Abstract
Purpose
Neuroretinal ischemic injury contributes to several degenerative diseases in the eye and the resulting pathogenic processes involving a series of necrotic and apoptotic events. This study investigates the time and extent of changes in acetylation, and whether this influences function and survival of neuroretinal cells following injury.
Methods
Studies evaluated the time course of changes in histone deacetylase (HDAC) activity, histone-H3 acetylation and caspase-3 activation levels as well as retinal morphology and function (electroretinography) following ischemia. In addition, the effect of two HDAC inhibitors, trichostatin-A and valproic acid were also investigated.
Results
In normal eyes, retinal ischemia produced a significant increase in HDAC activity within 2 hours that was followed by a corresponding significant decrease in protein acetylation by 4 hours. Activated caspase-3 levels were significantly elevated by 24 hours. Treatment with HDAC inhibitors blocked the early decrease in protein acetylation and activation of caspase-3. Retinal immunohistochemistry demonstrated that systemic administration of trichostatin-A or valproic acid, resulted in hyperacetylation of all retinal layers after systemic treatment. In addition, HDAC inhibitors provided a significant functional and structural neuroprtection at seven days following injury relative to vehicle-treated eyes.
Conclusions
These results provide evidence that increases in HDAC activity is an early event following retinal ischemia, and are accompanied by corresponding decreases in acetylation in advance of caspase-3 activation. In addition to preserving acetylation status, the administration of HDAC inhibitors suppressed caspase activation and provided structural and functional neuroprotection in model of ischemic retinal injury. Taken together these data provide evidence that decrease in retinal acetylation status is a central event in ischemic retinal injury, and the hyperacetylation induced by HDAC inhibition can provide acute neuroprotection.
Keywords: retina, neuroprotection, acetylation, ischemia, HDAC
1. INTRODUCTION
Retinal ischemia is associated with a number of vision impairing degenerative diseases including glaucoma, diabetic retinopathy, and retinopathy of prematurity. Knowledge of the cellular events occurring in retinal neurons after ischemic injury is important in understanding the pathophysiological response to ischemia and searching for new treatments for blinding diseases. Protein acetylation, like phosphorylation, plays a significant role in regulation of cellular activity and is controlled by the competing actions of two enzyme families, histone acetyltransferases and histone deacetylases (HDACs) (Crosson et al., 2010). The balance between the actions of these enzymes serves as a key regulatory mechanism for gene expression by modulating chromatin condensation, as well as several signaling events within cells (Haberland et al., 2009).
Dysregulation of acetylation contributes to the pathogenesis of a myriad of diseases, including oncogenic, cardiovascular, and inflammatory disorders (Zhang, McKinsey et al., 2002; Huang, 2006). In the central nervous system, in vitro studies have shown that the inhibition of HDACs can protect neurons from oxidative and nitrosative stress, and glutamate-induced excitotoxicity, as well as promote neuronal growth and prolong neuronal lifespan (Zhong and Kowluru, 2010; Hao et al., 2004; Kanai et al., 2004). In vivo studies have provided evidence that HDAC inhibition protected neurons exposed to intracerebral hemorrhage, ischemic injury and stroke (Kim et al., 2007; Sinn et al., 2007). These effects involve regulation of gene expression at the molecular level through epigenetic mechanisms, particularly in chromatin remodeling, via direct inhibition of HDACs preventing histone hypoacetylation of specific regions of the chromatin (Phiel et al., 2001).
This article focuses on how protein acetylation is an early event in the post-ischemic environment. Specifically, a rodent model of retinal ischemia was utilized to address changes in the acetylation state of histone-H3 at different time intervals following ischemic injury and to provide a direct comparison to changes in HDAC enzymatic activity levels as well as changes in an apoptotic marker, retinal cleaved caspase-3. This study expanded on previous studies from this laboratory, and evaluated how pharmacologically inhibiting these changes, using two structurally distinct HDAC inhibitors, trichostatin-A (TSA) and valproic acid (VPA), may provide similar structural and functional neuroprotection.
2. MATERIAL and METHODS
2.1 Animals
Adult male or female brown Norway rats (3-5 months of age, 150-200 grams; Charles River Laboratories, Inc., Wilmington, MA) were used in this study. Rats were maintained in an environmental cycle of 12-hours light and 12-hours dark. Animal handling was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research; and the study protocol was approved by the Animal Care and Use Committee at the Medical University of South Carolina. Previous studies from this laboratory have demonstrated effectiveness of TSA at a dose of 2.5 mg/kg i.p. (Crosson et al., 2010). Studies on VPA have shown neuroprotective effects at 200 mg/kg/day (Dou et al., 2003); however in the current study animals developed motor defects immediately after treatment with 200 mg/kg VPA. Subsequent preliminary dosing studies found that control animal treated twice daily with 100 mg/kg, did not exhibit any motor defects, and hyperacetylation was noted in the retina. Therefore, for neuroprotection studies, trichostatin-A (TSA) (2.5 mg/kg), valproic acid (VPA) (100 mg/kg), or vehicle (0.9% sodium chloride) was administered by intraperitoneal (i.p.) injection one hour prior and 3 hours following ischemic injury on the day studies were initiated. On post-ischemic days 1, 2, and 3, TSA, VPA or vehicle was administered twice daily. In animals receiving any i.p. treatment, functional and morphological results from contralateral eyes were used as control comparisons. For timing experiments, ischemic injury was induced in identical fashion, and retinal lysates were obtained at several early time points within the initial 24 hours after ischemia induction to analyze levels of acetylated histone-H3 and cleaved caspase-3 using Western blotting, and histone deacetylase (HDAC) activity using a fluorometric enzymatic assay.
2.2 Retinal Ischemia
Prior to the induction of retinal ischemia, rats were anesthetized by i.p. injection of ketamine (75 mg/kg) and xylazine (8 mg/kg) (Ben Venue Laboratories, Bedford, OH), and corneal analgesia created by the application of proparacaine (0.5%; 5 L; Akorn, Inc., Buffalo Grove, IL). Body temperature was maintained at 37°C by means of a heating pad (Harvard Apparatus; Holliston, MA). Retinal ischemia was created using methods previously described by Whitlock and colleagues (Whitlock et al., 2005). Briefly, the anterior chamber was cannulated with a 30-G needle that was connected to a container of sterile normal saline via polyethylene tubing (PE-50; Fischer, Atlanta, GA). To induce retina ischemia, the reservoir was elevated to raise the intraocular pressure (IOP) above systolic blood pressure to 160 mmHg for 45 minutes. The IOP was monitored by an in-line pressure transducer connected to a computer. Each pressure then returned to normal and the eye was examined to ensure that retinal blood flow was reestablished. The contralateral eye was left untreated, serving as control.
2.3 Electroretinograms
To quantitate baseline and post-ischemic neuroretinal function, electroretinograms (ERGs) were performed. Baseline values were obtained one day prior to ischemic injury and seven days post-injury. For these studies, rats were dark-adapted overnight. On the following day, rats were anesthetized with i.p. ketamine and xylazine administration as described above, and pupils dilated with a 10 L drop of a solution containing phenylephrine HCl (2.5%) and tropicamide (1%) (Akorn, Inc., Buffalo Grove, IL). A needle ground-electrode was placed subcutaneously in the back of the animal and a reference electrode on the tongue. A contact lens gold-ring electrode was held in place on the cornea with a drop of methylcellulose. A stimulus-intensity series of ERGs was recorded in response to single-flash intensities, from 40 dB attenuation (low-intensity flash), to no attenuation (high-intensity flash). Responses were an average of 2 flashes with an inter-stimulus interval of 2 minutes. Electroretinograms were recorded by means of an UTAS-2000 system (LKC Technologies, Gaithersburg, MD). Amplitudes of ERG a- and b-waves from ischemic eyes of VPA-treated animals were compared to contralateral control responses and corresponding responses from vehicle-treated animals.
2.4 Morphometric Analysis
For histological examination, rats were euthanized by an overdose of pentobarbital. Eyes were then enucleated and fixed for 1 hour in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) at 4°C. The eyes were opened at the ora serrata and fixation continued for 24 hours. Following fixation, the anterior segment was removed and the posterior eyecup dehydrated and embedded in paraffin. Retina cross-sections (5 m thick were then cut and stained with hematoxylin and eosin (Sigma Chemical Co., St. Louis, MO). Retina sections were photographed and measured 450 to 550 μm from the edge of the optic nerve head by means of a Zeiss Axioplan-2 fluorescent microscope (Maple Grove, MN).
For immunohistochemical analysis, selected eyes were fixed in 4% paraformaldehyde for 1 hour. Globes were washed in PBS and transferred into 15% sucrose solution for 1 hour, followed by 30% sucrose solution overnight at 4°C. Tissues were embedded in optimal cutting temperature (OCT) compound (Tissue Tek; Sakura Finetech, Torrance, CA) and 10 μm slices sectioned at -26°C. The sections were washed in PBS to remove OCT and blocked with 5% normal donkey serum, 3% bovine serum albumin, and 0.1% Triton X-100 in PBS for 1 hour at room temperature. Sections were incubated in primary antibody specific to acetyl histone-H3 (1:500 dilution) (Cell-Signaling Technologies) at 4°C overnight. Sections were then washed and incubated for two hours at room temperature with FITC-labeled secondary antibody (1:100 dilution) (Invitrogen). For negative controls, the staining with primary antibody was omitted and sections were stained with only FITC-labeled secondary antibody. Retina sections were observed and photographed by means of a Zeiss Axioplan-2 fluorescence microscope (Maple Grove, MN).
2.5 Western Blot Analysis
Western blot analysis was performed after homogenization of whole retina in lysis buffer (50 mM Tris-base; 10 mM EDTA; 0.5 mM sodium orthovanadate; 0.5% sodium deoxycholic acid; 1% Triton X-100) and protease inhibitors. Equivalent amounts of protein were loaded onto 10% SDS polyacrylamide gels; proteins were separated by PAGE and transferred to nitrocellulose paper. The membranes were blocked in 5% nonfat dry milk followed by incubation for 24 hours at 4°C with appropriate primary antibodies selective for acetyl histone-H3 (K9) (1:1000 dilution) (Cell-Signaling Technologies), β-actin (1:1000 dilution) (Cell-Signaling Technologies), cleaved caspase-3 (1:500) (Cell-Signaling Technologies), and the following HDAC isoforms: HDAC1 (1:500) (Millipore), HDAC2 (1:1000) (Invitrogen), HDAC3 (1:500) (Cell-Signaling Technologies) and HDAC6 (1:500) (Cell-Signaling Technologies). After washing, membranes were incubated for 1 hour at room temperature with appropriate secondary antibodies (horseradish peroxidase [HRP]-conjugated; 1:1000 dilution) (Sigma). Prestained molecular weight markers were run in parallel to identify the molecular weight of the proteins of interest. For chemiluminescent detection, the membranes were treated with enhanced chemiluminescence reagents, and the densitometric signal monitored using a Biorad Versadoc imaging system (Biorad, Hercules, CA).
2.6 HDAC Activity Assay
The deacetylase activities of Class-I and Class-II HDACs were measured by assaying enzyme activity using the peptidase, trypsin, and the fluorophore-conjugated synthetic substrate, t-butoxyacetyl-lysine aminomethoxy-cumarin (Boc-Lys(Ac)-AMC), as previously described (Wegener et al., 2003). Retinal lysates were obtained by sonicating whole retina in 400 L lysis buffer (50 mM Tris-base; 10 mM EDTA; 0.5 mM sodium orthovanadate; 0.5% sodium deoxycholic acid; 1% Triton X-100). Lysates were then centrifuged at 10,000 rpm for 10 minutes and the pellet discarded. All samples were were normalized to a protein concentration of 1.0 g/ L by addition of standard HDAC buffer (50 mM Tris-Cl pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2 and 0.1 mg/mL bovine serum albumin) and incubated with the conjugated-fluorophore acetylated lysine substrate Boc-Lys(Ac)-AMC in 96-well non-binding plates (Greiner Bio-one, NC) at room temperature for 2 hours. Baseline fluorescence was measured followed by treatment with the peptidase enzyme trypsin, freeing the fluorogenic 4-methylcoumarin-7-amide (AMC). The amount of fluorogenic AMC generated was then measured using an excitation wavelength of 355 nm and emission wavelength of 460 nm with a standard fluorospectrometer. The substrate for Class-I in this assay is specific to HDAC1, 2, 3 and 6.
2.7 Statistical Analysis
For all experiments, data were expressed as mean ± SEM. For comparison between two groups non-paired Student t-test was utilized. For comparing three or more treatment groups, analysis of variance (ANOVA) using the Dunnett posttest (GraphPad Software, Inc., San Diego, CA) was utilized. A P <0.05 was considered significant.
3. RESULTS
3.1 HDAC Activity Increases Early Following an Ischemic Event
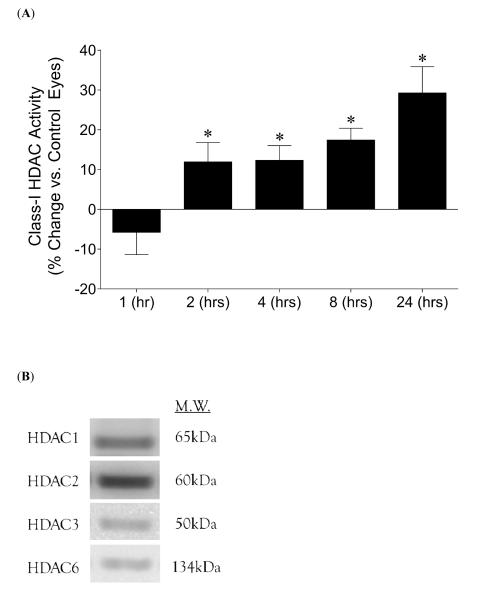
Total enzymatic activity for HDAC1, 2, 3, and 6 are summarized in Figure 1A. Significant increases in HDAC activity in ischemic eyes were observed as early as 2 hours following neuroretinal injury (11.9 ± 4.9%) (P <0.05). Furthermore, activity levels continued to rise reaching 31.0 ± 6.1% (P <0.05) at 24 hours following injury, demonstrating significant increases in HDAC activity relative to all earlier time periods. Figure 1B shows presence of retinal protein from the four HDACs evaluated by the assay. Class-II HDAC activity and HDAC8 activity demonstrated no changes relative to control values, and HDAC protein levels at 24 hours showed no change (data not shown). These results indicated that increased HDAC activity is an early event following retinal cell injury.
Figure 1.
Effect of 45 minutes of acute ischemia on retinal Class-I HDAC enzymatic activity. (A) Extent of HDAC activity was examined by fluorescent detection of aminomethoxy-cumarin (AMC) following cleavage from enzymatically-deacetylated lysines at different time points following ischemic injury. Using Student t-test, significant percentage increases (* P <0.05) in HDAC activity were observed as early as 2 hours (11.9 ± 4.9; n=4) post-ischemia initiation, and remained elevated at later time points when compared to contralateral control eyes (set at 100%): 4 hours (12.3 ± 6.6; n=5), 8 hours (17.4 ± 3.0; n=7), 24 hours (31.0 ± 6.1; n=10). The 24-hour data were also significantly elevated when compared to all lesser time periods. No significant changes were observed at 1 hour (-5.8 ± 6.0; n=4). (B) Representative Western blots indicating retinal presence of each of the four HDACs evaluated by the assay. Abbreviations: Hours post-ischemia initiation (hrs).
3.2 Changes in Acetylation Following Ischemic Retinal Injury
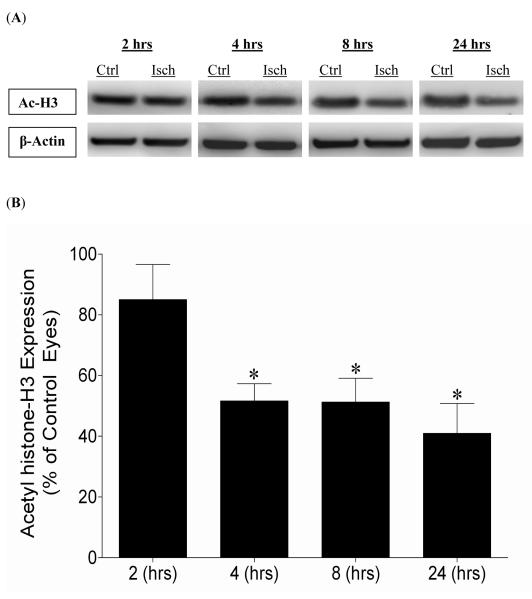
Global histone acetylation changes in ischemic eyes relative to contralateral control eyes were evaluated by comparison of acetyl histone-H3 protein. Figure 2 shows qualitative and quantitative changes in acetyl histone-H3 (K9) levels in ischemic eyes relative to control eyes of the same animal. While a trend toward lower acetylation levels were noted at 2 hours post-ischemic injury these changes were not significant. At 4 hours post-ischemic injury, acetyl histone-H3 levels were significantly reduced to 51.6 ± 5.7% compared to control contralateral eyes. These acetylation levels remained significantly reduced through 24 hours.
Figure 2.
Effect of 45 minutes of acute ischemia on early changes in acetylation status of histone-H3. Contralateral eyes that did not receive ischemia were designated as controls and were set to 100%. (A) Representative Western blots of retinal lysates for acetyl histone-H3 and β-actin at 24 hours after initiation of ischemic injury. (B) Ratio of acetyl histone-H3/β-actin measured at 2, 4, 8, and 24 hours from the initiation of 45 minutes of ischemia. Ratios are expressed as a mean percentage of the ischemic eyes relative to the control eyes (2 hours, 85.0 ± 11.6 (n=4); 4 hours, 51.6 ± 5.7 (n=5); 8 hours, 51.3 ± 7.9 (n=7); 24 hours, 41.0 ± 9.8 (n=9)). Student t-test was utilized (* P <0.05). Abbreviations: Acetyl histone-H3 (Ac-H3); Control eyes (Ctrl); Hours post-ischemia initiation (hrs); Ischemic eyes (Isch).
3.3 Activated Caspase-3 in the Ischemic Injury Process
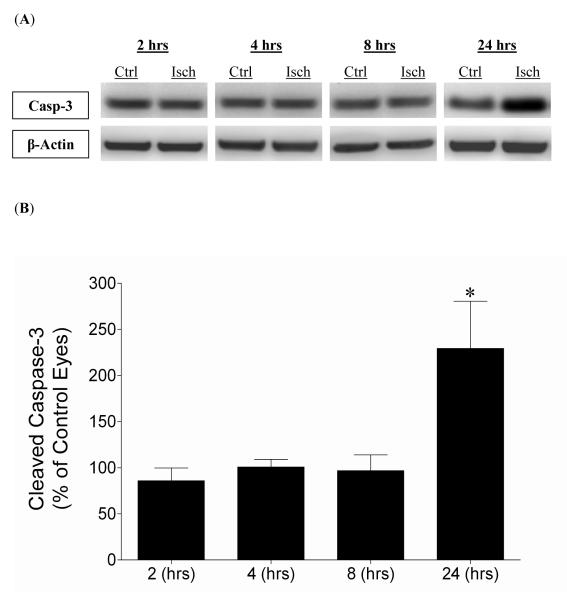
An estimation of apoptotic activity in ischemic eyes relative to contralateral control eyes was evaluated by comparing levels of cleaved caspase-3 protein. Figure 3 shows the levels of cleaved caspase-3 at 2, 4, 8, and 24 hours post-initiation of ischemia. No significant change in activated caspase-3 was measured at 2, 4, or 8 hours post-ischemic injury. However, at 24 hours post-ischemia, activated caspase-3 levels were elevated by 229% (P <0.05), when compared to contralateral eyes.
Figure 3.
Effect of 45 minutes of acute ischemia on retinal cleaved caspase-3 levels 2, 4, 8, and 24 hours from ischemia initiation. (A) Representative Western blot of retinal lysates for cleaved caspase-3 and β-actin at 2, 4, 8, and 24 hours after initiation of ischemic injury. (B) Levels are expressed as a mean percentage of the ischemic eyes relative to the control eyes with no significant changes at 2 hours (85.9 ± 13.6; n=4), 4 hours (100.8 ± 8.2; n=5), and 8 hours (96.8 ± 17.2; n=7). Significant increases (* P <0.05) in cleaved caspase-3 occurred at 24 hours (229.4 ± 51.1; n=9) after initiation of ischemia (Student t-test). Abbreviations: Cleaved caspase-3 (Casp-3); Control eyes (Ctrl); Hours post-ischemia initiation (hrs); Ischemic eyes (Isch).
3.4 HDAC Inhibition and Retinal Acetylation
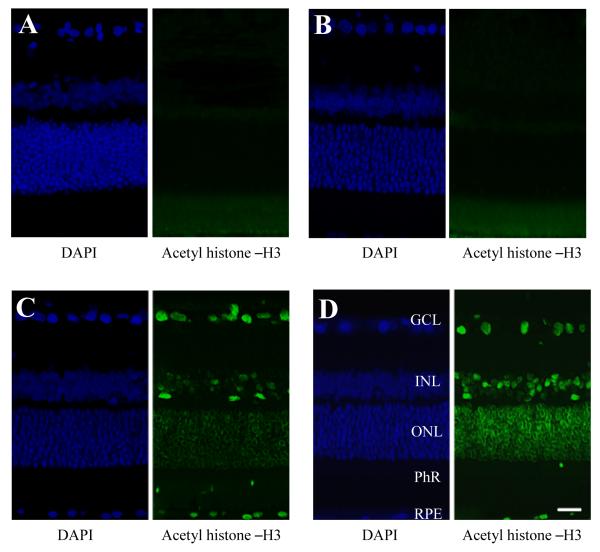
In order to assess if systemic administration of HDAC inhibitors can prevent the hypoacetylation of retinal chromatin, rats were treated with TSA (2.5 mg/kg), VPA (100 mg/kg), or vehicle and the levels of acetylated histone-H3 in the retina were evaluated by immunohistochemistry 24 hours later. As presented in Figure 4, labeled nuclei were observed in all retinal nuclear layers as well as the cell bodies of the RPE in animals treated with either HDAC inhibitor. Vehicle-treated animals showed some autofluorescence of the photoreceptor layer, but otherwise no detectable staining of other retinal layers. These observations indicate that both TSA and VPA have retinal bioavailability 24 hours post-i.p. administration.
Figure 4.
Effect of HDAC inhibition on retinal histone-H3 acetylation. Animals were treated with vehicle, TSA (2.5 mg/kg; i.p) or VPA (100 mg/kg; i.p.) 24 hours prior to tissue analysis. Immunohistochemical staining for retina acetylated histone-H3 from (A) negative-control (no primary antibody), (B) vehicle-treated, (C) TSA-treated, and (D) VPA-treated animals, with corresponding DAPI staining of cell bodies. Abbreviations: ganglion cell layer (GCL); inner nuclear layer (INL); outer nuclear layer (ONL); photoreceptor layer (PhL); retinal pigment epithelium (RPE). Scale bar = 20 μm.
3.5 HDAC Inhibition and Neuroprotection
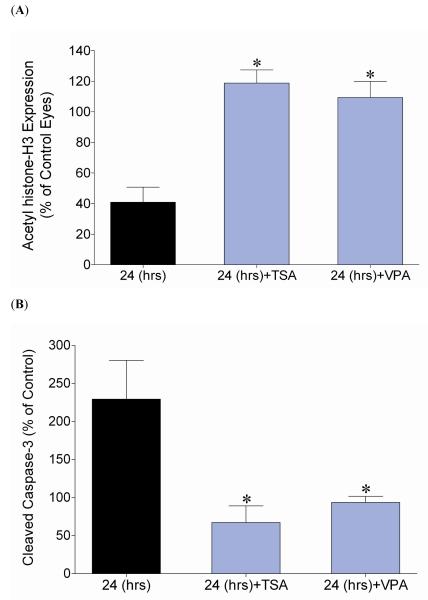
In animals that received HDAC inhibitors TSA or VPA one hour prior to injury, ischemic eyes showed no significant change in acetyl histone-H3 protein at 24 hours (Fig. 5A). The administration of TSA or VPA also blocked the elevation in activated caspase-3 at 24 hours in ischemic eyes when compared to contralateral control eyes (Fig. 5B). These results provide additional evidence that pharmacologically preventing early hypoacetylation changes can also inhibit levels of cleaved caspase-3. Values of control eyes did not change with drug treatment.
Figure 5.
Effect of HDAC inhibition on acetylation levels of histone-H3 and cleaved caspase-3, 24 hours after ischemia induction, using two structurally distinct HDAC inhibitors, TSA (2.5 mg/kg; i.p) and VPA (100 mg/kg; i.p.). (A) Ratio of acetyl histone-H3/β-actin measured at 24 hours from the initiation of 45-minutes acute ischemia and expressed as a mean percentage of the ischemic eyes relative to the control eyes. Significant differences (* P <0.05) were noted in both treatment groups, TSA (118.8 ± 8.6; n=4) and VPA (109.3 ± 10.6; n=4) when compared to the corresponding time point (24 hours, 41.0 ± 9.8; n=9) that did not receive HDAC inhibitor treatment (Student t-test). (B) Ratio of cleaved caspase-3/β-actin measured at 24 hours from the initiation of 45-minutes acute ischemia and expressed as a mean percentage of the ischemic eyes relative to the control eyes. Significant differences were noted in both treatment groups, TSA (67.1 ± 21.9; n=4) and VPA (93.7 ± 8.0; n=5) when compared to the corresponding time point (24 hours, 229.4 ± 51.1; n=9) that did not receive HDAC inhibitor treatment (* P <0.05) (Student t-test). Abbreviations: Hours post ischemia initiation (hrs); trichostatin-A (TSA); Valproic Acid (VPA).
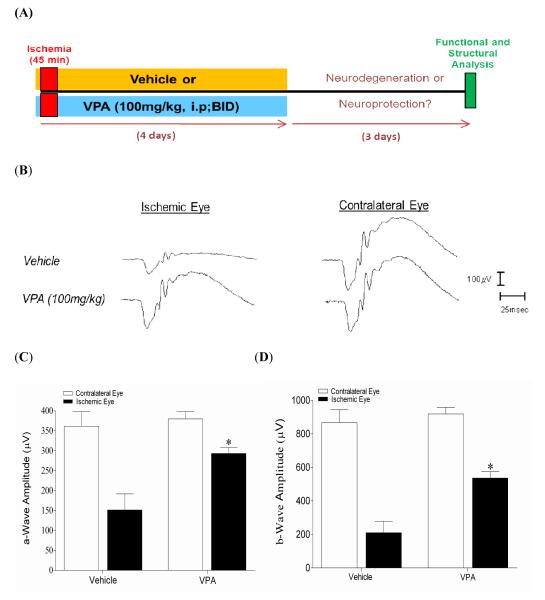
In vehicle-treated control animals at seven days following unilateral ischemic injury, there was a significant reduction in both a-wave and b-wave amplitudes of 58% and 76%, respectively in ischemic eyes when compared to contralateral eyes (Fig. 6). Direct comparison between ischemic eyes from vehicle- and VPA- treated animals demonstrated that a- and b-wave amplitudes were significantly preserved (P <0.05) by VPA treatment. In contralateral control eyes no significant difference between mean a- and b-wave amplitudes were measured between the VPA- and vehicle-treated animals.
Figure 6.
Effect of HDAC inhibition on functional neuroprotection using electroretinography. Animals were treated with vehicle or VPA (100 mg/kg; i.p.) twice-daily on days 0, 1, 2, and 3. ERGs were obtained by averaging two responses to full-intensity flashes with an inter-stimulus interval of two minutes. (A) Schematic representation of experimental procedure. (B) Representative ipsilateral and contralateral electroretinograms (ERGs) seven days following unilateral ischemic retinal injury. (C, D) Mean a- and b-wave amplitudes seven days following unilateral ischemic retina injury. Data are expressed as mean ± SEM. Asterisks denote a significant difference (* P <0.05) between ipsilateral ischemic responses in the vehicle- (n=9), and VPA-treated (n=5) animals. No significant differences in contralateral responses were measured. Abbreviations: intraperitoneal (i.p.); microvolts ( V); milliseconds (msec); twice-daily (BID).
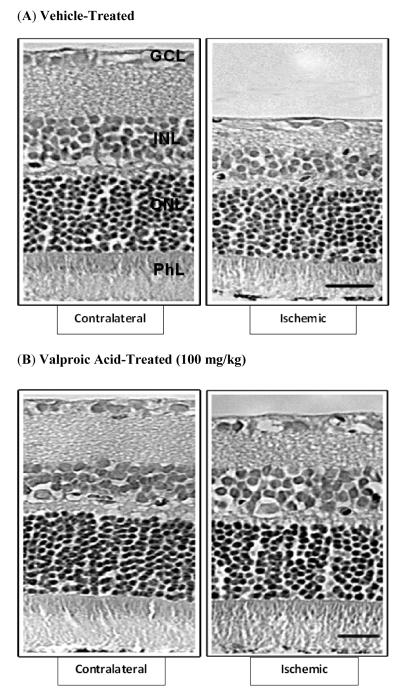
Morphological changes induced by retinal ischemia were evaluated seven days later (Fig. 7 and Table 1). In vehicle-treated rats, eyes that received ischemic injury demonstrated a significant reduction of 28% in overall retina thickness (Fig. 7A and Table 1). This reduction in thickness was primarily due to significant thinning of the inner plexiform and inner nuclear layers and the degeneration of cell bodies in the ganglion cell layer. In addition, mild loss and disorganization of the outer nuclear layers and photoreceptors were noted. In contrast, retinas from ischemic eyes that received VPA treatment displayed only limited thinning of the inner plexiform, and loss of retinal ganglion cells. No significant thinning of the deeper retinal layers was measured (Fig. 7B and Table 1). Contralateral eyes from both vehicle- and VPA-treated animals were normal in appearance.
Figure 7.
Effect of HDAC inhibition on retinal, morphological changes seven days following unilateral ischemia. Animals were treated with vehicle or VPA (100 mg/kg; i.p.) twice-daily on days 0, 1, 2, and 3. Photomicrographs of retina cross-sections of contralateral and ischemic eyes from (A) vehicle-treated, and (B) VPA-treated animals. All micrographs were taken 500 μm from the edge of the optic nerve head. Abbreviations: ganglion cell layer (GCL); inner nuclear layer (INL); outer nuclear layer (ONL); photoreceptor layer (PhL). Scale bar = 20 μm.
Table 1.
Thickness of retina and individual layers measured seven days post-ischemia.
| Retina (μm) | ONL (μm) | OPL (μm) | INL (μm) | IPL (μm) | RGCs (cells/200 μm) |
|
|---|---|---|---|---|---|---|
| Control | 186 ± 7.8 | 54.9 ± 2.6 | 10.9 ± 1.4 | 31.7 ± 1.2 | 39.8 ± 2.7 | 18.4 ± 1.0 |
| Ischemia | 133 ± 6.6 * | 51.9 ± 3.7 * | 9.2 ± 0.8 * | 20.4 ± 1.4 * | 15.3 ± 0.4 * | 11 ± 0.5 * |
| VPA | 184 ± 3.0 | 55.2 ± 2.5 | 11.3 ± 1.1 | 33.0 ± 0.7 | 40.9 ± 1.2 | 18.6 ± 0.7 |
|
VPA + Ischemia |
163 ± 7.4 * | 53.4 ± 2.6 | 9.9 ± 0.8 | 29.1 ± 2.3 | 29.9 ± 1.5 * | 15.4 ± 0.5 * |
Values are (mean ± SEM); n = 9. (Values were compared between groups using one-way ANOVA with Dunnett post-test.
indicates significant difference from control eye (P <0.05)).
Abbreviations: ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; RGCs, retinal ganglion cells; VPA, valproic acid.
4. DISCUSSION
Ischemic retinal injury depends on several factors including the vascular bed affected and the duration of ischemia, but perhaps of greatest consequence is the manner that cells respond to the ischemic and immediate post-ischemic environment. In the current study, there is a significant relationship between the extent of histone acetylation and the time after ischemia exposure. In fact, the acetylation state of histone-H3 (K9), which is found at active gene promoters and enhancers (Whyte et al., 2012), showed a 15% reduction at two hours after initiation of ischemia, rapidly followed by a 48% reduction at 4 hours, and decreasing to 59% of control levels at 24 hours. As acetylation of cytoplasmic proteins and histones have been proposed as neuroprotective strategies (Crosson et al., 2010; Majumdar et al., 2012), this study provides evidence that hypoacetylation is an early event in the pathogenesis of ischemic injury in the retina.
Studies related to the process of protein acetylation are in the midst of rapid evolution due to intimate association with epigenetic regulation, a process that has been linked to numerous high profile diseases including cancers, heart disease and inflammatory disorders (Huang, 2006). Recently, two histone deacetylase (HDAC) inhibitors, Vorinostat [suberoylanilide hydroxamic acid (SAHA)] and Romidepsin (Istodax, FK228), have been clinically approved for the treatment of cutaneous T-cell lymphoma (New et al., 2012). To date, 18 HDACs in four general classes have been identified in humans. These enzymes have been shown to modulate transcription (Walkinshaw et al., 2008), cell cycle progression (Zhang, Davies et al., 2002), differentiation (Kim et al., 1999), and apoptosis (Luo et al., 2000). Class-I HDACs (HDAC1, 2, 3, and 8) are found in all tissues, while Class-II HDACs (HDAC4, 5, 6, 7, 9, and 10) have a restricted tissue distribution. Class-III HDACs are homologous to yeast silent information regulator 2 (Sir2), are NAD+ dependent, and include SIRT1 – SIRT7. HDAC11 alone represents Class-IV HDACs. Both Class-I and Class-II HDACs are found in the nucleus and the cytosol (Yang et al., 2002). Although association of HDACs is commonly assumed to correlate with the repression of gene expression (McKinsey and Olson, 2005), a recent study shows that HDACs play a direct role in the activation of many of these genes reiterating the importance of cellular regulatory mechanisms between the processes of acetylation and deacetylation (Chandrasekaran et al., 2009).
The use of HDAC inhibitors is a reliable method for promoting acetylation in pathology, generally associated with neuroprotection (Fleiss et al., 2012; Petri et al., 2006). Despite some evidence that HDAC inhibitors are neuroprotective in the brain, their potential in improving outcomes of retinal degenerative changes has yet to receive much consideration. In fact, a previous study from this laboratory (Crosson et al., 2010) demonstrated that TSA, a pan-HDAC inhibitor (Majumdar et al., 2012), provided functional and structural protection to retinal neurons after ischemia. One of the goals of the current study was to determine if changes in histone acetylation contributed to the sequelae of events in ischemic retinal degeneration and the relative timing of these changes, when compared to other degenerative processes. This study provided the first evidence that increases in HDAC activity (Fig. 1) is an early event after retinal ischemia and continues to increase throughout the initial 24 hours of the post-ischemic period. The increase in Class-I HDAC activity appeared to be a primary contributor to the eventual hypoacetylated state found in retinal tissue following ischemic injury. However, a reduction to histone acetyltransferase activity could not be ruled out as an additional contributor.
Ischemia, similar to direct optic nerve injury, contributes to retinal cell damage primarily through apoptosis (Agudo et al., 2008). The main executioner molecule common to the intrinsic and extrinsic pathways in the apoptotic process is caspase-3, which is cleaved into the active 17 kDa moiety following proteolytic degradation. Studies have demonstrated that levels of cleaved caspase-3, which is also a direct measurement of apoptotic activity (Cor et al., 2004), are not significantly increased until 24 hours post ischemic injury (Produit-Zengaffinen et al., 2009). The process of apoptosis occurs through transcriptional alterations that favor the activity of proapoptotic genes and repression of neuroprotective genes (Boutillier et al., 2002). In the current study, apoptotic activity in ischemic eyes requires 24 hours in order to become significantly different (229.4 ± 51.1%) from control eyes (set at 100%) post-ischemic insult (Fig. 3). Since significant acetylation changes are noticeable within four hours, this study highlights the concept that apoptotic activity occurs downstream to initial deacetylation after injury. Furthermore, treatment with each HDAC inhibitor, VPA or TSA, prevented not only retinal hypoacetylation but also elevations in cleaved caspase-3 levels following ischemic injury (Fig. 5B). Together these data support the idea that the processes leading to retinal apoptosis are HDAC-dependent. Although HDAC inhibition is generally associated with promotion of gene expression, as observed here, many genes may actually be down regulated, partly due to variable changes in acetylation state of different transcription factors (Chandrasekaran et al., 2009). Further investigation is required to fully evaluate the mechanisms and pathways connecting deacetylation with apoptosis and hyperacetylation with neuronal survival.
In the developing retina, histone deacetylation is essential for the normal expression of photoreceptor and apoptotic genes (Chen and Cepko, 2007). However, in the mature retina HDAC inhibition does not lead to upregulation of genes in the apoptotic pathway (Wallace et al., 2006). Nevertheless, after ischemic reperfusion injury, results from this study and others demonstrate increases in cleaved caspase-3 and hence apoptotic activity (Produit-Zengaffinen et al., 2009). In opposition to apoptosis, acetylation contributes to differentiation and neuritogenesis of retinal cells, preserving retinal structure and function (Schwechter et al., 2007). Together, this evidence further pointed to the importance of protein acetylation playing a central role in regulating retina development and homeostasis.
Valproic acid, is an HDAC inhibitor (Santini et al., 2007) with multiple actions that can influence its efficacy in vivo. To support the concept that hyperacetylation can protect the retina from acute ischemic injury, we compared the in vivo responses in the retina of VPA to TSA. We have previously published that TSA can protect the retina from ischemic injury (Crosson et al., 2010) using the same model described in current study. The data presented in Figures 6 and 7, and Table 1 demonstrates that VPA treatment can provide functional and structural protection to the retina after severe acute ischemic retinal degeneration. The protection afforded by VPA pretreatment was not significantly different from our previously published data on TSA (Crosson et al., 2010). As a result, this study supported and expanded previous studies from this laboratory that inhibition of HDAC activity can ameliorate ischemic injury to both the outer and inner retinal segments, and supports the idea that VPA as an effective HDAC inhibitor and neuroprotective agent in the retina.
5. CONCLUSIONS
In untreated control animals, retinal ischemia induces a rapid increase in retinal HDAC activity leading to a sustained deacetylation in the neuroretina. Previous studies have demonstrated that HDAC inhibitors are neuroprotective. This study consolidated this idea, by demonstrating that two structurally distinct HDAC inhibitors, VPA and TSA, reverse not only the ischemic-induced retinal hypoacetylation but also the activation of caspase-3. In addition, comparison of the in vivo neuroprotective actions found that pretreatment with either inhibitor suppressed the retinal degeneration and functional loss associated with ischemic retinal injury. Taken together these data form the rational basis for developing HDAC inhibitors for the treatment of ischemic-related retinal degenerations. Future investigations need to address the mechanisms involved in the progression from ischemia to deacetylation to apoptosis, and whether these changes are due purely to an increase in HDAC activity, or whether there is a concurrent decrease in the activity of histone acetyltransferase enzymes.
HIGHLIGHTS.
Retinal hypoacetylation is an early event after ischemic injury.
Retinal hypoacetylation precedes increases in apoptotic activity after ischemia.
HDAC inhibition prevents early hypoacetylation changes associated with ischemia.
HDAC inhibition provides structural and functional neuroprotection in the retina.
ACKNOWLEDGEMENTS
Supported in part by National Institutes of Health grants NEI 5R01EY021368-02 (C.E.C.); and an unrestricted grant to Storm Eye Institute, Medical University of South Carolina, from Research to Prevent Blindness, New York, N.Y.
Special appreciation to Luanna Bartholomew, PhD, for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- Agudo M, Perez-Marin MC, et al. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol. Vis. 2008;14:1050–1063. [PMC free article] [PubMed] [Google Scholar]
- Boutillier AL, Trinh E, et al. Constitutive repression of E2F1 transcriptional activity through HDAC proteins is essential for neuronal survival. Ann. N.Y. Acad. Sci. 2002;973:438–442. doi: 10.1111/j.1749-6632.2002.tb04679.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S, Peterson RE, et al. Histone deacetylases facilitate sodium/calcium exchanger up-regulation in adult cardiomyocytes. FASEB J. 2009;23(11):3851–3864. doi: 10.1096/fj.09-132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Cepko CL. Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev. Biol. 2007;7:78. doi: 10.1186/1471-213X-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cor A, Pizem J, et al. Immunohistochemical analysis of pro- and active-caspase 3 in laryngeal squamous cell carcinoma. Virchows Arch. 2004;444(5):439–446. doi: 10.1007/s00428-004-0997-1. [DOI] [PubMed] [Google Scholar]
- Crosson CE, Mani SK, et al. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 2010;51(7):3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Birusingh K, et al. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis. J Neurosci. 2003;23(27):9162–9170. doi: 10.1523/JNEUROSCI.23-27-09162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss B, Nilsson MK, et al. Neuroprotection by the histone deacetylase inhibitor trichostatin A in a model of lipopolysaccharide-sensitised neonatal hypoxic-ischaemic brain injury. J Neuroinflammation. 9:70. doi: 10.1186/1742-2094-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, et al. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Creson T, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24(29):6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol. 2006;209(3):611–616. doi: 10.1002/jcp.20781. [DOI] [PubMed] [Google Scholar]
- Kanai H, Sawa A, et al. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. 2004;4(5):336–344. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, et al. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol. Exp. Ther. 2007;321(3):892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kim J, Sif S, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10(3):345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- Luo J, Su F, et al. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408(6810):377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- Majumdar G, Adris P, et al. Pan-histone deacetylase inhibitors regulate signaling pathways involved in proliferative and pro-inflammatory mechanisms in H9c2 cells. BMC Genomics. 13:709. doi: 10.1186/1471-2164-13-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin. Invest. 2005;115(3):538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New M, Olzscha H, et al. HDAC inhibitor-based therapies: can we interpret the code? Mol. Oncol. 6(6):637–656. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri S, Kiaei M, et al. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2006;22(1):40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol. Chem. 2001;276(39):36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Produit-Zengaffinen N, Pournaras CJ, et al. Retinal ischemia-induced apoptosis is associated with alteration in Bax and Bcl-x(L) expression rather than modifications in Bak and Bcl-2. Mol. Vis. 2009;15:2101–2110. [PMC free article] [PubMed] [Google Scholar]
- Santini V, Gozzini A, et al. Histone deacetylase inhibitors: molecular and biological activity as a premise to clinical application. Curr. Drug Metab. 2007;8(4):383–393. doi: 10.2174/138920007780655397. [DOI] [PubMed] [Google Scholar]
- Schwechter BR, Millet LE, et al. Histone deacetylase inhibition-mediated differentiation of RGC-5 cells and interaction with survival. Invest. Ophthalmol. Vis. Sci. 2007;48(6):2845–2857. doi: 10.1167/iovs.06-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn DI, Kim SJ, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol. Dis. 2007;26(2):464–472. doi: 10.1016/j.nbd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Walkinshaw DR, Tahmasebi S, et al. Histone deacetylases as transducers and targets of nuclear signaling. J Cell Biochem. 2008;104(5):1541–1552. doi: 10.1002/jcb.21746. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Donovan M, et al. Histone deacetylase activity regulates apaf-1 and caspase 3 expression in the developing mouse retina. Invest. Ophthalmol. Vis. Sci. 2006;47(7):2765–2772. doi: 10.1167/iovs.05-1383. [DOI] [PubMed] [Google Scholar]
- Wegener D, Hildmann C, et al. Improved fluorogenic histone deacetylase assay for high-throughput-screening applications. Anal. Biochem. 2003;321(2):202–208. doi: 10.1016/s0003-2697(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Whitlock NA, Agarwal N, et al. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest. Ophthalmol. Vis. Sci. 2005;46(3):1092–1098. doi: 10.1167/iovs.04-0043. [DOI] [PubMed] [Google Scholar]
- Whyte WA, Bilodeau S, et al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482(7384):221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WM, Tsai SC, et al. Functional domains of histone deacetylase-3. J Biol. Chem. 2002;277(11):9447–9454. doi: 10.1074/jbc.M105993200. [DOI] [PubMed] [Google Scholar]
- Zhang CL, McKinsey TA, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110(4):479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZK, Davies KP, et al. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol. Cell Biol. 2002;22(16):5975–5988. doi: 10.1128/MCB.22.16.5975-5988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Kowluru RA. Role of histone acetylation in the development of diabetic retinopathy and the metabolic memory phenomenon. J Cell Biochem. 110(6):1306–1313. doi: 10.1002/jcb.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]