Abstract
Primary synovial chondromatosis (PSC) of the shoulder is a rare condition and usually necessitates operative therapy. Arthroscopic partial synovectomy with removal of loose osteochondromas may be regarded as the current surgical treatment of choice. However, involvement of the biceps tendon sheath (BTS) occurs in almost half of the patients and required additional open surgery in all previously reported cases. We successfully performed tenoscopy of the BTS and long head of the biceps tendon during arthroscopic treatment of PSC in a 26-year-old male competitive wrestler. Biceps tenoscopy enabled minimally invasive partial (teno)synovectomy and removal of all osteochondromas within the BTS. The symptoms of PSC fully subsided within 2 postoperative weeks. There were no functional restrictions at the 3-month follow-up examination. These preliminary results support the feasibility, safety, and efficacy of biceps tenoscopy as a complement in arthroscopic treatment of PSC of the shoulder, dispensing with the need for additional open surgery. The spectrum of indications for biceps tenoscopy has still to be defined. Conceivable indications are proposed. This first report of a diagnostic and interventional biceps tenoscopy entails a detailed step-by-step description of the surgical technique.
Primary synovial chondromatosis (PSC) is a rare, idiopathic condition characterized by benign chondroid metaplasia of synovial tissue, growth of osteochondromas (OCs), and formation of loose bodies. The distribution is usually monoarticular, and the disease typically occurs in diarthrodial joints—in order of frequency, the knee, hip, elbow, wrist, ankle, and shoulder joint.1 However, bursae and tendon sheaths may also be affected. Several authors have presented cases of involvement of the biceps tendon sheath (BTS).2-5 Milgram6 histopathologically analyzed 30 cases of synovial chondromatosis and identified 3 phases: (1) active intrasynovial disease with no loose bodies, (2) transitional lesions with both active intrasynovial proliferation and free loose bodies, and (3) multiple osteochondral loose bodies without active intrasynovial involvement. Typically, the first stage of the disease causes no or only minor complaints. Formation of OCs and loose bodies during stages 2 and 3 is associated with rather unspecific clinical symptoms, for example, pain, limitation of range of motion, crepitation, and joint blockade.7 BTS involvement may cause additional pain and structural damage to the long head of the biceps tendon (LHBT). Even though there exist rare reports of spontaneous regression, surgical intervention is required in the vast majority of cases with advanced stages of disease.1 Beginning in the 1990s, arthroscopic treatment has increasingly replaced open surgery in operative therapy of PSC of the shoulder.8 However, BTS involvement necessitated additional open surgery for removal of loose OCs in all previously reported cases.3-5
We performed arthroscopic and tenoscopic treatment of PSC of the shoulder joint with BTS involvement in a 26-year-old male competitive wrestler, who presented to our shoulder service with increasing pain and discomfort in his right, dominant shoulder. Pain had occurred both at rest and on exertion. As a consequence, he had to abandon his sports activity. Internal rotation was found to be notably restricted compared with the contralateral side (third lumbar vertebra v tenth thoracic vertebra). In addition, internal rotation strength was impaired, and the results of subscapularis tests (belly-press and bear-hug tests) were mildly positive. There was local tenderness over the bicipital groove, and clinical LHBT signs were clearly positive. The Constant score was 91 points. Native magnetic resonance imaging (MRI) detected multiple free bodies inside the joint, most of them being located within the medial recess of the BTS and the subscapularis recess (Fig 1). A large mass adhered to the undersurface of the subscapularis tendon. There was evident glenohumeral joint effusion with fluid collection inside the enlarged BTS and around the normally dimensioned LHBT (“halo sign”). The clinical and radiologic findings were consistent with symptomatic and advanced (stage 2 and stage 3) PSC of the shoulder with BTS involvement. We suggested arthroscopic and tenoscopic partial (teno)synovectomy and removal of OCs. Biceps tenoscopy was supposed to enable direct visualization and elimination of all PSC-related pathologies, dispensing with the need for additional open surgery in case of an otherwise intact LHBT.
Fig 1.
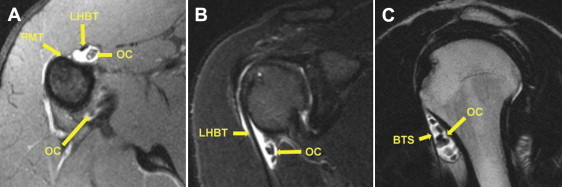
Preoperative MRI study of right shoulder. (A) Axial view (T1) at level of proximal insertion of pectoralis major tendon (PMT). OCs were detected in the medial recess of the BTS and in the axillary pouch. (B) Paracoronal view (T2). Regular course of LHBT appearing without signal alterations, with detection of OCs medial to LHBT. (C) Parasagittal view (T1). Multiple OCs were located within the medial recess of the BTS, which showed evident effusion and enlargement.
Surgical Technique
Surgery was performed by the first author (D.M.) with use of a standard 4.0-mm 30° arthroscope (Arthrex, Naples, FL). The patient was positioned in the lateral decubitus position with the arm held in 30° of abduction and neutral rotation (Fig 2). An axial traction weight of 5 kg was applied.
Fig 2.
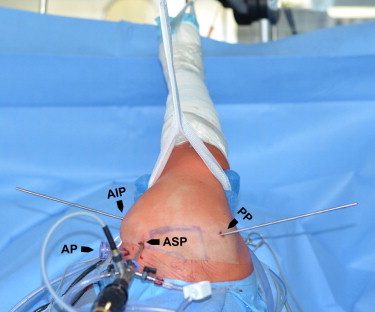
Patient positioning and arthroscopic approaches (right shoulder viewed from cranially). The patient was positioned in the lateral decubitus position with the arm held in 30° of abduction and neutral rotation. (AIP, anterior-inferior portal [rod inserted, working portal for LHBT tenoscopy]; AP, anterior portal [8.25-mm working cannula inserted]; ASP, anterior-superior portal [arthroscope inserted, arthroscope portal for LHBT tenoscopy]; PP, posterior portal [rod inserted]).
Biceps Tenoscopy
The entire procedure of biceps tenoscopy is described in a step-by-step fashion in Video 1, accompanied by an instructional commentary. An anterior-superior portal (ASP) was established directly anterior to the biceps tendon just above its entrance into the proximal BTS (Fig 2). Therefore a needle was used to mark the correct portal site. Then, a 5-mm longitudinal split was created in the rotator interval with a knife. Extreme care had to be taken to avoid injury to the LHBT or pulley complex. A blunt 4.0-mm rod was inserted into the BTS medial to the LHBT (Fig 3A). The rod was used as a guide for insertion of the shaft of the arthroscope. The rod was exchanged by the arthroscope. After verification of its correct placement within the BTS, fluid inflow was opened using a pump pressure of 30 mm Hg and 50% of the maximum flow. BTS tenoscopy showed 5 loose OCs located medial to the LHBT, just as detected by preoperative MRI (Figs 1 and 3A). There was focal villous synovitis indicating de novo formation of these loose bodies within the BTS itself. A needle was used to plan an additional anterior-inferior portal through the roof of the BTS directly anterior to the collection of OCs (Figs 2 and 3B). The portal was created by an outside-in technique without the use of a working cannula. The subcutaneous tissue and fascia should be carefully spread in a blunt fashion before use of the knife for creation of a 5-mm longitudinal split of the anterior BTS. Both the musculocutaneous nerve running medial to the BTS and the cephalic vein are at risk of iatrogenic injury. All loose bodies located within the BTS could be removed with an arthroscopic forceps or grasper, starting with the smaller loose bodies (Figs 3C and 3D). The anterior-inferior portal was dilated for removal of larger OCs. Figure 3D shows the empty biceps sheath completely cleared of loose bodies. Next, partial (teno)synovectomy of villous areas was performed with a 4.0-mm soft-tissue shaver (Arthrex) and an electrosurgical device (OPES Toothbrush; Arthrex) (Figs 3D and 3E). The suction intensity of the shaver was reduced by 50% to minimize the risk of injury to the LHBT. Injury to the branches of the anterior circumflex artery running along the floor of the bicipital groove may cause substantial bleeding. The LHBT itself appeared to be fully intact and therefore was left in situ (Fig 3E). The arthroscope was replaced into the joint for final control. After biceps tenoscopy, the intra-articular portion of the LHBT and the pulley complex were still found to be intact. Tables 1 and 2 summarize potential indications, technical key points, and potential risks of biceps tenoscopy.
Fig 3.
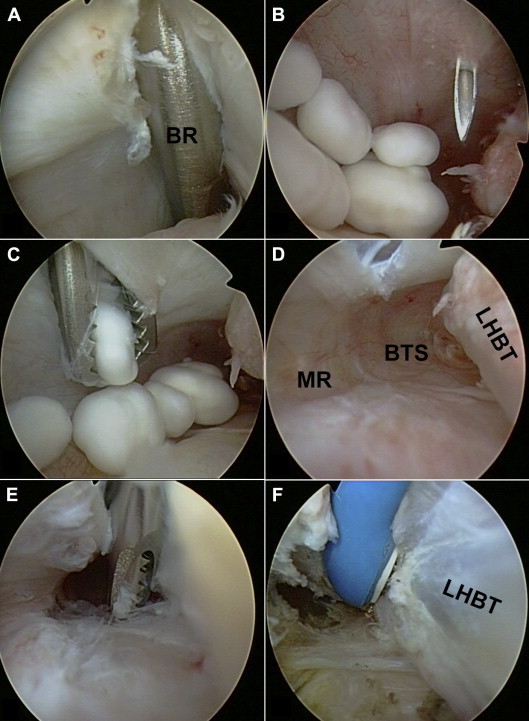
Biceps tenoscopy. (A) The blunt rod (BR) inserted through the ASP (Fig 2) into the BTS served as a guide for insertion of the arthroscope. (B) Tenoscopy showed 5 loose OCs. A needle was used to plan outside-in creation of an anterior-inferior portal (AIP) (Fig 2) for the tenoscopic procedure. (C) All loose OCs could be removed with a grasper. (D) The BTS showed villous tenosynovitis. (MR, medial recess.) (E, F) Partial tenosynovectomy was performed with a shaver and electrothermal device. The intact LHBT was left in situ.
Table 1.
Potential Indications for Biceps Tenoscopy (Preliminary Proposals)
| Underlying Pathology | Treating Disorder | Tenoscopic Procedure |
|---|---|---|
| Synovial disorders (e.g., rheumatoid, chondromatosis, villonodular synovitis) Post-traumatic Postseptic Calcifying tendinitis |
Tenosynovitis, adhesions of LHBT and/or BTS | Partial tenosynovectomy, removal of adhesions/calcifications |
| Synovial disorders (e.g., chondromatosis) Post-traumatic Degenerative arthritis |
Loose bodies within BTS | Removal of loose bodies |
| Bony pathology of bicipital groove (e.g., osteophyte, degenerative, post-traumatic) | LHBT entrapment | Tenoscopic decompression of bicipital groove |
| Proximal (arthroscopic) LHBT tenodesis due to intra-articular LHBT pathology | Concomitant LHBT pathology distal to tenodesis | Procedure selected according to tenoscopic findings (e.g., tenosynovectomy) |
| Proximal LHBT pathology | e.g., SLAP lesion, partial rupture, pulley lesion, tenosynovitis, hourglass biceps. | Tenoscopic suprapectoral biceps tenodesis |
Table 2.
Technical Key Points and Potential Risks of Biceps Tenoscopy
| Tenoscopic Procedure | Key Points | Potential Risks |
|---|---|---|
| Anterior-superior tenoscopy portal | Outside-in technique for exact portal placement anterior to LHBT, in continuity with bicipital groove Marking of correct portal site with needle Use of knife for 5-mm longitudinal split Insertion of blunt rod into BTS Rod should easily pass transverse ligament; consider use of smaller rod/arthroscope |
Injury to LHBT, pulley complex, transverse ligament, or BTS |
| Anterior-inferior working portal | Outside-in technique for exact portal placement through roof of BTS Abandonment of cannula facilitates tenoscopic procedure |
Injury to musculocutaneous nerve medial to BTS Injury to cephalic vein |
| Removal of loose bodies | Abandonment of cannula facilitates removal of loose bodies Thorough inspection of medial and lateral recess of BTS Preoperative MRI to locate and quantify loose bodies |
Persistence of loose bodies |
| Tenosynovectomy | Thorough inspection of BTS and LHBT Reduction of suction of shaver to avoid iatrogenic injuries Use of electrothermal ball probe to enable steep and perpendicular work |
Injury to LHBT or BTS Injury to branches of anterior circumflex artery on floor of bicipital groove (bleeding) Persistence of tenosynovitis |
Intra-Articular Procedure
A standard posterior portal was created for diagnostic glenohumeral arthroscopy (Fig 2). Several loose bodies were found to move freely inside the joint (Fig 4A). It was not possible to depict the intra-articular portion of the subscapularis tendon because it was covered by an adherent solid mass (Fig 4B). The LHBT showed neither structural damage (delamination, partial rupture) nor signs of instability (dislocation, subluxation). There was only mild tenosynovitis at its anterior aspects. The medial and lateral pulley complex was intact. An anterior portal was created above the solid mass in the rotator interval, and an 8.25-mm working cannula (Arthrex) was established by an outside-in technique (Fig 4B). Six OCs were floating inside the joint and were retrieved with a grasper through the anterior portal. The mass adherent to the subscapularis tendon proved to be a large calcified OC. A 5.75-mm working cannula (Arthrex) was inserted into the anterior-superior portal. The OC was cautiously dissected from the undersurface of the subscapularis tendon with use of electrosurgical devices (Fig 4C). The synovial adherence extended to the humeral insertion of the tendon. After complete detachment, the OC could be retrieved in toto through the anterior portal. For removal, the working cannula had to be extracted. The OC measured 28 × 20 × 15 mm at its largest dimension (Fig 5). Removal showed an intact subscapularis tendon, which now functioned without mechanical impediment (Fig 4D). A circular chondral lesion (diameter 30 mm; grade 2 to 3 according to International Cartilage Repair Society classification) had evolved at the anterior aspect of the humerus corresponding to the site of contact with the OC (Fig 4 C and E). Preoperative MRI had detected numerous free bodies within the subscapularis recess. Accordingly, the synovial lining of the recess showed villous transformation with multiple embedded OCs (Fig 4F). Another 8 loose bodies were removed from this recess with a grasper. Finally, partial synovectomy was performed in all suspicious areas. Thorough final glenohumeral inspection did not show any remaining loose or adherent OCs. In total, 19 loose bodies were removed throughout the procedure (Fig 5).
Fig 4.
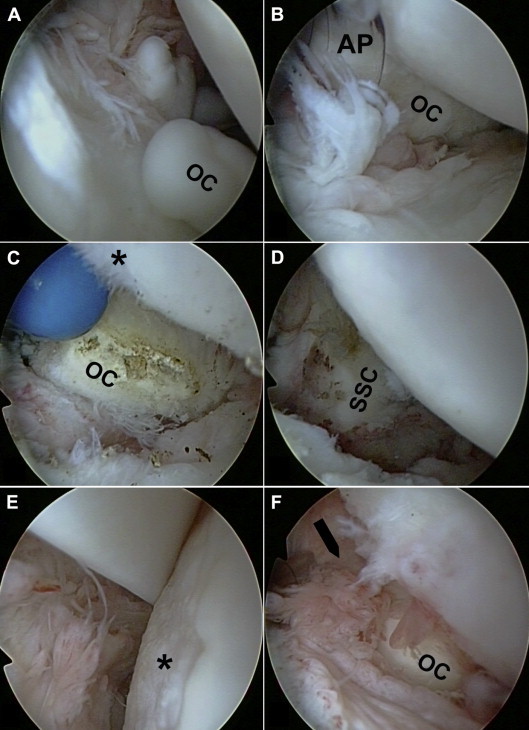
Glenohumeral arthroscopy (viewed from posterior standard portal). (A) Multiple loose OCs within joint space. (B) An anterior portal (AP) with an 8.25-mm working cannula was placed above a large OC, which adhered to the undersurface of the subscapularis tendon. (C) The OC was dissected from the undersurface of the subscapularis tendon using an electrothermal device. The inferior region of an extended humeral chondral lesion (asterisk) showed fibrillation of the cartilage. (D) After OC removal, the subscapularis (SSC) tendon became visible and then functioned without mechanical impediment. (E) Central and upper region of a distinct humeral chondral lesion (asterisk) that was grade 2 to 3 according to the International Cartilage Repair Society classification. The LHBT was found to be intact. (F) Multiple loose OCs were embedded within the subscapularis recess (arrow).
Fig 5.
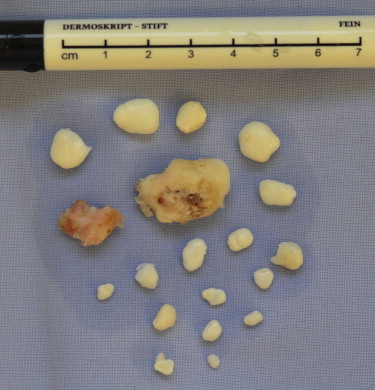
Macroscopic aspect of a total of 19 OCs, which were extracted throughout the procedure. The largest OC measured 28 × 20 × 15 mm at its largest dimension.
Postoperative Rehabilitation
The patient was discharged on the second postoperative day as scheduled. There were no complications. The diagnosis of PSC was confirmed by histopathologic examination of multiple biopsy specimens. The patient was allowed to perform unlimited range of motion and to return to sports as soon as he felt free of pain. The symptoms of PSC fully subsided within 2 postoperative weeks, and the patient started light sports activities. He was able to resume wrestling 6 weeks after surgery. At 3 months' follow-up, there were no functional restrictions and the Constant score had improved to 100 points, as compared with 91 points preoperatively.
Discussion
In 1990 Richman and Rose8 were the first authors to perform arthroscopic treatment of PSC of the shoulder. Lunn et al.3 reported a series of 18 arthroscopically treated patients with PSC (n = 7) and secondary synovial chondromatosis (n = 11). The mean Constant score improved from 70.9 points to 85.8 points after a mean follow-up period of 5.3 years (Table 3). In 9 patients (5 with PSC and 4 with secondary synovial chondromatosis), radiography and/or computed tomography detected loose bodies within the BTS. Seven of these patients underwent arthroscopic tenotomy, open bicipital debridement, and suture tenodesis to the pectoralis major tendon. The indications for tenodesis were inflammation and/or partial rupture of the LHBT. Werner et al.5 performed mini-open debridement of the BTS in 2 of 3 cases with intra-articular PSC (Table 3). BTS involvement required additional open surgery in all previous reports. The available data indicate that BTS involvement is much more common than previously thought. The few existing case series found loose OCs located within the BTS in approximately half of the patients (Table 3).3-5 Notable degenerative changes (chondromalacia, osteoarthrosis) existed in two-thirds of patients at the time of surgery. Recurrence and persistence represented the most common complications with the potential need for revision surgery. In the series of Lunn et al., recurrence occurred in 4 of 18 cases (22%). Of these patients, 2 had to undergo secondary surgery. OCs persisted in 3 of 18 patients (17%) with PSC. Two of these patients required mini-open surgical revision because of symptomatic BTS involvement. Table 3 summarizes the most relevant findings and results of arthroscopic treatment.
Table 3.
Findings and Results of Arthroscopic Treatment of PSC of Shoulder
| Author | No. of Patients | Age, yr | Mean FU Period, yr | BTS Involvement, n | CM, n | OA, n | Rec, n | Pers, n | Outcome | CS, points |
|---|---|---|---|---|---|---|---|---|---|---|
| Urbach et al.,4 2008 | 5 | 36.0 | 7.4 | 1 (detected at FU) | — | 3 | 2 | — | 5 of 5 good or excellent | 97 |
| Lunn et al.,3 2007 | 7 | 26.3 | 7.0 | 5 (4 mini-open with tenodesis) | — | 4 | 2 | 2 | — | 86 |
| Werner et al.,5 2002 | 6 | 46.6 | 3.0 | 2 (2 mini-open without tenodesis) | 5 | — | 0 | 1 | 4 of 6 good or excellent | — |
| Summary | 18 | — | — | 8 of 18 (44%) | CM or OA in 12 of 18 (67%) | 4 of 18 (22%) | 3 of 18 (17%) | — | — | |
CS, Constant score; CM, chondromalacia; FU, follow-up; OA, osteoarthrosis; Pers, persistence; Rec, recurrence.
To our knowledge, this is the first report of a diagnostic and interventional biceps tenoscopy. In our patient, tenoscopy of the BTS and LHBT enabled direct visualization and specific treatment of focal (teno)synovitis and loose bodies. In addition, the structural state of the LHBT could be assessed. These first experiences and preliminary results support the feasibility and efficacy of biceps tenoscopy as a complement in arthroscopic treatment of PSC of the shoulder, dispensing with the need for additional open surgery. However, the spectrum of indications for biceps tenoscopy might expand in the future. Diagnostic respective therapeutic biceps tenoscopy could be beneficial in tenosynovitis of various origins, for example, post-traumatic or postseptic conditions, calcifying tendinitis, rheumatoid arthritis, or specific types of synovitis such as (pigmented) villonodular synovitis. Partial tenosynovectomy could eliminate adhesions and restore the physiological gliding properties of the LHBT. The treatment of bony pathologies of the bicipital groove, for example, resection of mechanically relevant osteophytes, could reflect another potential application for biceps tenoscopy. From a technical point of view, correct placement of the arthroscopic anterior-superior approach (ASP) for biceps tenoscopy appears crucial. It is located just anterior to the LHBT just above the entrance and in continuity with the bicipital groove (Figs 2 and 4A). Depending on individual anatomic conditions, the ASP may be situated in the rotator interval or anterior supraspinatus tendon. This approach (5 mm longitudinal split) seems to be uncritical in terms of clinical morbidity. However, care must be taken not to damage the biceps reflection pulley or the LHBT itself. We did not observe any technical or surgical complications. In this case we used a standard 4.0-mm 30° arthroscope. However, the patient was rather tall, and the BTS was abnormally enlarged because of chondroma formation and chronic joint effusion. Obviously, smaller dimensioned arthroscopes (e.g., 2.7 mm or even smaller) should be used in patients with more narrow BTSs. The shaft of the arthroscope should easily pass the transverse ligament. In case of simultaneous biceps tenodesis, tenoscopic dissection of the transverse ligament could facilitate biceps tenoscopy distally. Table 1 outlines potential indications, and Table 2 outlines technical key points and potential risks of biceps tenoscopy. We preserved the LHBT in our patient because it did not show any pathologic changes. However, in case of fibrillation or partial rupture, LHBT tenotomy with or without subsequent tenodesis should be performed in an arthroscopic, tenoscopic, or mini-open fashion, according to the patient's and surgeon's preference. If an intra-articular arthroscopic tenodesis is performed, additional biceps tenoscopy might prove to be of value for the diagnosis and treatment of concomitant distal biceps pathologies. On the other hand, biceps tenoscopy could enable and facilitate biceps tenodesis performed in the extra-articular, suprapectoral region.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
Supplementary Data
Step-by-step illustration and description of entire procedure of biceps tenoscopy.
References
- 1.Christensen J.H., Poulsen J.O. Synovial chondromatosis. Acta Orthop Scand. 1975;46:919–925. doi: 10.3109/17453677508989279. [DOI] [PubMed] [Google Scholar]
- 2.Covall D.J., Fowble C.D. Arthroscopic treatment of synovial chondromatosis of the shoulder and biceps tendon sheath. Arthroscopy. 1993;9:602–604. doi: 10.1016/s0749-8063(05)80414-1. [DOI] [PubMed] [Google Scholar]
- 3.Lunn J.V., Castellanos-Rosas J., Walch G. Arthroscopic synovectomy, removal of loose bodies and selective biceps tenodesis for synovial chondromatosis of the shoulder. J Bone Joint Surg Br. 2007;89:1329–1335. doi: 10.1302/0301-620X.89B10.19545. [DOI] [PubMed] [Google Scholar]
- 4.Urbach D., McGuigan F.X., John M., Neumann W., Ender S.A. Long-term results after arthroscopic treatment of synovial chondromatosis of the shoulder. Arthroscopy. 2008;24:318–323. doi: 10.1016/j.arthro.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Werner A., Wild A., Mueller T., Borys A., Gohlke F., Krauspe R. Primary synovial chondromatosis of the shoulder. Z Orthop Ihre Grenzgeb. 2002;140:404–408. doi: 10.1055/s-2002-33400. [in German] [DOI] [PubMed] [Google Scholar]
- 6.Milgram J.W. Synovial osteochondromatosis: A histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59:792–801. [PubMed] [Google Scholar]
- 7.Imhoff A., Schreiber A. Synovial chondromatosis. Orthopade. 1988;17:233–244. [in German] [PubMed] [Google Scholar]
- 8.Richman J.D., Rose D.J. The role of arthroscopy in the management of synovial chondromatosis of the shoulder. A case report. Clin Orthop Relat Res. 1990:91–93. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Step-by-step illustration and description of entire procedure of biceps tenoscopy.


