Abstract
The placenta is essential to mammalian pregnancy with many roles beyond just nutrient supply, including both endocrine and immune functions. During the course of evolution, the placenta of higher primates has acquired some unique features, including the capacity to secrete corticotropin-releasing hormone (CRH). In addition, a placental receptor for IgG enables particularly high levels of protective maternal antibody to reach the fetus before birth. This paper reviews the placental biology of primates, and discusses its involvement in adrenocortical hormone activity during pregnancy, the transfer of maternal antibody, and finally the delivery of maternal iron to the fetus, which is needed for normal brain development. An understanding of these vital functions during a full-term, healthy pregnancy provides insights into the consequences of gestational disturbances, such as maternal stress, illness, and undernutrition, which have even larger ramifications if the infant is born premature.
Keywords: Placenta, pregnancy, primate, cortisol, antibody, immune iron, anemia, stress, prematurity, monkey
1. Introduction
The evolution of mammals with their long period of internal development before birth is intimately associated with the placenta, especially its capacity to nurture the fetus and sustain pregnancy (O’Leary et al., 2013). As mammals evolved from the marsupial to eutherian stage, and longer gestations became common, the placenta took on additional roles. In addition to being essential for connecting the implanting embryo with the maternal blood supply, the placenta initiates hormone and immune changes in the gravid female needed to prevent rejection, and then continues to have myriad actions during the remainder of pregnancy (Murphy et al., 2006). The significance of the placenta is particularly prominent in primates. In both concrete and symbolic ways, it initiates and presages the continued importance of the mother-infant relationship and nursing during the postnatal period. A consideration of placental functioning provides insight into the intimate communication between mother and fetus required for a successful outcome and the birth of a healthy, resilient infant.
Across the Order Primates, which is comprised of over 650 species, one can identify progressive changes in placental structure and function, trends that help to understand important aspects of human pregnancy. When comparing prosimians to monkeys and apes, the placenta becomes more invasive, penetrating deeper into the uterine endometrium, reflecting the change from an epitheliochoreal placenta into the hemochorial placenta (Montiel et al., 2013). The epithelial barrier between maternal and fetal blood thins to a point where the capillaries are sufficiently proximal to permit some fetal white blood cells to cross into the mother, where they remain and can be active for years after birth (Bianchi et al., 1996; Stevens et al., 2004). This proximity also exposes the mother’s own leukocytes to paternal antigens on fetal cells. That exposure required a more assertive regulation of the female’s immune responses during pregnancy, another process implemented by the placenta, in part through the synthesis of syncytin and the release of immunomodulatory hormones like progesterone. Moreover, in higher primates, it includes a placental capacity to secrete CRH, which provides a way for the fetus to regulate its own pituitary-adrenal axis, and thus cortisol levels in circulation (Margioris et al., 1988; Smith, 1999). In addition, placental CRH has some endocrine effects in the maternal compartment (Sasaki et al., 1989), is involved in immune tolerance early in gestation, and helps later to initiate labor (Makrigiannakis et al., 2003). An inappropriate or premature surge of CRH can also mediate some of the harmful effects of intrauterine infections (Mazoub and Karalis, 1999; Uh et al. 2008). Later in the review, we also illustrate the relationship between cortisol levels present in the maternal and fetal compartments of monkeys during pregnancy.
The hemochorial placenta is found in all monkeys, including the rhesus macaque, the primary species assessed in our research. This type of placenta is associated with a reduction in the number of offspring, with just one infant being common in monkeys and apes. The change in the placenta and decreased fecundity in the ancestors of Old and New World monkeys also coincided with a dramatic anatomical change in the reproductive tract, the shift from a bicornuate to a single, fused uterus. Even the subset of South American monkeys--the marmosets and tamarins that reverted back to having dizygotic twins-- do so with a unicornuate uterus and hemochorial placenta. But it should be reiterated that singleton pregnancies predominate among most monkeys and apes. For example, in our breeding colony of rhesus monkeys, twinning is exceedingly rare. It has occurred only 2 times in over 4000 pregnancies across a span of 50 years, a statistic far below the current prevalence of twinning in humans (3.3%). Another notable feature of the primate pregnancy is its long length. Gestation became more prolonged as the body size of primates increased: from the 2-month pregnancy in a small prosimian like the mouse lemur, to the 5.5 – 6.0 month duration in most monkeys, and finally reaching 8- months in the great apes.
In addition to permitting the mother to gestate a larger and more precocial infant with an elaborated brain by birth, the extension of pregnancy afforded the opportunity for some other adaptations to become established. In the monkeys of Africa and Asia, as well as in the great apes, the placenta acquired a receptor for one isotype of maternal antibody, the immunoglobulin G class (IgG) (Leach et al., 1996). This placental feature enables a receptor-mediated transfer of maternal IgG, which accelerates during the final weeks of pregnancy (Coe et al., 1994; Roopenian and Akilesh, 2007). Thus, the full-term infant monkey has as much or higher IgG levels than its mother at delivery. The pediatric significance of this transfer of maternal antibody is that it engenders an extended period of passive immunity, protecting the young infant against many bacterial and viral pathogens to which the mother had been exposed. The evolution of placental antibody transfer in primates is considered in the third section of our review.
Finally, we discuss another placental receptor, the transferrin receptor (TfR), which also has a transmission function that becomes prominent at the end of pregnancy. The TfR pulls increasing amounts of iron from the maternal blood stream into the fetal compartment. These iron stores in the neonate are critical for providing the iron needed to sustain rapid growth during the early postnatal period, and cannot be replaced after birth by the delimited iron available in breast milk. We have found that a low prenatal provision of maternal iron has many health consequences for the developing infant monkey. In addition to increasing the risk for iron-deficiency anemia, a low prenatal iron endowment will adversely impact the developing brain. The anemic infant monkey evinces reduced brain energetics, skewed monoaminergic neurotransmitter activity, and slower myelination due to the iron depletion in oligodendrocytes (Patton et al., 2012; Rao et al., 2013).
2. The placenta as an endocrine tissue
One of the first tasks for the implanting primate embryo, at not much more 100 cells, is to facilitate the survival of the corpus luteum and to ensure the continued secretion of progesterone in order to block menstruation. The syncytiotrophoblast, which will give rise to the placenta, is the source of the chorionic gonadotrophin (CG) that ‘rescues’ the corpus luteum. By extending the functional life span of the luteal body, it maintains high levels of progesterone until the placenta is large enough to produce sufficient amounts of its own estrogen and progesterone. Then, by mid-pregnancy, a luteoplacental shift will occur. Placental steroidogenesis becomes so pervasive at that point that a female monkey can be ovariectomized and her gravid state will be maintained normally until term. The primate placenta is also an active contributor to other hormone changes associated with pregnancy, including being the site for converting the dehydropiandrosterone-sulfate (DHEA-S) produced by the fetal adrenal into the estrogens found in the mother’s blood stream (Ramirez et al. 2004). This estrogen has many functions, including synergizing with prolactin later in pregnancy to prepare the breast tissue for lactation. But early in the primate pregnancy, estrogen has another important action, which is to stimulate the maternal adrenal to secrete more cortisol. In the squirrel monkey, a primate that has high cortisol levels even when not pregnant, this estrogenic effect is so pronounced and quick that a precipitous cortisol rise can be used to index conception (Coe et al., 1986). One can also mimic estrogen’s stimulatory action on the adrenal by administering it to adult males and tracking the resulting surge in cortisol and cortisol-binding globulin (CBG) levels over the next week.
Corticosteroids have important functions during pregnancy in many species. In some animals like sheep, the rising adrenal hormone levels at the end of gestation signal and initiate labor. The importance of cortisol in higher primates is also highlighted by the fact that the placenta acquired the ability to secrete CRH and thereby to directly influence pituitary-adrenal axis, primarily in the fetal compartment, but also in the maternal compartment (Sasaki et al., 1989). This unique ability to release CRH is associated with other critical placental actions, because it can stimulate the trophoblast production of syncytin. We have come to appreciate that syncytin helps to mediate the maternal immune tolerance for the fetus, and thus CRH indirectly contribute to the maintenance of pregnancy in this way. The evolutionary story behind placental syncytin is an interesting one -- a capacity acquired by incorporating DNA inserts from ancestral viral infections into the early mammalian and primate genome -- but those details go beyond the scope of this review (see: Dupressoir et al., 2012, Tolosa et al., 2012). Both CRH and cortisol levels in primates continue to rise significantly as pregnancy progresses and then serve other functions during the second half of pregnancy (Mastorakas and Ilias, 2003). Of clinical significance, an inappropriately early or large increase in CRH levels during the second or third trimester is a warning sign that the pregnancy will not go to term (Fahlbusch et al., 2012; Wadwha et al., 1997). Further, there is now evidence that in a normal term delivery, CRH synergizes with oxytocin, proinflammatory cytokines, and prostaglandins to stimulate the myometrial contractions of labor as progesterone’s tonic inhibition is withdrawn (Zoumakis et al. 1996; Jeschke et al., 2005, McLean and Smith, 2001).
The placenta fulfills another important role related to cortisol, which is to assist in the transfer of maternal cortisol to the fetal compartment and to regulate the amount that will cross (Campbell and Murphy, 1997). During the first half of gestation, the majority of the cortisol found in the circulation of fetal monkeys is actually of maternal origin (Althaus et al., 1986; Pepe and Albrecht, 1995). As pregnancy progresses, greater amounts are produced by the fetus, but that must wait until its tiny adrenal glands have increased to a sufficient size. Even when the developing adrenals can secrete enough cortisol and are responsive to ACTH from the fetal pituitary, this endogenous secretion by the fetus continues to be strongly influenced by how much corticosteroid is transferred. When the transfer of maternal cortisol is high, fetal cortisol synthesis and release are decreased as a counter-regulatory response. Although this reduction is adaptive, one collateral ramification of lessening fetal adrenal activity is that it simultaneously decreases the secretion of DHEAs, the placenta’s primary substrate for estrogen.
The placenta has one other way to regulate cortisol levels in the fetal compartment, which is to enzymatically modify maternal cortisol into a less active form, cortisone, a transformation mediated by 11-beta hydroxysteroid dehydrogenase type 2 (11β-HSD2) (Seckl, 2004; Wyrwoll et al., 2011). The importance of this modulatory pathway becomes evident if that placental enzyme is decreased or less active, as can happen in the context of a stressed pregnancy or when induced by high maternal catecholamine levels (Kajantie et al., 2003). Then, more maternal cortisol can reach the fetal compartment with a potential for eliciting deleterious effects on brain development (LeWinn et al., 2009). Moreover, because 11β-HSD2 cannot act on pharmacological glucocorticoids, some drugs commonly used in obstetrical practice, like dexamethasone (Dex), are able to have preferential access to the fetus (de Vries et al., 2007). We observed the potency of Dex in a study investigating maternal and fetal cortisol levels in the rhesus monkey (Coe et al., 1993).
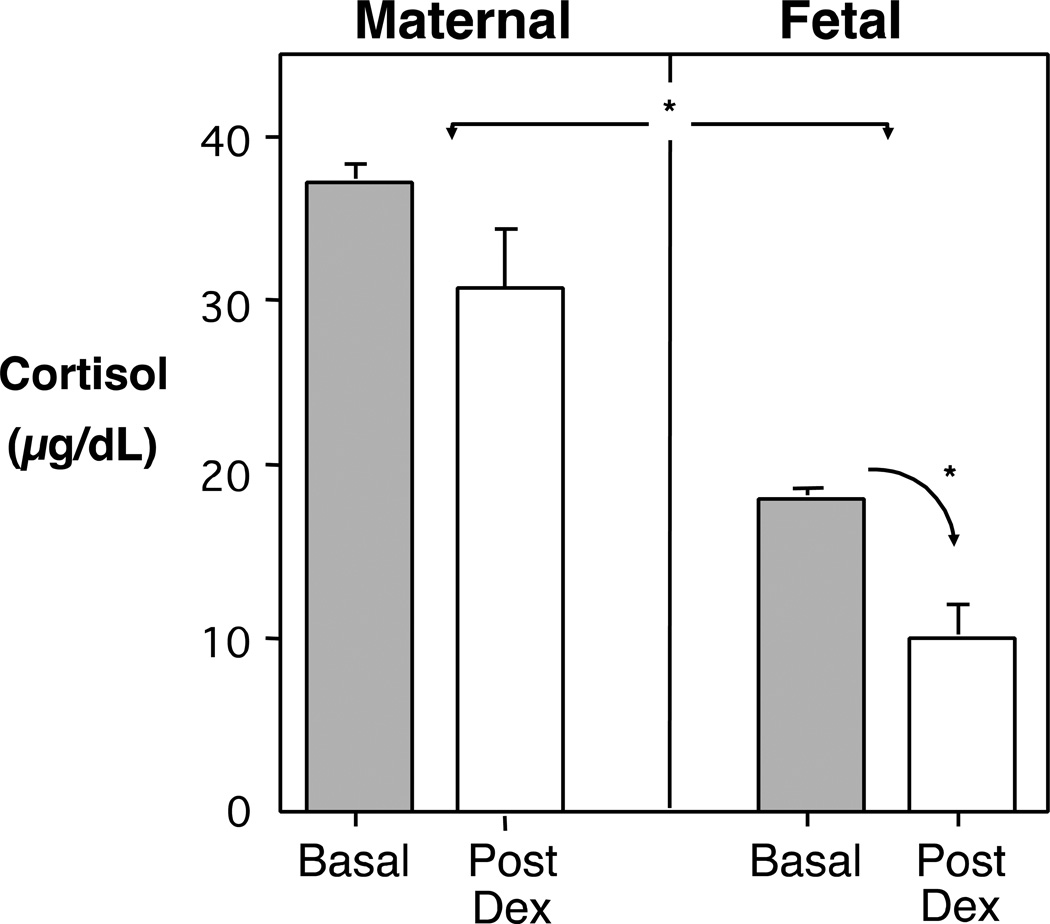
Figure 1 shows typical maternal and fetal cortisol levels in normal rhesus monkeys during the final 2 months of pregnancy, as well as the effect of a 2-day Dex treatment of the gravid female 5–7 days before the blood was collected. One can see the differential in cortisol levels between the mother and fetus, in keeping with the restricted placental transfer and the lower secretory capacity of the fetal adrenal. At Day 125 ga, the fetal/maternal ratio was 35%, and it increased progressively to approximately 68% in the monkeys by term. In the human fetus, the differential is even greater, with fetal cortisol levels typically being only 10% of maternal values. When Dex was administered to the gravid monkey, her endogenous secretion decreased due to the negative feedback on HPA axis. By 24 hours, her cortisol levels had declined from 32.7 to 4.0 µg/dL. However, by the 5–7 day time point shown in Fig 1, maternal cortisol was returning back toward normal pregnancy levels. In contrast, one can see that the Dex was still continuing to suppress cortisol in the fetal compartment to about ½ of normal levels. This more persistent suppression was indicative of both the placenta’s permissive transfer of Dex and the slower capacity of the fetus to metabolically degrade a synthetic glucocorticoid.
Figure 1.
Mean (SE) cortisol during mid-to-late pregnancy in rhesus monkeys, demonstrating significant differences between levels in the maternal and fetal compartments. Sixteen matched samples were obtained at caesarian delivery across Days 125–162 ga. Dexamethasone (0.5 mg/kg at 12 hr intervals) was administered to 4 other gravid females, and blood samples obtained 5–7 days later on Day 135 ga. The Dex inhib ition of fetal cortisol was significantly more prolonged, reflecting the permissive placental transfer of Dex and its persistence in the fetal compartment.
The long half-life of Dex we detected in the fetal compartment may also help to account for some of the undesired side effects of antenatal corticosteroid treatments on fetal organs and tissues, which have been found in many primate studies. We also determined that a 2-day exposure to Dex prenatally results in lingering postnatal effects on endocrine and immune responses in the infant monkey (Coe and Lubach, 2005). Many other detailed and elegant studies have been conducted on maternal-fetal hormone interactions in pregnant rhesus monkeys and also in baboons (Mesiano and Jaffe, 1997). From those investigations, we know that the placenta can passage cortisol in both directions, and that the placenta is a critical regulator of hormone levels in the fetal compartment (Walsh et al., 1979). For example, even with the time period of a single day, the placenta’s balancing act can be observed. When cortisol levels in the gravid female monkey are high in the morning, there is a greater inhibition of fetal adrenal activity than after her cortisol levels decline at night (Novy and Walsh, 1981).
3. The placenta as an immune gland
The primate placenta fulfills a number of equally important immune functions, and it is possible to identify critical actions at each stage of pregnancy. Along with secreting CG, the front edge of the implanting syncytiotrophoblast must manipulate the leukocytes in the mother’s endometrium in order for implantation to be successful (Slukvin et al., 1998). Some maternal cells, including the natural killer cells present in uterine tissue, are induced to assist with engulfment (Rai et al., 2005). Next, the monocyte/macrophage lineages in the maternal tissue are stimulated to produce angiogenesis factors to attract the initial blood supply and these cells help to sculpt the capillaries. Simultaneously, other maternal leukocytes, including the gamma/delta T cells, must be inhibited in order to prevent fetal rejection (Gonzalez et al., 2007). If the latter cells are primed for any reason, such as by a bacterial infection in the reproductive tract or are stimulated by a proliferative cytokine like interleukin-2, a miscarriage may result. These formative responsibilities of the placenta have been investigated in exquisite detail in primates, and are just the first of its many immune-related tasks.
Throughout pregnancy, the placenta must serve a barrier function, maintaining the fetal compartment free of viruses and bacteria. If this barrier is bridged and sterility compromised, or placental tissues become infected, such as in the case of chorioamnionitis, the pregnancy may be lost (Cardenas et al., 2010; Hisao and Patterson, 2011). In humans, bacterial and viral infections are thought to be a factor in about ½ of premature births (Menon et al., 2006). Some of these adverse obstetrical effects are induced by the placental release of proinflammatory cytokines into the fetal compartment (Fortunato et al, 1996). Even if the pregnancy does continue to term, research on prenatal infections in rodent and primate experimental models indicate that fetal development may be impaired. Placental cytokines that reach fetal circulation have ready access to the developing nervous system in the absence of an established blood-brain-barrier, often with teratogenic consequences for immature neurons, glia, and oligodendrocytes (Martinez et al., 1998; Rees et al., 2008; Shi et al., 2005).
The placenta must also guide the changes in systemic immunity taking place in the gravid female across pregnancy. Cellular immune processes that might lead to fetal rejection have to be dampened. This phasic shift can have one inadvertent benefit for a pregnant woman with a prior autoimmune condition, such as multiple sclerosis, which is that there may be a temporary remission of symptoms. Even in healthy pregnant women, the secretion of the proinflammatory cytokine, tumor necrosis factor (TNFalpha), will be down-regulated. Interestingly, this suppression is evident only in vivo, because if the mononuclear cells of pregnant women are cultured away from the endogenous modulatory factors, they actually show increased TNFalpha responses when stimulated in vitro with endotoxin (Rigo et al., 2004). Although an over-simplification, this shift in cytokine and immune balance across pregnancy has often been described as an inhibition of cellular immunity in favor of humoral immunity (Raghupathy, 1997; Wegmann et al., 1993).
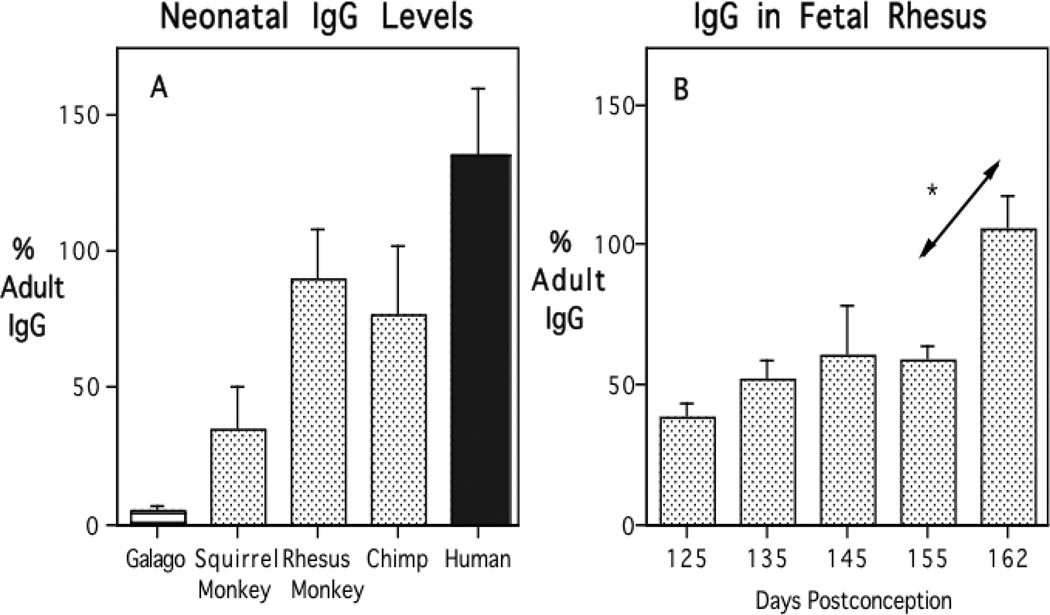
Many years ago, we investigated how one aspect of humoral immunity benefits from this shift. The findings reveal another novel aspect of placental biology in primates. In pediatrics it is well known that human infants are born with high levels of antibody of the G class, reflecting a large placental transfer of maternal antibody during the third trimester. That maternal antibody ensures a 3–6 month period of protective immunity against many bacterial and viral pathogens to which the mother had been exposed. But not all animals transfer maternal IgG in this way during the prenatal period. Instead, marsupials, many rodents, and domesticated farm animals provide IgG in breast milk after birth, along with another class of antibody, secretory IgA. We had the opportunity to investigate the process of placental antibody transfer in the mothers and neonates of 5 primate species, and thereby to evaluate what changes likely occurred during the shift from the prosimian to simian mode of antibody transfer (Coe et al., 1994). As can be seen in Figure 2A, the galago to this day still exemplifies the ancestral, mammalian pattern with minimal IgG present in the neonate at birth. The squirrel monkey, representing the South American monkeys, appears transitional with newborn IgG titers attaining approximately 40% of maternal levels. IgG levels in the infant rhesus monkey and chimpanzee reach equivalence with the mother by term. By comparison, the human placenta truly excels, engaging in such active transfer that our infants are often born with 150–200% of the mother’s values. This high capacity likely reflects a more effective internalization of maternal IgG by the placental IgG receptor (FcRn), which then transcytoses the antibody into fetal circulation at an accelerating rate during the final weeks of pregnancy (Leach et al., 1996; Roopenian and Akilesh, 2007).
Figure 2.
Placental transfer of maternal IgG to infant primates, revealing low levels in the prosimian galago, intermediate transmission in a New World monkey, and the equivalence with maternal values reached in Old World monkey and chimpanzee neonates (Panel A). All are less than the more active placental IgG transfer evident in the human newborn. Placental transfer of maternal IgG determined in fetal rhesus monkeys across late gestation, demonstrating the significant increment during the final 2 weeks before term (Panel B).
Beyond the interesting comparative biology across primate species, there are several important clinical ramifications of the evolutionary change in antibody transfer. Given that maternal antibody is provided so late in pregnancy, premature infants will be born without this endowment of maternal antibody. In the rhesus monkey, a premature birth or caesarian delivery more than 2-weeks before full-term is sufficient to result in a marked diminution of antibody (Coe et al., 1993). Figure 2B shows the accumulation of maternal IgG in fetal rhesus monkeys, and the significant increment that would normally occur during the last two weeks of pregnancy. There are also postnatal consequences. Given that the maternal IgG does not replicate in the infant, nor can it be synthesized de novo by the infant after birth, it declines progressively. He decrease follows the species-typical IgG half-life of 1–3 weeks (8 days in the monkey and 21 days in human, Challacombe and Rusell, 1979). Thus, a neonate born with low antibody will more quickly reach the nadir of its maternally derived IgG, which then requires an earlier initiation of its own antibody synthesis and immune defense. Here it is of clinical relevance that a child’s prenatal history and overall antibody titers are not usually taken into consideration by pediatricians when scheduling dates for routine immunizations, even though the presence or absence of maternal antibody does matter. When maternal antibody is still high, it will lessen a baby’s immune response to a vaccine, whereas for an infant born premature the more precipitous antibody decline may warrant administration of some immunizations earlier than the normally scheduled ‘well-baby’ visit.
We investigated several related issues in other experiments, including the transfer of virus-specific antibody from mother to infant. For example, in both monkeys and humans, antibody against influenza virus is readily transferred from the gravid female to her fetus (Short et al., 2010). In addition, we found that some vaccines can be administered to the gravid female to induce a transfer of antibody against infectious pathogens of potential concern for a young infant. Prenatal vaccination of rhesus monkeys against Haemophilus influenza type B was effective for transferring the mother’s HiB-specific antibody before birth. A similar prenatal immunization in humans could be used to modify the common postnatal regimen used in clinical practice, with the first HiB vaccine often given to newborns prior to leaving the hospital.
Although we have exclusively focused so far on the positive aspects of placental antibody transfer, there can be some deleterious consequences. For example, in the case of Rh incompatibility, a woman who is Rh-negative may produce antibody against the red blood cells of the fetus if those cells express Rh antigen (that is, a protein acquired because the father was Rh-positive). Then the placental transfer of this specific maternal antibody can cause hemolysis, premature birth, and even fetal death. Recent research in monkeys has also possibly implicated the placental transfer of another specific antibody in the etiology of autism spectrum disorder. A subset of women (12%) who gave birth to an autistic child may produce antibody against certain neural proteins required for normal brain development (Braunschweig et al., 2009; Singer et al., 2009). When this antibody was purified and administered to pregnant rhesus monkeys, it crossed the placenta and affected the neural and behavioral development of their offspring (Bauman et al., 2013).
In other monkey studies, we investigated whether total amount of maternal antibody transferred across the placenta is vulnerable or resistant to the effects of gestational stress. Here our prior knowledge of comparative primate biology proved insightful. In the squirrel monkey, a species with antibody transfer reaching only 40% of adult levels, disturbance of pregnant females did affect the amount of IgG reaching the infants (Coe and Crispen, 2000). The experimental stress manipulation involved moving gravid females into unfamiliar rooms and new social groups 3 times during their pregnancy. In contrast, the more active placental antibody transfer of the rhesus monkey buffered this process against an effect of stress. Neither maternal stress nor Dex administration reduced the antibody levels present in their infants at term (Coe et al., 1993). Thus, in the rhesus monkey, an effect on neonatal antibody levels would occur only if the maternal stress or drug treatment was sufficiently potent to shorten gestational length by 2 or more weeks (that is, premature birth). Although placental transmission of antibody proved to be fairly resilient, we found that similar stress and Dex manipulations can affect other aspects of fetal immunity and brain development (Bailey et al., 2004; Coe et al., 1999). This vulnerability is due both to direct effects on immune and neural tissue as well as via indirect pathways, including placental delivery of nutrients. For example, maternal stress can interfere with the placental delivery of iron, resulting in a greater risk for iron-deficiency and anemia after birth (Coe et al., 2007).
4. The placenta as a delivery vehicle for nutritional iron
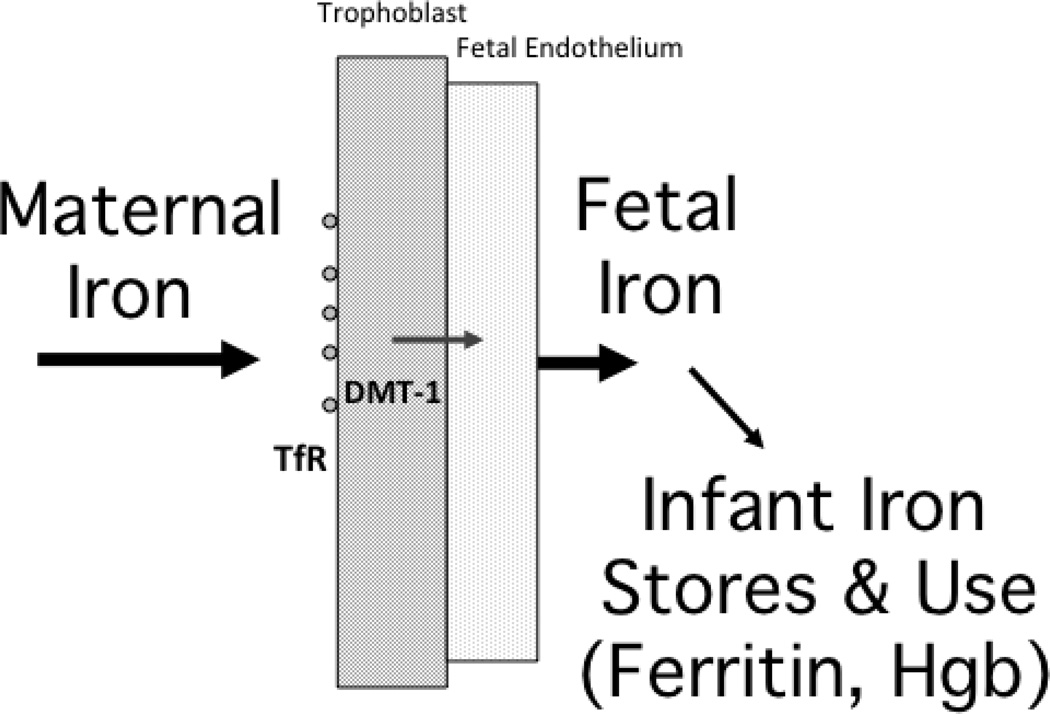
Clearly, a key function of the placenta is to provide nutrients and oxygen to the fetus and, in a coordinated manner, to facilitate the removal of fetal wastes. Our laboratory has been investigating how the placenta transfers one important micronutrient, maternal iron. Approximately 50% of the iron needed for postnatal growth in children, as well as in infant monkeys, must be acquired before birth. Figure 3 illustrates this placental transfer of iron is facilitated by two receptors, transferrin (TfR) and divalent metal transporter (DMT-1). They actively attract iron in maternal blood and transmit increasing amounts to the fetus by term. The lateness of the transfer means that any infant born premature will receive a small iron endowment. It is a concern not just for infant monkeys. The ramifications for premature human infants are well known in the Neonatal Intensive Care Unit (NICU), and there is an extensive clinical literature on the necessity and value of iron supplementation (Mills and Davies, 2012).
Figure 3.
Flow diagram illustrating placental transfer of maternal iron via the transferrin receptor (TfR) and divalent metal transporter (DMT-1), which ensures substantial iron stores, both in the human and monkey neonate. If born with low ferritin, there is a higher risk for anemia by 4–8 months of age, as growth-related needs exceed bioavailable iron in breast milk.
In order to further investigate the developmental consequences of being born with low iron, we refined a monkey model of iron-deficiency anemia (IDA) (Coe et al., 2013). It permitted us to assess prenatal risk factors, the impact on postnatal development, and also to evaluate iron treatment strategies. For example, we found that younger, primiparous monkeys and ones that were iron-deficient at the time of conception were more likely to birth infants prone to IDA (Lubach and Coe, 2006). In addition, by feeding monkeys a diet with marginal iron levels, we simulated the diet of under-nourished women in poor countries and increased the risk for birthing iron-deficient infants. If pregnant monkeys consumed a diet with 180–225 mg iron/kg body weight, then approximately 1/3 of their infants were born with insufficient iron stores and become anemic (Coe et al., 2013).
Although iron is present in breast milk, this lactoferrin is not sufficient to compensate for a low placental transfer and reduced stores at birth. In the monkey neonate, low serum ferritin is a sensitive prognostic of a looming risk for iron deficiency, especially for the infant doubles its birth weight quickly. By 2 months of age, the developmental trajectory towards anemia becomes more clearly evident, both by further declines in ferritin as well as by the infant’s low hemoglobin and smaller Mean Corpuscular Volumes (MCV). Given the significance of iron deficiency both for pediatrics and animal husbandry, we have used this monkey model of anemia to answer other questions, such as the effect on infant brain development. The extent of neural abnormalities caused by iron deficiency conveys a real need for earlier interventions and better supplements. The rapidly growing brain also becomes iron-depleted, with effects on its overall energetics, monoaminergic neurotransmitters and myelin synthesis (Coe et al., 2009; Lozoff et al., 2006). Thus, a placental process that involves the prenatal transfer of maternal iron continues to have many ramifications for the infant across the first year of life.
5. Conclusions
Our review covered three essential placental functions: 1) endocrine interactions between mother and fetus, 2) immune-related actions to prevent rejection and ensure some degree of immune competence by birth, and 3) nutrient delivery. The placenta serves these functions in other mammalian species, but with the prolongation of pregnancy and the evolution of a hemochorial placenta, there are some unique features evident in primates (Wildman et al., 2006). With respect to the HPA axis, the capacity of the primate placenta to synthesize CRH is distinctive. CRH directly influences hormone activity during pregnancy, especially in the fetal compartment, and also appears to participate in immune tolerance, and later to be involved in labor, synergizing with oxytocin, prostaglandins, and cytokines to stimulate the myometrium (Makrigiannakis et al., 2003; Markovic et al., 2013; Wadwha et al., 2004). Given these important actions, placental CRH has been implicated as one of the meditational pathways accounting for the effect of maternal stress on reproductive success (Kalantaridou et al., 2004). Other aspects of the placental hormone transfer and regulation, such as the enzymatic conversion of corticosteroids by 11β-HSD2, are found in both primates and rodent species (Wrywoll et al. 2011). But all processes in primates are influenced by the long duration of pregnancy. One further consequence of the extended gestational length is that the infant monkey is born more mature than the mouse or rat pup. At birth, the infant monkey has a large and functioning adrenal cortex. In fact, the adrenal of the newborn monkey is even relatively larger than the adult organ because of the special fetal zone for producing DHEAs during gestation. Moreover, the adrenal of the monkey does not a period hypo-responsiveness postpartum like the week-long period of suppression seen between days 3–10 in the mouse and rat pup (Levine, 1994).
The placenta also has immune-related roles in other mammals, but in primates the need for ensuring maternal tolerance takes on a special significance with the close proximity of the maternal and fetal blood supply for such an extended period of time. Both maternal and fetal immune tolerance must be induced. The gravid female is exposed to paternal antigens on fetal cells, and thus her immune reaction must be down-regulated. In turn, as the fetal thymus develops, any T cell lineages that start to respond to maternal antigens must be selected against and/or cellular apoptosis induced (Mold et al., 2008).
An appreciation of the placental biology of nonhuman primates is helpful for understanding human placenta and the maternal/fetal relationship. As described earlier, by comparing 5 primate species, it was possible to delineate the stages that must have preceded the active placental transfer of maternal IgG that occurs in humans. It requires a placental receptor for the Fc region of IgG, and an active transport process. During the final weeks of pregnancy, the accelerating placental transfer provides an antibody endowment that will persist for 3–6 months after birth. In doesn’t occur this way in all animals. Many species provide both IgG and sIgA primarily after birth in breast milk. But that also requires birthing an altricial infant with an immature intestinal lumen that remains permeable to IgG. That gut permeability to antibody does not occur in infant monkeys. Thus, the maternal transfer in higher primates is partitioned and sequenced: IgG is passaged almost exclusively before birth via the placenta, whereas the sIgA delivered subsequently in breast milk protects the epithelial and mucosal surfaces of the oral cavity and gut.
Our primate research has also focused on the maternal provision of iron, which involves some analogous processes. Placental TfR and DMT-1 facilitate the transfer of iron to the fetus. This transport occurs at an accelerating pace during the final weeks of pregnancy in order to ensure that iron reserves are sustained for a long time after birth. Iron is an essential micronutrient, needed for oxidative metabolism, growth, and normal brain and behavioral function (Lozoff and Georgeiff, 2006; Lubach and Coe, 2008). Thus, a shortfall at birth can impair normal development. Some of the maternal risk factors that we, and others, have identified are: young maternal age and being iron-deficient at the time of conception or during pregnancy. Monkey and human infants also share some of the same postnatal risk factors for anemia, including a rapid growth rate. Smaller infant monkeys are not the ones at most risk, but rather the large infant, or a prematurely born neonate that undergoes a compensatory growth spurt. These conclusions are also relevant for the husbandry of some other animals. Farmers who breed pigs know the critical importance of nutritional iron for piglets, especially given that maternal iron is partitioned among some many fast-growing members of the litter (Erickson et al., 1998; Starznski et al., 2013).
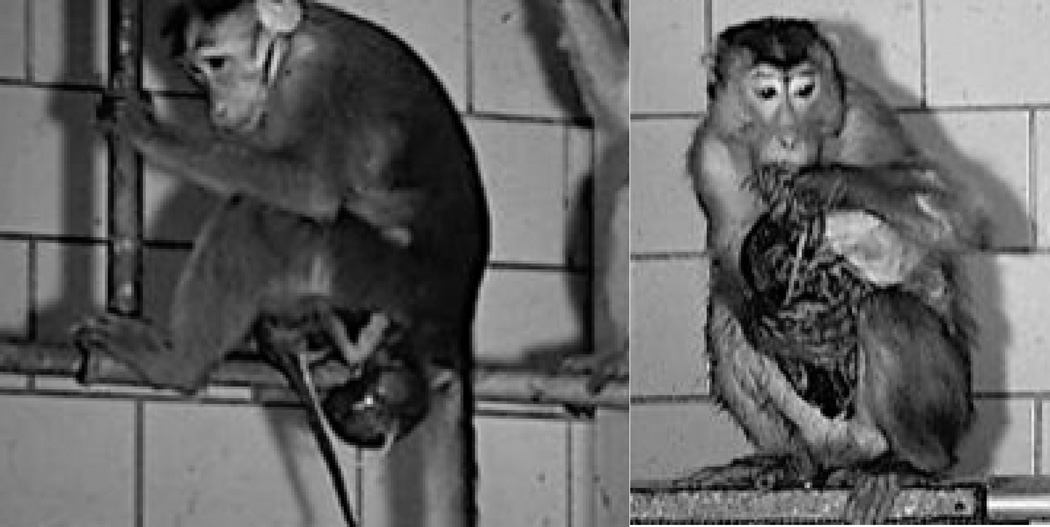
The topic of nutrition provides a good transition to one remaining point about the last function of the placenta in primates. After delivery, the placenta is involved in a final service of special significance (Kristal, 1980). All parturient primates, even the species that are normally leaf-eaters and not carnivorous, will consume the placenta (Figure 4). To accomplish this task, the mother raises the placenta over the infant’s head and compresses it, effectively inducing a large transfusion of blood into the newborn infant. There are many reasons why placentophagia is performed, but one benefit for the infant is that the pressure on the placenta and cord ensures that there is a rich source of red blood cells for the infant, which are then be scavenged for their iron.
Figure 4.
The placenta fulfills several additional functions after it passes soon after delivery in nonhuman primates (A). It is a source of energy and hormones for the parturient female. In addition, as female monkeys engage in placentophagia they raise the placenta above the infant and compress it, providing a substantial blood transfusion to the neonate (B). When those red blood cells die, iron is scavenged by the infant’s macrophages and recycled. In our research, we track placental delivery by administering stable iron isotopes to the gravid female and quantifying the iron incorporated into the infant’s red blood cells for months after birth.
Here is another primate example with relevance and inference for clinical practice and human health. Since the 1960s, obstetricians have routinely clamped the umbilical cord immediately at delivery, preventing a similar transfer of blood from the placenta to the human newborn. Many have suggested that the universal practice of rapid clamping be re-considered, because only a small subset of healthy infants are challenged by receiving the extra blood (McDonald et al., 2013). Midwives already take a different, more naturalistic approach, permitting the unclamped placenta to pulse, and thereby allowing the sanguine, cellular supply of future iron to be transferred. Although natural, this type of delivery is now referred to as ‘delayed cord clamping’, if an obstetrician or midwife delays for a few minutes before clamping and severing the cord. Our research with nonhuman primates indicates there are some functional benefits. Symbolically and concretely, it is the placenta’s parting benevolent gift. Although the placenta is a tissue of fetal origin, it has acquired many ways to ensure the long-term wellbeing of the infant: by modulating the pregnant female’s physiology, through helping to construct the optimal in utero milieu, and by preparing the infant for the transition into the external world.
Highlights.
Placental function is critical to a successful outcome at every stage of pregnancy from implantation to delivery.
The primate placenta has several unique features that maintain the gravid condition and promote fetal development, including the secretion of corticotropin-releasing hormone.
Specific placental receptors enable substantial amounts of maternal antibody and iron to be transferred to the fetus.
Acknowledgements
The research was enabled by support from NICHD (HD057064, HD39386), NIAID (AI067518), and a Grand Challenges Exploration Award to CLC as well as the primate resources of the WNPRC (P51 RR000167).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althaus ZR, Bailey JR, Leakey JEA, Slikker W. Transplacental metabolism of dexamethasone and cortisol in the late gestational age rhesus monkey (Macaca mulatta) Devel. Pharmacol. Therap. 1986;9:332–349. doi: 10.1159/000457112. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Iosif A-M, Ashwood P, Braunschweig D, Lee A, Schumann CM, Van de Water J, Amaral DG. Maternal antibodies from mothers of children with autism alter brain growth and social behavioral development in the rhesus monkey. Translat. Psychiatr. 2013;3:e278. doi: 10.1038/tp.2013.47. Doi:10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, Coe CL. Prenatal conditions alter the bacterial colonization of the gut in the infant monkey. J. Pediatr. Clin. Gastroenterol. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc. Nat. Acad. Sci. 1996;93(2):705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen L, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicol. 2009;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AL, Murphy BEP. The maternal-fetal cortisol gradient during pregnancy and at delivery. J. Clin Endocrinol. Metab. 1977;45:435–440. doi: 10.1210/jcem-45-3-435. [DOI] [PubMed] [Google Scholar]
- Cardenas I, Means ME, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J. Immunol. 2010;175(2):1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe SJ, Russell MW. Estimation of the intravascular half-life of normal rhesus monkey IgG, IgA, and IgM. Immunol. 1979;36:331–338. [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Crispen H. Social stress in pregnant monkeys differentially affects placental transfer of maternal antibody to male and female infants. Health Psych. 2000;19(6):554–559. [PubMed] [Google Scholar]
- Coe CL, Kemnitz JW, Schneider ML. Vulnerability of placental antibody transfer and fetal complement synthesis to disturbance of the pregnant monkey. J. Med. Primatol. 1993;22:294–300. [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Bianco L, Beard JL. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Devel. Psychobio. 2009;51(3):301–309. doi: 10.1002/dev.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci. BioBehav. Rev. 2005;29(2):227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Busbridge M, Chapman RS. Optimal iron fortification of maternal diet during pregnancy and nursing for investigating and preventing iron deficiency in young rhesus monkeys. Adv. Vet. Res. 2013;94(3):549–555. doi: 10.1016/j.rvsc.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Izard KM. Progressive improvement in the transfer of maternal antibody across the Order Primates. Am. J. Primatol. 1994;32(1):51–55. doi: 10.1002/ajp.1350320106. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Karaszewski J. Prenatal stress and immune recognition of self and nonself in the primate neonate. Biol. Neonate. 1999;76(5):301–310. doi: 10.1159/000014172. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff E. Maternal stress during pregnancy increases risk for iron deficient infants impacting innate immunity. Pediatr. Res. 2007;61:520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Coe CL, Murai JT, Wiener SG, Levine S, Siiteri P. Rapid cortisol and CBG response during pregnancy and estrogen administration in the squirrel monkey. Endocrinol. 1986;118:435–440. doi: 10.1210/endo-118-1-435. [DOI] [PubMed] [Google Scholar]
- De Vries A, Hlmes MC, Heijnis A, Seier JV, Heerden J, Lou J, Wofe-Coote S, Meaney M, Levitt NS, Seckl JR. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J. Clin. Invest. 2007;117(4):1058–1067. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupressoir A, Lavialle C, Heidmann T. From ancestral infectious retroviruses to to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta. 2012;33(9):663–671. doi: 10.1016/j.placenta.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Beard JL, Connor JR. Distribution of brain iron, ferritin, and transferrin in the 28-day-old piglet. J. Nutr. Biochem. 1998;9:276–284. [Google Scholar]
- Faeleabas AT, Kim JJ, Srinivasan S, Donnelly KM, Brudney A, Jaffe RC. Implantation in the baboon: Endometrial responses. Sem. Reprod. Endocrinol. 1999;17(3):257–265. doi: 10.1055/s-2007-1016233. [DOI] [PubMed] [Google Scholar]
- Fahlbusch FB, Ruebner M, Volkert G, Offergeld R, Hartner A, Menendez-Castro C, Rauh M, Rascher W, Dotsch J. Corticotropin-releasing hormone stimulates expression of letpin, 11beta-HSD2 and syncytin-1 in primary human trophoblasts. Reprod. Biol. Endocrinol. 2012 doi: 10.1186/1477-7827-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, Atterby C, Edqvist P-H, Ponten F, Zhang WW, Larsson E, Ryan FP. Detection of the human endogeneous retrovirus ERV3 encoded Env-protein in human tissues using antibody-based proteomics. J. Royal Soc Med. 2014;107(1):22–29. doi: 10.1177/0141076813509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato S, Meon RP, Swan KF, Menon R. Inflammatory cytokines (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am. J. Obstet. Gynecol. 1996;174:1855–1862. doi: 10.1016/s0002-9378(96)70221-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Xu H, Ofori E, Elovitz MA. Toll-like receptors in the uterus, cervix, and placenta: is pregnancy an immunosuppressed state? Am. J. Obstet. Gynecol. 2007;197:296. doi: 10.1016/j.ajog.2007.06.021. e1–6. [DOI] [PubMed] [Google Scholar]
- Grino M, Chrousos GP, Margioris AN. The corticotropin releasing hormone gene is expressed in human placenta. 1987;148:1208–1217. doi: 10.1016/s0006-291x(87)80261-9. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 2011;25(4):604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke U, Myulonas I, Richter D-U, Hocker I, Briese V, Makrigiannakis A, Friese K. 2005;272(1):7–12. doi: 10.1007/s00404-005-0728-0. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Dunkel L, Turpeinen U, Stenman U-H, Wood PJ, Nuutila M, Andersson S. Placental 11β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J. Clin. Endocrinol. Metab. 2003;88:493–500. doi: 10.1210/jc.2002-021378. [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. J. Reprod. Immunol. 2004;62(1–2):61–88. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kristal MB. Placentophagia: A biobehavioral enigma (or De gustibus nondisputandum est) Neurosci. Biobehav. Rev. 1980;4:141–150. doi: 10.1016/0149-7634(80)90012-3. [DOI] [PubMed] [Google Scholar]
- Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast. J. Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann. NY Acad. Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- LeWinn KA, Stroud LR, Molnar BE, Ware JH, Koenen KC, Buka SL. Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. Intern. J. Epidemiol. 2009;38:1700–1710. doi: 10.1093/ije/dyp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006;64(Suppl. 1):S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Sem. Pediatr. Neurol. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta) J. Nutr. 2006;136:2345–2349. doi: 10.1093/jn/136.9.2345. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL. Selective impairment of cognitive impairment in the young monkey following recovery from iron deficiency. Devel. Behav. Pediatr. 2008;29:11–17. doi: 10.1097/DBP.0b013e31815f24a9. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, Kalantaridou S, Mitsiades N, Margioris A, Chrousos GP, Gravanis A. Cortictropin-releasing hormone (CRH and immunotolerance of the fetus. Biochem. Pharmacol. 2003;65(6):917–921. doi: 10.1016/s0006-2952(02)01547-2. [DOI] [PubMed] [Google Scholar]
- Margioris AN, Grino M, Protos P, Gold PW, Chrousos GP. Corticotropin-releasing hormone and oxytocin simulate the release of placental proopiomelanocortin peptides. J. Clin Endocrinol. Metab. 1988;66(5):922–926. doi: 10.1210/jcem-66-5-922. [DOI] [PubMed] [Google Scholar]
- Markovic D, Bari MF, Lu B, Vatish M, Grammatopoulos DK. Corticotropin-releasing hormone interacts with interleukin-1β to regulate prostaglandin H synthase-2 in human myometrium during pregnancy and labor. J. Clin. Endocrinol. Metab. 2013;98(7):2864–2875. doi: 10.1210/jc.2013-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E, Figueroa R, Garry D, Visintainer P, Patel K, Verma U, et al. Elevated amniotic fluid interleukin-6 as a predictor of neonatal periventricular leukomalacia and intraventricular hemorrhage. J. Maternal Fetal Invest. 1998;8:101–107. [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N.Y. Acad. Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Mazoub JA, Karalis KP. Placental corticotropin releasing hormone: function and regulation. Am. J. Obstet. Gynecol. 1999;180:S242–S246. doi: 10.1016/s0002-9378(99)70708-8. [DOI] [PubMed] [Google Scholar]
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping on term infants on maternal and neonatal outcomes (Review) The Cochrane Library. 2013;7 doi: 10.1002/14651858.CD004074.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Smith R. Cortictrophin-releasing hormone and human parturition. Reproduction. 2001;121:493–501. doi: 10.1530/rep.0.1210493. [DOI] [PubMed] [Google Scholar]
- Menon R, Merialdi M, Betran AP, Dolan S, Jiang L, Fortunato SJ, et al. Analysis of association between maternal tumor necrosis factor-alpha promoter polymorphism (−308), tumor necrosis factor concentration and preterm birth. Am. J. Obstet. Gynecol. 2006;195:1240–1248. doi: 10.1016/j.ajog.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997;18(3):378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Davies MW. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD005095.pub2. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005095.pub2. [DOI] [PMC free article] [PubMed]
- Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel JF, Kaune H, Maliqueo M. Maternal-fetal unit interactions and eutherian neocortical development and evolution. Front. Neuroanat. 2013;7(22):1–14. doi: 10.3389/fnana.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- Novy MJ, Walsh SW. In: Regulation of fetoplacental steroidogenesis in rhesus macaques. Novy MJ, Resko JA, editors. New York: Fetal Endocrinology, Academic Press; 1981. pp. 65–94. [Google Scholar]
- O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo Z-H, et al. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 2013;339(6120):662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using an iTRAQ in a primate model of iron deficiency anemia. Devel. Neurosci. 2012;32:354–365. doi: 10.1159/000341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr. Rev. 1995;16(5):608–648. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- Rai R, Sacks G, Trew G. Natural killer cells and reproductive failure-theory, practice and prejudice. Human Reprod. 2005;20(5):1123–1126. doi: 10.1093/humrep/deh804. [DOI] [PubMed] [Google Scholar]
- Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today. 1997;18(10):478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Rainey W, Rehman K, Carr BR. The human fetal adrenal: Making adrenal androgens for placental estrogens. Semin. Reprod. Med. 2004;22:327–366. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- Rao R, Ennis K, Oz G, Lubach GR, Georgieff MK, Coe CL. Metabolomic analysis of cerebrospinal fluid indicates iron deficiency compromises cerebral energy metabolism in the infant monkey. Neurochem. Res. 2013;38:573–580. doi: 10.1007/s11064-012-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Intern. J. Devel. Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Rigo JR, Szelenyi J, Selmeczy Z, Papp Z, Vizi ES. Endotoxin-induced TNF production changes inversely to its plasma level during pregnancy. Eur. J. Obstet. Gynec. 2004;114:236–238. doi: 10.1016/j.ejogrb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-reasing hormone may be a stimulator of pituitary adrenocorticotropic hormone secretion in humans. J. Clin Invest. 1989;84:1997–2001. doi: 10.1172/JCI114390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoid programming. Ann. N.Y. Acad. Sci. 2004;1032:63084. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Stevens AM, McDonnell WM, Mullarkey ME, Pang JM, Leisenring W, Nelson JL, editors. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab. Invest. 2004;84:1603–1609. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal development indirectly. Int. J. Devel. Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore J, Coe CL. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol. Psychiat. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J. Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Slukvin I, Boyson JE, Watkins DI, Golos TG. The rhesus monkey analogue of human lymphocyte-antigen-G is expressed primarily in villous syncytiotrophoblasts. Biol. Reprod. 1998;58:728–738. doi: 10.1095/biolreprod58.3.728. [DOI] [PubMed] [Google Scholar]
- Smith R. The timing of human birth. Sci. Amer. 1999;3:68–75. doi: 10.1038/scientificamerican0399-68. [DOI] [PubMed] [Google Scholar]
- Starzynski RR, Laarakkers CM, Tjalsma H, Swinkels DW, Pieszka M, Stys A, Mickiewicz M, Lipinski P. Iron supplementation in suckling piglets: how to correct iron deficiency anemia without affecting plasma hepcidin levels. PLoS One. 2013;8(5):e64022. doi: 10.1371/journal.pone.0064022. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa JM, Schjenken JE, Clifton VL, Barbeau B, Lowry P, Maiti K, Smith R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta. 2013;33(11):933–941. doi: 10.1016/j.placenta.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Uh A, Nicholson RC, Gonzalez GV, Simmons CF, Gombart A, Smith R, Equils O. Lipopolysaccharide stimulation of trophoblasts induces cortcotropin-releasing hormone expression through MyD88. Am. J. Obstet. Gynecol. 2008;199:317. doi: 10.1016/j.ajog.2008.06.091. e1.−317.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Chicz-DeMet A, Porto M. Placental CRH modulates maternal pituitary-adrenal function in human pregnancy. Ann. NY. Acad. Sci. 1997;814:276–281. doi: 10.1111/j.1749-6632.1997.tb46163.x. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Corticotropin-releasing hormone (CRH), preterm birth and fetal growth restriction: a prospective investigation. Am. J. Obstet. Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Norman RL, Novy MJ. In utero regulation of rhesus monkey fetal adrenals: Effects of dexamethasone, adrenocorticotrophin, thyrotropin-releasing hormone, prolactin, human chorionic gonadotropin, and alpha-melanocyte-stimulating hormone on fetal and maternal plasma steroids. Endocrinol. 1979;104:1805–1813. doi: 10.1210/endo-104-6-1805. [DOI] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomeon? Immunol. Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R. Evolution of the mammalian placenta revealed by phylogenetic analysis. PNAS. 2006;103:3203–3208. doi: 10.1073/pnas.0511344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrywoll CS, Holmes MC, Seckl JR. 11β-hydroxysteroid dehydrogenases and the brain: From zero to hero, a decade of progress. Front. Neuroendocr. 2011;32(3):265–286. doi: 10.1016/j.yfrne.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumakis E, Makrigiannakis A, Margioris A, Stounaras C, Gravanis A. Corticotropin releasing hormone (CRH) in normal and pregnant uterus: physiological implications. Front. Biosci. 1996;1:e1–e8. doi: 10.2741/a137. [DOI] [PubMed] [Google Scholar]