Abstract
Focal chondral lesions of the glenohumeral joint, though less common than chondral defects in the knee or ankle, can be a significant source of pain in an active population. For patients in whom nonsurgical management fails, promising results have been reported after arthroscopic microfracture surgery to treat such lesions. However, microfracture leads to growth of fibrocartilage tissue and is biomechanically less durable than native hyaline cartilage. Recently, augmentation of the microfractured defect with micronized allogeneic cartilage and platelet-rich plasma has been described to restore hyaline-like cartilage and potentially protect the subchondral bone from postsurgical fracture biology within the base of the defect. We present a simple arthroscopic technique of implanting dehydrated, micronized allogeneic cartilage scaffold to treat an isolated chondral lesion of the glenoid.
Cartilage injury in the shoulder may be caused by trauma, shoulder instability, osteonecrosis, infection, chondrolysis, osteochondritis dissecans, inflammatory arthritis, rotator cuff arthropathy, and osteoarthritis. Although symptomatic glenohumeral chondral lesions in elderly and less active patients can be successfully treated with shoulder arthroplasty, focal chondral lesions in the younger, active patient population demand alternative treatment strategies that preserve the joint because of the high rate of glenoid component failure and need for revision surgery in younger patients treated with shoulder arthroplasty.1,2
For patients with continued pain and decreased function despite appropriate nonoperative management, surgical treatment can be considered. Non-arthroplasty options for young, active patients may include procedures such as debridement, microfracture, autologous chondrocyte implantation, and osteochondral transplantation. Microfracture is a frequently used reparative technique that is undertaken to access mesenchymal cells that are present within the subchondral bone.3 Despite the relative ease of the procedure, the repair tissue achieved with microfracture technique alone is fibrocartilaginous and lacks the mechanical properties and durability of normal hyaline articular cartilage. As an alternative, the use of micronized allogeneic cartilage and platelet-rich plasma (PRP) has been described as an attempt to restore the joint surface with hyaline-type cartilage. Animal studies support covering the microfractured area with micronized allograft cartilage tissue to serve as a scaffold to promote chondrogenesis and hyaline-like tissue formation.4
The purpose of this article is to describe an arthroscopic technique of implanting micronized allogeneic cartilage scaffold, BioCartilage (Arthrex, Naples, FL), to treat an isolated osteochondral lesion of the glenoid (Fig 1 and Video 1).
Fig 1.
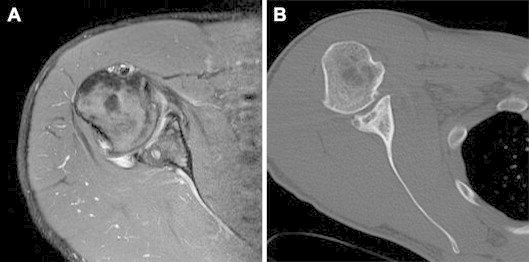
(A) Axial proton density-weighted magnetic resonance image and (B) axial computed tomography scan showing marked cartilaginous thinning and post-traumatic changes of posterior bony labrum in a right shoulder.
Surgical Technique
Patient Positioning and Diagnostic Arthroscopy
The shoulder is prepared and draped in standard fashion, and the patient is placed in the lateral decubitus position. Positioning the glenoid parallel to the floor avoids scaffold runoff due to gravity during implantation. A diagnostic arthroscopy is performed through the standard posterior glenohumeral viewing portal. The standard anterior rotator interval portal is used as a working portal.
Glenoid Defect Identification and Preparation
After the diagnostic arthroscopy, attention is turned to the glenoid defect. The defects most appropriate for augmented microfracture are contained and isolated high-grade chondral lesions of the glenoid without a significant opposing cartilage abnormality on the humeral head. Once the decision is confirmed to treat the defect using BioCartilage, blood is drawn from the patient by venipuncture (typically performed by the anesthesiologist or circulating nurse) and PRP is prepared (ACP Double Syringe system; Arthrex) according to the manufacturer's specifications for mixing with micronized allogeneic cartilage at a later stage.
A shaver is used to debride the chondral defect, and the calcified cartilage layer is removed with a curette. Vertical walls are created to contain the scaffold after placement.5 The defect is microfractured with an awl (Fig 2A). We prefer to use a PowerPick (Arthrex) to maintain consistency of subchondral penetration with each microfracture hole and to minimize the trauma to subchondral bone. The microfracture holes are spaced approximately 3 to 4 mm apart and should penetrate to a depth of approximately 3 to 4 mm into the subchondral surface.
Fig 2.
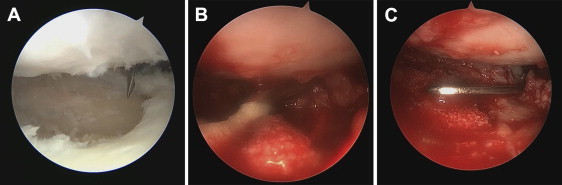
Arthroscopic visualization of right glenohumeral joint through anterior portal showing (A) glenoid microfracture after preparing defect. (B) BioCartilage paste being injected into microfractured defect. (C) Graft surface being smoothed with freer elevator.
Implantation of BioCartilage
The arthroscopic pump is shut off, and arthroscopic fluid is removed from the joint with suction; the defect bed is then dried thoroughly. A BioCartilage mixture is prepared in the Arthrex Mixing and Delivery Syringe (by mixing 1 mL of PRP with 1 mL of allograft powder). A thin layer of fibrin glue (Tisseel; Baxter, Deerfield, IL) is placed into the prepared bed. The BioCartilage mixture paste is then injected into the microfractured defect: The paste should fill the defect so that it is slightly recessed compared with the surrounding cartilage margins (Fig 2, B and C). A smooth graft surface is then created with a freer elevator. A thin layer of fibrin glue is applied over the graft and the neighboring cartilage to create a seal. All instruments are removed from the joint, and the fibrin glue is allowed to dry for 5 minutes. Tips and pearls of the surgical technique are summarized in Table 1. A list of surgical steps are outlined in Table 2.
Table 1.
Tips and Pearls
| Position the patient in the lateral decubitus position for treating glenoid defects because it eliminates the effects of gravity when implanting the scaffold. |
| Position the rotator interval portal slightly laterally. This allows a perpendicular trajectory to the defect when performing microfracture. |
| Debride the entire layer of calcified cartilage without penetrating the subchondral bone. An arthroscopic shaver may be used for this step. |
| Use a PowerPick to obtain short, uniform-diameter holes that are less likely to crack at the edges of the holes. |
| When the paste mixture of micronized allograft cartilage and PRP is too dry, making it difficult to handle, consider adding a small amount of additional PRP. |
Table 2.
Surgical Steps in Arthroscopic Augmented Microfracture of Glenoid
| 1. Diagnostic arthroscopy |
| 2. Platelet-rich plasma preparation |
| 3. Defect preparation |
| 4. Drying of field |
| 5. BioCartilage preparation |
| 6. BioCartilage paste application |
| 7. Smoothing of graft surface |
| 8. Application of fibrin glue to dry surface |
Postoperative Rehabilitation
After treatment of glenoid chondral defects with BioCartilage, we use a post-microfracture rehabilitation protocol.6 The patients are placed in a shoulder immobilizer (DJO, Vista, CA) immediately after surgery for 3 to 5 days to allow the bone marrow elements to fully infiltrate the BioCartilage mixture and form a stable clot. They remain in a sling for 4 to 6 weeks. During this period, they are allowed to come out of the sling for gentle active and active-assisted range-of-motion exercises including pendulums. Afterward, the patients are progressed to gentle strengthening and lifting exercises as tolerated. Heavy overhead lifting is restricted for 3 months, and by 4 months, full activity is allowed. After 6 months, the patients return to overhead competitive athletics. Rehabilitation protocols are altered as appropriate if other procedures are performed concomitantly with augmented microfracture.
Discussion
Focal chondral lesions of the glenohumeral joint can be a significant source of pain in an active population. The reported incidence of such defects in the shoulder ranges from 5% to 17%.3 Management of these lesions has been primarily reported in small case series using debridement, microfracture, and rarely, autologous chondrocyte implantation and osteochondral allografting procedures. Augmented microfracture techniques represent a minimally invasive strategy to approach isolated chondral defects; however, to our knowledge, no prior reports of this procedure as used in the glenohumeral joint are available. We have described an arthroscopic technique for application of micronized allogeneic cartilage matrix to treat glenoid chondral lesions.
The basic science behind microfracture technique has been thoroughly examined, and the reported clinical outcomes to date have been satisfactory.3,7 However, given the fibrocartilaginous fill resulting from standard microfracture techniques, alternative approaches that result in a more normal, hyaline-type cartilaginous fill are warranted. In our augmented technique, microfracture is used to create channels for mesenchymal stem cells in the subchondral bone to migrate and populate a scaffold that has been implanted over the defect.8 In vitro studies have shown that dehydrated, micronized allograft tissue promotes chondrogenesis.9 Moreover, the BioCartilage technique uses the anabolic and anti-inflammatory benefits of PRP. Several trials have reported superior functional outcome and improved histologic scores when comparing PRP-enhanced microfracture versus microfracture alone.10,11 Data regarding outcomes of BioCartilage use in human subjects are limited to expert opinion, but controlled human trials examining outcome differences between standard microfracture and BioCartilage techniques are currently under way. To date, an overall beneficial effect has been observed in more than 1,000 patients implanted with BioCartilage, with no adverse events related to this therapy.4
Augmented microfracture technique is contraindicated in uncontained, diffuse chondral lesions and in joints with advanced degenerative arthritis. Patients with other copathology of the shoulder, such as glenohumeral instability, should undergo concomitant treatment. Moreover, patients unable or unwilling to abide by postoperative protected weight-bearing and range-of-motion restrictions are not candidates for this technique.
In summary, the microfracture procedure augmented with dehydrated, micronized allogeneic cartilage scaffold is a safe single-stage solution that can be performed with an all-arthroscopic technique. The all-arthroscopic technique is minimally invasive and allows for short rehabilitation times. Ongoing clinical studies will determine the effectiveness of augmented microfracture compared with standard microfracture alone.
Footnotes
The authors report the following potential conflict of interest or source of funding: B.J.C. receives support from Arthrex, DJ Orthopaedics, Johnson & Johnson, Regentis, Zimmer, Medipost, Smith & Nephew, Carticept.
Supplementary Data
Arthroscopic visualization of a right glenoid defect. Once the intra-articular pathology is defined, microfracture is performed and the BioCartilage mixture is prepared on a side table. After the surgical field is dried, the paste is applied to the defect.
References
- 1.Elser F., Braun S., Dewing C.B., Millett P.J. Glenohumeral joint preservation: Current options for managing articular cartilage lesions in young, active patients. Arthroscopy. 2010;26:685–696. doi: 10.1016/j.arthro.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Gross C.E., Chalmers P.N., Chahal J. Operative treatment of chondral defects in the glenohumeral joint. Arthroscopy. 2012;28:1889–1901. doi: 10.1016/j.arthro.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Frank R.M., Van Thiel G.S., Slabaugh M.A., Romeo A.A., Cole B.J., Verma N.N. Clinical outcomes after microfracture of the glenohumeral joint. Am J Sports Med. 2010;38:772–781. doi: 10.1177/0363546509350304. [DOI] [PubMed] [Google Scholar]
- 4.Abrams G.D., Mall N.A., Fortier L.A., Roller B.L., Cole B.J. BioCartilage: Background and operative technique. Oper Tech Sports Med. 2013;21:116–124. [Google Scholar]
- 5.Steadman J.R., Rodkey W.G., Briggs K.K. Microfracture to treat full-thickness chondral defects: Surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170–176. [PubMed] [Google Scholar]
- 6.McCarty L.P., III, Cole B.J. Nonarthroplasty treatment of glenohumeral cartilage lesions. Arthroscopy. 2005;21:1131–1142. doi: 10.1016/j.arthro.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Millett P.J., Huffard B.H., Horan M.P., Hawkins R.J., Steadman J.R. Outcomes of full-thickness articular cartilage injuries of the shoulder treated with microfracture. Arthroscopy. 2009;25:856–863. doi: 10.1016/j.arthro.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Fortier L.A., Barker J.U., Strauss E.J., McCarrel T.M., Cole B.J. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng N.C., Estes B.T., Awad H.A., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc in press, available online 30 November 2013. doi:10.1007/s00167-013-2784-5. [DOI] [PubMed]
- 11.Hapa O., Çakici H., Yüksel H.Y., Fırat T., Kükner A., Aygün H. Does platelet-rich plasma enhance microfracture treatment for chronic focal chondral defects? An in-vivo study performed in a rat model. Acta Orthop Traumatol Turc. 2013;47:201–207. doi: 10.3944/aott.2013.2928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arthroscopic visualization of a right glenoid defect. Once the intra-articular pathology is defined, microfracture is performed and the BioCartilage mixture is prepared on a side table. After the surgical field is dried, the paste is applied to the defect.


