 and 12. Interleukin-2, IL-4, IL-5, TNF, and IFN-γ were not detected in any animals from day 0 to day 168. Interleukin-6 was found in three samples (one of each group) on day 0 before surgery and dosing. Apparent elevated levels of IL-6 were detected in all day 0 (after surgery and dosing) samples and most day 1 (before and after dosing) samples. From day 3 to day 168, IL-6 was sporadically detected in some animals. No clear difference was noted between the ASKP1240 and tacrolimus combination groups and the tacrolimus mono group. It was concluded that changes of serum IL-6 level were not related to the ASKP1240 administration under the condition of this study.
Histopathology
The details of histologic features of transplanted kidneys were summarized in Table 1. The percentage proportions of histologic types in each group are depicted in Figure 4. Acute rejections were found in all renal grafts of group 1 and group 2. The incidence of acute renal rejection, in ASKP1240 monotherapy groups, dropped to three of five in group 3 (ASKP1240, 2 mg/kg) and one of six in group 4 (ASKP1240, 5 mg/kg). ASKP1240 combined with tacrolimus 0.5 mg/kg (groups 5 and 6) did not show benefits in reducing acute allograft rejection compared with ASKP1240 monotherapy groups. When ASKP1240 combined with tacrolimus 1 mg/kg (groups 11 and 12) or MMF plus steroids (groups 8 and 9), no acute rejections were found in these groups. Varying degrees of chronic allograft nephropathy were the common pathologic changes observed in long-term survival animals, except for one animal in group 6 which displayed no rejection. The histopathologic results of the 19 animals that survived to the end of the study were the following: no rejection=1 (5.3%); nonspecific changes=6 (31.6%); grade I chronic nephropathy=8 (42.1%); grade II chronic nephropathy=3 (15.8%); and grade III chronic nephropathy=1 (5.3%).
FIGURE 4.
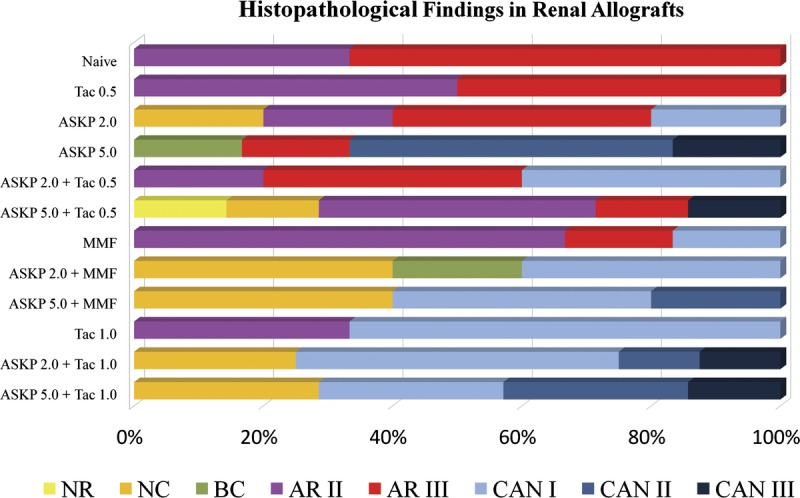
Proportions of histologic types in each group. Acute rejection (red and pink bars) was the only histologic type in Naive and Tac 0.5 groups, and was also the primary histologic type in ASKP 2.0, ASKP 2.0+Tac 0.5, ASKP 5.0+Tac 0.5, and MMF groups. Varying degree of chronic allograft nephropathy (bars with baby blue, cobalt blue or navy blue) constituted the main parts of pathologic findings in the remaining groups. NR, no rejection; NC, nonspecific changes; BC, borderline changes; AR, acute rejection; CAN, chronic allograft nephropathy; Tac 0.5, tacrolimus 0.5 mg/kg; Tac 1.0, tacrolimus 1.0 mg/kg; ASKP 2.0, ASKP1240 2.0 mg/kg; ASKP 5.0, ASKP1240 5.0 mg/kg; MMF, mycophenolate mofetil 15 mg/kg. 117×75 mm (300×300 DPI).
Renal artery thrombosis, a common technical complication in the kidney transplantation model (27), was found in some animals. The incidence of renal artery thrombosis between ASKP1240-treated (5.00%) and non-ASKP1240–treated (4.35%) groups was not significantly different (P=1.000; cases with technical complications were excluded from the analysis of survival and other parameters). No thromboembolism was found in other organs including the brain, lung, heart, liver, pancreas, and spleen in all animals at the time of necropsy and at the histologic assessments in obtained tissue specimens.
Hepatic abscess was developed in one monkey in group 9, and lymphoma was observed in another monkey in group 12 in kidney, mesentery, mediastinum, left lung, and heart.
DISCUSSION
Since the introduction of cyclosporine A into clinical practice in the early 1980s, calcineurin inhibitors (CNIs) have dramatically improved short-term outcomes of renal transplantation. However, the extensive adverse effects of CNIs including nephrotoxicity, neurotoxicity, posttransplantation diabetes, hypertension, and hyperlipidemia impact the long-term success rates of renal transplants. It has been known that most toxicities of CNIs are dose dependent. To minimize the side effects associated with CNI exposure, strategies applied in the clinic include CNI reduction, avoidance, and withdrawal (28, 29). In the Cynomolgus monkey kidney transplant model, tacrolimus 2 mg/kg was the therapeutic dose, whereas 1 mg/kg was considered to be a subtherapeutic dose (30). We show the notable additive effects when ASKP1240 in high dose (5 mg/kg) or in low dose (2 mg/kg) combines with subtherapeutic dose of tacrolimus. Median survival time s of renal allograft were also significantly prolonged in groups of ASKP1240 in combination with MMF and steroid. This implies that ASKP1240 can work in both CNI sparing and avoiding regimens. Previous report indicates the effect of anti-CD154 mAb can be abrogated when it is paired with CNIs (31). Our results demonstrate that it is not the same case with ASKP1240. Further studies to explore the effects of these regimens on immune components at the cellular and molecular level will be helpful to elucidate the exact mechanisms.
Histopathologic results of this study revealed that ASKP1240 monotherapy could successfully inhibit acute renal allograft rejection in a dose-dependent manner. When ASKP1240 combined with MMF or a subtherapeutic dose of tacrolimus, acute allograft rejections were abolished. Previous studies have shown that high-dose ASKP1240 can suppress the generation of donor-specific antibodies (23, 25) which are strongly associated with chronic allograft rejection (32). The effect of ASKP1240 on inhibiting acute rejection has been verified by this and other studies. To clarify the role of ASKP1240 in chronic allograft nephropathy will be an attractive topic for future.
It is notable that during maintenance phase, with 2 weeks dosing interval, serum concentration of ASKP1240 in most animals decreased to a low level. Correspondingly, ASKP1240 trough levels in normal monkeys, especially in the high-dose group, were apparently high. It also found that ASKP1240 half-life of the high-dose group during maintenance phase was markedly shorter than that of normal monkeys (26). These results imply that transplanted monkey may possess a different ASKP1240 metabolic profile compared with that of a normal monkey. One of the possibilities of these differences is caused by CD40 overexpression in transplanted monkey (26). These factors should be taken into consideration during designing optimal dosing regimens.
Previous studies showed that interference with the CD40-CD154 pathway by the administration of anti-CD154 mAbs was associated with thromboembolic events. The possible correlation between anti-CD40 mAbs and thromboembolism has been of concern although it is generally believed that these adverse effects are only associated with anti-CD154 mAbs (16, 33). To date, there have been no preclinical reports indicating that ASKP1240 or any other anti-CD40 mAbs are involved in the thromboembolic complications. In this study, except for renal artery thromboses which are common surgical complications and did not correlate to ASKP1240 treatment, there have been no thromboembolic findings in other organs.
We also measured serum IL-2, IL-4, IL-5, IL-6, TNF, and IFN-γ level in renal transplanted monkeys. The results indicate that ASKP1240 does not induced cytokine release. Anti-ASKP1240 antibodies were detected in some animals (mainly in low-dose groups). The partial reason for the production of these antibodies is because ASKP1240 is a human antibody and a foreign protein for monkey. On the other hand, anti-ASKP1240 antibody is rarely found in high-dose group. One of possible explanation is that higher-dose ASKP1240 may suppress antibody production.
ASKP1240 was shown to be a potent immunosuppressive agent in this NHP kidney transplantation model. Treatment of these animals for up to 180 days was not associated with a deterioration in the general clinical observational signs (activity or appetite) nor was it associated with weight loss. A recent phase I clinical trial supports and extends these animal findings by demonstrating that ASKP1240 is well tolerated in healthy subjects and is not associated with drug-induced cytokine release or with thromboembolic events (34).
In conclusion, ASKP1240, a fully human anti-CD40 mAb, prolongs renal allograft survival in a dose-dependent manner when administered as a monotherapy to renal allograft transplanted Cynomolgus monkeys. ASKP1240, when combined with subtherapeutic dose tacrolimus or MMF plus steroid shows the additive effect on prolonging renal graft survival compared with monotherapy. ASKP1240 seems to be a promising anti-rejection agent in solid organ transplantation. The present results provide concrete support for further clinical studies.
MATERIALS AND METHODS
Animals
Sixty-nine bred male Cynomolgus monkeys, with body weights ranging from 3.1 to 6.0 kg, hepatitis B virus-free, hepatitis C virus-free, simian immunodeficiency virus-free, and Herpes B virus-free, were obtained from laboratory animals center of the Academy of Military Medical Sciences, Beijing, China. All experimental procedures were approved by the Ethical Committee for Animal Experimentation at laboratory animals center of the Academy of Military Medical Sciences and were performed in accordance with the standards described in the Guide for the Care and Use of Laboratory Animals, National Institutes of Health Office of Animal Care and Use. Each animal was identified by number and randomly assigned to a dose group. All animals were screened for general health and quarantined for two weeks before study entry. They were housed in individual cages and were allowed free access to water, fruits, and monkey chow.
Life Supporting Kidney Transplantation
Renal transplantation was performed in ABO compatible, stimulation index of 2.5 or higher in the two-way mixed lymphocyte reaction monkey pairs. Each animal in this study acted as both donor and recipient. Left renal transplantations were performed as previously described (27, 35, 36). Briefly, all donor and recipient monkeys were anesthetized by intramuscular injection of ketamine and xylazine. The left kidneys were exchanged in paired monkeys by transplanting into the upper part of abdomen with end-to-side anastomoses of renal artery to aorta and renal vein to vena cava, and with end-to-end anastomosis of donor and recipient ureters. The right native kidney was removed after the transplanted kidney was reperfused.
Experimental Group and Treatment
Animals were divided into twelve groups as shown in Table 1, that is, naive control, tacrolimus monotherapy, ASKP1240 monotherapy, MMF monotherapy, ASKP1240 and tacrolimus combination, ASKP1240 and MMF combination. ASKP1240 (2 mg/kg or 5 mg/kg) was given intravenously on days 0 (before and after surgery), 3, 7, 11, 14, and then from day 28 to day 168 half dose (1 mg/kg or 2.5 mg/kg) biweekly. Tacrolimus (0.5 mg/kg or 1.0 mg/kg, nontherapeutic dose) (30) was orally administered immediately after kidney transplantation, then once daily, until day 179. Mycophenolate mofetil (15 mg/kg) was given subcutaneously twice daily from day 0 to day 14, and then once daily until day 179. Methylprednisolone (steroid) was administered subcutaneously once per day in a tapering manner. Recipients were observed for maximum of 180 days.
Biochemical and Hematologic Determinations
Serum creatinine was monitored at least twice per week for the first 2 postoperative months then weekly. Blood urea nitrogen, total protein, albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, creatinine kinase, and electrolyte levels (K+, Na+, Cl−) were measured using the auto analyzer.
Hematologic parameters including red blood cell, white blood cell, hemoglobin, hematocrit, platelets, and lymphocyte phenotypes (CD3, CD20) were examined in peripheral blood mononuclear cells using the automated hematology analyzer.
PK Evaluation
Serum ASKP1240 trough levels were measured using enzyme-linked immunosorbent assay as described previously (26) in groups 3 to 6, 8, and 9 on days 0, 7, 14, 28, 56, 84, 112, 140, and 168. The whole blood trough levels of tacrolimus in groups 2, 5, 6, 10 to 12 and plasma trough levels of MPA in groups 7 to 9 were determined using a validated LC-Tandem Mass Spectrometry (API 5000 LC/MS/MS System) assay on days 0, 7, 14, 28, 56, 84, 112, 140, and 168. In groups 11 and 12, the serum concentration transition of ASKP1240 was monitored on days 0, 7, 14, 28, 42, 56 (before and 1 hr after administration), 59, 63, 84, 112, 140, and 168.
Immunologic Assays
Anti-ASKP1240 Antibody
Monkey anti-ASKP1240 antibodies were detected using an enzyme-linked immunosorbent assay screening assay and further confirmed using immunodepletion assays at Shin Nippon Biomedical Laboratories (26).
Cytokine Assay
The serum concentrations of IL-2, IL-4, IL-5, IL-6, TNF, and IFN-γ were analyzed with cytometoric beads array NHP Th1/Th2 Cytokine Kit (BD Biosciences, San Jose, CA) using flow cytometer (FACSCalibur, BD Biosciences).
Histopathologic Determinations
All recipient monkeys were subjected to complete gross necropsies. Routine hematoxylin-eosin staining were performed on all paraffin-embedded samples sections including graft kidney, liver, pancreas, spleen, heart, lung, stomach, jejunum, thoracic aorta, and mesentery lymph nodes. Graft kidney tissues were stained with Masson’s Trichrome and Periodic acid Schiff as well. The Banff ’97 classification of kidney pathology was used for scoring the presence and degree of renal rejection (37).
Statistical Analysis
All results of body weight, biochemistry analyses, and drug concentrations were presented as mean±standard deviation. The statistical differences among groups were analyzed using one-way analysis of variance. Survival of renal allograft was presented as MST, with comparisons among groups performed by log rank test. A P value less than 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the expert technical support of the Shin Nippon Biomedical Laboratories, Ltd. Japan and Frontage Laboratories Co., Ltd. China.
Footnotes
This study was supported by Astellas Pharma Inc., Japan and Kyowa Hakko Kirin Co., Ltd., Japan.
The authors declare no conflicts of interest.
F.K., Y.S., Y.M., K.O., T.M., H.C. participated in the research design. L.S. participated in the writing of the article. L.S., A.M., H.D., Y.H., L.Z., J.B., G.Z., H.C. participated in the performance of the research. L.S., A.M. participated in data analysis. P.D. provided critical revision of the article for important intellectual content. H.C. and F.K. participated in the final approval of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1. Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med 1987; 165: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol 1988; 140: 3324. [PubMed] [Google Scholar]
- 3. Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest 2002; 109: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eliopoulos AG, Davies C, Knox PG, et al. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol 2000; 20: 5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pype S, Declercq W, Ibrahimi A, et al. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem 2000; 275: 18586. [DOI] [PubMed] [Google Scholar]
- 6. Li XL, Ménoret S, Le Mauff B, et al. Promises and obstacles for the blockade of CD40-CD40L interactions in allotransplantation. Transplantation 2008; 86: 10. [DOI] [PubMed] [Google Scholar]
- 7. Elgueta R, Benson MJ, de Vries VC, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009; 229: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996; 381: 434. [DOI] [PubMed] [Google Scholar]
- 9. Parker DC, Greiner DL, Phillips NE, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci U S A 1995; 92: 9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med 1999; 5: 686. [DOI] [PubMed] [Google Scholar]
- 11. Kawai T, Andrews D, Colvin RB, et al. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000; 6: 114. [DOI] [PubMed] [Google Scholar]
- 12. Schuler W, Bigaud M, Brinkmann V, et al. Efficacy and safety of ABI793, a novel human anti-human CD154 monoclonal antibody, in Cynomolgus monkey renal allotransplantation. Transplantation 2004; 77: 717. [DOI] [PubMed] [Google Scholar]
- 13. Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation 2004; 77: 460. [DOI] [PubMed] [Google Scholar]
- 14. Kirk AD, Knechtle SJ, Sollinger HW, et al. Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation [abstract]. Am J Transplant 2001; 1 (Suppl 1): 191 [Google Scholar]
- 15. André P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin-dependent mechanism. Nat Med 2002; 8: 247. [DOI] [PubMed] [Google Scholar]
- 16. Crow AR, Leytin V, Starkey AF, et al. CD154 (CD40 ligand)-deficient mice exhibit prolonged bleeding time and decreased shear-induced platelet aggregates. J Thromb Haemost 2003; 1: 850. [DOI] [PubMed] [Google Scholar]
- 17. Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation 2003; 75: 637. [DOI] [PubMed] [Google Scholar]
- 18. Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation 2002; 74: 933. [DOI] [PubMed] [Google Scholar]
- 19. Imai A, Suzuki T, Sugitani A, et al. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in Cynomolgus monkeys. Transplantation 2007; 84: 1020. [DOI] [PubMed] [Google Scholar]
- 20. Badell IR, Thompson PW, Turner AP, et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am J Transplant 2012; 12: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page A, Srinivasan S, Singh K, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant 2012; 12: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Pol W, van de Winkel JG. IgG receptor polymorphisms: risk factors for disease. Immunogenetics 1998; 48: 222. [DOI] [PubMed] [Google Scholar]
- 23. Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in Cynomolgus monkeys: Induction and maintenance therapy. Am J Transplant 2009; 9: 1732. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe M, Yamashita K, Suzuki T, et al. Long-term acceptance of islet allografts by ASKP1240 (4D11), a fully human anti-CD40 monoclonal antibody in Cynomolgus monkeys [abstract]. Am J Transplant 2011; 11 (Suppl 2): 80 [Google Scholar]
- 25. Oura T, Yamashita K, Suzuki T, et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant 2012; 12: 1740. [DOI] [PubMed] [Google Scholar]
- 26. Ma A, Dun H, Song L, et al. Pharmacokinetics and pharmacodynamics of ASKP1240, a fully human anti-CD40 antibody, in normal and renal transplanted Cynomolgus monkeys. Transplantation 2014; 97: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song L, Qi S, Dun H, et al. Surgical complications in kidney transplantation in nonhuman primates. Microsurgery 2010; 30: 327. [DOI] [PubMed] [Google Scholar]
- 28. Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant 2008; 22: 1. [DOI] [PubMed] [Google Scholar]
- 29. Barbari AG, Stephan AG, Masri MA. Calcineurin inhibitor-free protocols: risks and benefits. Saudi J Kidney Dis Transpl 2007; 18: 1. [PubMed] [Google Scholar]
- 30. Kinugasa F, Nagatomi I, Ishikawa H, et al. Efficacy of oral treatment with tacrolimus in the renal transplant model in Cynomolgus monkeys. J Pharmacol Sci 2008; 108: 529. [DOI] [PubMed] [Google Scholar]
- 31. Blaha P, Bigenzahn S, Koporc Z, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood 2003; 101: 2886 [DOI] [PubMed] [Google Scholar]
- 32. Mao Q, Terasaki PI, Cai J, et al. Extremely high association between appearance of HLA antibodies and failure of kidney grafts in a five-year longitudinal study. Am J Transplant 2007; 7: 864. [DOI] [PubMed] [Google Scholar]
- 33. Larsen CP, Knechtle SJ, Adams A, et al. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant 2006; 6: 876. [DOI] [PubMed] [Google Scholar]
- 34. Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjects. Am J Transplant 2013; 13: 1040. [DOI] [PubMed] [Google Scholar]
- 35. Qi S, Xu D, Peng J, et al. Effect of tacrolimus (FK506) and sirolimus (rapamycin) mono- and combination therapy in prolongation of renal allograft survival in the monkey. Transplantation 2000; 69: 1275. [DOI] [PubMed] [Google Scholar]
- 36. Chen H, Peng J, Luo H, et al. Compromised kidney graft rejection response in Vervet monkeys after withdrawal of immunosuppressants tacrolimus and sirolimus. Transplantation 2000; 69: 1555. [DOI] [PubMed] [Google Scholar]
- 37. Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


