Summary
Daily cycles of rest and activity are a common example of circadian control of physiology. In Drosophila rhythmic locomotor cycles rely on the activity of 150-200 neurons grouped in seven clusters [1, 2]. Work from many laboratories points to the small Lateral Neurons ventral (sLNvs) as essential for circadian control of locomotor rhythmicity [3-7]. sLNv neurons undergo circadian remodeling of their axonal projections opening the possibility for a circadian control of connectivity of these relevant circadian pacemakers [8]. Here we show that circadian plasticity of the sLNv axonal projections has further implications than mere structural changes. First, we found that the degree of daily structural plasticity exceeds that originally described [8] underscoring that changes in the degree of fasciculation as well as extension or pruning of axonal terminals could be involved. Interestingly, the quantity of active zones changes along the day, lending support to the attractive hypothesis that new synapses are formed while others are dismantled between late night and the following morning. More remarkably, taking full advantage of the GFP Reconstitution Across Synaptic Partners (GRASP) technique [9] we showed that, in addition to new synapses being added or removed, sLNv neurons contact different synaptic partners at different times along the day. These results lead us to propose that the circadian network, and in particular the sLNv neurons, orchestrates some of the physiological and behavioral differences between day and night by changing the path through which information travels.
Results and discussion
Temporal dynamics of the structural plasticity
Circadian remodeling of the sLNv dorsal terminals was first described at the peak and trough levels of the pigment dispersing factor (PDF) immunoreactivity, that is at zeitgeber time (ZT) 2 and 14 (2 h after lights ON and lights OFF, respectively), as well as their counterparts under constant darkness (DD) (circadian times (CT) 2 and 14, [8]). To more precisely examine the extent of structural remodeling a timecourse was carried out. An inducible GAL4 version termed GeneSwitch [10, 11] restricted to PDF neurons (pdf-GS, [12]) combined with a membrane tethered version of GFP (mCD8GFP) was used as control. As expected from our original observations, a significant reduction in complexity of the axonal arbor-measured as total axonal crosses-could be seen between CT2 and CT14, CT18 and CT22 (Figure 1A, B), which remained unchanged at nighttime. However, towards the end of the subjective night (CT22) the primary processes appeared shorter. To more precisely describe this additional form of plasticity, we measured the length of the maximum projection from the lateral horn towards the midbrain. This analysis revealed that towards the end of the subjective night (CT22) PDF projections are significantly shorter than at the beginning (CT2) of the day (Figure 1C). These observations imply that mechanisms other than the proposed changes in the degree of fasciculation are recruited during circadian plasticity [8, 13]. To get a deeper insight into the nature of the phenomena we monitored the changes in brain explants kept in culture for 48 h after dissection. Transgenic pdf-GAL4; UAS-mCD8RFP flies (from now on referred to as pdf>RFP) were dissected under safe red light and brains were maintained under constant darkness (DD). Imaging of individual brains at two different time points highlighted three types of changes experienced by axonal terminals: a) changes in the degree of fasciculation/defasciculation, more common in primary branches; b) the addition/retraction of new processes, mostly affecting those of secondary or tertiary order, and c) positional changes of minor terminals (Figure 1D-E), thus confirming and extending our previous observations. Altogether these results indicate that a rather complex remodeling process takes place on daily basis in the axonal terminals of PDF neurons.
Figure 1. Severe morphological and synaptic changes occur during the dark to light transition.
(A)Representative confocal images taken at CT2, CT14 and CT22. During early subjective day (CT2) axonal projections are more complex and extended, reaching further towards the medial region, while at CT22 PDF projections are less complex (as in CT14) and appear shorter. (B-C) Quantitation of total axonal crosses (B) and the longest axonal branch (C) at CT2, CT6, CT14, CT18 and CT22 of control brains (pdf-GS>mCD8GFP). Dissections were performed on the 4th day of constant darkness (DD4). Dark gray represents subjective night while light grey, subjective day. * indicates significant differences with p<0.05. Statistical analysis included Blocked ANOVA (Total axonal crosses p=0.0002; circuit length p=0.0417) with Tukey post-hoc test (p<0.05, (Total axonal crosses least significant difference = 3.40; circuit length least significant difference = 10.98 μm). (D) Representative confocal images of dorsal sLNvs projections from cultured brains. Brains were cultured 72 h and imaged 24 h post dissection (PD, left panel), which equals CT14, and 36 h PD (CT2, right panel). A fasciculation/defasciculation process could be appreciated in the principal branches (arrows), while in secondary neurites different phenomena were observed: addition/retraction (asterisk) and positional changes (arrowhead). (E) Quantitation of changes seen in different cultured brains (n=6). (F) Representative confocal images of fly brains stained for BRP-RFP (white) and PDF (magenta) dissected at CT2, CT14 and CT22 on DD4. (G-H) Quantitation of BRP+ active zones (G) and the total area covered by them (H). Control pdf-GS>brpRFP flies display circadian changes in BRP+ active zones as well as the area covered by BRP+ immunoreactivity. Significant differences were found in both variables between subjective day and night, but not between timepoints taken at nighttime. Same letters indicate no significant differences. Statistical analysis included one way ANOVA (BRP+ active zones p=0.0069; BRP+ area p<0.0001) with Tukey post-hoc test (p<0.05, BRP+ active zones least significant difference = 6.99; BRP+ area least significant difference = 3.35 μm2). In all cases the scale bar represents 10 μm.
Morphological plasticity correlates with changes in synaptic markers
The level of structural remodeling occurring at the dorsal terminals suggested that synapses themselves could undergo changes in a time-dependent fashion. We first examined the presynaptic protein SYNAPTOTAGMIN (SYT) at different times across the day as an indicator of vesicle accumulation. A GFP-tagged version of SYT was expressed in PDF neurons (pdf>sytGFP), and both, the number and area span by SYT+ puncta (likely describing the accumulation of several dense core vesicles [14]) were analyzed separately at the sLNv dorsal terminals (Figure S1A-C). No statistical differences were observed in the number of SYT+ puncta (although there is a tendency for higher numbers in the early morning), perhaps as a result of the nature of the signal, which is too diffuse for precise identification of individual spots (Figure S1B). On the other hand, SYT+ puncta were larger and, as a result, the area covered by SYT+ immunoreactivity was significantly different at CT2 compared to CT14 but not between CT22 and CT2, perhaps reflecting that vesicles started to accumulate at the end of the day in preparation for the most dramatic membrane change taking place between CT22 and the beginning of the following morning (Figure S1C).
The observation that a more complex structure correlated with a larger area covered by presynaptic vesicles reinforced the notion that indeed the number of synapses could be changing throughout the day, and prompted us to analyze BRUCHPILOT (BRP), a well-established indicator of active zones [15-18]. Expressing a tagged version of BRP in PDF neurons we quantitated the number of BRP+ puncta as a proxy for active zones [19] at times where the most dramatic changes in structure had been detected (i.e., CT2, CT14 and CT22, Figure 1F-H). Interestingly, the number of active zones was significantly larger at CT2 than at CT14 or CT22; in fact no statistical differences were observed between the last two timepoints, underscoring that axonal remodeling can occur (i.e., pruning of major projections taking place towards the end of the night) without significantly affecting overall connectivity. Thus, circadian structural plasticity is accompanied by changes in the number of synapses. Not only more vesicles are recruited towards CT2, but also a higher number of active zones are being established.
Circadian changes in the abundance of the presynaptic active zone BRP have also been shown in the first optic neuropil of the fly brain, although BRP abundance in the lamina increases in the early night under DD conditions [20], in contrast to the oscillations in BRP levels observed at the dorsal protocerebrum that peak in the early subjective day just described. In addition, rhythmic changes in the number of synapses have also been described in the terminals of adult motor neurons in Drosophila [21] examined through transmission electron microscopy as well as BRP+ light confocal microscopy, underscoring the validity of the approach employed herein. Interestingly, in different brain areas the level of presynaptic markers (such as BRP or SYT-GFP) also changes in response to the sleep/wake “state”, being high when the animals are awake and lower during sleep [19, 22, 23]; this observation led to the proposal that sleep could be involved in maintaining synaptic homeostasis altered during the awaking state. This trend coincides with our observation of higher levels during the subjective morning, and lower levels at the beginning of the subjective night; however, we could not detect changes through the night, suggesting that, at least in clock neurons, there is a circadian rather than an homeostatic control of synaptic activity. Given that clock outputs are predominantly regulated at the transcriptional level [24], and that there is circadian regulation of MEF2, a transcription factor that turns on a program involved in structural remodeling [13], this correlation opens the provocative possibility that the circadian clock is controlling the ability of assembling novel synapses in particularly plastic neurons, which might become stabilized/recruited or otherwise pruned (disassembled), towards the end of the day.
Activity dependent and independent mechanisms underlie structural plasticity
Adult-specific electrical silencing of PDF neurons reduces the complexity of dorsal arborizations while a certain degree of circadian remodeling of the axonal terminals still takes place [12]. To examine if electrical alterations could affect circadian changes in the number of active zones, either KIR2.1 or NaChBac (to hyperpolarize o depolarize PDF neurons, respectively) was expressed. To avoid any undesired developmental defects we used pdf-GS to drive expression of the channels only during adulthood. Interestingly, KIR2.1 expression abrogated circadian changes in the number of active zones. In fact, PDF neurons displayed a reduced number of active zones compared to controls at CT2 and remained at similar levels throughout the day, indistinguishable from nighttime controls (Figure S1D). On the other hand, when neurons were depolarized through NaChBac expression, the number of active zones did not change along the day, and were maintained at daytime levels even at CT14 and CT22 (Figure S1E).
It has recently been shown that MEF2, a transcription factor involved in activity-dependent neuronal plasticity and morphology in mammals [25], is circadianly regulated and mediates some of the remodeling of PDF dorsal terminals through the regulation of Fasciclin2 [13]. On the other hand, adult-specific silencing (and depolarization) of PDF neurons abolishes cycling in the number of BRP+ active zones (Figure S1D-E), despite that it does not completely obliterate the remodeling of the axonal terminals [12], suggesting that some of the mechanisms underlying structural plasticity are clearly activity-independent, and are likely the result of additional clock-controlled output pathways still to be identified.
Circadian changes in the sLNvs connectome
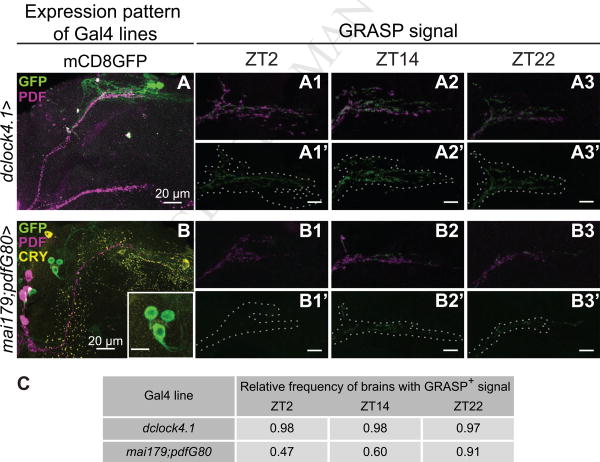
Since structural remodeling of PDF neurons results in the formation and disappearance of new synapses on daily basis we anticipated that not only the number but also the postsynaptic partners of these contacts could concomitantly be changing. To shed light on this possibility we employed “GRASP” (for GFP Reconstitution Across Synaptic Partners), which labels contacts between adjacent membranes [9, 26]. Briefly, two complementary fragments of GFP tethered to the membrane are expressed in different cells. If those cells are in contact, GFP is reconstituted and becomes fluorescent. GRASP has previously been employed to monitor synapses in adult flies [26-29]. Given the complex arborization at the dorsal protocerebrum we inquired whether specific subsets of circadian neurons projecting towards that area [1] could be contacting across the day. Perhaps not surprisingly, an extensive reconstituted GFP signal could be observed between the sLNv dorsal projections and those of the posterior Dorsal Neuron 1 cells (DN1ps, lighted up by the dClk4.1-Gal4 line, [30, 31]), suggesting contacts along the entire area (Figure 2A and 2C), which are detectable across all timepoints analyzed (ZT2, 14 and 22). Consistent with our observations, extensive physical contact between the sLNv projections and those of the DN1p neurons has just been reported at the dorsal protocerebrum with no clear indication of the time of day examined [32, 33]. We next examined whether a subset of dorsal LN neurons (LNds), projecting both towards the accessory medulla as well as the dorsal protocerebrum (through the combined expression of Mai179-Gal4; pdf-Gal80), could also contact the profuse dorsal arborization of sLNv neurons; this genetic combination enables expression of split-GFP in a restricted number of circadian cells (which are part of the evening oscillator [4], i.e. up to 4 LNds, including at least a CRYPTOCHROME positive one, and the 5th sLNv), as well as others located within the Pars intercerebralis (PI), a neurosecretory structure recently identified as part of the output pathway relevant in the control of locomotor behavior [32]. In contrast to the extensive connections between DN1p and sLNv clusters, only very discreet reconstituted puncta were detected; quite strikingly, the degree of connectivity appeared to change across the day reaching a maximum (when almost every brain exhibited reconstituted signal) at ZT22 (Figure 2B and 2C); however, due to the nature of the signal no quantitation of its intensity was attempted. Although a more detailed analysis is required to define the identity (i.e., whether it is one or several LNds, the 5th sLNv, or both groups that directly contact the sLNvs) this finding highlights a potentially direct contact between the neuronal substrates of the morning and evening oscillators. In sum, through GRASP analysis we have begun to map the connectivity within the circadian network; commensurate with a hierarchical role, the sLNvs appear to differentially contact specific subsets in a distinctive fashion.
Figure 2. GRASP analysis on putative clock partners reveals constant and plastic changes in sLNv connectivity.
Images represent examples of putative synaptic partners of PDF neurons. Expression profiles of (A) dClock4.1-Gal4 to light up DN1p neurons and (B) Mai179Gal4;pdfG80 expression on a restricted subset of circadian-relevant neurons including the 5th sLNv, up to 4 LNds (and PI cells). PDF and GFP signals are shown in magenta and green, respectively. (A1-A3) Representative confocal images of a pdf-lexA>lexAop-CD4::GFP11/dClock4.1-Gal4>UAS-CD4GFP110 brain dissected during early day (ZT2, A1), early night (ZT14, A2) and late night (ZT22, A3). (Al′-A3′) Reconstituted GFP+ signal is shown; the structure of PDF projections is outlined by a dashed line (encircling the PDF signal) to improve visualization of the reconstituted GFP. GFP+ signal was observed at all timepoints analyzed. (B1-B3 and Bl′-B3′) Intersection between PDF and Mai179Gal4;pdfG80 neurons (the so-called evening oscillator, [8]). The reconstituted signal changes across the day, becoming more pervasive at nighttime. Scale bar represents 10 μm unless otherwise noted. (C) Quantitative analysis confirms constant contacts between sLNvs and DN1p clusters, but plastic ones between sLNvs and the evening oscillator, with a statistically significant increase at ZT22 (Kruskal-Wallis test p<0.01).
Transmitting time of day information to non-circadian targets
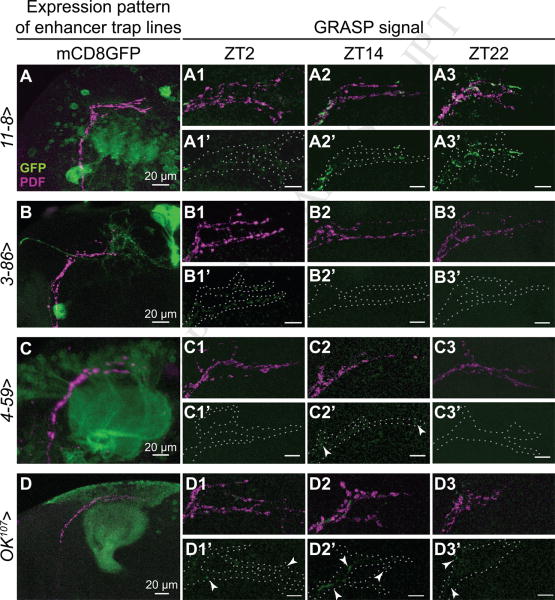
To address the possibility that PDF neurons could be contacting non circadian targets at different times across the day, an enhancer trap screen was carried out employing a subset of GAL4 enhancers selected on the basis of their expression pattern in the adult brain, i.e. known to drive expression in the dorsal protocerebrum; an additional requirement imposed was that none of the selected GAL4 lines could direct expression to the sLNv neurons to avoid internal GFP reconstitution. Reconstitution of the GFP signal at the sLNvs dorsal terminals by recognition through specific antibodies was assessed at three different timepoints for each independent GAL4 line (ZT2, ZT14 and ZT22). Some of the GAL4 lines showed reconstituted GFP signal at every timepoint analyzed (see for example the 11-8 line shown in Figure 3A, or 4-93 in Supplementary Figure 2D), suggesting that those neuronal projections are indeed in close contact across the day and might represent stable synaptic contacts. No GFP signal was detected in the negative parental controls (Supplementary Figure 2A-B). Despite several GAL4 drivers directed expression to the proximity of the PDF dorsal terminals some of the selected lines did not result in reconstituted GFP signal (about 20% of the samples analyzed, Supplementary Figures 2C and 2D).
Figure 3. A GRASP screen uncovers changes in connectivity to non-circadian targets.
Images represent examples of putative synaptic partners of PDF neurons contacting them in different time-windows; throughout the day (A), during ZT2 (B) or during ZT14 (C). (A-C). Expression profiles of 11-8 (A), 3-86 (B), 4-59 (C), and OK107 (D) neuronal clusters. PDF and GFP signals are shown in magenta and green, respectively. 3-86 is expressed in the PI and sends neurites proximal to sLNvs dorsal projections. 4-59 and 11-8 are both expressed in the calyx of the MBs, although different subgroups of Kenyon cells appear to be included in each line. OK107 is a widely used MB driver. (A1-A3). Representative confocal images of pdf-lexA>lexAop-CD4::GFP11/11-8-GAL4>UAS-CD4GFP110 brains dissected during early day (ZT2, A1), early night (ZT14, A2) and late night (ZT22, A3). (A1′-A3′) Reconstituted GFP+ signal is shown; the overall structure is outlined by a dashed line (encircling PDF signal) to improve visualization of the reconstituted GFP. GFP+ signal was observed at the 3 analyzed timepoints. (B1-B3 and B1′-B3′) Intersection between PDF and 3-86 neurons. Reconstitution signal was observed only at ZT2. (C1-C3 and C1′-C3′) A similar analysis was carried out with the 4-59 enhancer-trap line. Reconstitution was observed at ZT14. (D1-D3 and D1′-D3′) Synaptic contacts between PDF neurons and the mushroom bodies evidenced by GRASP at ZT2, ZT14 and ZT22. Arrows indicate synaptic reconstitution. Scale bar represents 10 μm unless otherwise indicated.
Quite remarkably, a proportion of the GAL4 lines showed GFP+ signal only at a specific timepoint. One such example is line 3-86, where reconstitution was detected in most of the brains analyzed at ZT2, but not at nighttime (Figure 3B). Being able to identify putative postsynaptic contacts to the sLNvs in the early morning is consistent with the observation of a higher number of BRP+ active zones in the early day (Figure 1F-H). This enhancer trap spans different neuropils such as the mushroom body (MBs) lobes, the lateral horn, and directs expression to particularly high levels in the PI (Figure 3B and Supplementary Figure 2E), a structure that has recently been implicated in the rhythmic control of locomotor activity [32]; in fact, some yet unidentified somas in the PI appear to arborize profusely near the PDF dorsal terminals, underscoring a potential link between the two neuronal groups; these direct contacts are unlikely to be the ones reported by Mai179; pdfGal80 since those connect to the sLNv neurons preferentially at night (Figure 2). Interestingly, a subset of neurons in the PI is relevant in mediating the arousal promoting signal from octopamine [34]; in addition, sleep promoting signals are also derived from a different subset of neurons in the PI [35], opening the attractive possibility that both centers could be under circadian modulation.
GRASP analysis also uncovered a different neuronal cluster (4-59) that contacts PDF neurons preferentially during the early night (ZT14), which is in itself striking, since this timepoint corresponds to that with fewer arborizations and an overall decrease in the number of synapses (Figure 3C). This enhancer trap is expressed in the MBs, the subesophagic ganglion, antennal lobes and accessory medulla (Supplementary Figure 2G). Among those structures, the MBs are important for higher order sensory integration and learning in insects [36]. Interestingly, circadian modulation of short-term memory [37] and memory retrieval after sleep deprivation [38] was reported; short-term memory was found to peak around ZT15-17, coinciding with the window of GFP reconstitution, providing a functional connection to the synaptic plasticity observed. To corroborate if there is a direct contact between the two neuronal clusters, the extensively used Gal4 driver OK107, which is expressed in the α′/β′ and the γ lobes of the MB and to a lower extent in the PI ([39] and Supplementary Figure 2H), was employed for GRASP analysis. Surprisingly, reconstituted GFP signal could be observed at every timepoint analyzed, suggesting that MB lobes contact PDF neurons throughout the day, but specific clusters (for example those highlighted by the 4-59 line) establish plastic, time-of day-dependent physical contact with PDF neurons (Figure 3D).
We next inquired whether these prospective postsynaptic targets of PDF neurons could play a role in the output pathway controlling rhythmic locomotor activity. To address this possibility we examined the impact of adult-specific alteration of excitability of distinct neuronal groups through expression of TRPA1. Interestingly, adult-specific depolarization of specific neuronal populations triggered a clear deconsolidation of the rhythmic pattern of activity, which resulted in less rhythmic flies accompanied by a significant decrease in the strength of the underlying rhythm (Table 1). These results lend support to the notion that the underlying neuronal clusters are relevant in the control of rest-activity cycles.
Table 1. Deconsolidation of rhythmic activity upon adult-specific activation of specific neuronal clusters.
Circadian rhythmicity is affected when non-circadian contacting neurons are depolarized. Average period, percentage of rhythmicity and FFT in control and TrpA1-expressing groups at 28 °C (activated condition); the period under free-running conditions is shown. Statistically significant differences could be observed in FFT for control groups and treatments. “n” refers to the number of individuals analyzed per experimental group. Two to four locomotor activity experiments were carried out. Statistical analysis included a Kruskal-Wallis test with pair-wise comparisons. Different letters indicate statistically significant differences (p<0.01).
| Genotype | τ (h) | Rhythmicity (%) | Power FFT | n |
|---|---|---|---|---|
| UAS-TrpA1/+ | 23.34 | 91.54 | 0.06B | 124 |
| OK107-GAL4/+ | 23.36 | 100.00 | 0.10C | 42 |
| 4-59-GAL4/+ | 23.67 | 100.00 | 0.07B | 37 |
| 3-86-GAL4/+ | 23.70 | 98.08 | 0.06B | 54 |
| OK107>TrpA1 | 22.55 | 47.62 | 0.04A | 58 |
| 4-59>TrpA1 | 23.52 | 68.95 | 0.03A | 71 |
| 3-86>TrpA1 | 23.43 | 73.22 | 0.04A | 79 |
Over the years it has become increasingly clear that the circadian clock modulates structural properties of different cells (reviewed in [40]). In fact, a number of years ago it was reported that the projections of a subset of core pacemaker fly PDF+ [8] and mammalian VIP+ [41] neurons undergo structural remodeling on daily basis. The work presented herein lends support to our original hypothesis that circadian plasticity represents a means of encoding time-of-day information. By changing their connectivity PDF neurons could drive time-specific physiological processes. As new synapses assemble while others are dismantled the information flux changes, allowing PDF neurons to promote or inhibit different processes at the same time. This type of plasticity adds a new level to the complex information encoded in neural circuits, where PDF neurons could not only modulate the strength in the connectivity between different partners, but also define which neuronal groups could be part of the circadian network along the day. While further analysis of the underlying process is ensured, evidence so far supports the claim that structural plasticity is an important circadian output.
Experimental procedures
Strains and Fly Rearing
Flies were reared and maintained at 22 (locomotor activity assays) or 25 °C in vials containing standard cornmeal medium under 12:12 h light:dark (LD) cycles, with the exception of those including RU486 (mifepristone, Sigma) which were treated as previously described [12]. A list of the stocks employed throughout this work is included in the Supplementary information.
Brain cultures
For brain cultures we used the protocol previously described [42], with minor changes. Briefly, flies reared in LD were cold anesthetized and washed with 70% ethanol. Brains were quickly dissected in ice-cold Schneider medium (Invitrogen) and placed on a millicell low height culture plate insert (Millipore), previously coated with laminin (BD Biosciences) and polylysine (Sigma), on a Petri dish with culture medium, which were kept at 25 °C under DD conditions. The first observation was made 24 h post dissection (PD). The culture medium was supplemented with penicillin, streptomycin, fetal bovine serum (Natocor, Córdoba, Argentina), and insulin, and was replaced on daily basis.
Locomotor behavior analysis
Flies were crossed and maintained at 22 °C while entrained to a 12 h LD cycle. Newly eclosed adult males were placed in glass tubes containing standard food and monitored for locomotor activity using the DAM system (TriKinetics). Isolated males were kept in LD conditions for 3 days, followed by 6 days at 22 °C on DD. On day 7, the temperature was raised to 28 °C, flies were transferred to fresh tubes under red light and kept in the incubator for additional 7 days. Period, FFT and rhythmicity were estimated using ClockLab software (Actimetrics) as previously described [12, 43].
Dissection and Immunofluorescence
Dissection and immunostaining of adult fly brains was performed as previously described [12]. The primary antibodies employed were rabbit anti-GFP 1:500 (Invitrogen), rabbit anti-RFP 1:500 (Rockland), chicken anti-GFP 1:500 (Upstate), rabbit anti-PDF 1:1500 (custom-made by NeoMPS, France) and homemade rat anti-Drosophila-PDF 1:500 [12]. Secondary antibodies used were Cy2-and Cy3-conjugated anti-rabbit, Cy2-conjugated anti-chicken and Cy5- and Cy3-conjugated anti-rat (Jackson ImmunoResearch). Images were taken on either a Zeiss Pascal LSM, a Zeiss LSM 510 Meta confocal or a Zeiss LSM 710 two-photon microscope. After acquisition, images were processed employing Fiji, an ImageJ-based image processing environment [44].
Quantitation of the axonal branching and axonal length
Structural plasticity was analyzed as reported [8]. The Zeiss LSM Image Browser software was used to measure the length of the sLNvs dorsal arborization. The starting point was set at the lateral horn and the maximal length was measured towards the mid brain, following the path of the largest neurite.
BRP and SYT quantification
Images were processed employing Fiji. First, a Z projection of the stacks is made. Then, a region of interest around the dorsal arborization of the sLNvs was selected. Threshold image was adjusted in order to mark most of the BRPRFP or SYTGFP puncta. Finally, the “Analyze particles” tool was employed to measure the total area and number of fluorescent puncta.
Grasp
A GRASP screen was carried out with a subset of the Heberlein's enhancer trap collection [45] and the analysis was performed at three timepoints (ZT2, 14 and 22). The mouse monoclonal anti-GFP from Sigma recognized the reconstituted GFP molecule but not the GFP1-10 or GFP11 fragments alone and was employed for GRASP analysis. A minimum of 15 brains were analyzed per genotype and timepoint. A positive GFP signal at a given timepoint was considered only if more than half of the brains presented reconstituted GFP signal. Only in those GAL4 lines that supported GFP reconstitution at some of the timepoints studied we confirmed that parental strains (pdf-lexA>lexAop-CD4GFP11 and X-GAL4>UAS-CD4GFP1-10) do not present GFP+ signal.
Statistical analysis
Statistical analyses were performed with InfoStat (Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina). Whenever possible, ANOVA was performed. Normality was tested using Shapiro-Wilks test, and the homogeneity of variance was assessed with Levene's test. p<0.05 was considered statistically significant. When in a two way ANOVA an interaction between factors was significant, interaction contrast was performed and p-values were informed.
Supplementary Material
Acknowledgments
The authors would like to thank M.P. Fernández for invaluable help with TRP experiments. We are indebted to E. Beckwith, N. Muraro and A. Schinder for critical reading of the manuscript, and to members of the Ceriani lab for helpful discussion. We would like to specially thank U. Heberlein (now at HHMI, Janelia Farm, VA) for providing access to the GAL4 collection kept at UCSF. We also thank K. Scott (UC Berkeley, US), J. Dubnau (CSHL, US), J. Berni (Univ. of Cambridge, UK), P. Emery (Univ. of Massachusetts, US) and the Bloomington Stock Center for fly stocks, and the Developmental Studies Hybridoma Bank for antibodies. MFC, LF and NP are members of the Argentine Research Council (CONICET). EAG and AD-C were supported by graduate fellowships from CONICET and ANPCyT. This work was supported by a grant from the ANPCyT, Argentina (PICT2011-2185) and by a FIRCA-NIH grant (1R03TW008342) to MFC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helfrich-Forster C. The neuroarchitecture of the circadian clock in the brain of Drosophila melanogaster. Microsc Res Tech. 2003;62:94–102. doi: 10.1002/jemt.10357. [DOI] [PubMed] [Google Scholar]
- 2.Shafer OT, Helfrich-Forster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 4.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 5.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 6.Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- 7.Blanchardon E, Grima B, Klarsfeld A, Chelot E, Hardin PE, Preat T, Rouyer F. Defining the role of Drosophila lateral neurons in the control of circadian rhythms in motor activity and eclosion by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci. 2001;13:871–888. doi: 10.1046/j.0953-816x.2000.01450.x. [DOI] [PubMed] [Google Scholar]
- 8.Fernández MP, Berni J, Ceriani MF. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depetris-Chauvin A, Berni J, Aranovich EJ, Muraro NI, Beckwith EJ, Ceriani MF. Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol. 2011;21:1783–1793. doi: 10.1016/j.cub.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivachenko A, Li Y, Abruzzi KC, Rosbash M. The transcription factor mef2 links the Drosophila core clock to fas2, neuronal morphology, and circadian behavior. Neuron. 2013;79:281–292. doi: 10.1016/j.neuron.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuyama K, Meinertzhagen IA. Synaptic connections of PDF-immunoreactive lateral neurons projecting to the dorsal protocerebrum of Drosophila melanogaster. J Comp Neurol. 2010;518:292–304. doi: 10.1002/cne.22210. [DOI] [PubMed] [Google Scholar]
- 15.Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 17.Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyhersmuller A, Hallermann S, Wagner N, Eilers J. Rapid active zone remodeling during synaptic plasticity. J Neurosci. 2011;31:6041–6052. doi: 10.1523/JNEUROSCI.6698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorska-Andrzejak J, Makuch R, Stefan J, Gorlich A, Semik D, Pyza E. Circadian expression of the presynaptic active zone protein Bruchpilot in the lamina of Drosophila melanogaster. Dev Neurobiol. 2013;73:14–26. doi: 10.1002/dneu.22032. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz S, Ferreiro MJ, Menhert KI, Casanova G, Olivera A, Cantera R. Rhythmic changes in synapse numbers in Drosophila melanogaster motor terminals. PLoS One. 2013;8:e67161. doi: 10.1371/journal.pone.0067161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozkaya O, Rosato E. The circadian clock of the fly: a neurogenetics journey through time. Adv Genet. 2012;77:79–123. doi: 10.1016/B978-0-12-387687-4.00004-0. [DOI] [PubMed] [Google Scholar]
- 25.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Q, Xiang Y, Yan Z, Han C, Jan LY, Jan YN. Light-induced structural and functional plasticity in Drosophila larval visual system. Science. 2011;333:1458–1462. doi: 10.1126/science.1207121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang Y, Haynes P, Pirez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci. 2011;14:889–895. doi: 10.1038/nn.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pech U, Pooryasin A, Birman S, Fiala A. Localization of the contacts between Kenyon cells and aminergic neurons in the Drosophila melanogaster brain using SplitGFP reconstitution. J Comp Neurol. 2013;521:3992–4026. doi: 10.1002/cne.23388. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr Biol. 2010;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanaugh DJ, Geratowski JD, Wooltorton JR, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, Sehgal A. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seluzicki A, Flourakis M, Kula-Eversole E, Zhang L, Kilman V, Allada R. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS Biol. 2014;12:e1001810. doi: 10.1371/journal.pbio.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 36.Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 37.Lyons LC, Roman G. Circadian modulation of short-term memory in Drosophila. Learn Mem. 2009;16:19–27. doi: 10.1101/lm.1146009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Glou E, Seugnet L, Shaw PJ, Preat T, Goguel V. Circadian modulation of consolidated memory retrieval following sleep deprivation in Drosophila. Sleep. 2012;35:1377–1384B. doi: 10.5665/sleep.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements J, Hens K, Merugu S, Dichtl B, de Couet HG, Callaerts P. Mutational analysis of the eyeless gene and phenotypic rescue reveal that an intact Eyeless protein is necessary for normal eye and brain development in Drosophila. Dev Biol. 2009;334:503–512. doi: 10.1016/j.ydbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frenkel L, Ceriani MF. Circadian Plasticity: From structure to behavior. International Review of Neurobiology. 2011;99:107–138. doi: 10.1016/B978-0-12-387003-2.00005-7. [DOI] [PubMed] [Google Scholar]
- 41.Becquet D, Girardet C, Guillaumond F, Francois-Bellan AM, Bosler O. Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia. 2008;56:294–305. doi: 10.1002/glia.20613. [DOI] [PubMed] [Google Scholar]
- 42.Ayaz D, Leyssen M, Koch M, Yan J, Srahna M, Sheeba V, Fogle KJ, Holmes TC, Hassan BA. Axonal injury and regeneration in the adult brain of Drosophila. J Neurosci. 2008;28:6010–6021. doi: 10.1523/JNEUROSCI.0101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science. 2014;343:1516–1520. doi: 10.1126/science.1251285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.