Abstract
Background and Purpose
The Secondary Prevention of Small Subcortical Stroke trial (SPS3) recruited participants meeting clinical and radiologic criteria for symptomatic lacunes. Individuals randomized to dual antiplatelet therapy with clopidogrel and aspirin had an unanticipated increase in all-cause mortality compared with those assigned aspirin. We investigated the factors associated with mortality in this well characterized population.
Methods
We identified independent predictors of mortality among baseline demographic and clinical factors by Cox regression analysis in participants of the SPS3 trial. Separately, we examined the effect on mortality of non-fatal bleeding during the trial.
Results
During a mean follow-up of 3.6 years, the mortality rate was 1.78%/yr for the 3020 participants, mean age 63 years. Significant independent predictors of mortality at study entry were age, diabetes, history of hypertension, systolic blood pressure (HR = 1.3 per 20 mmHg increase), serum hemoglobin < 13 g/dl (HR = 1.6), renal function (HR = 1.3 per eGFR decrease of 20 mL/min), and body mass index (HR = 1.8 per 10kg/m2 decrease). Participants with ischemic heart disease (p=0.01 for interaction) and normotensive/pre-hypertensive participants (p=0.03 for interaction) were at increased risk if assigned to dual antiplatelet therapy. Non-fatal major hemorrhage increased mortality in both treatment arms (HR 4.5, 95% CI 3.1, 6.6, p <0.001).
Conclusions
Unexpected interactions between assigned antiplatelet therapy and each of ischemic heart disease and normal/pre-hypertensive status accounted for increased mortality among patients with recent lacunar stroke given dual antiplatelet therapy. Despite extensive exploratory analyses, the mechanisms underlying these interactions are uncertain.
Keywords: lacunar stroke, mortality, antiplatelet therapy, clinical trials
Background
Lacunar infarcts, or small subcortical strokes, represent 25% of all ischemic strokes, are associated with cognitive and functional impairment, and are frequently discovered on brain imaging without a prior clinical presentation (i.e. covert infarcts). (1-4) Hypertension is associated, and the pathology is often in the small penetrating arteries of the brain (5) (6). Little is known about the predictors of mortality in those with lacunar infarcts. The Secondary Prevention of Small Subcortical Strokes (SPS3) trial tested two levels of blood pressure control and dual antiplatelet therapy (aspirin plus clopidogrel) vs. monotherapy (aspirin plus placebo) in patients with a recent symptomatic lacunar stroke verified by MRI. All-cause mortality was increased in those assigned dual antiplatelet therapy with higher mortality for vascular and nonvascular causes of death. (7) A meta-analysis of trials of dual antiplatelet therapy vs. aspirin monotherapy did not show a similar association suggesting that the SPS3 results were unique. (8) While fatal hemorrhage was more common in the dual antiplatelet arm (9 cases vs. 4), the number of fatal bleeds was insufficient to explain the difference in mortality. (7) We sought to determine the predictors of mortality in this population and hypothesized that the increased mortality might be the result of higher rates of non-fatal hemorrhage in the dual antiplatelet therapy group.
Methods
The rationale, design, participant characteristics, and main results of the SPS3 trial have been reported elsewhere. (7, 9-11) In brief, SPS3 was a randomized, multicenter clinical trial conducted in 81 clinical centers in North America, Latin America, and Spain. Patients >30 years with a recent (<180 days) symptomatic lacunar stroke who were without surgically-amenable ipsilateral carotid artery disease (hemispheral infarcts) or major-risk cardioembolic sources (all infarcts) were eligible and randomized simultaneously in a 2-by-2 factorial design, either to single or dual antiplatelet therapy (aspirin + placebo vs. aspirin + clopidogrel, double-blind) and to one of two target levels of systolic blood pressure control (130-149 mmHg vs. <130 mmHg, open-label). Participants with a clinical lacunar syndrome were required to meet MRI criteria. (7) MRI eligibility was determined by local investigators, with images submitted for central interpretation by a neuroradiologist. Disabling strokes (modified Rankin Scale ≥4) were excluded.
Prior lacunar stroke or TIA was identified only when a clinical episode antecedent to the qualifying event consistent with a classic subcortical ischemic stroke syndrome was documented and was not based solely on neuroimaging findings. Severity of hypertension was determined from the systolic blood pressure at entry adjusted by the number of antihypertensive medications. Specifically, the average of two screening systolic blood pressure measurements (3 measurements each visit) taken at least one week apart, were adjusted by adding 5 mmHg for each antihypertensive medication (up to a maximum of 4) at the time of the measurement. Normotensive was defined as <120 mmHg, pre-hypertensive as 120-<140 mmHg, stage I hypertension as 140-<160 mmHg, and stage II hypertension as ≥160 mmHg. For these analyses, we combined normotensives and pre-hypertensives into one group. Participants with a history of myocardial infarction, angina pectoris as well as those who had undergone coronary artery bypass, coronary angioplasty or stenting were identified as having ischemic heart disease (IHD). Estimated glomerular filtration rate (eGFR) was computed using the Chronic Kidney Disease Epidemiology Collaboration equation. (12)
Hemorrhage during the trial was defined as major if serious or life-threatening if it met any of the following criteria: urgent hospitalization or emergency medical treatment, transfusion of red blood cells (excluding that associated with coronary artery bypass graft, open-heart, or elective surgery), residual functional sequelae, or surgical therapy for bleeding.
The antiplatelet arm of SPS3 was stopped on recommendation of the data and safety monitoring committee after a planned interim analysis when 2/3 of the primary events had occurred due to futility and evidence of harm (increased mortality in patients assigned dual antiplatelet therapy). For this analysis, we considered deaths that occurred until the time that the antiplatelet trial was terminated; 16 additional deaths that occurred during continuation of the blood pressure trial were not considered. One additional death was discovered after reporting of the main results. (7) The cause of death was adjudicated by a central events committee blinded to treatment assignment and classified as vascular cerebral, vascular non-cerebral, probable vascular, nonvascular, or uncertain. Disagreements were resolved by consensus.
All analyses followed the intention to treat principle. Patient demographic and clinical characteristics were summarized by treatment group using proportions for categorical variable and means and standard deviations (SD) for continuous. The distributions of the continuous variables were assessed for normality and found to be approximately normally distributed with the exception of the body mass index (BMI). BMI was further classified as: < 18.5 kg/m2 (underweight), 18.5–24.9 (normal), 25–29.9 (overweight), and ≥30 (obese). Crude (univariate) hazard ratios (HR) and the 95% confidence intervals (CI) for each of the patient demographic and clinical characteristics were computed separately by assigned antiplatelet treatment groups. To determine independent predictors of mortality, variables univariately associated with increased risk of death (p < 0.05) in one or both treatment groups along with assigned antiplatelet treatment group were then considered in a multivariable Cox proportional hazards regression model. Forward stepwise regression was used to identify independent predictors, and interaction terms between assigned antiplatelet treatment and patient characteristic were investigated if the main effect term for the patient characteristic was statistically significant. HR estimates and 95% CIs were reported for variables independently predictive of mortality. Mortality and other event rates were computed by dividing the total number of deaths (or other event) by the total number of patient years of exposure for each assigned treatment group. Confidence intervals for event rates were computed assuming a Poisson distribution. The time to major hemorrhage was calculated as the time to the first one in the case of multiple hemorrhages and analyzed as a time dependent variable. Exposure for patients without a major hemorrhage was censored at the time of termination of study participation or at death. Statistical significance was accepted at the 0.05 level, and all tests were two-sided. Analyses were done using SPSS 20.0 (Armonk, NY).
Results
Among the 3,020 participants with recent lacunar stroke, the mean age was 63 years and the frequencies of hypertension, diabetes and ischemic heart disease were 80%, 37% and 10% respectively. During 10,758 years of follow-up for the antiplatelet trial (mean 3.6 years, range 0-8.2 years), there were 191 deaths (annualized rate 1.78%). All-cause mortality was increased in the group assigned dual antiplatelet therapy compared with those assigned aspirin monotherapy (113 deaths vs. 78, HR 1.5, 95% CI 1.1 to 2.0). Mortality did not vary by assigned blood pressure target at the time the antiplatelet trial was terminated. Major bleeding was increased in the dual antiplatelet therapy arm but was not affected by blood pressure target assignment. (Table 1)
Table 1.
Mortality and major bleeding by assigned interventions. The 3020 participants were randomized in a 2 × 2 factorial design to aspirin and higher systolic blood pressure target, n = 755; aspirin and lower target, n = 748; clopidogrel + aspirin and higher target, n = 764, and clopidogrel + aspirin and lower target, n = 753.
| Higher systolic blood pressure target | Lower systolic blood pressure target | Overall | ||
|---|---|---|---|---|
| Death, n | Aspirin | 37 | 41 | 78 |
| Clopidogrel + aspirin | 51 | 62 | 113* | |
| Overall | 88 | 103^ | ||
| Major Bleed, n | Aspirin | 26 | 30 | 56 |
| Clopidogrel + aspirin | 50 | 55 | 105 | |
| Overall | 76 | 85 |
Hazard ratio 1.5 for clopidogrel plus aspirin vs. aspirin monotherapy; 95% CI, 1.1 to 2.0, p = 0.006.
Hazard ratio 1.1 for lower vs. higher blood pressure target, 95% CI 0.86 to 1.5, p = 0.4.
Factors at study entry associated with mortality
Patient demographic and clinical characteristics examined for an association with mortality are shown in Table 2. Participants in either assigned antiplatelet group who were older (an average of 7 years), had lower body mass index, were diabetic, a history of hypertension or reduced renal function had increased risk of death. Those assigned to dual antiplatelet therapy with a history of ischemic heart disease, lower diastolic blood pressure, less severe hypertension, aspirin use at the time of the qualifying event, and lower hemoglobin were also at higher risk.
Table 2.
Baseline features associated with all cause death by assigned antiplatelet
| Prevalence, % or mean (sd) | Crude Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| aspirin + clopidogrel | aspirin + placebo | aspirin + clopidogrel | aspirin + placebo | |
| Age, years | 63 (11) | 63 (11) | 1.8 (1.5, 2.1)* per 10 year increase | 1.8 (1.4, 2.2)* per 10 year increase |
| Male | 62 | 64 | 1.1 (0.73, 1.6) | 1.2 (0.75, 2.0) |
| Race/ethnic group | ||||
| Non-Hispanic White | 51 | 51 | reference group | reference group |
| Hispanic | 30 | 30 | 1.0 (0.66, 1.6) | 0.60 (0.33, 1.1) |
| Black | 16 | 16 | 0.79 (0.46, 1.3) | 0.64 (0.34, 1.2) |
| Other/multiple | 3 | 2 | 0.81 (0.25, 2.6) | 0.86 (0.21, 3.5) |
| Region | ||||
| United States or Canada | 65 | 65 | reference group | reference group |
| Latin America | 23 | 23 | 1.5 (0.94, 2.3) | 0.70 (0.36, 1.4) |
| Spain | 12 | 12 | 1.4 (0.74, 2.7) | 0.90 (0.38, 2.1) |
| Body mass index, kg/m2 | 29 (6) | 29 (6) | 2.1 (1.5, 3.1)* per 10 unit increase | 1.7 (1.1, 2.6)* per 10 unit increase |
| Body mass index, kg/m2 | * | * | ||
| < 25# | 24 | 23 | reference group | reference group |
| 25-29.9 | 41 | 41 | 0.66 (0.44, 1.0) | 0.60 (0.36, 1.0) |
| ≥ 30 | 35 | 36 | 0.44 (0.27, 0.71) | 0.41 (0.23, 0.72) |
| Current tobacco smoker | 20 | 21 | 0.75 (0.45, 1.2) | 1.4 (0.85, 2.4) |
| Alcohol use (≥7 drinks/week) | 12 | 13 | 0.70 (0.37, 1.3) | 1.2 (0.64, 2.3) |
| History of hypertension | 76 | 74 | 1.7 (1.0, 2.8)* | 2.7 (1.3, 5.7)* |
| Screening blood pressure, mmHg | ||||
| Systolic | 143 (19) | 143 (19) | 1.1 (0.98, 1.2) per 10 unit increase | 1.2 (1.1, 1.3)* per 10 unit increase |
| Diastolic | 78 (11) | 78 (11) | 1.3 (1.1, 1.6)* per 10 unit decrease | 1.1 (0.86, 1.3) per 10 unit decrease |
| Severity of hypertension^ | * | |||
| normotensive | 2 | 2 | reference group | reference group |
| pre-hypertensive | 18 | 18 | ||
| stage I | 40 | 42 | 0.55 (0.33, 0.91) | 1.3 (0.67, 2.5) |
| stage II | 39 | 38 | 1.2 (0.77, 1.9) | 1.8 (0.93, 3.3) |
| Diabetes | 35 | 38 | 2.0 (1.3, 2.8)* | 1.6 (1.0, 2.5)* |
| Ischemic heart disease | 10 | 11 | 2.9 (1.9, 4.4)* | 1.2 (0.65, 2.3) |
| Prior symptomatic lacunar stroke or transient ischemic attack | 15 | 15 | 1.3 (0.82, 2.1) | 1.9 (1.1, 3.3)* |
| Aspirin use at time of qualifying event | 30 | 29 | 1.5 (1.1, 2.2)* | 0.93 (0.57, 1.5) |
| Statins at time of randomization | 68 | 69 | 0.91 (0.62, 1.3) | 0.69 (0.44, 1.1) |
| Hemoglobin (g/dL) | 14 (2) | 14 (2) | 0.75 (0.67, 0.84)* per 1 unit decrease | 0.92 (0.80, 1.1) per 1 unit decrease |
| Hemoglobin < 13 g/dL | 34 | 33 | 2.4 (1.7, 3.5)* | 1.6 (1.0, 2.4)* |
| HbA1c (%) (n = 987 of 1105 diabetics) | 8.2 (2) | 8.4 (3) | 1.0 (0.92, 1.2) per 1 unit increase | 1.0 (0.86, 1.2) per 1 unit increase |
| eGFR (ml/min/1.73m2) | 81 (20) | 82 (20) | 1.2 (1.1, 1.4)* per 10 unit decrease | 1.4 (1.2, 1.5)* per 10 unit decrease |
| LDL-c (mg/dL) | 113 (40) | 112 (39) | 1.0 (0.98, 1.1) per 10 unit decrease | 1.1 (1.0, 1.1) per 10 unit decrease |
| Assigned intensive blood pressure control | 50 | 50 | 1.1 (0.82, 1.7) | 1.1 (0.70, 1.7) |
Indicates a significant increase in mortality risk (p < 0.05)
13 patients had body mass index < 18.5 kg/m2
See Methods section for definition of severity of hypertension.
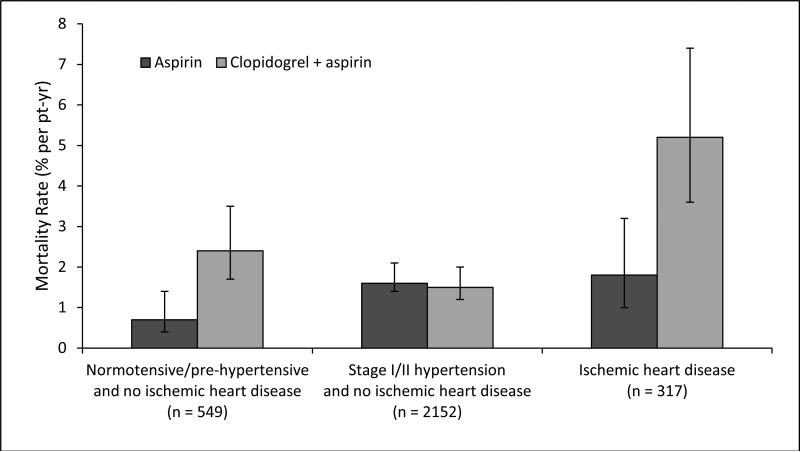
By multivariable analysis, independent predictors at study entry of death were increasing age, lower body mass index, history of hypertension, higher systolic blood pressure, diabetes, hemoglobin <13g/dL, and lower eGFR with no statistically significant interaction between any of these factors and antiplatelet therapy assignment. (Table 3) However, patients assigned to dual antiplatelet therapy with ischemic heart disease (p=0.01 for interaction), and normotensive/prehypertensive patients (p=0.03 for interaction) were at increased risk of death. (Table 3, Figure 1) For the 71% of participants who were stage I or stage II hypertensive but without ischemic heart disease, the mortality rates were equal between antiplatelet arms. There was no significant imbalance in these factors between the antiplatelet assignments. Estimates of HRs were not appreciably changed by the exclusion of history of hypertension as a variable.
Table 3. Multivariable predictors of all cause death - final model.
Variables considered for MV model were those in Table 1 with p ≤ 0.05 for one or both treatment groups. If main effect term was significant, then an interaction term with assigned AP was investigated.
| Hazard Ratio (95% CI) | Statistical significance | |
|---|---|---|
| Age, per 10 y increase | 1.6 (1.3, 1.8) | < 0.001 |
| Body mass index per 10 kg/m2 decrease | 1.8 (1.4, 2.4) | < 0.001 |
| History of hypertension | 1.7 (1.1, 2.7) | 0.02 |
| Systolic blood pressure per 20 mmHg increase | 1.3 (1.1, 1.5) | 0.003 |
| Diabetes | 2.0 (1.5, 2.7) | < 0.001 |
| Hemoglobin < 13 g/dL | 1.6 (1.2, 2.1) | 0.001 |
| eGFR per 20 mL/min/1.73m2 decrease | 1.3 (1.1, 1.5) | 0.02 |
| Normotensive/pre-hypertensive | * | |
| assigned mono antiplatelet therapy | 1.0 (0.56, 2.0) | |
| assigned dual antiplatelet therapy | 2.5 (1.5, 4.0) | |
| Ischemic heart disease | ** | |
| assigned mono antiplatelet therapy | 0.99 (0.53, 1.8) | |
| assigned dual antiplatelet therapy | 2.7 (1.8, 4.1) | |
p = 0.03 for interaction
p = 0.01 for interaction
Abbreviations: eGFR = estimated glomerular filtration rate
Figure 1.
Mortality rates with bars for 95% confidence intervals by hypertension and ischemic heart disease status and assigned antiplatelet group.
Nonfatal hemorrhage and mortality
We hypothesized that nonfatal major hemorrhage would be associated with death and that assignment to dual antiplatelet therapy would be associated with a higher rate of nonfatal major hemorrhage. A major hemorrhage during the study period was independently predictive of mortality (HR 4.5, 95% CI 3.1, 6.6, p <0.001) when added to the model with patient characteristics at study entry (Table 3). No important effect on hazard ratio estimates in that model was observed, and the increased risk of death with major hemorrhage was not increased for patients assigned dual antiplatelet therapy (p = 0.7 for interaction). Of the 161 patients who had a major hemorrhage, the first was fatal for 13 patients (9 assigned dual antiplatelet therapy). Those assigned dual vs. monotherapy had a higher rate of non-fatal major hemorrhage (n=96, 1.9% /pt-yr vs. n=52, 1.0%, p < 0.001). Regardless of assigned antiplatelet treatment, the mortality rate was higher for patients with a non-fatal major hemorrhage compared to those with no major hemorrhage (dual: 4.2%/pt-yr vs. 1.8%, p=0.002; mono: 3.1% vs. 1.3%, p=0.03). (Table 4)
Table 4.
Relationship of severity of first bleeding event during trial and death according to antiplatelet assignment
| Aspirin | Clopidogrel + aspirin | |||
|---|---|---|---|---|
| # Deaths / n | Rate (% per pt-yr) | # Deaths / n | Rate (% per pt-yr) | |
| Fatal hemorrhage | 4/4 | 9/9 | ||
| Non-fatal major hemorrhage | 8/52 | 3.1 | 18/96 | 4.2 |
| No major hemorrhage | 66/1447 | 1.3 | 86/1412 | 1.8 |
Discussion
The observation of increased mortality in individuals with lacunar stroke assigned dual antiplatelet therapy was unexpected
We undertook data-driven analyses to assess the differential mortality observed with dual antiplatelet therapy in SPS3. Participants with a history of ischemic heart disease had a significantly higher mortality rate when assigned to dual antiplatelet therapy compared to those assigned to aspirin, despite evidence that dual antiplatelet therapy prevents myocardial infarction better than aspirin in populations with vascular disease. (8) Those assigned dual antiplatelet therapy were at increased risk of death if they were normotensive/pre-hypertensive and had no history of ischemic heart disease. While this may be a chance association, the definition of lacunar stroke in SPS3 was rigorous, requiring MRI confirmation, and may have selected for a unique mixture of vascular pathology with an unexpected response to the trial interventions.
Bleeding was associated with mortality in the SPS3 cohort. There were more fatal and non-fatal major bleeds in the dual treatment arm and major nonfatal bleeds correlated with mortality. The association between non-fatal major bleeding and mortality is plausible as it may lead to physician discontinuation of medications and/or nonadherence by patients. (13)The proportion of patients with non-fatal major bleeding in SPS3 was 6.3% in the dual antiplatelet group and 3.5% in the monotherapy group over a mean follow up of 43 months. These rates are similar to those observed in the CHARISMA trial which compared dual therapy to aspirin in individuals at high vascular risk. (14)
We lacked detailed data on exposure time for non-assigned treatments in participants who withdrew permanently from assigned antiplatelet therapy and cannot exclude antiplatelet non-adherence as an explanation of the excess mortality associated with nonfatal major hemorrhage. Non-adherence is unlikely though to be the complete explanation for the difference in mortality as the increase in vascular mortality in those assigned dual antiplatelet therapy was insufficient to explain the mortality difference between antiplatelet arms. (Table 5)This likely reflects an overlap of factors which affect vascular and non-vascular mortality. In addition, the assignment of cause of death by record review is inherently imprecise and a limitation of our analysis.
Table 5.
Cause of death by treatment assignment
| Number of deaths | Hazard ratio (95%CI) | ||
|---|---|---|---|
| Aspirin | Clopidogrel + aspirin + | ||
| All participants | |||
| All deaths | 78 | 113 | 1.5 (1.1, 2.0) |
| Vascular* | 25 | 45 | 1.9 (1.1, 3.0) |
| Non-vascular | 31 | 39 | |
| Uncertain cause | 22 | 29 | |
| Ischemic heart disease ± stage I/ II hypertension at entry | |||
| All deaths | 11 | 30 | 2.9 (1.4, 5.8) |
| Vascular* | 4 | 14 | 3.8 (1.2, 12) |
| Non-vascular | 4 | 9 | |
| Uncertain cause | 3 | 7 | |
| Normotensive/pre-hypertensive and no ischemic heart disease at entry | |||
| All deaths | 8 | 28 | 3.3 (1.5, 7.3) |
| Vascular* | 0 | 11 | >60 (0.51,> 1000) |
| Non-vascular | 6 | 9 | |
| Uncertain cause | 2 | 8 | |
| Stage I/II hypertension and no ischemic heart disease at entry | |||
| All deaths | 59 | 55 | 0.98 (0.68, 1.4) |
| Vascular* | 21 | 20 | 1.0 (0.54, 1.8) |
| Non-vascular | 21 | 21 | |
| Uncertain cause | 17 | 14 | |
Includes definite and probable vascular deaths (defined in supplementary appendix)
In general populations, obese individuals experience significantly higher all-cause mortality when compared to normal weight individuals while those overweight have significantly lower mortality suggesting a J shaped relationship. (15) In SPS3 we found a linear, inverse relationship between BMI and mortality with no interaction with treatment assignment. This may be partially explained by confounding due to smoking or pre-existing disease. (16) While we cannot exclude pre-existing disease, adjustment for smoking did not weaken the association between lower body mass index and mortality. A similar association has been observed in recent observational studies of stroke cohorts. (17, 18) The mechanism for this observation, termed the obesity paradox, remains unexplained.
The overall mortality rate in SPS3 (1.78%/yr) is lower than in other recent trials of secondary prevention. (PRoFESS 3%/yr, VITATOPS 4.7%/yr, PERFORM 2.6%/yr, ESPRIT 2.3%/yr) which may be explained by a distinctive type of vascular disease underlying stroke or younger participant age. (19-22) Mortality risk following lacunar infarct was 8% in the first year in a recent systematic review (N = 544). (23)While this is substantially higher than we observed there are several limitations in the observational cohorts which include relatively small numbers, poor and variable definition of lacunar stroke, variable follow up and a mean age of 71 (SPS3 mean age 63). Our analyses have been done in a population which met trial inclusion criteria with aggressive management of blood pressure and high prevalence of statin therapy which likely contributed to lower mortality than found in the systematic review. Independent predictors of mortality in patients with MRI defined lacunar infarcts have not been previously defined. We identified seven independent predictors at study entry of mortality: age, lower body mass index, hemoglobin <13 g/dL, lower eGFR, history of hypertension, increased systolic blood pressure, and diabetes. Nonfatal major hemorrhage during the course of the trial was also associated with increased mortality.
A systematic review and meta-analysis of the effect of the addition of clopidrogel to aspirin on mortality identified SPS3 results as an outlier and noted that overall adding clopidogrel to aspirin does not increase mortality. (8) An interaction between dual therapy and mortality has been observed though in the asymptomatic and primary prevention subsets of the CHARISMA population with increased mortality risk in those assigned dual therapy. (24) The SPS3 population behaves similar to the low risk population in CHARISMA suggesting that individuals with lacunar infarct form a distinct subset of those with established vascular disease and have increased risk of mortality on dual antiplatelet therapy.
Our study has several limitations. The population included in the study was relatively healthy stroke survivors recruited a median of 62 days after the qualifying stroke. Classification of death as vascular or nonvascular was dependent on the narrative supplied by the trial sites. We did not correct for multiple comparisons and the results must be interpreted with caution.
In conclusion, participants in SPS3, while experiencing a lower mortality rate than previously reported lacunar stroke populations, had higher mortality when assigned dual antiplatelet therapy which was not explained by fatal hemorrhage. It is unlikely to be explained by non-adherence to antiplatelet therapy as vascular mortality did not differ significantly between groups. Unexpected interactions were observed between dual antiplatelet therapy and absence of hypertension and history of ischemic heart disease with increased mortality in both subgroups. This observation, if confirmed in other datasets, has implications for the design of trials testing combination antiplatelet therapy and its broader use in stroke populations.
Supplementary Material
Acknowledgments
Funding Source: National Institute of Neurological Disorders and Stroke (U01 NS38529-04A1)
Footnotes
Clinical Trial Registration Information: URL: http://www.SPS3ClinicalTrials.gov Unique identifier: NCT00059306
Disclosures: Mukul Sharma, Lesly A. Pearce, Santiago Palacio and David C. Anderson report no disclosures; Oscar R. Benavente and Robert G. Hart report received grant support from NIH/NINDS as co-principal investigators of the SPS3 trial; Christopher S. Coffey is a consultant for ZZ Biotech LLC and received grant support from NIH/NINDS and the Michael J. Fox Foundation; Stuart J. Connolly reports honoraria for Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Portola and Bayer. Stuart J. Connolly is also a consultant for Boehringer Ingelheim, Bristol Myers Squibb, Pfizer and Portola.
Contributor Information
Mukul Sharma, Department of Medicine (Neurology) McMaster University / Population Health Research Institute 237 Barton Street East, C4-120 Hamilton, Ontario, Canada, L8L 2X2 Phone: (905) 521-2100 x40367 Fax: (905) 577-1427 Mike.Sharma@phri.ca
Lesly A. Pearce, Biostatistics consultant Minot, North Dakota, USA lpearce@minot.com
Oscar R. Benavente, Department of Medicine (Neurology) University of British Columbia Vancouver, British Columbia, Canada oscar.benavente@ubc.ca
David C. Anderson, Department of Neurology University of Minnesota Minneapolis, Minnesota, USA ander012@umn.edu
Stuart J. Connolly, Department of Medicine (Cardiology) McMaster University / Population Health Research Institute Hamilton, Ontario, Canada Stuart.Connolly@phri.ca
Santiago Palacio, Department of Neurology University of Texas Health Science Center San Antonio, Texas, USA PALACIOS0@uthscsa.edu
Christopher S. Coffey, Department of Biostatistics University of Iowa Iowa City, Iowa, USA christopher-coffey@uiowa.edu
Robert G. Hart, Department of Medicine (Neurology) McMaster University / Population Health Research Institute Robert.Hart@phri.ca
References
- 1.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083, 92. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 2.Bejot Y, Caillier M, Ben Salem D, Couvreur G, Rouaud O, Osseby G- V, et al. Ischaemic stroke subtypes and associated risk factors: a French population based study. J Neurol Neurosurg Psychiatry. 2008;79:1344–8. doi: 10.1136/jnnp.2008.150318. [DOI] [PubMed] [Google Scholar]
- 3.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of Ischemic Stroke Subtypes According to TOAST Criteria: Incidence, Recurrence, and Long-Term Survival in Ischemic Stroke Subtypes: A Population-Based Study. Stroke. 2001;32:2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe CDA, Rudd AG, Howard R, Coshall C, Stewart J, Lawrence E, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2002;72:211–6. doi: 10.1136/jnnp.72.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacco S, Marini C, Totaro R, Russo T, Cerone D, Carolei A. A population-based study of the incidence and prognosis of lacunar stroke. Neurology. 2006;66:1335–8. doi: 10.1212/01.wnl.0000210457.89798.0e. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1969;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 7.The SPS3 Investigators. Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. The N Engl J Med. 2012;367:817–25. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palacio S, Hart RG, Pearce LA, Benavente OR. Effect of addition of clopidogrel to aspirin on mortality: systematic review of randomized trials. Stroke. 2012;43:2157–62. doi: 10.1161/STROKEAHA.112.656173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–75. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White CL, Szychowski JM, Roldan A, Benavente MF, Pretell EJ, Del Brutto OH, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. J Stroke Cerebrovasc Dis. 2013;22:764–74. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The SPS3 Study Group. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–15. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Int Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 17.Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011;42:30–6. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 18.Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body Mass index and poststroke mortality. Neuroepidemiology. 2008;30:93–100. doi: 10.1159/000118945. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–51. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The VITATOPS Trial Study Group B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–65. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 21.Bousser MG, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 2011;377:2013–22. doi: 10.1016/S0140-6736(11)60600-4. [DOI] [PubMed] [Google Scholar]
- 22.ESPRIT Study Group. Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–73. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 23.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128:2507–17. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TH, Bhatt DL, Fox KA, Steinhubl SR, Brennan DM, Hacke W, et al. An analysis of mortality rates with dual-antiplatelet therapy in the primary prevention population of the CHARISMA trial. Eur Heart J. 2007;28:2200–7. doi: 10.1093/eurheartj/ehm274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.