Abstract
White adipose tissue (WAT) is innervated by the sympathetic nervous system (SNS) and its activation is necessary for lipolysis. WAT parasympathetic innervation is not supported. Fully-executed SNS-norepinephrine (NE)-mediated WAT lipolysis is dependent on β-adrenoceptor stimulation ultimately hinging on hormone sensitive lipase and perilipin A phosphorylation. WAT sympathetic drive is appropriately measured by electrophysiological and neurochemical (NE turnover) in non-human animals and this drive is fat pad-specific preventing generalizations among WAT depots and non-WAT organs. Leptin-triggered SNS-mediated lipolysis is weakly supported, whereas insulin or adenosine inhibition of SNS/NE-mediated lipolysis is strongly supported. In addition to lipolysis control, increases or decreases in WAT SNS drive/NE inhibit and stimulate white adipocyte proliferation, respectively. WAT sensory nerves are of spinal-origin and sensitive to local leptin and increases in sympathetic drive, the latter implicating lipolysis. Transsynaptic viral tract tracer use revealed WAT central sympathetic and sensory circuits including SNS-sensory feedback loops that may control lipolysis.
Keywords: pseudorabies virus, herpes simplex virus-1, Siberian hamsters, rats, mice, humans, denervation, norepinephrine turnover, proliferation, insulin, leptin, tract tracing
1. Introduction
Despite abundant data to the contrary, many textbooks, reviews and research papers continue to assert that adrenal medullary catecholamines, especially epinephrine (EPI), as the primary stimulator of lipolysis by white adipose tissue (WAT). This dogma belies the conclusive data showing that the removal of the sole source of circulating EPI by bilateral adrenal demedullation (ADMEDx) does not block lipolysis (e.g., [220;283]). By contrast, the sympathetic nervous system (SNS) innervation of WAT is sufficient and necessary for the initiation of WAT lipolysis and performs a commanding function in the adjustment of lipid energy stores. Thus, this review will focus on the support for the direct SNS innervation of WAT as the principal initiator of lipolysis in mammals, including, of course, humans, as well as the role of the SNS in another WAT functions – fat cell proliferation. The typical counterpart to the SNS is the parasympathetic nervous system (PSNS) and despite some suggestion to the contrary (e.g., [155]), the PSNS innervation of WAT and a function to oppose lipolysis is unfounded or trivial at best [28; 108; 109], as will be briefly discussed below. We also will describe the sensory innervation of WAT and its potential functions in the control of lipid metabolism. Finally, we will not describe in detail, the SNS innervation of brown adipose tissue and its role in energy balance, except in its role to act in concert, or separately from the SNS innervation of WAT in response to several challenges to energy balance, because we have recently reviewed the literature on this topic (for review see: [25]). Because we also have reviewed the SNS innervation of WAT recently and across the years (e.g., [20–24]), we will make every attempt not to repeat this information, although some reiteration is necessary, but we will add to this growing body of knowledge, embellishing previous topics with new findings, suggest new areas of further research to deepen our understanding, and to speculate about the various mysteries of this area that remain to be solved.
Before delving into the heart of the review, we feel it would helpful to indicate the location of the WAT depots we will be discussing primarily in rodent model research, as well as to compare and contrast them with WAT depots in humans. Tchkonia et al. [287] nicely demonstrate the location of WAT depots in rodents and humans (Fig. 1). Although there are some similarities in WAT depots [e.g., mesenteric WAT (MWAT), perirenal WAT, retroperitoneal WAT (RWAT) -the latter not clearly shown; Fig. 1], there also are striking differences including the absence of omental WAT in rodents, the presence of perigonadal WAT in rodents [epididymal WAT (EWAT) or parametrial WAT (PWAT)], but their absence in humans, and differences in the extent of the subcutaneous WAT with restriction of these depots around the haunches in rodents [inguinal WAT (IWAT) and dorso subcutaneous WAT (DWAT)], but underlying most of the skin in humans, and the absence of leg WAT in rodents. We feel these differences are not trivial distinctions and that conclusions based on rodent models, whether for functions of the dichotomy between visceral and subcutaneous WAT as well as individual WAT depots, should be made with more caution that historically or currently is the practice.
Figure 1.
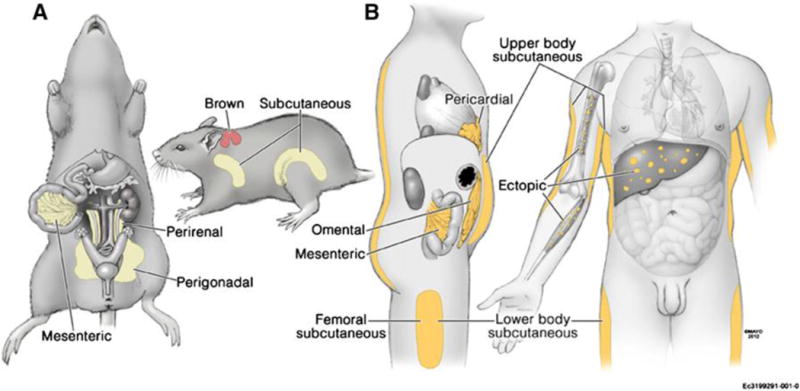
From: Tchkonia et al. [287]
Location of major WAT depots in: A (left) – rodents (laboratory rats and mice, hamsters). Mesenteric WAT (MWAT); perigonadal [epididymal WAT (EWAT) or parametrial WAT (PWAT); perirenal WAT, retroperitoneal WAT (RWAT, not shown located in back of the peritoneal cavity); brown [interscapular brown adipose tissue (IBAT), the largest BAT depot in rodents]; subcutaneous [dorsosubcutaneous WAT (DWAT) located around front haunch; inguinal WAT (IWAT) located around rear haunch]; and B (right) – humans. Note the differences in that humans have no gonadal WAT, but rodents do, humans have omental WAT (visceral) but rodents do not but have MWAT as their only visceral WAT), rodents have subcutaneous WAT located only around the haunch area whereas subcutaneous WAT underlies the skin in most of the human body, and humans have appreciable leg WAT, but rodents do not.
1.1 Why We Initiated Studies of the SNS Innervation of WAT
Our interest in the study of SNS innervation of WAT was driven by our core focus on the naturally-occurring decrease in the body fat of Siberian hamsters (Phodopus sungorus) resulting from changes in daylength. During the long days (LDs) of summer, their impressive obesity (e.g., ~50% of body mass as body fat [18;296]) is at its peak and is reversed to a more moderate level of adiposity (~20% of body mass as body fat) in the short days (SDs) of winter in the field naturally [298]. Fortunately, these naturally-occurring changes in lipid mass can be mimicked in the laboratory by changing only the photoperiod from LDs to SDs, while holding all other environmental factors constant such as temperature and food ([296]; for review see: [15;26]). This is because for Siberian hamsters, and many other species exhibiting seasonal changes in adiposity and reproductive status, the daylength (photoperiod) cue is translated into a neuroendocrine signal via the duration of the nocturnal secretion of melatonin (MEL) from the pineal gland that occurs in direct proportion to the length of the dark period (for review see: [16;20;113]) stimulating the MEL1a receptor (a.k.a. MT1-R) subtype that mediates photoperiodic responses (e.g., [238]). Because MEL does not affect lipolysis in vivo even at ‘industrial strength’ doses [216], an intermediary must exist. Even though there was nearly 100 years of suggestive, indirect evidence for the SNS innervation of WAT and its role in lipid mobilization (lipolysis), we fell into the trap of most researchers of the 1980’s and focused on circulating factors and in the case of Siberian hamsters, those that changed with changes in the daylength that also had been implicated in altering lipolysis (e.g., epinephrine (EPI), glucocorticoids, prolactin, thyroid hormones, gonadal steroids, insulin; for review see: [15]). None of these factors could account for the photoperiod-induced reversal of obesity by Siberian hamsters; therefore, there appeared to be a non-circulating factor initiating WAT lipolysis – perhaps a neural one. In addition, another factor favoring a ‘neural hypothesis’ was that in our initial and follow-up studies of the photoperiodic reversal of seasonal obesity, the intra-abdominal WAT pads (EWAT, RWAT) had the greatest degree of lipid mobilization, with the IWAT pad showing a lesser and later degree of lipid mobilization [12;13;18;296], a feat that could be accomplished by a circulating factor if its receptor number/affinity/signaling cascade varied accordingly among the WAT depots, or more simply by differential SNS drive to pads via its innervation and the release of norepinephrine (NE), the principal sympathetic nerve neurotransmitter. Indeed, in vitro lipolysis increases in isolated white adipocytes incubated with physiological concentrations of NE (e.g., [86;227]).
2.2 Circulating Adrenal Medullary Epinephrine or Pancreatic Glucagon Are Not Primary Initiators of Lipolysis in WAT
As noted in brief above, historically, but also unfortunately presently, adrenal medullary EPI often is ascribed as the primary stimulator of WAT lipolysis. Perhaps this is due to the profound lipolysis engendered by application of physiological concentrations of the monoamine to WAT fragments ex vivo or isolated adipocytes in vitro (e.g., [38;177;234;239;242;303;307]). The role of adrenal medullary EPI for in vivo lipolysis has been discredited, however, because ADMEDx, which removes of the sole source of circulating EPI, does not block fasting-, exercise-, electrical stimulation of the hypothalamus- or glucoprivation-induced lipid mobilization in laboratory rats and mice or lipid mobilization in SD-exposed Siberian hamsters [71;158;220;283;288].
Glucagon has long been implicated in mediating WAT lipolysis [111;179–181;190;293], but its effects on lipolysis are independent of CNS action because WAT SNS denervation does not block glucagon-induced glycerol release (an index of lipolysis), although it does decrease free fatty acid (FFA) release [179]. The latter effect is not due to a blockade of the effects of glucagon on lipolysis, rather it is attributed to re-esterification [179]. Indeed, exogenously administered i.v. glucagon that results in physiological concentrations of the hormone does not stimulate lipolysis in vivo as assessed by intra-WAT microdialysis [117].
2. Neuroanatomical Evidence for the SNS Innervation of WAT
When we finally turned to the SNS as a possible mediator, we realized direct evidence (tract tracing) of the sympathetic innervation of WAT had not been demonstrated. Previous studies used histological approaches at the level of the WAT pad. To our knowledge the first was by Dogiel 115 years ago [78] where he reported visible nerves of unknown type/origin entering WAT depots. The sympathetic innervation of WAT was then demonstrated at the level of the fat pad using histofluorescence methods (e.g., [73;235;267;306]), electron microscopy [267] and immunohistochemical identification of the proven sympathetic nerve marker tyrosine hydroxylase (TH) immunoreactivity (ir) (e.g., [90;106;108;263]). Although relatively sparse compared with the SNS innervation of brown adipose tissue (BAT), immunohistochemical [90;106;108;263] and electron microscopic [267] studies revealed sympathetic nerve endings in the parenchymal of WAT depots that appear to be of the en passant variety, where the release of NE at each ‘terminal’ or axonal swelling would affect many surrounding cells. ‘Direct’ evidence, however, awaited tract tracing of the postganglionic sympathetic nerves inferred as innervating the fat pad.
To unambiguously define the SNS innervation of WAT, we used conventional single neuron tract tracing, as opposed to multi-neuron transneuronal circuit tracing (see below), to demonstrate for the first time the postganglionic sympathetic innervation of WAT and did so bidirectionally [316]. Specifically, we injected a retrograde tracer (Fluorogold) into WAT to label the sympathetic ganglia innervating WAT and injected an anterograde tracer (DiI) into the sympathetic ganglia to label the sympathetic nerves surrounding the adipocytes (DiI; [316]).
2.1 Neuroanatomical and Functional Evidence for the mediation of SD-induced lipid mobilization via the SNS Innervation of WAT
Importantly for the reversal of seasonal obesity in Siberian hamsters by SD exposure [18;296], we established that surgical sympathetic WAT denervation blocked SD-induced lipid mobilization [317], whereas ADMEDx did so incompletely [71]. We then determined that MEL1a mRNA, detected by emulsion autoradiographic in situ hybridization, was co-localized in the central sympathetic circuits ultimately innervating WAT across the neuroaxis (brain, spinal cord and sympathetic chain), the latter defined using a transneuronal viral tract tracer [pseudorabies virus (PRV)] and immunohistochemistry to label PRV [272]. Specifically, MEL1a gene expression was extensively co-localized with PRV labeled SNS outflow forebrain neurons to WAT [i.e., paraventricular hypothalamic nucleus (PVH), zona incerta and sub zona incerta (ZI, subZI), reuniens/xiphoid area, dorsomedial hypothalamic nucleus and to a lesser degree, in the periventricular nucleus, thalamic paraventricular nucleus, anterior hypothalamus, perifornical area, and periventricular fiber system [272]]; we did not assess midbrain or hindbrain contributions. We also found that SD exposure increases: a) the SNS drive to WAT [shown by increases in NE turner (NETO), a neurochemical measure of sympathetic drive [316]], b) NE-induced cyclic adenosine 3′,5′-monophosphate (cAMP) accumulation in isolated adipocytes from SD-housed hamsters, c) the potency of the β3-adrenoceptor [(AR) a.k.a. adrenergic receptor] agonist BRL37344 to stimulate lipolysis (glycerol release) from SD vs LD isolated adipocytes [32] and d) adipocyte β3-AR mRNA [72]. Collectively, these data support the scenario that the SD-associated increase in nightlength triggers increases in the duration of nocturnal secretion of pineal MEL [131] that increases the stimulation of MEL1a receptors on SNS outflow neurons thereby enhancing the sympathetic drive to WAT to increase lipid mobilization and reverse the LD-associated obese state, an effect blocked by surgical and selective chemical WAT sympathetic nerve denervation [71;317], but not ADMEDx [71].
3. Histological Studies of the SNS Innervation of WAT
3.1 PRV, a Transneuronal Viral Tract Tracer, Can be used to Define Central SNS Outflow Circuitry to WAT
As noted in brief above, the Bartha’s K strain of PRV is a viral transneuronal tract tracer that provides the ability to define multi-synaptic circuits within the same animal [for review see: [82;83;274]]. Some neurotropic viruses, such as PRV, are endocytosed at axon terminal membranes after binding to viral attachment protein molecules that act as ‘viral receptors’. They then are transported retrogradely [65] specifically from the dendrites of these infected neurons to axons making synaptic contact with them where they replicate (self-amplifying) in the neuronal soma and continue their specific backward journey resulting in an infection along the neuronal chain from the periphery to higher CNS sites (for review see: [82;83;274]). PRV-infected cells can be readily visualized using standard immunohistochemical methods or genetically engineered PRV mutants expressing fluorescent reporters (GFP PRV 152, mRFP PRV 614 [9]).
Using this PRV methodology, we initially defined the CNS sympathetic outflow to WAT in Siberian hamsters and laboratory rats for IWAT and EWAT [8]. Dozens of sites across the neuroaxis were revealed as part of the CNS-SNS-WAT circuitry in this seminal study [8], results that were verified and expanded in a more detailed analysis in subsequent Siberian hamster WAT studies [31;260;272;275] as well as studies in laboratory rats [1]. In brief, the CNS efferents to these WAT tissues were more similar than different in terms of the brain sites comprising these outflow circuits and included nuclei at all brain levels [e.g., a partial list: intermediolateral (IML) horn of the spinal cord, raphe midline nuclei, gigantocellular nuclei, reticular nuclei/areas, rostral ventrolateral/ventromedial nuclei of the medulla, A5 cell group, and nucleus of the solitary tract (NTS) in the hindbrain, central gray and parabrachial nuclei of the pons/midbrain, and the PVH, suprachiasmatic nucleus (SCN), dorsomedial hypothalamic nucleus (DMH) septal and medial preoptic areas of the forebrain] [8]. It is especially noteworthy that despite various manipulations (stimulation and lesion) of the ventromedial hypothalamic area (VMH) resulting in changes in WAT lipid mobilization and metabolism demonstrated by others previous (e.g., [10;33]), few neurons within the VMH became infected and those very few are located at the peripheral edges of the ventromedial hypothalamic nucleus (VMN distinguished from the VMH area) and retrochiastmatic area; [8] (for review of this issue see: [14]). Despite the largely similar brain sites comprising the neuronal nodes in these central circuits ultimately projecting to these WAT depots, some sites contained greater degrees of infection than others suggesting the probability of some fat pad-specific sympathetic circuitry [8]. As for some notable examples, there were relatively fewer infected cells in the SCN, DMH, arcuate nuclei, C1 adrenaline cell group and NTS in the EWAT central sympathetic circuits than for IWAT. By contrast, IWAT central sympathetic circuits had greater labeling in the lateral and gigantocellular reticular nuclei [8]. There were no striking differences in the pattern of PRV infected neurons between Siberian hamsters and laboratory rats after virus inoculation in EWAT or IWAT. Note, however, that at the time of this initial report of SNS outflow circuitry to EWAT and IWAT, the PRV mutant with distinct fluorescent reporters were being developed and not available for critical studies whereby two WAT pads could be injected in the same animal to define shared and unshared neurons/circuits. Others, however, claim ‘viscerotopic’ SNS innervation of WAT using two strains of PRV with unique reporters after inoculation of separate WAT depots [156]; however quantification of these innervations was not performed. Such critical studies for understanding the control of WAT pads by the CNS have recently been performed in our laboratory with quantification [217] (discussed in 10 below).
3.2 There is a Lack of Evidence Supporting the Parasympathetic Innervation of WAT
Most peripheral tissues are dually innervated by the two arms of the autonomic nervous system, the SNS and the PSNS. A notable and important physiological exception – peripheral blood vessels (except for facial blood vessels involved in peripheral cutaneous vasodilation – the blushing response – only are sympathetically innervated [142;246]). There are species differences in that in some species cholinergic sympathetic innervation of skeletal musculature vasculature exists, whereas laboratory rats and mice have noradrenergic innervation as demonstrated by TH-ir in addition to neuropeptide Y (NPY)-ir [106;189]. Thus, archetypal cholinergic nerve markers such as vasoactive intestinal peptide (VIP), vesicular acetylcholine transporter (VAchT) or acetylcholinesterase does not occur in laboratory rat and mouse skeletal muscle vasculature [121]. Therefore, evidence for PSNS innervation of peripheral blood vessels in skeletal muscle is non-existent except for a few, to our knowledge, uncorroborated reports (e.g., [248]). Analogously, there are unconfirmed assertions of PSNS innervation of WAT [155]. We [108;109] and others [27;28] critically challenged the claim of WAT PSNS innervation on several grounds including the viral tract tracing methodology used by these researchers [155] as well as several classic aspects of PSNS innervation that are missing including demonstration of parasympathetic ganglia in or near the WAT pads and biochemical indicators of PSNS innervation [7]. Moreover, we found no evidence of known neurochemical markers of PSNS innervation (VIP, neuronal nitric oxide synthase, VAchT) in three WAT depots [IWAT, RWAT, EWAT] from three rodent models (C57BL6 mice, ob/ob mice, Sprague-Dawley rats) from all samples [108]. For a more neuroanatomically functional test of PSNS innervation, we reasoned that selective local sympathetic denervation could be accomplished by intra-IWAT microinjections of the catecholamine-specific neurotoxin 6-hydroxy-dopamine (6OHDA) that we used successfully previously for local SNS denervations [90;108;241] and thereby would spare any PSNS innervation. When the NE depletion was maximal based on our previous work, we injected PRV to label any PSNS innervation that existed. We found, however, no labeling of any neurons or fibers in the sympathetic ganglia, spinal cord or across the central neuroaxis [108], but control (6OHDA-vehicle injected animals) had the typical pattern of SNS outflow labeling from brain to WAT [108]. Collectively, these data suggest no PSNS innervation of WAT, or that the PSNS innervation of WAT occurs without the classic neurochemicals found in other PSNS innervated tissues despite the unsubstantiated claims to the contrary [154;155]).
3.3 Innervation of blood vessels and its possible relation to lipolysis
By contrast with the histological and functional studies conducted on WAT SNS parenchymal innervation, innervation of the vasculature supplying WAT by the SNS is scant. Much of what is presumed rests on the sympathetic innervation of skeletal muscle in a number of species. In laboratory rats, the SNS innervation of the spinotrapezius muscle as a representative skeletal muscle suggests that the arterial side of the vasculature is densely innervated by the sympathetic nerves, but not the venous side [193] and other than some of the blood vessels to the face [143], there is no PSNS innervation of the vasculature as also is apparent for WAT itself [108;109]. In skeletal muscle, furthermore, with increases in sympathetic drive, constriction of the proximal arterioles appears stronger than that of the distal arterioles and thereby is the principal controller of skeletal muscular vascular resistance whereas the distal arterioles only transiently do so thereby restoring the surface area of the capillaries for oxygen/nutrient-carbon dioxide/metabolite byproduct exchange [193]. In terms of the function of SNS innervation of the vasculature in WAT and its relevance to lipolysis, the pioneering studies of Rosell (for review see: [244]) encompass most of the literature. Using primarily an unanaesthetized dog model whereby the IWAT depot had its arterial and venous supplies catheterized and often using electrical stimulation of the sympathetic innervation of this pad, Rosell and associates addressed the issue of the SNS innervation on blood flow and possible consequential alterations in lipolysis. First, stimulation of the SNS innervation to this pad increases FFA concentrations in the venous drainage [225], an effect not due to changes in blood flow when this is controlled [243]. Because electrical stimulation of WAT sympathetic innervation of blood vessels increases vascular permeability, an effect mediated by alpha adrenergic receptors [140;222], as well as because of other data from his laboratory, Rosell concludes [244] that a role of the SNS innervation of WAT vasculature in lipolysis could be to increase capillary permeability needed to allow liberated FFA to leave the interstitial space reducing their extracellular concentration thereby promoting lipolysis by decreasing FFA end product inhibition, the latter a well-known in vitro effect [39;240]. These data and the SNS innervation of WAT vasculature are important in the interpretation of both the neuroanatomical and functional data implicating the SNS in lipid mobilization. Regarding the former, although there clearly is parenchymal sympathetic innervation of WAT [51;267;306] in addition to its innervation of WAT vasculature [267;305;306], it seems likely that some of the PRV tract tracing of the SNS outflow to WAT also includes this sympathetic blood vessel innervation. From a functional standpoint, the ability of the sympathetic WAT denervation to decrease or block lipolysis also could have, as part of its mechanism, a decrease in SNS-induced capillary permeability [185;186]. Thus, although the sympathetic innervation of WAT vasculature might appear to contribute caveats to the interpretations of the viral transneuronal tract tracing of the SNS outflow from the brain to WAT and to sympathetic denervation-induced decreases in lipid mobilization, neither caveat creates an interpretational nightmare. This is because of the general relation between increases in blood flow and tissue metabolic activity (e.g., glucose uptake [125]) that includes glucose uptake into the brain and peripheral tissues including BAT [265;284].
4. AR Subtype, Number and Affinity Involved with WAT Lipid Mobilization
The control of lipolysis by the SNS relies on many factors at the cellular level, not the least of which are the number, affinity and type of ARs on the adipocyte membranes (for review see: [55;173]). The classic studies of Lafontan, Langin and associates [44;166;174;199] discovered, clarified and highlighted the role of white adipocyte receptors in catecholamine and thus principally, SNS-induced lipolysis (for review see: [162;163]). From these and subsequent confirmatory studies, they unequivocally demonstrated that the balance between the stimulation of lipolysis by β-ARs (subtypes β-AR1–3) and its inhibition by α2-ARs dictates the degree of lipolysis if all other factors affecting lipolysis are equivalent [56;161;165]. Therefore, when β-AR activation predominates, lipolysis increases and when α2-AR activation predominates, lipolysis is inhibited [160;162]. As we noted previously [21], the lipolytic contribution of the β-AR subtypes and antilipolytic contribution of the α2 ARs in lipolysis can differ with WAT depot, species, sex, age and adiposity (e.g.,[43]). Activation of β1–3 AR agonists engages the GTP-binding protein Gsα, thereby activating cAMP production via adenylyl cyclase (Fig. 2). Adenylyl cyclase, in turn, stimulates protein kinase A (PKA) which phosphorylates two proteins critical for lipolysis (Fig. 2; and see directly below). In a converse manner, stimulation of α2-ARs decreases cytosolic cAMP because adenylyl cyclase is inhibited; therefore, PKA is not stimulated and HSL and perilipin A are not phosphorylated ([6;42;173] and Fig. 2).
Figure 2.
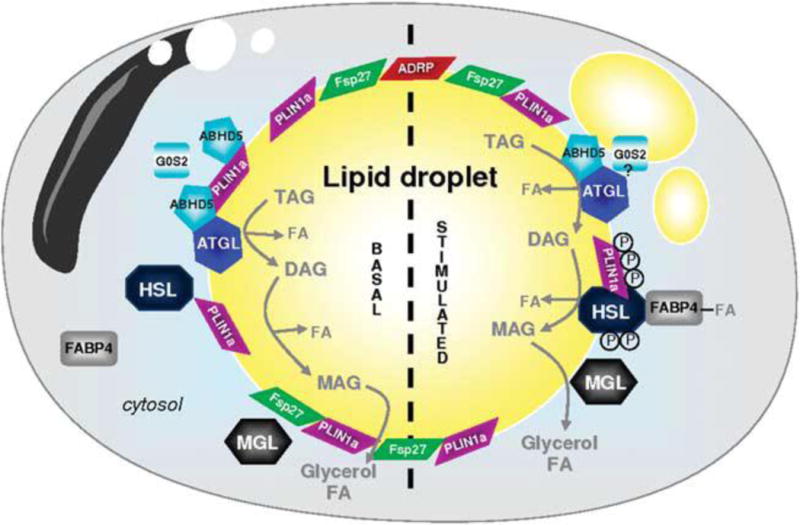
From: A Girousse and D Langin [110]
Adipocyte ‘lipolysome’ in basal and stimulated state. Lipid droplets (LDs) are surrounded by a phospholipid monolayer in which different proteins are anchored: PLIN1a (perilipin A), PLIN2 (ADRP) and Fsp27/Cidec. In the unstimulated basal state, ATGL, perilipin and ABHD5 are forming a complex at the surface of the LD. These protein interactions maintain ABHD5 inactive and as a consequence limit basal ATGL-mediated lipolysis. HSL and FABP4 are in the cytosol. In the stimulated state, HSL is phosphorylated by protein kinases (PKs); the active form of HSL migrates to the surface of the LD. PK also phosphorylate PLIN1a that undergoes structural modification and rearrangement leading to fragmentation of the LD. ABHD5 released from phosphorylated PLIN1a activates ATGL to initiate TAG hydrolysis. G0S2 can limit ATGL enzyme activity. DAGs are then transformed into MAG by active HSL. MGL ends the lipolytic process and releases glycerol and FA. FA-complexed FABP4 can interact with HSL to modulate lipolysis. ABHD5, abhydrolase domain containing; ADRP, adipose differentiation-related protein; ATGL, adipose triglyceride lipase; DAG, diacylglycerol; FA, fatty acid; FABP4, fatty acid-binding protein 4; Fsp27, fat-specific protein 27, also called Cidec; G0S2, G0/G1 switch gene 2; HSL, hormone-sensitive lipase; MAG, monoacylglycerol; MGL, monoglyceride lipase; PLIN1a, perilipin A; TAG, triacylglycerol.
Recently a new possible feedback system within the adipocyte related to cAMP has been described [277]. In addition to the cAMP stimulation of PKA, cAMP also stimulates cAMP response element-binding protein (CREB) regulated transcriptional coactivators [(Crtc); [277]]. Specifically, Crtc3 inhibits cAMP production via regulator of G-protein signaling 2 (RGS2), a protein that activates GTPase inhibiting Gqα cell signaling thereby dampening β-AR activation of the lipolytic cascade ([277]; Fig. 2). Moreover, in high fat diet-fed wild-type mice, Rgs2 mRNA increases (implying decreases in lipolysis), but not in CRT3−/− mice [277]. In addition, a Crtc3 variant allele that encodes a missense variant (S72N) is increased in obese Mexican-Americans humans [277]. These data, and others showing a diminished lipolytic response in situ in obese versus non-obese humans with electrical intraneural stimulation of the femoral sympathetic nerves, suggests that inadequate SNS/NE stimulation of lipolysis [76] could promote lipid accumulation in WAT and ultimately obesity or hamper obesity reversal [277].
Finally, an additional neurochemical that affects lipolysis has been located in sympathetic nerves – VGF, at least in perigonadal WAT (EWAT and parametrial WAT; [232]). VGF is co-localized with TH-ir (sympathetic) neurons in these WAT depots and, moreover, a VGF-derived peptide, TLQP-21 also is co-localized in these WAT sympathetic neurons, although the extent of the co-localization is unclear [232]. TLQP-21 binds with saturability to adipocytes and exaggerates β-AR-induced lipolysis (isoproterenol) and phosphorylated HSL (pHSL) in isolated adipocytes [232]. Interestingly, TLQP-21 does not affect lipolysis itself, but appears to enhance lipolysis via augmented phosphorylated 5′AMP-activated protein kinase (pAMPK) and pHSL [232]. When given systemically, infusion of TLQP-21 decreases adiposity as seen by reduced fat cell size in diet-induced obese mice and the chronic stress-induced obesity exhibited by subordinate mice [232]. The importance of VGF/TLQP-21 for control of SNS/NE-induced lipolysis will require further demonstration of its pervasiveness across and within WAT pads. Whether these findings can be extended to humans or not, it could provide a pharmacological target for obesity treatment.
5. A Series of Lipases Work Together to Fully Hydrolyze Triacylglycerol (TAG) in White Adipose Tissue
Lipid is stored primarily as TAG and primarily in white adipocytes. Some distinction should be made between basal and stimulated lipolysis in terms of the relative importance of the series of enzymatic steps involved in complete TAG hydrolysis into three fatty acids (FAs) and glycerol. In brief, regarding basal lipolysis (i.e., non-SNS/NE-stimulated lipolysis), adipose triglyceride lipase (ATGL; a.k.a. desnutrin) has a preferential affinity for hydrolysis of TAG compared with diacylglycerol (DAG) and monoacylglycerol (MAG) (for review see: [110]; Fig. 2). In basal lipolysis, ATGL activity is considered the rate limiting step. The interaction of ATGL with TAG results in a liberated FFA and DAG. ATGL is located in the cytosol during this basal condition of lipolysis, whereas perilipin A and ATGL activator CGI-58 [a.k.a. abhydrolase domain containing 5 (Abhd5)], two other key players in TAG hydrolysis, are bound to the lipid droplet ([110]; Fig. 2). Because Abhd5 and perilipin A are bound together, the level of ATGL-induced lipolysis is limited in the non-SNS/NE-stimulated lipolytic state (for review see: [110]; Fig. 2). This should not, however, diminish the apparent importance of ATGL in basal and stimulated lipolysis. For example, in mice where ATGL is knocked out (ATGL-KO), NE-stimulated lipolysis is severely blunted and inhibited in 3T3L1 murine cell line where ATGL was knockdown by short interfering RNA (siRNA) [150], whereas ATGL overexpression increases basal and isoproterenol (pan β AR agonist; [150])-induced lipolysis. Thus, ATGL is important in TAG hydrolysis which provides the substrate for HSL. In addition to reduced basal- and β-AR agonist stimulated-lipolysis, ATGL KO mice cannot maintain normal rates of exercise [138], are cold intolerant [123] and accumulate lipid deposits in the heart [123]. In a cautionary note, some species differences exist in the control of lipolysis. In human primary adipocyte culture ATGL seems important for basal lipolysis, but not isoproterenol (β-AR) -stimulated lipolysis [247], whereas in the murine 3T3L1 cell line ATGL is important for both basal and isoproterenol-stimulated lipolysis [150].
For the SNS/NE-stimulated complete lipolytic response, HSL and perilipin A in conjunction with one additional lipase, monoglyceride lipase are necessary (Fig. 2). In brief, in vivo, NE released from sympathetic nerve terminals stimulates the G-protein coupled β3 ARs in rodents and β1 and β2 receptors in humans, respectively (for review see: [165;173]), leading to the canonical intracellular signaling sequence that ultimately results in lipolysis (Fig. 2). Specifically, activated PKA phosphorylates HSL at three serine sites 563, 659 and 660 in rodents, with the latter two necessary for translocation of HSL to lipid droplet (for review see: [136]; Fig. 2). The necessity of HSL for SNS/NE-induced lipolysis is demonstrated by severe reduction, but not complete blockade, of glycerol and FFA release by NE in isolated adipocytes from HSL KO mice [212]. This prompted the search for other lipases culminating with the discovery of ATGL (see directly above: [144;294;322]; Fig. 2).
pHSL translocates to the lipid droplet where PKA phosphorylated perilipin A is thought to function as a scaffolding protein exposes the lipid droplet to HSL [259] thereby facilitating lipolysis (for review see: [110]; Fig. 2). Note that in the basal state, perilipin A is not phosphorylated thus, preventing hydrolysis [205]. The Abhd5-perilipin A complex dissociates when PKA phosphorylates perilipin A thereby freeing Abhd5 to activate ATGL [150;175;280;311] by phosphorylating on residues Ser492 or Ser517 ([116]; Fig. 2). Next, ATGL removes a FA from TAG leaving DAG whereupon DAG lipase removes another FA leaving behind monoacylglycerol (MAG) which is hydrolyzed by MAG lipase yielding glycerol and the final FA (for review see: [110]; Fig. 2).
5.1 pHSL and pPerilipin A Can Serve as Intracellular Markers of Lipolysis in a Fat Pad-Specific Manner
Lipolysis can be inferred from increases in plasma glycerol and/or FFAs; however, the origin of these lipolytic products is unclear. There are numerous WAT depots as well as non-WAT tissues that undergo lipolysis, the latter including BAT, liver, muscle any or all of which could contribute to the circulating TAG metabolites. Therefore, specific intracellular makers for NE-stimulated lipolysis is necessary to determine which WAT pads are undergoing lipolysis because, in terms of SNS-stimulated lipolysis, various metabolic stimuli (e.g., food deprivation, glucoprivation, or cold exposure) produce unique neurochemical patterns of differential SNS drive across the WAT pads (for review see:[21]). As noted above, because phosphorylation of HSL and perilipin A are necessary for catecholamine-induced lipolysis in adipocytes (Fig. 2), we postulated that pHSL and pPerilipin A could serve as in vivo intracellular indicators of fat pad-specific SNS/NE-stimulated lipolysis. Indeed, we found that a single injection of melanotan II (MTII), the melanocortin 3 and 4-receptor (MC3-R and MC4-R) agonist, into the 3rd ventricle (3V) increased NETO only to the subcutaneous WAT pads (DWAT and IWAT), but not the intraabdominal pads (EWAT and RWAT; [34]) and only DWAT and IWAT, but not EWAT or RWAT, had increased ratios of the pHSL/HSL and pPerilipin A/perilipin [266]. These findings make it likely that the previous determined increase in circulating FFA and glycerol by the same treatment with central MTII was due to lipolysis from these subcutaneous WAT pads [34].
6. Preclinical and Clinical Measures of SNS Activity
A pervading assumption that likely has done more harm than good in our understanding of the SNS control of WAT lipolysis is that the SNS activity can be generalized from measures of one of its effected tissues such as measuring suspected indices of the SNS drive to the heart, skeletal muscle, or BAT and generalizing this to WAT. Perhaps this type of thinking stems from Walter Cannon’s ‘fight-or-flight’ response [40] where the SNS is turned on uniformly with stress/fear (e.g., a bear is chasing you) and off when relatively serene. In an electrical analogy, if a volt-ohm meter is placed in one electrical outlet in the house and voltage measured, it will be quite similar if not identical to that measured from another electrical outlet elsewhere within the house. By contrast, the SNS outflow can vary across tissues (e.g., WAT, BAT, heart) and across the same tissue type with different anatomical locations (e.g., EWAT vs IWAT vs RWAT; for general review see: [141]). Therefore, as an important cautionary note, when reading the literature in this and related fields, one cannot surmise that ‘tapping into’ one part of the SNS has any generality to any other part. Moreover, as is discussed below and reviewed recently [292], there are no surrogates for SNS activity – direct measures involve electrophysiological assay of sympathetic nerve activity or neurochemical analysis of sympathetic terminal release of NE (i.e., NETO). Assays of heart rate variability(HRV), TH protein by western blot or immunohistochemistry or gene expression, tissue NE content, or plasma or urine NE concentrations or its metabolites are not valid measures (as discussed below and in [292]) as they are not necessarily correlated with SNS activity to WAT.
6.1 HRV, NE spillover (NES) and Muscle SNS Activity (MSNA) as Measures of SNS Activity
HRV, plasma NES and MSNA often are used clinically to assess SNS activity in human patients for several SNS-associated pathologies [3;99;152;285]. HRV is the favored assessment measure because it is relatively simple to employ, convenient in terms of required equipment and noninvasive. Unlike NES and MSNA, HRV is touted as a measure of both SNS and PSNS control of cardiac activity and in terms of the latter, it is theorized that the beat-to-beat interval is well-controlled by both vagal (PSNS) and sympathetic nerves [206]. Both time domain and frequency domain analyses of HRV are used to analyze the data. Frequency domain analyses are preferred because, according to the theory, the high frequency (HF) and low frequency (LF) components, when normalized, can represent PSNS and SNS activity, respectively [102]. Unfortunately, this is not as simple and straightforward an assay tool of the autonomic nervous system as some would like to believe and certainly is not a direct measure of SNS or PSNS activity. Regarding inconsistencies with the theoretical basis for HRV as a measure of SNS and PSNS activity, atropine, a muscarinic acetylcholine receptor competitive antagonist for the PSNS reduces the absolute level of both HF and LF and propranolol, the β1 and β2 AR SNS antagonist, only inhibits LF suggesting that both PSNS and SNS contribute to the LF component [230]. It also should be noted that humoral factors and intrinsic variability in sinoatrial pacemaker cells also may influence HRV (e.g., [231]). Regardless, using the HRV to conclude that SNS activity is increased in obese versus lean humans [e.g., [4;319]] is unsubstantiated. To our knowledge, the SNS activity to WAT has not been examined in obese or lean humans, yet general statements often are made that obese people have increased sympathetic drives. Increases in MSNA in the obese versus lean are generally, although not completely, supported (for review see: [66]). Extending this to increases in the SNS drive to WAT would suggest increases in lipolysis in the obese decreasing adiposity if the mobilized FFAs are not re-esterified into TAG within the adipocytes and assuming normal β-AR number, affinity, and the intracellular cascade resulting in TAG catabolism. These are not trivial assumptions of normality in the obese. In addition, any purported increase in SNS drive to WAT could be countered by the normal ability of insulin to inhibit lipolysis (e.g., [100;115;234]); however, this ability is greatly diminished in obesity/metabolic syndrome perhaps by insulin resistance [169;236]. Under the condition of WAT insulin resistance, therefore, the presumed, but not documented, increases in SNS/NE mediated lipolysis could be the culprit in the increases in circulating FFAs purported to be exhibited by the obese versus lean. Indeed in an early study, increases in circulating FFAs in the obese vs lean were reported [224], the findings of which have achieved dogmatic status. A recent analysis of the literature, however, suggests that this fiercely held notion of increases in circulating FFAs with obesity is often not found or can even be opposite [for review see: [149]] and it may be that increases in FFAs do not produce insulin resistance [149]. Moreover, the possibility that the increase in FFAs in the basal state that sometimes is seen in obesity is due to increases in the SNS drive to WAT is unlikely as basal lipolysis appears under the control of ATGL as shown in the mouse embryonic fibroblasts adipocyte cell system [204]. Thus, it appears that ATGL can trigger lipolysis in the non-stimulated state (basal state) acting as a TG hydrolyase, although this has not, to our knowledge, been demonstrated in a primary adipocyte culture.
The notion that sympathetic drive to WAT is increased in the obese state is not on solid experimental ground. MSNA is a direct measure of sympathetic drive and has been applied to humans effectively including obese and lean humans [for review see: [66]. Collectively, these studies of postganglionic direct nerve recordings show that the increases in adiposity of obese humans is associated with ~50–100% higher MSNA compared with their lean counterparts (for review see: [66]). Thus, claims of increases in SNS activity of obese humans should be made cautiously and appear only to be clearly applicable to the SNS drive to skeletal muscle, likely not all skeletal muscle as well and contradictory findings have been reported [for review see: [66].
6.2 Measures of NES in Urine or Plasma as Indicators of SNS Activity
Some researchers have and continue to use plasma or urine concentrations of NE as indicators of SNS activity with the underlying premise that NE in these fluids represents a fraction of that released by the sympathetic nerve terminals in SNS innervated tissues. As reviewed in detail by Davy [66], this method has severe drawbacks as it is affected by NE clearance rate from blood, blood flow, NE degradation and suffers most greatly from a lack of organ specificity [66;84]. Thus, one does not know the origin, nor the proportion of NE released by the sympathetic nerve terminals represented by blood or urine NE concentrations nor how much of the NE is derived from the adrenal medulla. In terms of the latter, both EPI and NE are synthesized by separate chromaffin cells [133] that are separately innervated by the preganglionic sympathetic neurons in the IML [81] and are released in varying proportions depending upon the stimulus [295].
6.3 NETO as a Direct Measure of SNS Activity
NETO as a neurochemical measure of SNS activity has its origins in the findings of Julius Axelrod [130;302]. Specifically, Axelrod and co-workers demonstrated that the sympathetic nerve terminals have mechanisms for NE uptake and storage in the so-called ‘readily releasable pool’ located close to the terminal bouton until released. In addition, a longer term storage pool located more distantly from the nerve terminal exists [57]. The normal reuptake system of interstitially released NE from sympathetic nerve terminals permitted the use of tracer 3NE to be injected and taken up by SNS terminals with its release allowing estimation of endogenous NE to calculate NETO and was initially applied to laboratory rat heart ([171]; for review see: [170]). This method was used extensively by Landsberg and Young in their seminal studies of sympathetic drive under various metabolic challenges, diets and hormonal states (e.g., [172;312–314]). More specifically, the method is based on the uptake and subsequent decline in 3NE such that fractional turnover (slope of the decline) and NETO [NE released per gram of tissue per hour [170]] can be determined. Although we learned considerable information on the activity of the SNS under a variety of these states, the data have often been extended from the tissue of interest, often BAT, more generally including implications for that of WAT, the topic at hand here. As noted above and below, this is problematic in that the rule is differential SNS drive across tissues rather than that being the exception. Another problem with this method is that it is assumed 3NE mixes fully with both pools of endogenous NE, but it apparently does not do so with the longer term storage pool [57]. Finally, there is the practical matter of injecting radioactive substances into live animals which is being more difficult to do with the increasing biosafety and animal care and use regulations in many countries.
The alternative approach is the alpha-methyl-para-tyrosine (AMPT) method (for detailed description see: [292]). AMPT is a competitive inhibitor of TH [278] and thus stops the production of new NE such that only the currently synthesized NE (in both pools) is available for measurement. Therefore, using steady state kinetics [36] the decline in tissue content of NE from time zero to usually 2–4 hr is represented by a slope (fractional turnover) that can be expressed as per gram tissue or whole WAT pad (or other tissue of interest). With this method many different WAT and other SNS innervated tissues can be assayed, as with the 3NE method, but without the problem of measuring only one of the two pools of NE and without using radioactivity [292]. The downside is that AMPT is a severe behavioral depressant that therefore also would be expected to markedly affect many physiological/biochemical responses. Therefore, only NETO should be measured in AMPT-treated animals with a parallel set of animals used for non-NETO measures. With this method we [34;35;211;215;262;316] and others [104;126;176;202;229] have measured differential NETO across WAT depots and BAT showing the fat pad-specific patterns of sympathetic drive that are triggered by various energy demanding challenges. Thus, for measures of SNS drive in non-human animals we see the AMPT method as preferable to electrophysiological measures because with the latter usually only innervation to one or two tissues can be measured at a time because of electrical interference, whereas with the AMPT method the number of tissues that can be simultaneously assayed are only limited by one’s interest. In addition the AMPT method seems and preferable to the 3NE method because it does not underestimate NETO and is not radioactive. The method, of course, cannot be used in humans where the only direct method to date is intraneural electrophysiological measures [66], but these have not been done in WAT to our knowledge.
7. Functional Evidence for in vivo Lipolysis in WAT mediated via the SNS Innervation
7.1 Lipolysis is Blocked by SNS Denervation and Increased by SNS Electrical Stimulation
The first functional indication that activation of WAT SNS innervation is the primary initiator of lipolysis was reported nearly one hundred years ago by Mansfeld [192] whose hemiplegic patient with cancer cachexia only mobilized lipid from their neurally intact leg. Laboratory findings functionally implicating the SNS innervation of WAT mediating lipid mobilization date back to 1926 ([301], for review see: [14]), where dogs with damage to the spinal cord did not mobilize WAT. Surgical denervation of the SNS innervation of WAT at the fat pad level blocks/attenuates lipolysis occurring with food deprivation in laboratory rats, cats, rabbits, and dogs [29;33;41;53;301], as well as blocking estradiol-induced lipolysis in ovariectomized laboratory rats [176]. These and other data (for review see: [14;21]) provide incontrovertible evidence for the necessity of WAT SNS innervation for lipolysis triggered by a wide range of stimuli (photoperiod, estradiol, glucoprivation, food deprivation, cold exposure and others (except for one possible exception noted below).
Support for the sufficiency of the activation of the SNS innervation of WAT for lipid mobilization comes from ex vivo studies where EWAT pads with intact sympathetic nerves were electrically stimulated and concentrations of FFAs in the incubation medium increased [58], an effect blocked by pre-incubation with a β-AR antagonist [299]. In an in vivo preparation in humans, intraneural electrical stimulation of the lateral femoral cutaneous nerve innervating subcutaneous WAT increases lipolysis as measured by glycerol concentrations in the perfusate of microdialysis probes located in subcutaneous leg fat [75], an effect diminished in obese but not in lean humans [76]. Together, these denervation and stimulation studies demonstrate the necessity and sufficiency of activation of the WAT SNS innervation on lipid mobilization, respectively, in rodents and possibly in humans (for review see: [77]).
8. Potential and Existing Inhibitors of SNS-Mediated WAT Lipolysis
8.1 Insulin is a Major SNS Inhibitory Factor for Lipolysis
NE-induced lipolysis is countered by pancreatic insulin, a powerful and prevalent inhibitor of lipolysis (e.g., [100;115;234]). This insulin-induced lipolysis inhibition can promote adiposity in humans because overeating triggers large increases in circulating insulin concentrations postprandially – a situation that currently prevails during waking hours given the availability of inexpensive and calorically dense foods [297]. In brief, the classic explanation of the inhibition of lipolysis by insulin is via phosphatidylinositoI-3-kinase (PI3-kinase)-dependent activation of phosphodiesterase 3B (PDE3B) thereby increasing the catabolism of cAMP and consequently inhibiting phosphorylation of PKA (for review see: [173]). Recently, however, another mechanism has been uncovered that builds on the pioneering work of Mario DiGirolamo [64;92] who championed the importance of white adipocyte lactate production (for review see: [74]). Because the postprandial increase in lactate production is so pronounced and can be mimicked by in vitro glucose and insulin administration [64], the obvious interpretation of those data is that lactate acts as a possible fuel for liver gluconeogenesis and glycogen production (for review see: [74]). Recently, the production of lactate and the control of lipolysis have been linked via the orphan Gi-coupled receptor GPR81 that is highly expressed in adipocytes in both humans and mice and is activated in vitro by lactate [2;187]. When circulating glucose concentrations increase, lactate production increases (as noted directly above) which is associated with decreases in non-esterified fatty acid (NEFA) probably mobilized from WAT [2;187]. Moreover, GPR81 KO mice have diminished lipolysis in vivo and the ability of insulin to decrease cAMP levels, although insulin-triggered lactate release is normal [2;187]. Collectively, these new data show that lactate acts on adipocytes via the GPR81 and that the inhibition of lipolysis by insulin is dependent on this response, suggesting an unappreciated autocrine role of lactate as well as an alternate mechanism by which insulin inhibits lipolysis ([2;187]; for review see: [173]).
8.2 Purinergic inhibition of the SNS control of lipolysis
Adenosine is a purine nucleoside present in all cells including adipocytes and exerts an antilipolytic effect via paracrine action on the A1-adenosine receptor [79]. Stimulation of the A1-adenosine receptor inhibits adenylyl cyclase activity, thereby reducing cyclic AMP and hence inactivating its downstream effectors such PKA, HSL and perilipin A resulting in a decrease in lipolysis [87]. Adenosine also can enhance the antilipolytic effect of insulin [252]. The antilipolytic effect of adenosine is not due to altered NE release from sympathetic nerve terminals [269], but instead adenosine inhibits cAMP production and consequently the downstream signaling cascade of the lipolytic process as described above [94;97;145]. Endogenous adenosine tonically activates the A1-adenosine receptor thereby actively inhibiting lipolysis due to the extreme sensitivity of the receptor to nanomolar concentrations of adenosine which, even at these extremely low amounts, occupies ~50% of all A1-adenosine receptors [184]. Endogenous adenosine, however, is incapable of completely inhibiting catecholamine-induced lipolysis [289]. Using the dog subcutaneous in situ model, electrical stimulation of the sympathetic nerves innervating IWAT or injections of NE into the vasculature supplying this depot increases purine and purine metabolites in the stimulated WAT tissue, including adenosine, inosine, xanthine and uric acid [98]. Moreover, blockade of α-ARs on adipocytes during the NE stimulation inhibits adenosine release suggesting its origin from these cells and importantly functions downstream of catecholamine stimulation on [93]. Thus, adenosine and or its metabolites inosine, xanthines might be thought of as potential mediators of lipolysis and in this case as effectors of SNS-NE stimulated lipolysis.
9. Possible Non-SNS Lipid Mobilization from WAT
9.1 Leptin and Sympathetically-and Non-Sympathetically-Mediated Lipolysis
There a few exceptions to this conclusion that the SNS is the principal stimulator of lipolysis in WAT. Leptin was initially reported to increase lipolysis in vitro by adipocytes [101] and to increase rat SNS activity to WAT when administered intravenously at very high doses yielding a relatively small lipolytic response compared with equimolar NE injections [257]. The relatively small in vivo lipolytic response to both peripheral and central leptin administration at doses that produce non-physiological concentration of the adipokine is surprising in that leptin given via both routes does markedly increase the SNS drive to peripheral tissues including WAT [229]. When peripheral leptin is given at doses that produce physiological concentrations, selective chemical sympathetic denervation accomplished by intra-WAT 6OHDA injections, does not block lipid mobilization in laboratory mice or rats [241]. Therefore, the SNS innervation of WAT is not required for low, physiological doses of leptin to reduce body fat. The mechanism triggering lipid mobilization in the face of sympathetic denervation is unknown. One possible explanation for the increases in lipid mobilization after WAT SNS denervation, beyond an obvious incomplete denervation, is that decreases/elimination of one arm of the SNS can result in compensation by the remaining intact arm [315]. For example, although not a measure/surrogate of SNS drive, the NE content of the sympathetically innervated intact IWAT increases after ADMEDx compared with that of their sham-operated counterparts [71]. Conversely, NETO increases in both IBAT and pancreas following ADMEDx [282] and more to the point, EPI turnover increases in the adrenal medulla after systemic chemical sympathectomy with 6OHDA [282]. Thus, although not in the case of leptin-induced increases in lipid mobilization in vitro [101], WAT sympathetic denervation may trigger increases in adrenal medullary EPI or NE secretion to stimulate lipolysis, despite the role of adrenal catecholamines being either not important or greatly less important for normal SNS/ne-stimulated lipolysis in WAT (for review see: [14;21]).
9.2 Natriuretic Peptides and Non-Sympathetically-Mediated Lipolysis
In humans and non-human primates, in addition to the SNS-mediated lipolysis [59;75;76;161;165], a family of peptides – natriuretic peptides – appear to promote lipolysis (e.g., [164;208;253]). Three natriuretic peptides have been identified to date: 1) atrial natriuretic peptide (ANP), a 28 amino acid peptide predominantly synthesized and released by atrial myocytes [67;69], 2) brain natriuretic peptide (BNP), a 32 amino acid peptide secreted by atria and heart ventricle cells, but first isolated from the brain [191] and 3) C-type natriuretic peptide (CNP), a 22 amino acid peptide that is extensively produced in the vascular endothelium, but also was first discovered in the brain ([281]; for review see: [68;255]). ANP and BNP are associated with cardiac and renal functions, whereas CNP in an autocrine/paracrine factor affecting vascular tone. Importantly, ANP is a strong lipolytic stimulus for human and non-human primate macaque adipocytes, but perhaps not in laboratory rats or mouse adipocytes ([253;254]; for review see [167] – although see further directly below). Three classes of NP receptors exist. The first, termed the NPRA (a.k.a. NPR1 or GC-A) is the signaling form of the NP receptors and ANP and BNP are preferentially bound to NPRA with a lesser binding by CNP. The second class of NP receptors is termed NPRB and conversely preferentially binds to CNP, but with lesser affinity to ANP and BNP. The third, NPRC is an interesting receptor as its role is to clear circulating NPs, especially ANP [5] thereby in this unique manner controlling NP interactions with its receptors at its site of action [194].
Regarding NP roles in lipolysis, large human adipocytes have significantly more NPRA receptors than smaller adipocytes and consequently increase lipolysis (as measured by glycerol release) to a greater degree after NPRA stimulation than do small adipocytes [318]. ANP also stimulates lipid mobilization in vivo in humans as assessed by increases in intercellular glycerol using in situ microdialysis of abdominal subcutaneous WAT following iv ANP infusion. Apparently this effect occurs independent of SNS activation as evidenced by its continued efficacy with the addition of the β-AR antagonist propranolol to the microdialysis infusate [103]. Moreover, in exercising humans, in situ lipolysis, measured via microdialysis from subcutaneous adipose tissue, is partially blocked by β-AR antagonism and it is assumed that the remaining unblocked lipolysis is due to ANP from the heart [207]. Most recently, the role of the heart as a bona fide endocrine organ involved in energy metabolism has emerged, albeit slowly given the discovery of ANP in 1985, ~30 years ago [67;69]. As noted by Collins and Bordicchia [54], the absence of a response to ANP in rat adipocytes is odd because they possess NP receptors and in food-deprived animals there is a large decrease in NPRC which makes sense if NPs stimulate lipolysis and NPRCs counteract that by clearing NPs from the circulation. Therefore, the Collins laboratory reasoned that in rodent adipocytes, as with humans, the ratio of the NPRs to NPRCs may be the critical factor for lipolytic stimulation. Indeed, NPR-C gene null mice (NPR-C−/−mice) are lean likely because of the absence of the clearing receptor NPRC and their decrease in body fat is accompanied by browning of the adipocytes [i.e., increased brown adipocyte markers PPARgamma coactivator-1alpha (PGC-1alpha) and uncoupling protein-1 (UCP1) expression]. As above, adipocytes from NPR-C+/+ mice are refractory to ANP-induced lipolysis, but ANP induces a lipolytic response in NPR-C−/− mice having significant increases in NPRA expression suggesting that under conditions of repressed NPRC expression ANP could induce lipolysis via NPRA in rodents [30].
9.3 Forkhead Box Gene Group Other-1 (FOXO1) Stimulates Lipolysis In Vitro
Among the plethora of transcription factors involved in energy balance, FOXO1 appears to play an important role in both glucose and lipid metabolism (for review see: [120]). As noted above, ATGL is the rate limiting enzyme in the lipolytic cascade and a decrease in expression of ATGL and HSL is associated with insulin-resistance and obesity [159]. FOXO1 binds to the promoter region of ATGL thereby increasing ATGL expression and promoting lipolysis in 3T3L-1 cells, and conversely, its knockdown diminishes both basal and isoproterenol-stimulated lipolysis [47]. Because of abundant caveats concerning 3T3L-1 cells as a model of true adipocytes for a variety of its functions (e.g., [178;233]), the relevance of this mechanism of lipolysis in vivo for normal physiology is unclear although the data discussed above strongly infer its potential involvement in the increases in circulating FFA in the pathophysiology accompanying insulin insufficiency/resistance via direct stimulation of ATGL activity and independently of SNS/NE and β-AR activation.
9.4 Epinephrine as Initiator of Lipolysis with Exercise in Humans
Despite the dominant role of the activation of WAT SNS innervation as the principal initiator of lipolysis, rather than adrenal medullary EPI as noted above (for review see: [14;21]), it is possible that adrenal medullary EPI, not NE, triggers lipolysis during exercise in humans [70]. The vast majority of the data across species and treatments, however, shows that activation of the SNS innervation of WAT is the principal initiator of lipolysis in mammals.
9.5 Other Factors Affecting SNS/NE-Induced Lipolysis
Recently, short chain FAs and ketone bodies have been shown to either stimulate or inhibit activation of postganglionic sympathetic neurons, respectively, via GPR41 receptors found in abundance in these cells [151]. Stimulation of GPR41 receptors is mediated via Gβγ complex and mitogen-activated protein kinase (MAPK), triggered by short chain fatty acids and opposed by the ketone β-hydroxybutyrate. These data, however, are based on cardiac-related indirect measures of SNS activity [151] and therefore remain to be tested in SNS/NE-induced lipolysis preferably in adipose tissues.
10. Sympathetic Drive to White Adipose Tissue is Not Uniform
As noted above, it is critical to realize that the SNS drive to peripheral tissues is not uniform. Moreover, across WAT depots we find that the pattern of SNS drive, as measured by NETO, is seemingly unique for each lipolytic stimulus tested to date prompting us to term this a ‘neurochemical fingerprint’ due to this individuality and the neurochemical nature of this measure of SNS drive. As examples, cold exposure increases NETO to IWAT, EWAT, RWAT and especially IBAT, but not DWAT [35], whereas food deprivation increases NETO to IWAT and EWAT, but not RWAT, DWAT or IBAT [35]. Glucoprivation, by contrast, produced by systemic injection of the non-metabolizable glucose analog, 2-deoxy-glucose (2DG), increases NETO to IWAT, RWAT and DWAT, but not to EWAT or IBAT [35]. Broad stimulation of central MC4-Rs resulting from 3V injection of MTII increases NETO only to the subcutaneous WAT pads (IWAT, and DWAT), but not the intraabdominal WAT pads (RWAT or EWAT [34]). By contrast, the longer term increase in SNS outflow produced by SD exposure of Siberian hamsters has the opposite effect, increasing NETO to the intraabdominal WAT pads (RWAT and EWAT), but much less so to the subcutaneous WAT (e.g., IWAT [316]). Others using laboratory rats find fat pad-specific sympathetic drives to WAT depots as well. For example, with prolonged food deprivation RWAT and EWAT NETO increases, but not IBAT [202], whereas with acute cold exposure RWAT, EWAT and IBAT NETO increases [104]. Clearly, these data exemplify the differential nature of SNS drive to WAT and BAT and emphasize the danger in generalizing the degree of SNS drive from one fat pad (or other tissue type) to another fat pad. The underlying mechanisms responsible for SNS trafficking with these various lipolytic stimuli remains a biological mystery to some extent (for review see: [209]), although the use of distinct reporter-producing versions of the transneuronal tract tracer PRV to label convergent and divergent central to peripheral circuits innervating WAT has helped to begin to solve the enigma, at least at the neuroanatomical level. Specifically, we have recently injected PRV 152 (eGFP) into IWAT and PRV 614 (mRFP) into MWAT and vice versa to test for common and uncommon neurons across the neuroaxis. Note that MWAT is the only visceral WAT in rodents because it is the only WAT depot that drains into the hepatic portal vein (the definition of ‘visceral WAT’), whereas the other intra-abdominal WAT depots drain into the vena cava. With food deprivation, there are significant increases in SNS (NETO) to IWAT, but not to MWAT [217]; therefore there must be some separate circuitry to each pad and indeed, at the level of the postganglionic (sympathetic chain) and preganglionic (IML of the spinal cord) there are occasional doubly-infected neurons, but these are few and far between suggesting separate neurologies for those sympathetic neurons. There are, however, some shared (doubly-infected) neurons at various sites in the forebrain, midbrain and brainstem ranging from ~20–55 for any nucleus [217]. Clearly, we are only beginning to get a glimpse at the intricacies of the control of SNS outflow to peripheral tissues including across WAT depots and BAT.
10.1 Some Central Factors Associated with Alterations in SNS/NE-Induced Lipolysis
It is beyond the scope of this review to summarize and integrate the many studies of central effectors that have been reported to alter body fat most of which use nebulous surrogate measures of SNS drive to WAT (see discussion of SNS surrogate measures above in 6 Preclinical and Clinical Measures of SNS Activity). There are, however several studies where the methodologies yield strong inferential conclusions concerning the role of these neurochemicals in the control of the SNS outflow to WAT.
As noted above, Siberian hamsters go from ~50% body fat to ~20% body fat when transferred from long ‘summer-like’ day photoperiods (LDs) to short ‘winter-like’ day photoperiods (SDs) in the laboratory [19;195;296], a process due to the coding of the duration of the photoperiod by the nocturnal duration of MEL secretion from the pineal gland (for review see: [20;114]). Because surgical denervation of WAT blocked SD-induced lipid mobilization [71;317], we labeled the SNS outflow from the brain to WAT using the transneuronal viral tract tracer PRV and labeling MEL1a gene expression using in situ hybridization [272] to yield MEL1a mRNA+PRV-ir neurons as noted above. In subsequent functional studies [182], we gave central timed melatonin treatments to duplicate the endogenous durational melatonin signals in pinealectomized hamsters doing so only to areas showing PRV+MEL1a double-labeled cells [272] including the SCN, SubZI, DMH, nucleus reunions and PVT via inserting and removing MEL filled cannulae into these sites. A long duration, SD-like melatonin signal applied site-specifically for 5 wks only to the SCN or only to the SubZi decreased WAT mass [182]. After these tests of sufficiency, we conducted tests of necessity by making lesions of the areas and giving systemic timed melatonin treatments that mimicked endogenous SD-like melatonin durations. We found that only the SCN and the DMH were necessary for the increased lipid mobilization (decreased WAT mass; [17;183;270;271]).
Using a similar neuroanatomical strategy to that of the PRV labeling of SNS outflow to WAT visualized by immunohistochemistry and combined with localization of the MEL1a mRNA by in situ hybridization, we tested for co-localization of PRV-ir cells with gene expression for the melanocortin receptor thought to be most responsible for the effects on body and lipid mass, the MC4-R [275]. We found high concentrations of double-labeled cells (PRV+MC4-R) across the neuroaxis with an approximate average of 60% of the SNS outflow (PRV-ir) cells expressing MC4-R mRNA. This level of colocalization suggests an important role of MC4-Rs in the control of the SNS outflow to WAT [275]. As noted above, central MC4-R stimulation increases the SNS drive (NETO) to WAT doing so primarily in the subcutaneous WAT depots (IWAT, DWAT but not at all or lesser in EWAT and RWAT; [34]) and, moreover, only in these depots showing significant increases in pHSL and pPerilipin A suggesting lipolysis in these WAT pads [266]. Consistent with these results is an increase in TAG synthesis in WAT when central MC4-Rs are antagonized via SHU9119 through 3V infusion, but no change in WAT lipid oxidation factors [221]. Moreover, chronic stimulation of MC3/4-Rs with MTII increases gene expression of ATGL (protein not measured) and HSL mRNA (protein not phosphorylation measured [221]). In addition, single 3V injection of MTII appears to increase sympathetic nerve electrophysiological activity to WAT [221]. These latter data are puzzling because of the extremely long onset of the increase in electrophysiological activity after the injection (~4 h with 5 h maximum effect post injection; [221]), whereas we found increases in pHSL/HSL and pPerilipin A/Perilipin A 10 min after a single 3V MTII injection [266]. Such a long delay questions whether this was a direct effect of CNS MC4-R activation instead of reflecting CNS activation of potential peripheral effects that, in turn, could have central effects that subsequently increase the SNS activity to WAT. Analogously to the 3V MTII-induced increase in SNS drive (NETO; [34]) and lipolysis (pHSL and pPerilipin A; [266]), humans with single-nucleotide polymorphisms (SNPs) near the MC4-R gene have increases only in the subcutaneous WAT depots (IWAT and DWAT) with their obesity [48;128;188] and, interestingly, those with the rs17782313 SNP appear to differentially deposit lipid in subcutaneous WAT [128]. Thus, stimulation of central MC4-Rs increases the SNS drive and intracellular markers of lipolysis in subcutaneous WAT only [34;266] and conversely, malfunctioning MC4-Rs in humans results in lipid accumulation in subcutaneous, but not visceral WAT [128]. Additional complementary data come from MC4-R deficient humans who have smaller decreases in fasting-induced respiratory exchange ratio (suggesting lesser lipid oxidation) than their normal counterparts [221]. Of additional clinical importance is that the MC4-R agonist α-MSH/ACTH(4–10) administered intranasally to non-obese, healthy males significantly increases abdominal subcutaneous WAT lipolysis (>50%; measured by interstitial sampling of glycerol via microdialysis) with no changes in local forearm skeletal muscle concentrations of glycerol nor resting MSNA suggesting CNS activation of MC4-Rs selectively stimulates WAT lipolysis in humans [300]. In sum, these data strongly implicate central MC4-Rs in the activation of SNS/NE-induced lipolysis and as potential targets for obesity treatment, although given the wide range of effects of MC4-R activation on several systems including the cardiovascular system (for review see: [124]), harnessing the sympathetic activity to only WAT may be difficult if not impossible.
Several other CNS factors have been implicated in the control of the SNS outflow to WAT. Orexin (a.k.a. hypocretin) that comes in two isoforms, orexin A and B, is synthesized exclusively in the lateral hypothalamus (LH). LH neurons have abundant projetions throughout the neuroaxis and have been implicated in the control of SNS outflow to a number of tissues including WAT (for review see: [80]). Exogenously applied orexin A has a biphasic effect on WAT sympathetic nerve activity with high doses of orexin-A (1 μg) increasing WAT SNS activity (measured electrophysiologically from sympathetic nerves innervation the tissue) and circulating FFAs, whereas a low dose (10 ng) decreases WAT SNS activity and does not stimulate circulating FFAs [258]. These effects of the high dose of orexin A were inhibited by histamine 1-receptor blockade [258]. Collectively, these data suggest orexin A affects sympathetic drive to WAT and consequent lipolysis, but in an apparent inverted ‘U’ function.
In addition to the profound inhibition of SNS/NE-triggered lipolysis by peripheral insulin, as discussed above, central insulin also inhibits lipolysis [250]. Specifically, chronic insulin infusion into the mediobasal hypothalamus of laboratory rats increases WAT lipogenic proteins while simultaneously inhibiting activation of WAT HSL phosphorylation thereby decreasing lipolysis apparently by decreasing the SNS drive to WAT as suggested by decreases in pHSL [250]. Moreover, mice with a neural specific-knockout of insulin receptors have the converse lipid profile, with decreases in lipogenesis and marked increases in lipolysis as suggested by increases in pHSL [250]. The exact neuronal population within the mediobasal hypothalamus responsible for the effects on WAT lipolysis is unknown, but recent studies showing that most AgRP neurons lay outside the blood brain barrier [223] leads us and others [250] to speculate that insulin receptors on these neurons may be involved. This also would explain a possible route of access to the brain for WAT-released leptin as indicated by the hypersensitivity of this population of neurons to leptin [223] as well as the opposing effects of mediobasal infused leptin on lipid metabolism versus insulin (for review see: [249]). That is, mediobasal hypothalamic leptin infusion decreases lipogenesis, inhibits FA uptake into WAT doing so via the SNS innervation of WAT as both surgical and 6OHDA-induced sympathetic WAT denervation blocks these effects [37]. Mediobasal leptin also increases WAT lipolysis, inferred by pHSL increases, an effect also blocked by sympathetic denervation [37].
11.1 Role of SNS in fat cell proliferation
Despite being the hallmark of obesity, white adipocyte proliferation is studied relatively infrequently compared with the vast literature on white adipocyte differentiation (for review see: [129]). In 1992, Roy Martin’s laboratory [146] showed that in the presence of NE, cultured rat stromovascular fraction does not exhibit the normal increases in fat cell proliferation. Moreover, application of the β-AR antagonist propranolol before NE application disinhibited the proliferation of white adipocytes suggesting the NE inhibition was via the β-ARs on the stromal vascular cells destined to become adipocytes [146]. Soon thereafter, Penicaud’s group found in vivo evidence of the role of the SNS/NE in WAT cell proliferation showing that denervation of laboratory rat RWAT triggers an increase in WAT pad mass, DNA (suggesting cell number) and A2COL6, a preadipocyte marker ([63]; for review see: [228]).
Independently in our search for the role of the SNS innervation of WAT in lipid mobilization by the SDs in Siberian hamsters, we [317] surgically denervated IWAT unilaterally and sham denervated the contralateral pad in Siberian hamsters with the initial purpose of blocking the short photoperiod-induced lipid mobilization in this species [18;296]. We found, however, a more remarkable effect. Siberian hamsters remaining in LDs where they are in an anabolic condition or curiously even hamsters transferred to SDs where they are in a catabolic condition [18;296] both increase fat pad mass (~200 %) in the denervated, but not the contralateral mate. Moreover, fat cell number (FCN) increased by ~250% in LDs and ~180% in SDs with the contralateral mate, not showing changes in FCN in either photoperiod [317]. Thus, this profound effect of denervation on increasing FCN occurs even under the catabolic SD condition. Although in our study [317] FCN increased with denervation, the method used to count cells, only detects cells >20μm [134]. Thus, if SNS denervation simply decreased basal lipolysis, the <20μm adipocytes could accumulate enough lipid to be detected (become >20 μm) thereby causing what would appear to be a bona fide increase in FCN when actually the increase was based on an increase in cell size, allowing previously undetected preadipocytes to be counted. In addition, our denervation method was surgical ablation, destroying both sympathetic and sensory nerves and leaving a possibility, although unlikely, that the lack of sensory innervation promoted some or all of the proliferation. Therefore, to produce a more selective SNS denervation, we developed the intra-WAT injection of 6OHDA, the catecholaminergic toxin, to selectively ablate only the sympathetic nerves that innervate WAT sparing the sensory nerves [241]. We also tested the seemingly unlikely contribution of the sensory innervation of WAT by killing the small diameter, unmyelinated (C) and some thin myelinated (A delta) fibers that innervate WAT [263] by the selective sensory neurotoxin, capsaicin [45;214]. To more accurately assess fat cell proliferation, these treatments were combined with systemic injection of the bromodeoxyuridine (5-bromo-2′-deoxyuridine; BrDU) to label dividing cells coupled with immunohistochemistry for AD3, using an antibody against this preadipocyte/adipocyte-specif membrane protein [308]. Therefore, doubly-labeled cells would indicate cells that they had proliferated and that were preadipocytes [90]. Fat cell proliferation (BrDU+AD3-ir cells) was remarkably increased by ~400% only 10 days after selective sympathetic chemically-denervated IWAT (intra-WAT 6OHDA injection; significantly decreased TH-ir, a marker of sympathetic nerves), but not by selective sensory chemical WAT denervation (intra-WAT capsaicin injection – toxin for small unmyelinated and some thin myelinated sensory nerves; significantly decreased calcitonin gene-related peptide (CGRP), a marker of sensory nerves [90]). Thus, the SNS denervation-induced increase in FCN appears to be bona fide, massive and rapid increase in fat cell proliferation.
Using the power of murine molecular genetics, transgenic mice that lack the neural transcription factor neurological stem cell leukemia (Nscl-2; a.k.a. Nhlh2) found in brain and peripheral nerves. [157], have impressive decreases in both the sympathetic and sensory innervation of EWAT and IWAT accompanied by an increase in small fat cells [245]. The small adipocytes of these Nscl-2 KO mice could be the result of SNS denervation-induced fat cell proliferation adding converging evidence to the role of the innervation of WAT, especially the sympathetic innervation in fat cell proliferation. The mechanisms underlying the NE inhibition of proliferation is currently under investigation in our laboratory. The phenomenon makes sense from an energetic perspective given that increasing adipocyte number under conditions where the SNS drive to WAT is increased to mobilize needed lipid fuels would seem counterproductive, whereas it might be advantageous to create additional lipid storage receptacles (fat cells) in times of food surfeit.
12 WAT has Sensory Innervation
12.1 Neuroanatomical Evidence for the Sensory Innervation of WAT
The presence of sensory nerve-associated peptides in neural fibers innervating WAT were the earliest suggestions of WAT sensory innervation seen as immunohistochemical labeling of the sensory nerve-associated peptide substance P [95] and later another sensory-nerve associated peptide CGRP [132] in laboratory rat WAT [107] and CGRP in Siberian hamster IWAT and EWAT [90;261;263]. Direct evidence (tract tracing) for WAT sensory innervation is seen in the labeling of the pseudounipolar neurons of the dorsal root ganglia (DRG) after application of the retrograde neural tract tracer True Blue into laboratory rat IWAT or DWAT [89]. Co-culturing the murine adipocyte cell line 3T3L1 cells with dispersed DRG cells reveal en passant neural connections by electron microscopy [153] reminiscent of the sympathetic nerve terminal type ‘connections’ to white adipocytes shown by electron microscopy noted above [267]. Collectively, these in vitro and in vivo data support the neuroanatomical reality of WAT sensory innervation, but are limited to the first order sensory neurons that project rostrally via unknown circuits ultimately providing sensory inflow to the brain from WAT.
We used viral transneuronal tract tracing with the H129 strain of herpes simplex virus-1 to test for the central sensory circuits from WAT [276] adapting the approach Rinaman and Schwartz [237] used to define the central sensory circuits from the stomach to the brain – an approach we also extended recently to define central sensory circuits from BAT [291]. This was possible because H129 travels only in an anterograde manner [320], whereas PRV travels strictly in a retrograde direction; thus, only sensory nerves would be labeled. H129 injected into IWAT of Siberian hamsters labeled the pseudounipolar sensory neurons in the DRG, then the anterior horn of the spinal cord and on to the hindbrain, midbrain (to the least extent) and forebrain, with no labeling of the nodose ganglia [PSNS equivalent of DRGs; [273]]. There was a striking similarity in the brain sites comprising the central sensory circuits from WAT to brain [273] compared with the SNS outflow from brain to WAT we previously found [8;272;275]. Indeed, we have recently discovered via dual injections of the sensory nerve-labeling H129 and the sympathetic nerve-labeling PRV into the same IWAT pad doubly-infected (labeled) neurons across the neuroaxis including a few in the DRGs (~1–3 neurons associated with each vertebrate at levels T11-L3) suggesting an ultra-short neural feedback loop from WAT between its sympathetic and sensory innervations (Ryu and Bartness, submitted).
It may seem somewhat remarkable that the sensory innervation is via spinal afferents [273] as these typically are associated with ascending spinothalamic and spinocerebellar circuits thought to relay pain and proprioceptive information, respectively [304]. As appears to be the case for most brain areas, the orinal discovery of function tends to become dogma thereby often leading to an oversimplification of the multiple functions of most brain sites as they interact in circuits by contrast to the rudimentary attribution of a single function per brain site. In the present case, there are sympathetic afferent soma residing within the DRGs of the thoracic and more rostral lumbar spinal levels [46]. These are considered non-nociceptive (i.e., non-pain) visceral sensory neurons involved in the reflexive control and/or homeostasis [46]. The possible functions of the WAT sensory nerves are addressed directly below.
12.2 Functional Evidence for Role of the Sensory Innervation of WAT
Although the histological evidence for the presence of sensory nerves in WAT has been known for several decades or more (see directly above), the function of these nerves is a topic of more recent investigations except for the first, relatively startling finding by one of the fathers of peripheral nerve electrophysiology, Akira Niijima [218]. Niijima, four years after the discover of the largely white adipocyte-derived cytokine, leptin [321], injected leptin intra-EWAT in laboratory rats resulting in increases in the afferent nerve multiunit electrophysiological activity emanating from that fat depot. We and others in the field turned a blind eye to these findings because the zeitgeist at this time (e.g., [147]) and persisting today (e.g., [105]) is that adipose tissue conveys information to the brain, perhaps to apprise it of the level of adiposity, but does so via the circulation. Subsequently, Niijima [219] demonstrated an apparent ‘adipose reflex arc’ whereby intra-EWAT leptin injection triggers an increase in sympathetic nerve multiunit electrophysiological activity to its contralateral WAT mate [218;219]. This was hypothesized to be a compensatory response to the apparent increase in adiposity that was experimentally signaled by the intra-WAT leptin injections resulting in increases in extracellular leptin that, in turn, increase SNS-induced lipolysis to counter the apparent increase in WAT lipid stores [218;219].
12.3 Sensory Innervation of White Adipose Tissue May Alter the SNS Drive to WAT by Monitoring Lipolysis/Lipolysis-Associated Factors, Leptin and Other Adipose Factors
These seminal studies by Niijima [218;219] involving the notion of an adipose reflex afferent-efferent arc to WAT has been termed the ‘adipose afferent reflex’ [AAR; [264]; for review see: [310]]. The ability of intra-leptin to be sensed by WAT afferents is bolstered by our finding that labeling the pseudounipolar neurons innervating WAT using Fluorogold (FG) combined with a newly developed, selective and characterized antibody for the long form of the leptin receptor (ObRb; chicken anti-rat Ob-Rb; Neuromics, Edina MN, [11;52;137;201] revealed FG-labeled DRG pseudounipolar neurons providing afferents from IWAT that possessed ObRbir [213]. Moreover, we replicated the ability of intra-WAT leptin to increase WAT afferent electrophysiological activity in Siberian hamsters and in a different WAT depot (IWAT; [213]). Thus, the spinal sensory neurons from WAT appear to respond to locally-released (or at least applied) leptin, perhaps with leptin serving as a paracrine factor using the spinal sensory nerve circuitry as a conduit to inform the brain of lipid stores in WAT and do so in a fat pad-specific manner [213].
The importance of transmitting fat pad-specific information to the brain rests on the accumulating data that ‘all WAT pads are not created equally’. That is, by contrast to thinking about adiposity as a general characteristic or even considering the dichotomy of subcutaneous versus visceral WAT, it may be more than coincidental that at least intra-abdominal WAT depots are located adjacent to non-adipose organs (e.g., EWAT and parametrial WAT located next to the gonads, perirenal WAT next to the kidneys) and thereby may serve specific functions beyond a local source of lipid energy. An excellent example of this notion is EWAT. EWAT pads appear to have a special role supporting male reproductive status because EWAT lipectomy (EWATx) inhibits spermatogenesis in frogs [49], laboratory rats [88;279] and mice [88] and we demonstrated and extended the phenomenon to Syrian hamsters [50]. Moreover, when the partially EWAT lipectomized pads regrow, spermatogenesis returns to near normal levels in laboratory rats [88;279] and mice [88]. This EWATx-induced aspermatogenesis is not due to disruption of the innervation of the testes, circulating testosterone, or luteinizing hormone concentrations as the production of testosterone and reproductive behavior is normal [50]. A larger lipid deficit produced by IWATx has no effect on spermatogenesis and the EWATx-induced aspermatogenesis is not rescued with transplant of the removed EWAT to a different location [50]. The mechanism for this effect remains elusive, but is unlikely to be due to a factor released from all lipid depots such as leptin or adiponectin because they or other adipokines would carry no fat pad-specific information. Thus, such a pad-specific effect suggests sensory spinal nerve participation [108;109]]. Indeed the increases in body lipid mass induced by the surgical lipid deficit produced by EWATx (e.g., [196–198]) occur without a lipid deficit if the sensory nerves coming from EWAT are selectively ablated by intra-EWAT injection of the sensory nerve toxin, capsaicin [261].
Returning to the ability of intra-WAT leptin administration to increase WAT sensory nerve activity (see above and [213;218;219]), in addition to the ability of intra-WAT leptin injection to stimulate WAT afferents and trigger increases in SNS drive to WAT, intra-EWAT leptin injection increases SNS drive to BAT, adrenal medulla, pancreas and liver [219]. Intraperirenal WAT leptin injection increases renal sympathetic nerve activity (RSNA), but without effecting possible circulating factors that can modify SNS activity such as leptin itself, but also not insulin, lactate or glucose [286]. Intra-RWAT and intra-IWAT capsaicin injection at low doses that are not toxic to sensory nerves, but stimulate the transient receptor potential cation channel subfamily V member 1-receptors or vanilloid receptor-1 [(TRPV-1Rs; initially cloned and described as a heat sensitive receptor [45]], trigger similar increases in RSNA [264;286]. Consistent with a hypothesized role of TRPV-1Rs in sensory feedback from WAT is that TRPV-1R deficient mice are lean [210] perhaps suggesting that, in the absence of sensory feedback from WAT under conditions of stimulated or perhaps basal lipolysis, there may be inappropriately exaggerated SNS drive resulting in abnormal increases in lipid mobilization until other feedback mechanisms are engaged [21]. Collectively, these data demonstrate that stimulation of sensory terminals in WAT transmits information to the CNS that, in turn, increases SNS drive to several peripheral tissues including WAT (for review see: [310]). Intra- WAT injection of substances other than leptin or capsaicin, such as bradykinin or adenosine increases RSNA as well as blood pressure, whereas intra-WAT ATP did not [264]. Furthermore, reports suggest that activation of the AAR may contribute to the hypertension associated with obesity [309].
As noted earlier, intra-WAT adenosine injection can stimulate the AAR ([264], and 8.2 Purinergic inhibition of the SNS control of lipolysis]) and serve as an inhibitory function for lipolysis after release from adipocytes [96;135;251]. Because adenosine activates afferent nerve fibers in one forearm and triggers increases in sympathetic drive via the peroneal nerve in the contralateral leg, an effect separate from its abilities to inhibit neuronal activity and cause vasodilation [60], these data infer a possible role of adenosine as one of the mediators of the AAR WAT.
One central site showing promise for involvement in the AAR is the PVH [264], an area showing substantial numbers of neurons that are part of the SNS outflow to WAT and that also receive sensory inflow from WAT (V. Ryu and T. Bartness, submitted). Injection of the neurotoxin kainic acid into the PVH blocks the ability of low dose capsaicin intra-IWAT injectn to trigger the AAR suggesting involvement of the PVH in this response [264]. If replicable, this is a remarkable finding in that the modern conceptualization of brain function for homeostatic and other functions is a distributed circuit model [112;118;119]. Indeed, conventional lesions of the PVH do not block basal or food deprivation-induced lipid mobilization from WAT in Siberian hamsters [91] suggesting other components of the SNS outflow to WAT are engaged when the PVH is eliminated.
It should not be forgotten that relatively ultra-short feedback SNS-sensory circuits could be in play when central sites are not engaged. For example spinal cord-severed patients have decentralized sympathetics and afferents below the level of the lesion, yet with stimulation of the bladder by abdominal pressure in these patients, there is a striking increase in sympathetic activity below, but not above the level of the lesion suggesting the engagement of a spinal reflex arc producing this response – a response that could affect lipolysis [148]. It should be noted, however, that the measure of SNS activity was NES which was discussed above (6 Preclinical and Clinical Measures of SNS Activity) with the associated caveats of the source of the NE and its reuptake by SNS terminals [66;85]. Nevertheless, we have identified SNS-sensory feedback neurons in several spinal regions innervating IWAT as indicated by the presence of H129+PRV colocalized neurons (V. Ryu and Bartness, submitted) that could account for this potential neural mediation of lipolysis in patients with decentralized peripheral nervous systems. Clearly forebrain areas are not necessary for WAT SNS-mediated lipolysis because we found that food-deprived, chronic decerebrate rats mobilize WAT lipid stores using their truncated neuroaxis [127].
Just as the sensory nerve bundle emanating from fingers contains fibers sensitive to touch pain, temperature and vibration, at present there is an unknown range of sensory functions for the afferents leaving WAT. As detailed above, WAT sensory nerves respond to leptin, adenosine, capsaicin and several other substances. We initially focused on the possibility that WAT spinal sensory nerves sense some aspect of lipolysis to transmit feedback on the degree/effectiveness of lipid mobilization to the brain, sparked by our findings in separate studies using viral transneuronal tract tracers showing the preponderance of CNS sites involved in the sympathetic outflow circuits to WAT [8] also are nodes in the sensory inflow circuits to the CNS [276]. The suggestion [21] that there were individual neurons within these sites that were part of the SNS outflow and sensory inflow from brain and WAT, respectively, was confirmed when we injected the sensory nerve-associated H129 and sympathetic-associated PRV into the same WAT depot (V. Ryu and T. J. Bartness, submitted). Thus, the neurochemical underpinnings of the notion of a SNS-sensory feedback loop were demonstrated, but the identity of what was actually being sensed was unknown. We previously demonstrated a rapid (within 5 min) and intense increase in sensory multiunit electrophysiological activity after glucoprivation created by systemic administration of 2DG [276], a stimulus that markedly increases sympathetic drive to WAT [35], suggesting the possibility that the afferent nerve activity may be sensing some aspect of lipolysis (see directly below).
There are a number of possible consequences of lipolysis that could be sensed by these WAT afferents, the obvious two being the products of lipolysis – glycerol and FFAs. Regarding the latter, in other tissues, sensory nerves are responsive to FAs; for example, the gastrointestinal extrinsic afferent nerves in sheep [61;62], laboratory rats [168] and cats [200]. Cat gut afferents have subpopulations of fibers activated by short chain FAs and by glycerol, whereas other afferents are activated by long chain FAs [200] implying that at least two distinct receptors for these lipolytic products exist in the gastrointestinal system of cats.
Although the identification, location and function of FA receptors is far from complete or well understood, progress has been made (for review see: [290]). FFAs come in different chain lengths and therefore it is not surprising that there are identified FFA receptors that respond to FFAs of different lengths including GPR40 (a.k.a. FFA1), GPR41, (FFA3), GPR43 (FFA2) GPR84, and GPR120, and FFA1, FFA2 and FFA3 [290]. Of these FFA1 is activated by FAs of 12 carbons or more (long chain FAs) and much less so by FAs of 7–12 carbons (medium chained FAs). GPR120 deficient mice and humans are obese [139], which might at first blush suggest altered sensory feedback from lipolysis, and that is possible, but has not been demonstrated. GPR120 deficient mice have decreases in energy expenditure that could be due to an inability to mobilize stored lipid normally, but they also have increases in lipogenesis in WAT and liver suggesting a contribution of increases in FA synthesis [139]. Clearly functional evidence for the role of this or other FA receptors in the afferent feedback with lipolysis are unknown and at this point is highly speculative.
Another possible SNS/NE lipolysis-associated potential factor that could reflect the degree of SNS activity and/or lipolysis is prostaglandin E2 (PGE2). Early in the study of the consequences of WAT sympathetic nerve stimulation, PGE2 was shown to be released in an in vitro sympathetic nerve-WAT preparation and when isolated adipocytes taken from food deprived laboratory rates are incubated with NE [256]. Although WAT does not possess PSNS innervation (see above), PGE2 and other prostanoids increase the firing of vagal afferent nerve fibers [268] and perhaps could do so for WAT spinal afferents potentially reflecting SNA and/or lipolysis.
Finally, as we suggested previously [21], substances released from sensory nerves could modulate the SNS drive to WAT. CGRP and its structurally-related counterpart adrenomedullin are present in sensory nerves [203], although the latter has not been identified to our knowledge in WAT sensory nerves. CGRP and adrenomedullin bind to the calcitonin receptor-like receptor (CLR) thereby modifying receptor activity-modifying protein-1 (RAMP1). All three substances are found in sensory nerves, but also in preadipocytes and adipocytes harvested from abdominal subcutaneous WAT [122]. Although the function of these substances is not precisely known it has been speculated [226] that at least for CGRP in BAT, CGRP inhibits NE-induced increases in BAT thermogenesis. Perhaps they might similarly do so for SNS/NE triggered lipolysis [21].
In summary, although we do not know what information is being conveyed to the brain via WAT spinal afferents, leptin, capsaicin, bradykinin, adenosine and perhaps PGE2, and the products of lipolysis (FAs and glycerol) activate or potentially may active WAT sensory nerve endings thus informing the brain via this neural conduit of aspects of lipid metabolism (e.g., lipolysis) and/or a corollary of lipid reserves and do so in a fat pad-specific manner. Thus, the more recent data on responses of WAT afferents (for review see: [310]) and the seminal data of Niijima [218;219] show the multi-modal afferent sensitivities of the spinal sensory nerves innervating WAT and demonstrate the relative infancy of our understanding of the function of WAT sensory nerves and their role in SNS outflow.
13. Conclusions and Perspectives
This review summarizes the current knowledge of the roles of the sympathetic and sensory innervation of WAT. We have attempted to be comprehensive and at the same time not reiterative of our previous reviews. For what we consider to be an interesting historical perspective on the sympathetic innervation of WAT going back more than 100 years, see our initial review on the topic from 1998 [14].
Clearly, investigations of the SNS innervation of WAT occupy the vast majority of the literature with little attention paid to its sensory innervation. We hope that our review of what is known of the neuroanatomy and function of the sensory innervation of WAT will stimulate further investigations by others. In addition, the clear interaction between the SNS and sensory innervation of WAT in the form of SNS-sensory ‘feedback loops’, as revealed through the use of transneuronal viral tract tracers, highlights the apparent importance of the sensory innervation for the control of the SNS innervation. As with all the studies of WAT sensory innervation, the question that is begged is ‘what is being sensed’? Indications of some aspect of SNS activity including SNS/NE stimulated-lipolysis and perhaps a signal of lipid reserves such as leptin head the factors that have begun to be explored.
Regarding the sympathetic innervation of WAT, it is irrefutable that activation of the SNS innervation of WAT is the principal initiator of stimulated lipolysis. In addition, the sympathetic innervation of WAT via NE has marked effects on adipocyte proliferation inhibiting the increase in FCN when the SNS innervation is activated and releasing a profound explosion of new adipocytes in its absence or likely with a turning down of sympathetic drive.
It is apparent that our understanding of both innervations of WAT has nearly an embryonic status, but for both, notions of the predominance of circulating factors controlling lipolysis (EPI) and informing the brain of lipid stores (leptin) belies the accumulating data, especially for the former, and should refocus at least some of the fields efforts towards the ‘wiring’ of WAT and for that matter BAT given it also has both SNS and sensory innervation as well as the apparent ‘SNS-sensory’ feedback loops (Ryu, Yang, and Bartness, in preparation).
HIGHLIGHTS.
WAT has SNS and sensory innervation
WAT SNS activation is the principal initiator of lipolysis
WAT SNS drive inhibits white adipocyte proliferation
WAT sympathetic drive is fat pad-specific
WAT sensory nerves are spinal and sensitive to leptin and sympathetic drive
Central WAT SNS-sensory feedback loops exist
Highlights.
Principal stimulator of lipolysis in mammals is the sympathetic nervous system (SNS)
White adipose tissue (WAT) has sensory innervation of spinal origin
Alterations in SNS drive affects adipocyte proliferation in addition to lipolysis
WAT does not appear to possess parasympathetic innervation
Insulin and adenosine inhibit SNS-induced lipolysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci. 2012;32:15913–15921. doi: 10.1523/JNEUROSCI.2591-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 4.Altuncu ME, Baspinar O, Keskin M. The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome. Cardiol J. 2012;19:501–506. doi: 10.5603/cj.2012.0091. [DOI] [PubMed] [Google Scholar]
- 5.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–482. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne B. Histochemical and biochemical aspects of cholinesterase activity of adipose tissue. Arch Int Pharmacodyn Ther. 1968;173:343–350. [PubMed] [Google Scholar]
- 8.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of th e sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 9.Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J Virol. 2003;77:10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkai A, Allweis C. Effect of electrical stimulation of the hypothalamus on plasma levels of free fatty acids and glucose in rats. Metabolism. 1972;21:921–927. doi: 10.1016/0026-0495(72)90026-1. [DOI] [PubMed] [Google Scholar]
- 11.Barnes MJ, Rogers RC, Van Meter MJ, Hermann GE. Co-localization of TRHR1 and LepRb receptors on neurons in the hindbrain of the rat. Brain Res. 2010;1355:70–85. doi: 10.1016/j.brainres.2010.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartness TJ. Short day-induced depletion of lipid stores is fat pad- and gender-specific in Siberian hamsters. Physiol Behav. 1995;58:539–550. doi: 10.1016/0031-9384(95)00082-t. [DOI] [PubMed] [Google Scholar]
- 13.Bartness TJ. Photoperiod, sex, gonadal steroids and housing density affect body fat in hamsters. Physiol Behav. 1996;60:517–529. doi: 10.1016/s0031-9384(96)80027-8. [DOI] [PubMed] [Google Scholar]
- 14.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: Implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 15.Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones and the sympathetic nervous system. Exp Biol Med. 2002;227:363–376. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- 16.Bartness TJ, Goldman BD. Mammalian pineal melatonin: A clock for all seasons. Experientia. 1989;45:939–945. doi: 10.1007/BF01953051. [DOI] [PubMed] [Google Scholar]
- 17.Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- 18.Bartness TJ, Hamilton JM, Wade GN, Goldman BD. Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol. 1989;257:R1533–R1540. doi: 10.1152/ajpregu.1989.257.6.R1533. [DOI] [PubMed] [Google Scholar]
- 19.Bartness TJ, Morley JE, Levine AS. Photoperiod-peptide interactions in the energy intake of Siberian hamsters. Peptides. 1986;7:1079–1085. doi: 10.1016/0196-9781(86)90137-3. [DOI] [PubMed] [Google Scholar]
- 20.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 21.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol Behav. 2007;91:343–351. doi: 10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartness TJ, Song CK. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Bartness TJ, Song CK, Shi H, Bowers RR, Foster MT. Brain-adipose tissue cross talk. Proc Nutr Soc. 2005;64:53–64. doi: 10.1079/pns2004409. [DOI] [PubMed] [Google Scholar]
- 25.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartness TJ, Wade GN. Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR, Fox EA, Neuhuber WL. Vagaries of adipose tissue innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1240–R1242. doi: 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- 28.Berthoud HR, Fox EA, Neuhuber WL. Controversial white adipose tissue innervation by the vagus nerve: Seeing is believing. Am J Physiol. 2007;293:R553–R554. [Google Scholar]
- 29.Beznak ABL, Hasch Z. The effect of sympathectomy on the fatty deposit in connective tissue. Quart J Exptl Physiol. 1937;27:1–15. [Google Scholar]
- 30.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 32.Bowers RR, Gettys TW, Prpic V, Harris RBS, Bartness TJ. Short photoperiod exposure increases adipocyte sensitivity to noradrenergic stimulation in Siberian hamsters. Am J Physiol. 2005;288:R1354–R1360. doi: 10.1152/ajpregu.00792.2004. [DOI] [PubMed] [Google Scholar]
- 33.Bray GA, Nishizawa Y. Ventromedial hypothalamus modulates fat mobilisation during fasting. Nature. 1978;274:900–901. doi: 10.1038/274900a0. [DOI] [PubMed] [Google Scholar]
- 34.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–53347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 35.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1445–R1452. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- 36.Brodie BB, Costa E, Dlabar A, Neff H, Smooker HH. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966;154:494–498. [PubMed] [Google Scholar]
- 37.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rosseti L, Buettner C. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bukowiecki L, Lupien J, Follea N, Paradis A, Richard D, LeBlanc J. Mechanism of enhanced lipolysis in adipose tissue of exercise-trained rats. Am J Physiol. 1980;239:E422–E429. doi: 10.1152/ajpendo.1980.239.6.E422. [DOI] [PubMed] [Google Scholar]
- 39.Burns TW, Langley PE, Robison GA. Site of free-fatty-acid inhibition of lipolysis by human adipocytes. Metabolism. 1975;24:265–276. doi: 10.1016/0026-0495(75)90108-0. [DOI] [PubMed] [Google Scholar]
- 40.B W. Cannon Bodily Changes in Pain, Hunger, Fear and Rage. Appleton; New York: 1915. [Google Scholar]
- 41.Cantu RC, Goodman HM. Effects of denervation and fasting on white adipose tissue. Am J Physiol. 1967;212:207–212. doi: 10.1152/ajplegacy.1967.212.1.207. [DOI] [PubMed] [Google Scholar]
- 42.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Carpene C, Bousquet-Melou A, Galitzky J, Berlan M, Lafontan M. Lipolytic effects of beta 1-, beta 2-, and beta 3-adrenergic agonists in white adipose tissue of mammals. Ann NY Acad Sci. 1998;839:186–189. doi: 10.1111/j.1749-6632.1998.tb10756.x. [DOI] [PubMed] [Google Scholar]
- 44.Carpene C, Galitzky J, Collon P, Esclapez F, Dauzats M, Lafontan M. Desensitization of beta-1 and beta-2, but not beta-3, adrenoceptor-mediated lipolytic responses of adipocytes after long-term norepinephrine infusion. J Pharmacol Exp Ther. 1993;265:237–247. [PubMed] [Google Scholar]
- 45.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 46.Cervero F, Foreman RD. Sensory Innervation of the Viscera. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 104–125. [Google Scholar]
- 47.Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, Balding D, Scott J, Kooner JS. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 49.Chieffi G, Rastogi RK, Iela L, Milone M. The function of fat bodies in relation to the hypothalamo-hypophyseal-gonadal axis in the frog, Rana esculenta. Cell Tissue Res. 1975;161:157–165. doi: 10.1007/BF00220364. [DOI] [PubMed] [Google Scholar]
- 50.Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology. 2010;151:5669–5779. doi: 10.1210/en.2010-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cinti S. The Adipose Organ. Editrice Kurtis; Milano: 1999. [Google Scholar]
- 52.Ciriello J, Moreau JM. Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat. Neuroscience. 2013;229:88–99. doi: 10.1016/j.neuroscience.2012.10.065. [DOI] [PubMed] [Google Scholar]
- 53.Clement G. La mobilisation des glycerides de reserve chez le rat. II. Influence due systeme nerveux sympathique. Etablissement d’un procede de mesure de I’intensite de la mobilisation. Arch Sci Physiol. 1950;4:13–29. [PubMed] [Google Scholar]
- 54.Collins S, Bordicchia M. Heart hormones fueling a fire in fat. Adipocyte. 2013;2:104–108. doi: 10.4161/adip.22515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18:2123–2131. doi: 10.1210/me.2004-0193. [DOI] [PubMed] [Google Scholar]
- 56.Collins S, Migliorini RH, Bartness TJ. Mechanisms controlling adipose tissue metabolism by the sympathetic nervous system: anatomical and molecular aspects. In: Sibley D, Hanin I, Kuhar M, Skolnick P, editors. Handbook of Contemporary Neuropharmacology. John Wiley; New York: 2007. pp. 785–814. [Google Scholar]
- 57.Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York: 1982. [Google Scholar]
- 58.Correll JW. Adipose tissue: ability to respond to nerve stimulation in vitro. Science. 1963;140:387–388. doi: 10.1126/science.140.3565.387. [DOI] [PubMed] [Google Scholar]
- 59.Correll JW. Central neural structures and pathways important for free fatty acid mobilization demonstrated in chronic animals. Fed Proc. 1963;22:547. [Google Scholar]
- 60.Costa F, Biaggioni I. Adenosine activates afferent fibers in the forearm, producing sympathetic stimulation in humans. J Pharmacol Exp Ther. 1993;267:1369–1374. [PubMed] [Google Scholar]
- 61.Cottrell DF, Iggo A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. J Physiol. 1984;354:497–522. doi: 10.1113/jphysiol.1984.sp015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cottrell DF, Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cousin B, Casteilla L, Lafontan M, Ambid L, Langin D, Berthault MF, Penicaud L. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology. 1993;133:2255–2262. doi: 10.1210/endo.133.5.8404678. [DOI] [PubMed] [Google Scholar]
- 64.Crandall DL, Fried SK, Francendese AA, Nickel M, DiGirolamo M. Lactate release from isolated rat adipocytes: influence of cell size, glucose concentration, insulin and epinephrine. Horm Metab Res. 1983;15:326–329. doi: 10.1055/s-2007-1018710. [DOI] [PubMed] [Google Scholar]
- 65.Curanovic D, Enquist L. Directional transneuronal spread of alpha-herpesvirus infection. Future Virol. 2009;4:591. doi: 10.2217/fvl.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2008 doi: 10.1016/j.neubiorev.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 68.de Bold AJ. Thirty years of research on atrial natriuretic factor: historical background and emerging concepts. Can J Physiol Pharmacol. 2011;89:527–531. doi: 10.1139/y11-019. [DOI] [PubMed] [Google Scholar]
- 69.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 70.de Glisezinski I, Larrouy D, Bajzova M, Koppo K, Polak J, Berlan M, Bolow J, Langin D, Marques MA, Crampes F, Lafontan M, Stich V. Epinephrine but not norepinephrine is a determinant of exercise-induced lipid mobilization in human subcutaneous adipose tissue. J Physio. 2009;1 doi: 10.1113/jphysiol.2009.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Demas GE, Bartness TJ. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol. 2001;281:R1499–R1505. doi: 10.1152/ajpregu.2001.281.5.R1499. [DOI] [PubMed] [Google Scholar]
- 72.Demas GE, Bowers RR, Bartness TJ, Gettys TW. Photoperiodic regulation of gene expression in brown and white adipose tissue of Siberian hamsters (Phodopus sungorus) Am J Physiol. 2002;282:R114–R121. doi: 10.1152/ajpregu.2002.282.1.R114. [DOI] [PubMed] [Google Scholar]
- 73.Diculescu I, Stoica M. Fluorescence histochemical investigation on the adrenergic innervation of the white adipose tissue in the rat. J Neurovisc Relat. 1970;32:25–36. doi: 10.1007/BF02324328. [DOI] [PubMed] [Google Scholar]
- 74.DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 1992;6:2405–2412. doi: 10.1096/fasebj.6.7.1563593. [DOI] [PubMed] [Google Scholar]
- 75.Dodt C, Lonnroth P, Fehm HL, Elam M. Intraneural stimulation elicits an increase in subcutaneous interstitial glycerol levels in humans. J Physiol. 1999;521(Pt 2):545–552. doi: 10.1111/j.1469-7793.1999.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dodt C, Lonnroth P, Fehm HL, Elam M. The subcutaneous lipolytic response to regional neural stimulation is reduced in obese women. Diabetes. 2000;49:1875–1879. doi: 10.2337/diabetes.49.11.1875. [DOI] [PubMed] [Google Scholar]
- 77.Dodt C, Lonnroth P, Wellhoner JP, Fehm HL, Elam M. Sympathetic control of white adipose tissue in lean and obese humans. Acta Physiol Scand. 2003;177:351–357. doi: 10.1046/j.1365-201X.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- 78.Dogiel AS. Die sensiblen Nervenendigungen im Herzen und in den Blutgefassen der Saugethiere. Arch mikr Anat. 1898;52:44–70. [Google Scholar]
- 79.Dole VP. Effect of nucleic acid metabolites on lipolysis in adipose tissue. J Biol Chem. 1961;236:3125–3130. [PubMed] [Google Scholar]
- 80.Dun NJ, Le Dun S, Chen CT, Hwang LL, Kwok EH, Chang JK. Orexins: a role in medullary sympathetic outflow. Regul Pept. 2000;96:65–70. doi: 10.1016/s0167-0115(00)00202-0. [DOI] [PubMed] [Google Scholar]
- 81.Edwards SL, Anderson CR, Southwell BR, McAllen RM. Distinct preganglioinic neurons innervate noradrenaline and adrenaline cells in the cat adrenal medulla. Neurosci. 1996;70:825–832. doi: 10.1016/s0306-4522(96)83019-3. [DOI] [PubMed] [Google Scholar]
- 82.Ekstrand MI, Enquist LW, Pomeranz LE. The alpha-herpesviruses: molecular pathfinders in nervous system circuits. Trends Mol Med. 2008;14:134–140. doi: 10.1016/j.molmed.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Enquist LW. Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: insights to pathogenesis and circuit tracers. J Infect Dis. 2002;186(Suppl 2):S209–S214. doi: 10.1086/344278. [DOI] [PubMed] [Google Scholar]
- 84.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 85.Esler MD, Hasking GJ, Willett IR, Leonard PW, Jennings GL. Noradrenaline release and sympathetic nervous system activity. J Hypertens. 1985;3:117–129. doi: 10.1097/00004872-198504000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Fain JN, Garcija-Sainz JA. Adrenergic regulation of adipocyte metabolism. J Lipid Res. 1983;24:945–966. [PubMed] [Google Scholar]
- 87.Fain JN, Pointer RH, Ward WF. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972;247:6866–6872. [PubMed] [Google Scholar]
- 88.Faust IM, Johnson PR, Hirsch J. Noncompensation of adipose mass in partially lipectomized mice and rats. Am J Physiol. 1976;231:538–544. doi: 10.1152/ajplegacy.1976.231.2.538. [DOI] [PubMed] [Google Scholar]
- 89.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;253:R942–R944. doi: 10.1152/ajpregu.1987.253.6.R942. [DOI] [PubMed] [Google Scholar]
- 90.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1630–R1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 91.Foster MT, Song CK, Bartness TJ. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 2010;18:682–689. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Francendese AA, DiGirolamo M. Alternative substrates for triacylglycerol synthesis in isolated adipocytes of different size from the rat. Biochem J. 1981;194:377–384. doi: 10.1042/bj1940377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fredholm BB. Release of adenosine-like material from isolated perfused dog adipose tissue following sympathetic nerve stimulation and its inhibition by adrenergic alphareceptor blockade. Acta Physiol Scand. 1976;96:122–130. doi: 10.1111/j.1748-1716.1976.tb10211.x. [DOI] [PubMed] [Google Scholar]
- 94.Fredholm BB. Adenosine and lipolysis. Int J Obes. 1981;5:643–649. [PubMed] [Google Scholar]
- 95.Fredholm BB. Nervous control of circulation and metabolism in white adipose tissue. In: Cryer A, Van RLR, editors. New Perspectives in Adipose Tissue: Structure, Function and Development. Butterworth; Boston: 1985. pp. 45–64. [Google Scholar]
- 96.Fredholm BB, Hjemdahl P. Uptake and release of adenosine in isolated rat fat cells. Acta Physiol Scand. 1979;105:257–267. doi: 10.1111/j.1748-1716.1979.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 97.Fredholm BB, Johansson S, Wang YQ. Adenosine and the regulation of metabolism and body temperature. Adv Pharmacol. 2011;61:77–94. doi: 10.1016/B978-0-12-385526-8.00003-5. [DOI] [PubMed] [Google Scholar]
- 98.Fredholm BB, Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol. 1981;313:351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freeman R, Saul JP, Roberts MS, Berger RD, Broadbridge C, Cohen RJ. Spectral analysis of heart rate in diabetic autonomic neuropathy. A comparison with standard tests of autonomic function. Arch Neurol. 1991;48:185–190. doi: 10.1001/archneur.1991.00530140079020. [DOI] [PubMed] [Google Scholar]
- 100.Froesch ER, Burgi H, Bally P, Labhart A. Insulin inhibition of spontaneous adipose tissue lipolysis and effects upon fructose and glucose metabolism. Mol Pharmacol. 1965;1:280–296. [PubMed] [Google Scholar]
- 101.Fruhbeck G, Aguado M, Martinez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun. 1997;240:590–594. doi: 10.1006/bbrc.1997.7716. [DOI] [PubMed] [Google Scholar]
- 102.Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial presure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 103.Galitzky J, Sengenes C, Thalamas C, Marques MA, Senard JM, Lafontan M, Berlan M. The lipid-mobilizing effect of atrial natriuretic peptide is unrelated to sympathetic nervous system activation or obesity in young men. J Lipid Res. 2001;42:536–544. [PubMed] [Google Scholar]
- 104.Garofalo MAR, Kettelhut IC, Roselino JES, Migliorini RH. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J Auton Nerv Syst. 1996;60:206–208. doi: 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- 105.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giordano A, Frontini A, Murano I, Tonello C, Marino MA, Carruba MO, Nisoli E, Cinti S. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53:679–687. doi: 10.1369/jhc.4A6566.2005. [DOI] [PubMed] [Google Scholar]
- 107.Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol. 1996;25:125–136. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 108.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 109.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. No sympathy for the claim of parasympathetic innervation of white adipose tissue. Am J Physiol. 2007;293:R550–R552. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 110.Girousse A, Langin D. Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obes (Lond) 2012;36:581–594. doi: 10.1038/ijo.2011.113. [DOI] [PubMed] [Google Scholar]
- 111.Gjedsted J, Gormsen LC, Nielsen S, Schmitz O, Djurhuus CB, Keiding S, Orskov H, Tonnesen E, Moller N. Effects of a 3-day fast on regional lipid and glucose metabolism in human skeletal muscle and adipose tissue. Acta Physiol (Oxf) 2007;191:205–216. doi: 10.1111/j.1748-1716.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 112.Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- 113.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- 114.Goldman BD. Pattern of melatonin secretion mediates transfer of photoperiod information from mother to fetus in mammals. Sci STKE. 2003;2003:E29. doi: 10.1126/stke.2003.192.pe29. [DOI] [PubMed] [Google Scholar]
- 115.Goodridge AG, Ball EG. Studies on the metabolism of adipose tissue. 18. In vitro effects of insulin, epinephrine and glucagon on lipolysis and glycolysis in pigeon adipose tissue. Comp Biochem Physiol. 1965;16:367–381. doi: 10.1016/0010-406x(65)90303-8. [DOI] [PubMed] [Google Scholar]
- 116.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gravholt CH, Moller N, Jensen MD, Christiansen JS, Schmitz O. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J Clin Endocrinol Metab. 2001;86:2085–2089. doi: 10.1210/jcem.86.5.7460. [DOI] [PubMed] [Google Scholar]
- 118.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 119.Grill HJ, Kaplan j, Kaplan JM. Caudal brainstem participates in the distributed neural control of feeding. In: Stricker EM, editor. Neurobiology of Food and Fluid Intake. Plenum Press; New York: 1990. pp. 125–149. [Google Scholar]
- 120.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 121.Guidry G, Landis SC. Absence of cholinergic sympathetic innervation from limb muscle vasculature in rats and mice. Auton Neurosci. 2000;82:97–108. doi: 10.1016/S0165-1838(00)00094-1. [DOI] [PubMed] [Google Scholar]
- 122.Gupta P, Harte AL, da Silva NF, Khan H, Barnett AH, Kumar S, Sturdee DW, McTernan PG. Expression of calcitonin gene-related peptide, adrenomedullin, and receptor modifying proteins in human adipose tissue and alteration in their expression with menopause status. Menopause. 2007;14:1031–1038. doi: 10.1097/gme.0b013e31803c56b6. [DOI] [PubMed] [Google Scholar]
- 123.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 124.Hall JE, Brands MW, Hildebrandt DA, Kuo J, Fitzgerald S. Role of sympathetic nervous system and neuropeptides in obesity hypertension. Braz J Med Biol Res. 2000;33:605–618. doi: 10.1590/s0100-879x2000000600001. [DOI] [PubMed] [Google Scholar]
- 125.Hall WG, Swithers-Mulvey SE, Agrawal CM, Burka NR, Horner J, Menacherry S. Analysis of 2-DG autoradiograms using image-averaging and image-differencing procedures for systems-level description of neurobehavioral function. Physiol Behav. 1991;50:109–119. doi: 10.1016/0031-9384(91)90506-j. [DOI] [PubMed] [Google Scholar]
- 126.Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring) 2012;20:1355–1364. doi: 10.1038/oby.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology. 2006;147:1365–1376. doi: 10.1210/en.2005-1156. [DOI] [PubMed] [Google Scholar]
- 128.Haupt A, Thamer C, Heni M, Tschritter O, Machann J, Schick F, Machicao F, Haring HU, Staiger H, Fritsche A. Impact of Variation Near MC4R on Whole-body Fat Distribution, Liver Fat, and Weight Loss. Obesity (Silver Spring) 2009;17:1942–1945. doi: 10.1038/oby.2009.233. [DOI] [PubMed] [Google Scholar]
- 129.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 130.HERTTING G, Axelrod J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature. 1961;192:172–173. doi: 10.1038/192172a0. [DOI] [PubMed] [Google Scholar]
- 131.Hiebert SM, Green SA, Yellon SM. Daily timed melatonin feedings mimic effects of short days on testis regression and cortisol in circulation in Siberian hamsters. Gen Comp Endocrinol. 2006;146:211–216. doi: 10.1016/j.ygcen.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 132.Hill B, Ralevic V, Crowe R, Burnstock G. Innervation and nitric oxide modulation of mesenteric arteries of the Golden hamster. Eur J Pharm. 1996;317:275–283. doi: 10.1016/s0014-2999(96)00727-3. [DOI] [PubMed] [Google Scholar]
- 133.Hillarp NA, Hokfelt B. Evidence of adrenaline and noradrenaline in separate adrenal medullary cells. Acta Physiol Scand. 1953;30:55–68. doi: 10.1111/j.1748-1716.1954.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 134.Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968;9:110–119. [PubMed] [Google Scholar]
- 135.Hjemdahl P, Sollevi A. Antilipolytic effect of adenosine in isolated perifused fat cells. Acta Physiol Scand. 1978;103:270–274. doi: 10.1111/j.1748-1716.1978.tb06214.x. [DOI] [PubMed] [Google Scholar]
- 136.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. BiochemSoc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 137.Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab. 2009;297:E505–E513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- 139.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Korner A, Kiess W, Pigeyre M, Caiazzo R, Van HW, Van GL, Horber F, Balkau B, Levy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 140.Intaglietta M, Rosell S. Capillary permeability and sympathetic activity in canine subcutaneous adipose tissue. Nature. 1974;249:481–482. doi: 10.1038/249481a0. [DOI] [PubMed] [Google Scholar]
- 141.Iriki M, Simon E. Differential control of efferent sympathetic activity revisited. J Physiol Sci. 2012;62:275–298. doi: 10.1007/s12576-012-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Izumi H. Reflex parasympathetic vasodilatation in facial skin. Gen Pharmacol. 1995;26:237–244. doi: 10.1016/0306-3623(94)00155-g. [DOI] [PubMed] [Google Scholar]
- 143.Izumi H. Nervous control of blood flow in the orofacial region. Pharmacol Ther. 1999;81:141–161. doi: 10.1016/s0163-7258(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 144.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 145.Johansson SM, Lindgren E, Yang JN, Herling AW, Fredholm BB. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol. 2008;597:92–101. doi: 10.1016/j.ejphar.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 146.Jones DD, Ramsay TG, Hausman GJ, Martin RJ. Norepinephrine inhibits rat pre-adipocyte proliferation. Int J Obesity. 1992;16:349–354. [PubMed] [Google Scholar]
- 147.Kaiyala K, Woods SC, Schwartz MW. New model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. 1995;62:1123S–1134S. doi: 10.1093/ajcn/62.5.1123S. [DOI] [PubMed] [Google Scholar]
- 148.Karlsson AK, Friberg P, Lonnroth P, Sullivan L, Elam M. Regional sympathetic function in high spinal cord injury during mental stress and autonomic dysreflexia. Brain. 1998;121(Pt 9):1711–1719. doi: 10.1093/brain/121.9.1711. [DOI] [PubMed] [Google Scholar]
- 149.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- 151.Kimura R, Takahashi N, Murota K, Yamada Y, Niiya S, Kanzaki N, Murakami Y, Moriyama T, Goto T, Kawada T. Activation of peroxisome proliferator-activated receptor-alpha (PPARalpha) suppresses postprandial lipidemia through fatty acid oxidation in enterocytes. Biochem Biophys Res Commun. 2011;410:1–6. doi: 10.1016/j.bbrc.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 152.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 153.Kosacka J, Nowicki M, Kacza J, Borlak J, Engele J, Spanel-Borowski K. Adipocyte-derived angiopoietin-1 supports neurite outgrowth and synaptogenesis of sensory neurons. J Neurosci Res. 2006;83:1160–1169. doi: 10.1002/jnr.20811. [DOI] [PubMed] [Google Scholar]
- 154.Kreier F, Buijs RM. Evidence for parasympathetic innervation of white adipose tissue, clearing up some vagaries. Am J Physiol. 2007;293:R548–R549. doi: 10.1152/ajpregu.00890.2006. [DOI] [PubMed] [Google Scholar]
- 155.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat -functional implications. J Clin Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kreier F, Kap YS, Mettenleiter TC, van HC, van d, V, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 157.Kruger M, Braun T. The neuronal basic helix-loop-helix transcription factor NSCL-1 is dispensable for normal neuronal development. Mol Cell Biol. 2002;22:792–800. doi: 10.1128/MCB.22.3.792-800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumon A, Takahashi A, Hara T, Shimazu T. Mechanism of lipolysis induced by electrical stimulation of the hypothalamus in the rabbit. J Lipid Res. 1976;17:551–558. [PubMed] [Google Scholar]
- 159.Kuo YT, Lin TH, Chen WL, Lee HM. Alpha-lipoic acid induces adipose triglyceride lipase expression and decreases intracellular lipid accumulation in HepG2 cells. Eur J Pharmacol. 2012;692:10–18. doi: 10.1016/j.ejphar.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 160.Lafontan M. Differential recruitment and differential regulation by physiological amines of fat cell β-1, β-2 and β-3 adrenergic receptors expressed in native fat cells and in transfected cell lines. Cell Signal. 1994;6:363–392. doi: 10.1016/0898-6568(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 161.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057–1091. [PubMed] [Google Scholar]
- 162.Lafontan M, Berlan M. Fat cell α2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocrine Rev. 1995;16:716–738. doi: 10.1210/edrv-16-6-716. [DOI] [PubMed] [Google Scholar]
- 163.Lafontan M, Berlan M, Carpene C. Fat cell adrenoceptors: inter- and intraspecific differences and hormone regulation. Int J Obesity. 1985;9(Suppl. 1):117–127. [PubMed] [Google Scholar]
- 164.Lafontan M, Berlan M, Stich V, Crampes F, Riviere D, de G, I, Sengenes C, Galitzky J. Recent data on the regulation of lipolysis by catecholamines and natriuretic peptides. Ann Endocrinol (Paris) 2002;63:86–90. [PubMed] [Google Scholar]
- 165.Lafontan M, Bousquet-Melou A, Galitzky J, Barbe P, Carpene C, Langin D, Valet P, Castan I, Bouloumie A, Saulnier-Blache JS. Adrenergic receptors and fat cells: differential recruitment by physiological amines and homologous regulation. Obesity Res. 1995;3:507S–514S. doi: 10.1002/j.1550-8528.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 166.Lafontan M, Dang-Tran L, Berlan M. Alpha-adrenergic antilipolytic effect of adrenaline in human fat cells of the thigh: comparison with adrenaline responsiveness of different fat deposits. Eur J Clin Invest. 1979;9:261–266. doi: 10.1111/j.1365-2362.1979.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 167.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009 doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 169.Landin K, Lonnroth P, Krotkiewski M, Holm G, Smith U. Increased insulin resistance and fat cell lipolysis in obese but not lean women with a high waist/hip ratio. Eur J Clin Invest. 1990;20:530–535. doi: 10.1111/j.1365-2362.1990.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 170.Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26:497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Landsberg L, Axelrod J. Reduced accumulation of 3H-norepinephrine in the rat heart following hypophysectomy. Endocrinology. 1968;82:175–178. doi: 10.1210/endo-82-1-175. [DOI] [PubMed] [Google Scholar]
- 172.Landsberg L, Young JB. Diet-induced changes in sympathetic nervous system activity. In: Beers RF, Bassett EG, editors. Nutritional Factors: Modulating Effects on Metabolic Processes. Raven Press; New York: 1981. pp. 155–174. [Google Scholar]
- 173.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 174.Langin D, Portillo MP, Saulnier-Blache JS, Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharm. 1991;199:291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- 175.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 176.Lazzarini SJ, Wade GN. Role of sympathetic nerves in effects of estradiol on rat white adipose tissue. Am J Physiol. 1991;260:R47–R51. doi: 10.1152/ajpregu.1991.260.1.R47. [DOI] [PubMed] [Google Scholar]
- 177.Leboeuf B, Flinn RB, Cahill GF., Jr Effect of epinephrine on glucose uptake and glycerol release by adipose tissue in vitro. Proc Soc Exp Biol Med. 1959;120:527–529. doi: 10.3181/00379727-102-25306. [DOI] [PubMed] [Google Scholar]
- 178.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296:E1230–E1238. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lefebvre P, Luyckx A, Bacq ZM. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Horm Metab Res. 1973;5:245–250. doi: 10.1055/s-0028-1093959. [DOI] [PubMed] [Google Scholar]
- 180.Lefebvre PJ. Glucagon and adipose tissue. Biochem Pharmacol. 1975;24:1261–1266. doi: 10.1016/0006-2952(75)90333-0. [DOI] [PubMed] [Google Scholar]
- 181.Lefebvre PJ, Luyckx AS. Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia. 1969;5:195–197. doi: 10.1007/BF01213680. [DOI] [PubMed] [Google Scholar]
- 182.Leitner C, Bartness TJ. Distributed forebrain sites mediate melatonin-induced short-day responses in Siberian hamsters. Endocrinology. 2010;151:3133–3140. doi: 10.1210/en.2010-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Leitner C, Bartness TJ. An intact dorsomedial hypothalamic nucleus, but not the subzona incerta or reuniens nucleus, is necessary for short-day melatonin signal-induced responses in Siberian hamsters. Neuroendocrinology. 2011;93:29–39. doi: 10.1159/000320474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liang HX, Belardinelli L, Ozeck MJ, Shryock JC. Tonic activity of the rat adipocyte A1-adenosine receptor. Br J Pharmacol. 2002;135:1457–1466. doi: 10.1038/sj.bjp.0704586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Linde B. Effect of sympathetic nerve stimulation on net transvascular movement of fluid in canine adipose tissue. Acta Physiol Scand. 1976;97:166–174. doi: 10.1111/j.1748-1716.1976.tb10249.x. [DOI] [PubMed] [Google Scholar]
- 186.Linde B, Chisolm G, Rosell S. The influence of sympathetic activity and histamine on the blood-tssue exchange of solutes in canine adipose tissue. Acta Physiol Scand. 1974;92:145–155. doi: 10.1111/j.1748-1716.1974.tb05731.x. [DOI] [PubMed] [Google Scholar]
- 187.Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 188.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf F, I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O’Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CI, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lundberg JM, Terenius L, Hokfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- 190.Luyckx AS, Dresse A, Cession-Fossion A, Lefebvre PJ. Catecholamines and exercise-induced glucagon and fatty acid mobilization in the rat. Am J Physiol. 1975;229:376–383. doi: 10.1152/ajplegacy.1975.229.2.376. [DOI] [PubMed] [Google Scholar]
- 191.Maekawa K, Sudoh T, Furusawa M, Minamino N, Kangawa K, Ohkubo H, Nakanishi S, Matsuo H. Cloning and sequence analysis of cDNA encoding a precursor for porcine brain natriuretic peptide. Biochem Biophys Res Commun. 1988;157:410–416. doi: 10.1016/s0006-291x(88)80062-7. [DOI] [PubMed] [Google Scholar]
- 192.Mansfeld G, Muller F. Der Einfluss der Nervensystem auf die Mobilisierung von Fett. Arch Physiol. 1913;152:61–67. [Google Scholar]
- 193.Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mauer MM, Bartness TJ. Body fat regulation following partial lipectomy in Siberian hamsters is photoperiod-dependent and fat pad-specific. Am J Physiol. 1994;266:R870–R878. doi: 10.1152/ajpregu.1994.266.3.R870. [DOI] [PubMed] [Google Scholar]
- 196.Mauer MM, Bartness TJ. Photoperiod-dependent fat pad mass and cellularity changes following partial lipectomy in Siberian hamsters. Am J Physiol. 1996;270:R383–R392. doi: 10.1152/ajpregu.1996.270.2.R383. [DOI] [PubMed] [Google Scholar]
- 197.Mauer MM, Bartness TJ. Short day-like body mass changes do not prevent fat pad compensation after lipectomy in Siberian hamsters. Am J Physiol. 1997;272:R68–R77. doi: 10.1152/ajpregu.1997.272.1.R68. [DOI] [PubMed] [Google Scholar]
- 198.Mauer MM, Bartness TJ. Temporal changes in fat pad mass following lipectomy in Siberian hamsters. Physiol Behav. 1997;62:1029–1036. doi: 10.1016/s0031-9384(97)00233-3. [DOI] [PubMed] [Google Scholar]
- 199.Mauriege P, DePergola G, Berlan M, Lafontan M. Human fat cell beta-adrenergic receptors: Beta-agonist-dependent lipolytic responses and characterization of beta-adrenergic binding sites on human fat cell membranes with highly selective beta1-antagonists. J Lipid Res. 1988;29:587–601. [PubMed] [Google Scholar]
- 200.Melone J. Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst. 1986;17:231–241. doi: 10.1016/0165-1838(86)90060-3. [DOI] [PubMed] [Google Scholar]
- 201.Messenger SA, Moreau JM, Ciriello J. Intermittent hypoxia and systemic leptin administration induces pSTAT3 and Fos/Fra-1 in the carotid body. Brain Res. 2012;1446:56–70. doi: 10.1016/j.brainres.2012.01.074. [DOI] [PubMed] [Google Scholar]
- 202.Migliorini RH, Garofalo MAR, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol. 1997;272:R656–R661. doi: 10.1152/ajpregu.1997.272.2.R656. [DOI] [PubMed] [Google Scholar]
- 203.Miriel VA, Chen Y, Rivers RJ. The involvement of CGRP, adrenomedullin, and sensory nerves in remote vasomotor responses within the hamster cheek pouch microcirculation. Microvasc Res. 2009;77:192–197. doi: 10.1016/j.mvr.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 204.Miyoshi H, Perfield JW, Obin MS, Greenberg AS. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J Cell Biochem. 2008;105:1430–1436. doi: 10.1002/jcb.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miyoshi H, Perfield JW, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 206.Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, Iellamo F. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev. 2009;33:71–80. doi: 10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 207.Moro C, Crampes F, Sengenes C, de G, I, Galitzky J, Thalamas C, Lafontan M, Berlan M. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J. 2004;18:908–910. doi: 10.1096/fj.03-1086fje. [DOI] [PubMed] [Google Scholar]
- 208.Moro C, Galitzky J, Sengenes C, Crampes F, Lafontan M, Berlan M. Functional and pharmacological characterization of the natriuretic peptide-dependent lipolytic pathway in human fat cells. J Pharmacol Exp Ther. 2004;308:984–992. doi: 10.1124/jpet.103.060913. [DOI] [PubMed] [Google Scholar]
- 209.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–R698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 210.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mul JD, O’Duibhir E, Shrestha YB, Koppen A, Vargovic P, Toonen PW, Zarebidaki E, Kvetnansky R, Kalkhoven E, Cuppen E, Bartness TJ. Pmch-deficiency in rats is associated with normal adipocyte differentiation and lower sympathetic adipose drive. PLoS ONE. 2013;8:e60214. doi: 10.1371/journal.pone.0060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mulder H, Sorhede-Winzell M, Contreras JA, Fex M, Strom K, Ploug T, Galbo H, Arner P, Lundberg C, Sundler F, Ahren B, Holm C. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. J Biol Chem. 2003;278:36380–36388. doi: 10.1074/jbc.M213032200. [DOI] [PubMed] [Google Scholar]
- 213.Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304:E1338–E1347. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nakagawa H, Hiura A. Capsaicin, transient receptor potential (TRP) protein subfamilies and the particular relationship between capsaicin receptors and small primary sensory neurons. Anat Sci Int. 2006;81:135–155. doi: 10.1111/j.1447-073X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 215.Nautiyal KM, Dailey MJ, Brito NA, Brito MN, Harris RBS, Bartness TJ, Grill HJ. Energetic responses to cold temperatures in rats lacking forebrain-caudal brainstem connections. Am J Physiol. 2008;295:R789–R798. doi: 10.1152/ajpregu.90394.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ng TB, Wong CM. Effects of pineal indoles and arginine vasotocin on lipolysis and lipogenesis in isolated adipocytes. J Pineal Res. 1986;3:55–66. doi: 10.1111/j.1600-079x.1986.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 217.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central Sympathetic Innervations to Visceral and Subcutaneous White Adipose Tissue. Am J Physiol Regul Integr Comp Physiol. 2014 doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst. 1998;73:19–25. doi: 10.1016/s0165-1838(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 219.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett. 1999;262:125–128. doi: 10.1016/s0304-3940(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 220.Nishizawa Y, Bray GA. Ventromedial hypothalamic lesions and the mobilization of fatty acids. J Clin Invest. 1978;61:714–721. doi: 10.1172/JCI108984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oberg B, Rosell S. Sympathetic control of consecutive vascular sections in canine subcutaneous adipose tissue. Acta Physiol Scand. 1967;71:47–56. doi: 10.1111/j.1748-1716.1967.tb03708.x. [DOI] [PubMed] [Google Scholar]
- 223.Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci U S A. 2013;110:E697–E706. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.OPIE LH, WALFISH PG. Plasma free fatty acid concentrations in obesity. N Engl J Med. 1963;268:757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- 225.Oro L, Wallenberg LA, Rosell S. Circulatory and metabolical processes in adipose tissue. Nature. 1965;205:178–179. doi: 10.1038/205178a0. [DOI] [PubMed] [Google Scholar]
- 226.Osaka T, Kobayashi A, Namba Y, Ezaki O, Inoue S, Kimura S, Lee TH. Temperature- and capsaicin-sensitive nerve fibers in brown adipose tissue attenuate thermogenesis in the rat. Pflugers Arch. 1998;437:36–42. doi: 10.1007/s004240050743. [DOI] [PubMed] [Google Scholar]
- 227.Pecquery R, Leneveu MC, Giudicelli Y. Characterization of the beta-adrenergic receptors of hamster white fat cells: Evidence against an important role for the alpha2-receptor subtype in the adrenergic control of lipolysis. Biochim Biophys Acta. 1983;731:397–405. doi: 10.1016/0005-2736(83)90034-2. [DOI] [PubMed] [Google Scholar]
- 228.Penicaud L. The neural feedback loop between the brain and adipose tissues. Endocr Dev. 2010;19:84–92. doi: 10.1159/000316900. [DOI] [PubMed] [Google Scholar]
- 229.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–R1621. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- 230.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 231.Ponard JG, Kondratyev AA, Kucera JP. Mechanisms of intrinsic beating variability in cardiac cell cultures and model pacemaker networks. Biophys J. 2007;92:3734–3752. doi: 10.1529/biophysj.106.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Possenti R, Muccioli G, Petrocchi P, Cero C, Cabassi A, Vulchanova L, Riedl MS, Manieri M, Frontini A, Giordano A, Cinti S, Govoni P, Graiani G, Quaini F, Ghe C, Bresciani E, Bulgarelli I, Torsello A, Locatelli V, Sanghez V, Larsen BD, Petersen JS, Palanza P, Parmigiani S, Moles A, Levi A, Bartolomucci A. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J. 2012;441:511–522. doi: 10.1042/BJ20111165. [DOI] [PubMed] [Google Scholar]
- 233.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood) 2010;235:1185–1193. doi: 10.1258/ebm.2010.010063. [DOI] [PubMed] [Google Scholar]
- 234.Prigge WF, Grande F. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. Comp Biochem Physiol. 1971;39B:69–82. doi: 10.1016/0305-0491(71)90254-9. [DOI] [PubMed] [Google Scholar]
- 235.Rebuffe-Scrive M. Neuroregulation of adipose tissue: molecular and hormonal mechanisms. Int J Obesity. 1991;15:83–86. [PubMed] [Google Scholar]
- 236.Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest. 1994;93:2590–2599. doi: 10.1172/JCI117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rinaman L, Schwartz GJ. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Roca AL, Godson C, Weaver DR, Reppert SM. Structure, characterization, and expression of the gene encoding the mouse Mel1a melatonin receptor. Endocrinology. 1996;137:3469–3477. doi: 10.1210/endo.137.8.8754776. [DOI] [PubMed] [Google Scholar]
- 239.Rochon L, Bukowiecki LJ. Alterations in adipocyte response to lipolytic hormones during cold acclimation. Am J Physiol. 1990;258:C835–C840. doi: 10.1152/ajpcell.1990.258.5.C835. [DOI] [PubMed] [Google Scholar]
- 240.Rodbell M. Modulation of lipolysis in adipose tissue by fatty acid concentration in fat cell. Ann N Y Acad Sci. 1965;131:302–314. doi: 10.1111/j.1749-6632.1965.tb34798.x. [DOI] [PubMed] [Google Scholar]
- 241.Rooks CR, Penn DM, Kelso E, Bowers RR, Bartness TJ, Harris RB. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol. 2005;289:R92–102. doi: 10.1152/ajpregu.00858.2004. [DOI] [PubMed] [Google Scholar]
- 242.Rosak C, Hittelman KJ. Characterization of lipolytic responses of isolated white adipocytes from hamsters. Biochim Biophys Acta. 1977;496:458–474. doi: 10.1016/0304-4165(77)90328-2. [DOI] [PubMed] [Google Scholar]
- 243.Rosell S. Release of free fatty acids from subcutaneous adipose tissue in dogs following sympathetic nerve stimulation. Acta Physiol Scand. 1966;67:343–351. doi: 10.1111/j.1748-1716.1966.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 244.Rosell S. Neuronal control of microvessels. Ann Rev Physiol. 1980;42:359–371. doi: 10.1146/annurev.ph.42.030180.002043. [DOI] [PubMed] [Google Scholar]
- 245.Ruschke K, Ebelt H, Kloting N, Boettger T, Raum K, Bluher M, Braun T. Defective peripheral nerve development is linked to abnormal architecture and metabolic activity of adipose tissue in Nscl-2 mutant mice. PLoS ONE. 2009;4:e5516. doi: 10.1371/journal.pone.0005516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ruskell GL. Facial parasympathetic innervation of the choroidal blood vessels in monkeys. Exp Eye Res. 1971;12:166–172. doi: 10.1016/0014-4835(71)90085-6. [DOI] [PubMed] [Google Scholar]
- 247.Ryden M, Jocken J, van H, V, Dicker A, Hoffstedt J, Wiren M, Blomqvist L, Mairal A, Langin D, Blaak E, Arner P. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am J Physiol Endocrinol Metab. 2007;292:E1847–E1855. doi: 10.1152/ajpendo.00040.2007. [DOI] [PubMed] [Google Scholar]
- 248.Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- 249.Scherer T, Buettner C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev Endocr Metab Disord. 2011;12:235–243. doi: 10.1007/s11154-011-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Scherer T, O’hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schwabe U, Ebert R, Erbler HC. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3′,5′-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276:133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- 252.Schwabe U, Schonhofer PS, Ebert R. Facilitation by adenosine of the action of insulin on the accumulation of adenosine 3′:5′-monophosphate, lipolysis, and glucose oxidation in isolated fat cells. Eur J Biochem. 1974;46:537–545. doi: 10.1111/j.1432-1033.1974.tb03647.x. [DOI] [PubMed] [Google Scholar]
- 253.Sengenes C, Berlan M, de G, I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 254.Sengenes C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumie A, Lafontan M, Galitzky J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R257–R265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 255.Sergeeva I, Christoffels VM. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 256.Shaw JE, Ramwell PW. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J Biol Chem. 1968;243:1498–1503. [PubMed] [Google Scholar]
- 257.Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett. 2007 doi: 10.1016/j.neulet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 258.Shen J, Tanida M, Yao JF, Niijima A, Nagai K. Biphasic effects of orexin-A on autonomic nerve activity and lipolysis. Neurosci Lett. 2008;444:166–171. doi: 10.1016/j.neulet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 259.Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50:2306–2313. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull. 2001;54:375–385. doi: 10.1016/s0361-9230(00)00455-x. [DOI] [PubMed] [Google Scholar]
- 261.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol. 2005;289:R514–R520. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 262.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav. 2004;81:535–543. doi: 10.1016/j.physbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 263.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol. 2005;288:R1028–R1037. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 264.Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol. 2012;112:1008–1014. doi: 10.1152/japplphysiol.01164.2011. [DOI] [PubMed] [Google Scholar]
- 265.Shimizu Y, Nikami H, Tsukazaki K, Machado UF, Yano H, Seino Y, Saito M. Increased expression of glucose transporter GLUT-4 in brown adipose tissue of fasted rats after cold exposure. Am J Physiol. 1993;264:E890–E895. doi: 10.1152/ajpendo.1993.264.6.E890. [DOI] [PubMed] [Google Scholar]
- 266.Shrestha YB, Vaughan CH, Smith BJ, Jr, Song CK, Baro DJ, Bartness TJ. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–R149. doi: 10.1152/ajpregu.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Slavin BG, Ballard KW. Morphological studies on the adrenergic innervation of white adipose tissue. Anat Rec. 1978;191:377–389. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- 268.Smith JA, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br J Pharmacol. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sollevi A, Hjemdahl P, Fredholm BB. Endogenous adenosine inhibits lipolysis induced by nerve stimulation without inhibiting noradrenaline release in canine subcutaneous adipose tissue in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:112–119. doi: 10.1007/BF00505303. [DOI] [PubMed] [Google Scholar]
- 270.Song CK, Bartness TJ. The effects of anterior hypothalamic lesions on short-day responses in Siberian hamsters given timed melatonin infusions. J Biol Rhythms. 1996;11:14–26. doi: 10.1177/074873049601100102. [DOI] [PubMed] [Google Scholar]
- 271.Song CK, Bartness TJ. Dorsocaudal SCN microknife-cuts do not block short day responses in Siberian hamsters given melatonin infusions. Brain Res Bull. 1998;45:239–246. doi: 10.1016/s0361-9230(97)00234-7. [DOI] [PubMed] [Google Scholar]
- 272.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol. 2001;281:R666–R672. doi: 10.1152/ajpregu.2001.281.2.R666. [DOI] [PubMed] [Google Scholar]
- 273.Song CK, Bartness TJ. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Central projections of the sensory nerves innervating brown adipose tissue. Online. [Google Scholar]
- 274.Song CK, Enquist LW, Bartness TJ. New developments in tracing neural circuits with herpesviruses. Virus Res. 2005;111:235–249. doi: 10.1016/j.virusres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 275.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 276.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R501–R511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, Hogan MF, Singh PK, Goebel N, Vera L, Miller N, Cui J, Jones MR, Chen YD, Taylor KD, Hsueh WA, Rotter JI, Montminy M. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468:933–939. doi: 10.1038/nature09564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Spector S, Sjoerdsma A, Udenfriend S. Blockade of endogenous norepinephrine synthis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther. 1965;147:86–95. [PubMed] [Google Scholar]
- 279.Srinivasan V, Thombre DP, Lakshmanan S, Chakrabarty AS. Effect of removal of epididymal fat on spermatogenesis in albino rats. Indian J Exp Biol. 1986;24:487–488. [PubMed] [Google Scholar]
- 280.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–1991. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 281.Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- 282.Takahashi A, Ikarashi Y, Ishimaru H, Maruyama Y. Compensation between sympathetic nerves and adrenal medullary activity: effects of adrenomedullation and chemical sympathectomy on catecholamine turnover. Life Sci. 1993;53:1567–1572. doi: 10.1016/0024-3205(93)90565-k. [DOI] [PubMed] [Google Scholar]
- 283.Takahashi A, Shimazu T. Hypothalamic regulation of lipid metabolism in the rat: effect of hypothalamic stimulation on lipolysis. J Auton Nerv Syst. 1981;4:195–205. doi: 10.1016/0165-1838(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 284.Takahashi A, Shimazu T, Maruyama Y. Importance of sympathetic nerves for the stimulatory effect of cold exposure on glucose utilization in brown adipose tissue. Jpn J Physiol. 1992;42:653–664. doi: 10.2170/jjphysiol.42.653. [DOI] [PubMed] [Google Scholar]
- 285.Tan CO, Cohen MA, Eckberg DL, Taylor JA. Fractal properties of human heart period variability: physiological and methodological implications. J Physiol. 2009;587:3929–3941. doi: 10.1113/jphysiol.2009.169219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tanida M, Iwashita S, Ootsuka Y, Terui N, Suzuki M. Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci Lett. 2000;293:107–110. doi: 10.1016/s0304-3940(00)01490-7. [DOI] [PubMed] [Google Scholar]
- 287.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Teixeira VL, Antunes-Rodrigues J, Migliorini RH. Evidence for centers in the central nervous system that selectively regulate fat mobilization in the rat. J Lipid Res. 1973;14:672–677. [PubMed] [Google Scholar]
- 289.Turpin BP, Duckworth WC, Solomon SS. Perifusion of isolated rat adipose cells. Modulation of lipolysis by adenosine. J Clin Invest. 1977;60:442–448. doi: 10.1172/JCI108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 2012;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Vaughan CH, Bartness TJ. Anterograde Transneuronal Viral Tract Tracing Reveals Central Sensory Circuits From Brown Fat and Sensory Denervation Alters Its Thermogenic Responses. Am J Physiol. 2012;302:R1049–R1058. doi: 10.1152/ajpregu.00640.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods in Enzymology. 2013 doi: 10.1016/B978-0-12-411619-1.00011-2. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Vaughan M, Steinberg D. Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. J Lipid Res. 1963;4:193–199. [PubMed] [Google Scholar]
- 294.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 295.Vollmer RR, Baruchin A, Kolibal-Pegher SS, Corey SP, Stricker EM, Kaplan BB. Selective activation of norepinephrine- and epinephrine-secreting chromaffin cells in rat adrenal medulla. Am J Physiol. 1992;263:R716–R721. doi: 10.1152/ajpregu.1992.263.3.R716. [DOI] [PubMed] [Google Scholar]
- 296.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight and body composition in Siberian hamsters. Am J Physiol. 1984;246:R26–R30. doi: 10.1152/ajpregu.1984.246.1.R26. [DOI] [PubMed] [Google Scholar]
- 297.Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–126. doi: 10.1016/j.ymgme.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 298.Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp zool Soc Lond. 1987;57:167–187. [Google Scholar]
- 299.Weiss B, Maickel RP. Sympathetic nervous control of adipose tissue lipolysis. Int J Neuropharmacol. 1965;7:393–403. doi: 10.1016/0028-3908(68)90023-3. [DOI] [PubMed] [Google Scholar]
- 300.Wellhoner P, Welzel M, Rolle D, Dodt C. In vivo effects of corticotropin-releasing hormone on femoral adipose tissue metabolism in women. Int J Obes (Lond) 2006 doi: 10.1038/sj.ijo.0803463. [DOI] [PubMed] [Google Scholar]
- 301.Wertheimer E. Stoffwechselregulationen. I. Regulation des Fettstoffwechsels. Die zentrale Regulierung der Fettmobilisierung. Pflugers Arch ges Physiol. 1926;213:262–298. [Google Scholar]
- 302.WHITBY LG, Axelrod J, WEIL-MALHERBE H. The fate of H3-norepinephrine in animals. J Pharmacol Exp Ther. 1961;132:193–201. [PubMed] [Google Scholar]
- 303.White JE, Engel FL. A lipolytic action of epinephrine and norepinephrine on rat adipose tissue in vitro. Proc Soc Exp Biol Med. 1958;99:375–378. doi: 10.3181/00379727-99-24355. [DOI] [PubMed] [Google Scholar]
- 304.Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. Plenum Press; New York: 1991. [Google Scholar]
- 305.Wirsen C. Adrenergic innervation of adipose tissue examined by fluorescence microscopy. Nature. 1964;202:913. doi: 10.1038/202913a0. [DOI] [PubMed] [Google Scholar]
- 306.Wirsen C. Studies in lipid mobilization with special reference to morphological and histochemical aspects. Acta Physiol Scand. 1965;65:1–46. [PubMed] [Google Scholar]
- 307.Wool IB, Goldstein MS, Ramey ER, Levine B. Role of epinephrine in the physiology of fat mobilization. Am J Physiol. 1954;178:427–432. doi: 10.1152/ajplegacy.1954.178.3.427. [DOI] [PubMed] [Google Scholar]
- 308.Wright JT, Hausman GJ. Monoclonal antibodies against cell surface antigens expressed during porcine adipocyte differentiation. Int J Obesity. 1990;14:395–409. [PubMed] [Google Scholar]
- 309.Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension. 2012;60:1280–1286. doi: 10.1161/HYPERTENSIONAHA.112.198002. [DOI] [PubMed] [Google Scholar]
- 310.Xiong XQ, Chen WW, Zhu GQ. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiologica. 2013 doi: 10.1111/apha.12182. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- 311.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 312.Young JB, Burgi-Saville ME, Burgi U, Landsberg L. Sympathetic nervous system activity in rat thyroid: potential role in goitrogenesis. Am J Physiol Endocrinol Metab. 2005;288:E861–E867. doi: 10.1152/ajpendo.00292.2004. [DOI] [PubMed] [Google Scholar]
- 313.Young JB, Landsberg L. Suppression of the sympathetic nervous system during fasting. Science. 1977;196:1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- 314.Young JB, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in pancreas and liver. Am J Physiol. 1979;236:E524–E533. doi: 10.1152/ajpendo.1979.236.5.E524. [DOI] [PubMed] [Google Scholar]
- 315.Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247:E35–E40. doi: 10.1152/ajpendo.1984.247.1.E35. [DOI] [PubMed] [Google Scholar]
- 316.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in the Siberian hamster. Am J Physiol. 1995;268:R744–R751. doi: 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- 317.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol. 1998;275:R1488–R1493. doi: 10.1152/ajpregu.1998.275.5.R1488. [DOI] [PubMed] [Google Scholar]
- 318.Yu J, Yu HC, Kim KA, Kwon KB, Park JW, Kim SZ, Kim SH, Park BH. Differences in the amount of lipolysis induced by atrial natriuretic peptide in small and large adipocytes. J Pept Sci. 2008;14:972–977. doi: 10.1002/psc.1035. [DOI] [PubMed] [Google Scholar]
- 319.Zahorska-Markiewicz B, Kuagowska E, Kucio C, Klin M. Heart rate variability in obesity. Int J Obes Relat Metab Disord. 1993;17:21–23. [PubMed] [Google Scholar]
- 320.Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci U S A. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 322.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]


