Abstract
Copper (Cu) is an essential metal for growth and development that has the potential to be toxic if levels accumulate beyond the ability of cells to homeostatically balance uptake with detoxification. One system for Cu acquisition is the integral membrane Cu+ transporter, Ctr1, which has been quite well characterized in terms of its function and physiology. The mammalian Ctr2 protein has been a conundrum for the copper field, as it is structurally closely related to the high affinity Cu transporter Ctr1, sharing important motifs for Cu transport activity. However, in contrast to mammalian Ctr1, Ctr2 fails to suppress the Cu-dependent growth phenotype of yeast cells defective in Cu+ import, nor does it appreciably stimulate Cu acquisition when over-expressed in mammalian cells, underscoring important functional dissimilarities between the two proteins. Several roles for the mammalian Ctr2 have been suggested both in vitro and in vivo. Here, we summarize and discuss current insights into the Ctr2 protein and its interaction with Ctr1, its functions in mammalian Cu homeostasis and platinum-based chemotherapy.
Keywords: copper transporter, Ctr; copper trafficking; regulation; structure; platinum-based chemotherapy; copper homeostasis
Introduction
Copper (Cu) is an essential metal to all aerobic life that is used in a wide range of enzymatic reactions owing to its ability to undergo redox reactions (see Cu reviews (1-3)). Our knowledge of how Cu traverses cell membranes, is trafficked intracellularly by its chaperone proteins, and is transported out of cells or incorporated into Cu-dependent proteins has drastically increased over the last few decades. We now recognize that the evolutionary conserved Cu+ transporter 1, Ctr1, is part of a major pathway for cellular Cu uptake (4-10) and that Cu chaperones hand over Cu to Cu, Zn superoxide dismutase (SOD1) via direct ligand-exchange interactions between the Cu chaperone CCS and SOD1 (11-14), and between the Cu chaperone Atox1 and the two Cu-transporting ATPases, Atp7A and Atp7B (15-18), which pump Cu into both the secretory- and export pathways (19, 20). The discovery of an existing protein homologous to Ctr1, presented the field with another potential player in mammalian Cu homeostasis; the Cu transporter 2, Ctr2 (5). Although, seventeen years have passed since Zhou and Gitschier made the discovery of a cDNA encoding this protein related to Ctr1, in contrast to the wealth of information regarding the structure, physiological function and mechanism of action of mammalian Ctr1, comparatively little is known about Ctr2 in mammalian Cu homeostasis. Here, we summarize and discuss what is currently known about the roles the enigmatic mammalian Ctr2 protein plays in Cu uptake, intracellular Cu distribution, Cu utilization, and platinum-based chemotherapy and its functional interaction with Ctr1.
Gene and protein organization and structure
By DNA sequence analysis Zhou and Gitschier (5) identified a human gene, hCTR2 (SLC31A2), which is highly homologous to the hCTR1 gene (SLC31A1). Both hCTR2 and hCTR1 are located on chromosome 9 on the positive strand, separated by approximately 60 kb that encompasses the FKBP15 gene on the negative strand. The SLC31A2 gene covers 17 kb, with its encoded mRNA possessing four exons, while the SLC31A1 gene covers 43 kb encoding an mRNA which has five exons. However, the consensus coding DNA sequences (CDS) are 50% identical between the two genes (CCDS, Clustal Omega). Like human Ctr1, the human Ctr2 mRNA is ubiquitously expressed in all tissues evaluated, with the highest levels found in brain, spleen, placenta, pancreas, and testis, and lower levels in liver, thymus, ovary, intestine and colon (5). Mouse Ctr2 is also ubiquitously expressed but show a somewhat different mRNA expression pattern with the highest levels found in heart, liver, kidney, and testis and lower levels in muscle and brain (21). Interestingly, the steady state levels of mouse Ctr1 mRNA has a similar expression profile as mouse Ctr2 (6, 21), possibly indicating that the encoded proteins may act in the same biological process. The discrepancies between the rodent and human data can be due to species differences, but also possibly explained by alterations in Cu status. While, we know the Cu content in the standardized diet for laboratory mice, and that they are maintained in a controlled environment regarding water, pathogens, day and night cycles, and housing, we know very little about the Cu status and potential pathologies in the human tissue samples evaluated. The tissue expression profile of Ctr2 mRNA needs to be further investigated in several species under controlled conditions.
In contrast to Ctr1, Ctr2 is not conserved from yeast to humans, though, as described below, both the yeast and mammalian Ctr2 proteins function in pathways that serve to mobilize vesicular Cu stores into the cytoplasm (21-23). When and how Ctr2 evolved is currently unknown. Possibly the Ctr2 gene arose from a gene duplication event, giving rise to a new protein by neofunctionalization. However, whether this is a plausible course of events remains to be further explored.
When translated, the human SLC31A2 mRNA encodes a Ctr2 protein of 143 amino acid residues, compared with the human SLC31A1 (Ctr1) protein consisting of 190 amino acid residues. The amino acid sequences between these two proteins are 30% identical (Clustal Omega, Figure 1) and the two proteins share several common topological features that are conserved in the Ctr1 family of high affinity Cu+ transporters from yeast to humans. Ctr2 is computationally predicted to harbor three trans-membrane domains, which is the same number as both predicted in silico for Ctr1 and which is supported by the cryo electron microscopy structure of hCtr1 (24, 25). Ctr1 and Ctr2 also share a conserved Met–X3–Met motif in the second transmembrane domain that is critical for efficient Cu+ transport by all known members of the Ctr1 family (26, 27), and the Gly-X3-Gly motif in the third trans-membrane domain that is known to be important for proper helix packing, localization, and oligomerization of the Ctr1 protein (28). Moreover, in line with Ctr1, evidence suggests that Ctr2 homo-multimerizes to form a complex with nine total transmembrane domains (23), but whether this occurs in vitro, and the significance of Ctr2 oligomerization is currently unknown. Both proteolytic mapping and epitope-access experiments indicate that Ctr1 and Ctr2 have the same topological orientation, with the amino-termini located outside of the plasma membrane or inside of an endosomal/lysosomal vesicle, and the carboxyl-terminus facing the cytoplasm (21, 26, 29-31). In contrast to Ctr2, Ctr1 has a significantly longer amino-terminus, with several metal binding motifs consisting of Met and His that, while not essential, are important for full activity of the high affinity import of Cu+ (26, 32). The mammalian Ctr2 protein also lacks the His-Cys-His motif that Ctr1 harbors at the carboxyl-terminus, which is thought to act as a sink for the Cu+ traversing the pore (24), and which may function in trafficking the Cu to the intracellular chaperones CCS and Atox1.
Figure 1.
Alignment of human Ctr1 and Ctr2 showing trans-membrane domains in yellow and glycosylation sites in the ecto-domain of Ctr1 in orange. Cleavage sites of Ctr1 protein ecto-domain are indicated with vertical black arrowheads. The Met-X3-Met motif in second trans-membrane domain, crucial for Cu+ transport activity, is boxed in red and the Gly-X3-Gly in third trans-membrane domain, involved in helix packing, is boxed in blue. The Cys-His-Cys motif at the carboxyl-terminus of Ctr1, involved in trafficking Cu to the chaperones CCS and Atox1, is boxed in green.
Post-transitional modifications, expression and interactions
The Ctr1 protein undergoes post-translational modifications that may influence its function. Two glycan chains are added to the amino-terminal ecto-domain of Ctr1, one N-linked modification at Asn15 and one O-linked glycosylation at Thr27 (Figure 1) (31, 32). The glycosylation shows differential tissue patterns in mice (21), which is in line with other membrane proteins modified by glycans. Currently it is unknown whether alterations in glycosylation can function as a regulatory mechanism for Ctr1 abundance, localization, and/or function Cu+ import in different tissues.
The human Ctr1 O-linked glycosylation at Threonine 27 has, by mutational studies, been suggested to protect the Ctr1 amino-terminal ecto-domain from proteolytic cleavage (32). When this O-linked glycosylation event is prevented, the Ctr1 ecto-domain is cleaved more frequently, generating a shorter form of Ctr1. This cleaved form of Ctr1 has been observed in both cell lines and mouse tissues (32, 33), and like the full length Ctr2 protein, lacks a large portion of the Cu+ binding ecto-domain (Figure 1). The Ctr1 ecto-domain Ctr1 harbors the capacity to bind multiple Cu+ atoms via the Met- and His-rich motifs and through the amino-terminal copper/nickel (ATCUN) motif (34, 35). Recently it was demonstrated by mass spectrometry that the truncated form of Ctr1 initiates within the Met-rich motif closest to the first transmembrane domain (Figure 1) (21). Initial studies evaluating the function of truncated Ctr1 suggested that this form is still localizes to the plasma membrane and is competent to transport Cu, albeit with approximately 50% the efficiency of the full-length protein (32). Thus, changes in Ctr1 truncation would be predicted to alter cellular Cu import.
The mouse Ctr2 protein, in line with its mRNA, is ubiquitously expressed in a wide range of tissues, showing the highest protein expression in liver, kidney, and testis, which is very similar to the protein expression pattern for mouse Ctr1 (Unpublished data). In mammalian cells Ctr2 protein localizes to intracellular vesicular compartments including endosomes and lysosomes (21, 23), and the protein also contains both di-leucine and tyrosine-based motifs, which are potential lysosomal targeting sequences. However, when overexpressed via transfection with an epitope tag attached to the protein, Ctr2 has also been observed at the plasma membrane (29). Whether Ctr2 localizes to the plasma membrane under normal physiological conditions or if the protein can be recruited to the plasma membrane under certain circumstances is presently unknown. Further studies examining the precise localization and possible cellular and tissue relocalization of Ctr2 are warranted. On the contrary, numerous studies demonstrate that Ctr1 localizes to both the plasma membrane and to intracellular vesicles, having different location depending upon cell lines examined or within a cell line upon exposure to exogenous Cu (36-39). In enterocytes, Ctr1 is highly expressed on the apical membrane facing the lumen of the intestine, where it functions in dietary Cu uptake under low Cu conditions, and under elevated Cu condition Ctr1 localizes to a higher degree in intracellular vesicles (33, 40). Ctr1 has also been demonstrated to undergo rapid endocytosis that is enhanced by Cu+ (36, 37), perhaps as a mechanism for reducing the capacity for Cu+ uptake to mitigate toxicity. Furthermore, internalization and recycling of Ctr1 from and to the plasma membrane could constitute a regulatory mechanism for both Ctr1-dependent Cu transport across the plasma membrane and across intracellular membranes from an endosome, thereby increasing cytosolic Cu levels.
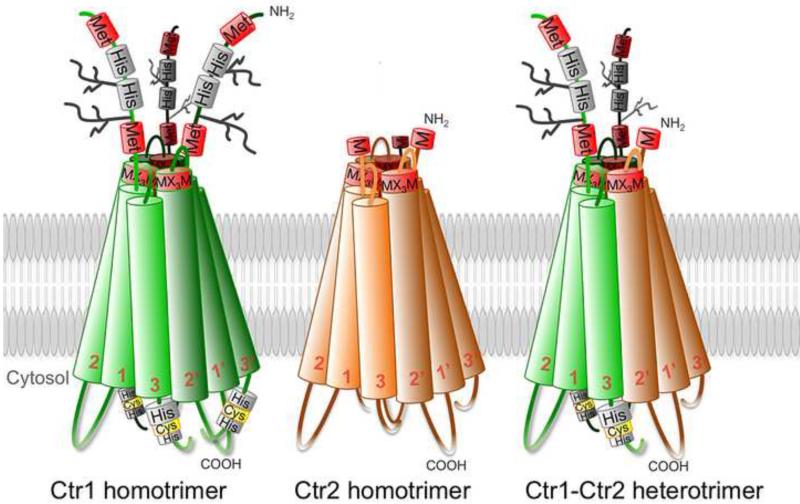
Consistent with the partial co-localization of Ctr1 and Ctr2 to endosomal compartments, recent co–immuno precipitation and bimolecular fluorescence complementation studies provide strong evidence that Ctr2 physically interacts with Ctr1 (21). These observations give rise to the possibility that, in addition to forming homo-trimers, the two proteins may also form hetero-trimers or that Ctr1 and Ctr2 homo-trimers engage in a complex (Figure 2). Whether Ctr2 and Ctr1 proteins together can form a functional unit, such as a pore for Cu ions to traverse, is currently unknown. This is an intriguing idea and would add an additional regulatory layer to cellular Cu homeostasis.
Figure 2.
Schematic figure depicting Ctr1 and Ctr2 homotrimers, and a possible way for their interaction; heterotrimerization. Adapted and modified from Öhrvik, H. & D.J. Thiele. 2014. How Copper Traverses Cellular Membranes Through the Mammalian Copper Transporter 1, Ctr1. Ann. N.Y. Acad. Sci. (In press).
The role of Ctr2 in Cu homeostasis
In baker’s yeast cells lacking the two major Cu importers, Ctr1 and Ctr3, complementary DNAs encoding Ctr1 from a variety of sources including humans (4, (41) mice (6), amphibians (42, 43), plants (44, 45) and other fungi (46, 47) are able to complement the Cu deficient phenotype, demonstrating the conserved role of Ctr1 as a high affinity Cu importer. In contrast, we, and others have been unable to demonstrate that the mouse or human Ctr2 protein is able to complement the growth defect of yeast cells lacking the high affinity Cu+ uptake machinery (5, 21). In mammalian cells, some studies have suggested a role for Ctr2 analogous to the yeast Ctr2, which functions as a vacuolar Cu exporter, mobilizing Cu from the vacuolar lumen into the cytosol during times of extracellular Cu scarcity (22, 23). Further, based on over expression and RNAi-mediated knock down studies in cultured mammalian cells, Ctr2 has been suggested to function as a low affinity Cu importer at the plasma membrane when exposed to extracellular Cu (29). The low affinity for Cu may partially be linked to the fact that Ctr2 lacks the His- and Met-rich motifs that bind Cu with high affinity to the ecto-domain of the full-length Ctr1 protein. However, when comparing the ability to transport Cu between the short truncated form of Ctr1 and Ctr2, which both lack the Cu binding ecto-domain, our studies indicated that the Ctr2 protein has virtually undetectable Cu transport ability, suggesting that the Ctr2 protein is not an efficient Cu transporter under normal conditions. Moreover, studies in Cu deficient rats have shown that Ctr2 mRNA and protein levels decrease in the liver and heart compared to rats fed a normal diet (48), which would be inconsistent with a role for Ctr2 as a Cu importer. Still it is possible that Ctr2 might act as a Cu transporter under certain conditions, though, at the moment we can conclude that in a physiological context the endogenous Ctr2 protein is not a general Cu importer of Cu+ through the plasma membrane. Additionally, cells in which CTR2 mRNA has been knocked down by RNAi exhibited an overall increased rate of macropinocytosis, suggesting that Ctr2 might also function as a regulator of macropinocytosis (49). However, as these studies have been performed in cell culture, they have yielded limited information about the physiological roles for Ctr2.
Based on the generation of a systemic knock out of the mouse CTR2 gene, recent experiments suggest a new role for the Ctr2 protein as an important factor for the biogenesis of the cleaved form of Ctr1 (21). Unexpectedly, Ctr2−/− mice, and their corresponding mouse embryonic fibroblasts (MEFs), show higher steady state Cu levels than that of the wild type littermates and MEFs, with Cu concentrated in endosomal and/or lysosomal compartments as suggested by both subcellular fractionation studies followed by inductively coupled plasma mass spectrometry (ICP-MS) Cu measurements, and X-ray fluorescence microscopy of brain tissue sections. Compared to wild type mice, Cu concentrations were increased 2 to 3-fold in brain, spleen and testis from Ctr2−/− animals, with several tissues showing an age-dependent accumulation of Cu. Moreover, loss of Ctr2 resulted in a severe, though not total defect in the generation or stability of the truncated form of Ctr1, consistent with the Cu accumulation phenotype observed in Ctr2−/− mice and MEFs. This phenotype, and other experimental data, suggests that Ctr2 plays a role in the biogenesis of the cleaved Ctr1 lacking a large portion of the metal-binding ecto-domain. As the truncated form of Ctr1 has been shown to import Cu less efficiently than the full-length protein, the reduction in the levels of truncated Ctr1 would explain why the Ctr2−/− animals have increased cellular Cu accumulation. No impact on the cellular Cu status biomarkers CCS and CoxIV was observed between wild type and Ctr2−/− cells and mice, suggesting that the accumulated Cu is not readily exchangeable, consistent with the accumulated Cu residing, at least in large part, within the lumen of endosomal compartments. Additionally, the concentration of Cu in endosomal compartments in Ctr2−/− MEFs, and its mobilization by transient expression of truncated Ctr1, imply that the cleaved form of Ctr1 plays a novel role in the mobilization of endosomal Cu into the cytosol. Together these data support a role for Ctr2 as a regulator of Ctr1 function by affecting the biogenesis of cleaved Ctr1, lacking the bulk of the metal binding Met- and His-rich motifs, thereby indirectly altering both Cu uptake and intracellular flux.
Ctr2 and platinum-based chemotherapy
Studies from over a decade ago revealed that Ctr1 is a significant pathway for the import of the cancer chemotherapeutic drug cisplatin, into both yeast and mammalian cells (50-53). Similar to Cu+, platinum binds to synthetic peptides encompassing the Met-rich motifs within the Ctr1 ecto-domain (27, 51, 54). Interestingly, Fluorescence Resonance Energy Transfer (FRET) -based studies, and the clever use of Cu+ transport mutants of Ctr1 suggest that Ctr1 might function as a receptor for platinum, bringing platinum into the cells via endocytosis rather than conductance through a membrane channel as for Cu+ (36, 53, 55). Platinum-based drugs, such as cisplatin and carboplatin, are highly effective and often used chemotherapeutics in a number of cancers including ovarian, pancreatic, testicular, melanoma, and lung. The success rate of platinum treatment has been shown to associate with high levels of Ctr1 expression. Consequently, lower Ctr1 levels have also been observed to associate with increased cisplatin resistance in tumors and higher Ctr1 expression associate with higher sensitivity to the cisplatin treatment (56). Although the mechanisms underlying the causes for intrinsic or acquired resistance to cisplatin are not fully understood, studies demonstrate that cisplatin-resistant mammalian cell lines exhibit reduced uptake of the drug (53). In non-small cell lung cancer patients, better survival rates have been reported in patients having higher expression of Ctr1 (57). Interestingly, and in contrast to Ctr1, low levels of Ctr2 have been reported to associate positively with the success rate of platinum treatment in patients (56, 58). Experimental studies have shown that cells lacking Ctr2, either by RNAi-mediated knock down or Ctr2 knock out MEFs, exhibit increased whole-cell platinum accumulation (21, 59). Moreover, in a panel of ovarian carcinoma cell lines an association between CTR2 levels and sensitivity to platinum has been demonstrated (60). In chemo-resistant patients the CTR2/CTR1 ratio is increased and patients with higher CTR2 or a CTR2/CTR1 ratio >1 had a poorer disease prognosis (58). Together with studies of the Ctr2 mouse knockout and the corresponding Ctr2−/− MEFs (21), these studies support the notion that Ctr2 affects platinum accumulation via a Ctr1-dependent mechanism involving the biogenesis of the cleaved form of Ctr1 lacking the metal binding ecto-domain. When Ctr2 levels are high the cells have more of the cleaved Ctr1 form that will have lower affinity for both Cu and platinum and, subsequently, import less platinum. This is an interesting area, where Ctr2 and Ctr1, and their ratio, could be used as prognostic markers for individual treatment responsiveness and as predictors of platinum-based chemotherapy resistance.
Conclusions
While we are beginning to have an understanding of the structure, localization and some of the roles Ctr2 plays in mammalian Cu homeostasis, our knowledge regarding the mechanisms that regulate Ctr2 abundance, trafficking and function is still in its early stages. New insights into how Ctr2 and Ctr1 functionally interact will be crucial for a better comprehension of mammalian Cu homeostasis, and for insights into how Ctr1 and Ctr2 may cause and modify human Cu-related diseases and platinum-based chemotherapeutic efficacy.
Acknowledgements
The authors gratefully acknowledge members of the Thiele laboratory for creating a stimulating scientific environment. Support is gratefully acknowledged from Swedish Research Council K2012-77PK-21938-01-2 and the Throne-Holst Foundation to H.Ö., and the National Institutes of Health grants GM041840 and DK07492 to D.J.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors declare no competing financial interests.
References
- 1.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 2.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 3.Nevitt T, Öhrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim Biophys Acta. 2012;1823(9):1580–1593. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancis A, et al. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A. 1997;94(14):7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254(1-2):87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci U S A. 2001;98(12):6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A. 2001;98(12):6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Wu X, Lee J. SLC31 (CTR) family of copper transporters in health and disease. Mol Aspects Med. 2013;34(2-3):561–570. doi: 10.1016/j.mam.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope CR, Flores AG, Kaplan JH, Unger VM. Structure and function of copper uptake transporters. Curr Top Membr. 2012;69:97–112. doi: 10.1016/B978-0-12-394390-3.00004-5. [DOI] [PubMed] [Google Scholar]
- 11.Culotta VC, et al. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272(38):23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 12.Wong PC, et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2000;97(6):2886–2891. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763(7):747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pope CR, De Feo CJ, Unger VM. Cellular distribution of copper to superoxide dismutase involves scaffolding by membranes. Proc Natl Acad Sci U S A. 2013;110(51):20491–20496. doi: 10.1073/pnas.1309820110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pufahl RA, et al. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science. 1997;278(5339):853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 16.Klomp LW, et al. Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J Biol Chem. 1997;272(14):9221–9226. doi: 10.1074/jbc.272.14.9221. [DOI] [PubMed] [Google Scholar]
- 17.Flores AG, Unger VM. Atox1 contains positive residues that mediate membrane association and aid subsequent copper loading. J Membr Biol. 2013;246(12):903–913. doi: 10.1007/s00232-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A. 2003;100(3):1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsivkovskii R, Eisses JF, Kaplan JH, Lutsenko S. Functional properties of the copper-transporting ATPase ATP7B (the Wilson’s disease protein) expressed in insect cells. J Biol Chem. 2002;277(2):976–983. doi: 10.1074/jbc.M109368200. [DOI] [PubMed] [Google Scholar]
- 20.Lutsenko S, LeShane ES, Shinde U. Biochemical basis of regulation of human copper-transporting ATPases. Arch Biochem Biophys. 2007;463(2):134–148. doi: 10.1016/j.abb.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Öhrvik H, et al. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc Natl Acad Sci U S A. 2013;110(46):E4279–4288. doi: 10.1073/pnas.1311749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rees EM, Lee J, Thiele DJ. Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem. 2004;279(52):54221–54229. doi: 10.1074/jbc.M411669200. [DOI] [PubMed] [Google Scholar]
- 23.van den Berghe PV, et al. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J. 2007;407(1):49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci U S A. 2009;106(11):4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci U S A. 2006;103(10):3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277(29):26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 27.Larson CA, Adams PL, Blair BG, Safaei R, Howell SB. The role of the methionines and histidines in the transmembrane domain of mammalian copper transporter 1 in the cellular accumulation of cisplatin. Mol Pharmacol. 2010;78(3):333–339. doi: 10.1124/mol.110.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aller SG, Eng ET, De Feo CJ, Unger VM. Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem. 2004;279(51):53435–53441. doi: 10.1074/jbc.M409421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’Abbe MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409(3):731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 30.Klomp AE, et al. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J. 2003;370(Pt 3):881–889. doi: 10.1042/BJ20021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277(32):29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 32.Maryon EB, Molloy SA, Kaplan JH. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem. 2007;282(28):20376–20387. doi: 10.1074/jbc.M701806200. [DOI] [PubMed] [Google Scholar]
- 33.Nose Y, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285(42):32385–32392. doi: 10.1074/jbc.M110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas KL, Putterman AB, White DR, Thiele DJ, Franz KJ. Model peptides provide new insights into the role of histidine residues as potential ligands in human cellular copper acquisition via Ctr1. J Am Chem Soc. 2011;133(12):4427–4437. doi: 10.1021/ja108890c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du X, et al. Kinetics and thermodynamics of metal binding to the N-terminus of a human copper transporter, hCTR1. Chem Commun (Camb) 2013;49(80):9134–9136. doi: 10.1039/c3cc45360j. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem. 2004;279(17):17428–17433. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- 37.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278(11):9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 38.Eisses JF, Chi Y, Kaplan JH. Stable plasma membrane levels of hCTR1 mediate cellular copper uptake. J Biol Chem. 2005;280(10):9635–9639. doi: 10.1074/jbc.M500116200. [DOI] [PubMed] [Google Scholar]
- 39.Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364(Pt 2):497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136(1):21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moller LB, Petersen C, Lund C, Horn N. Characterization of the hCTR1 gene: genomic organization, functional expression, and identification of a highly homologous processed gene. Gene. 2000;257(1):13–22. doi: 10.1016/s0378-1119(00)00394-2. [DOI] [PubMed] [Google Scholar]
- 42.Haremaki T, Weinstein DC. Xmc mediates Xctr1-independent morphogenesis in Xenopus laevis. Dev Dyn. 2009;238(9):2382–2387. doi: 10.1002/dvdy.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riggio M, et al. High affinity copper transport protein in the lizard Podarcis sicula: molecular cloning, functional characterization and expression in somatic tissues, follicular oocytes and eggs. Biochim Biophys Acta. 2002;1576(1-2):127–135. doi: 10.1016/s0167-4781(02)00337-8. [DOI] [PubMed] [Google Scholar]
- 44.Sancenon V, Puig S, Mira H, Thiele DJ, Penarrubia L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol. 2003;51(4):577–587. doi: 10.1023/a:1022345507112. [DOI] [PubMed] [Google Scholar]
- 45.Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Van Montagu M. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J Biol Chem. 1995;270(47):28479–28486. doi: 10.1074/jbc.270.47.28479. [DOI] [PubMed] [Google Scholar]
- 46.Knight SA, Labbe S, Kwon LF, Kosman DJ, Thiele DJ. A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev. 1996;10(15):1917–1929. doi: 10.1101/gad.10.15.1917. [DOI] [PubMed] [Google Scholar]
- 47.Zhou H, Thiele DJ. Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J Biol Chem. 2001;276(23):20529–20535. doi: 10.1074/jbc.M102004200. [DOI] [PubMed] [Google Scholar]
- 48.Bertinato J, Duval S, L’Abbe MR. Copper transporter 2 content is lower in liver and heart of copper-deficient rats. International journal of molecular sciences. 2010;11(11):4741–4749. doi: 10.3390/ijms11114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blair BG, et al. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol Pharmacol. 2011;79(1):157–166. doi: 10.1124/mol.110.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99(22):14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Y, Smith K, Petris MJ. Cisplatin stabilizes a multimeric complex of the human Ctr1 copper transporter: requirement for the extracellular methionine-rich clusters. J Biol Chem. 2004;279(45):46393–46399. doi: 10.1074/jbc.M407777200. [DOI] [PubMed] [Google Scholar]
- 52.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70(4):1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 53.Song IS, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3(12):1543–1549. [PubMed] [Google Scholar]
- 54.Crider SE, Holbrook RJ, Franz KJ. Coordination of platinum therapeutic agents to met-rich motifs of human copper transport protein1. Metallomics. 2010;2(1):74–83. doi: 10.1039/b916899k. [DOI] [PubMed] [Google Scholar]
- 55.Sinani D, Adle DJ, Kim H, Lee J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem. 2007;282(37):26775–26785. doi: 10.1074/jbc.M703973200. [DOI] [PubMed] [Google Scholar]
- 56.Lee YY, et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol Oncol. 2011;122(2):361–365. doi: 10.1016/j.ygyno.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Xu X, et al. Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012;77(2):438–442. doi: 10.1016/j.lungcan.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida H, et al. Association of copper transporter expression with platinum resistance in epithelial ovarian cancer. Anticancer Res. 2013;33(4):1409–1414. [PubMed] [Google Scholar]
- 59.Blair BG, et al. Regulation of copper transporter 2 expression by copper and cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2010;77(6):912–921. doi: 10.1124/mol.109.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin Cancer Res. 2009;15(13):4312–4321. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]