Abstract
Background
Experimental studies suggest that visceral adiposity and adipose tissue dysfunction play a central role in obesity-related cardiometabolic complications. Impaired angiogenesis in fat has been implicated in the development of adipose tissue hypoxia, capillary rarefaction, inflammation, and metabolic dysregulation, but pathophysiological mechanisms remain unknown. In this study, we examined the role of a novel anti-angiogenic isoform of vascular endothelial growth factor-A (VEGF-A), VEGF-A165b, in human obesity.
Methods and Results
We biopsied paired subcutaneous and visceral adipose tissue in 40 obese subjects (BMI 45±8 kg/m2, age 45±11 yr) during bariatric surgery and characterized depot-specific adipose tissue angiogenic capacity using an established ex vivo assay. Visceral adipose tissue exhibited significantly blunted angiogenic growth compared to subcutaneous fat (p<0.001) which was associated with marked tissue up-regulation of VEGF-A165b (p=0.004). Extent of VEGF-A165b expression correlated negatively with angiogenic growth (r= -0.6, p=0.006). While recombinant VEGF-A165b significantly impaired angiogenesis, targeted inhibition of VEGF-A165b with neutralizing antibody stimulated fat pad neovascularization and restored VEGF receptor activation. Blood levels of VEGF-A165b were significantly higher in obese subjects compared to lean controls (p=0.02), and surgical weight loss induced a marked decline in serumVEGF-A165b (p=0.003).
Conclusions
We demonstrate that impaired adipose tissue angiogenesis is associated with over- expression of a novel anti-angiogenic factor VEGF-A165b that may play a pathogenic role in human adiposopathy. Moreover, systemic up-regulation of VEGF-A165b in circulating blood may have wider ranging implications beyond the adipose milieu. VEGF-A165b may represent a novel area of investigation to gain further understanding of mechanisms that modulate cardiometabolic consequences of obesity.
Keywords: Obesity, VEGF-A, VEGF-A165b, angiogenesis, visceral fat, metabolism
INTRODUCTION
Obesity and its associated metabolic complications has emerged as one of the most critical health care problems in the US and worldwide with nearly 70% of the US population currently overweight or obese.1, 2 Angiogenesis, the generation of new blood vessels, is critical for adequate fat expansion and adipose tissue remodeling.3 Clinical studies show that adipose tissue angiogenic responses are blunted in human obesity, particularly in visceral depots, and associated with inflammation and metabolic dysfunction.4–7 Strategies aimed toward therapeutic modulation of adipose vascularization to improve metabolism have focused on stimulating the primary regulator of tissue angiogenesis, vascular endothelial growth factor-A (VEGF-A).8–10 In experimental models, adipose tissue over-expression of VEGF-A promotes neovascularization and improves insulin sensitivity and glucose metabolism.9, 10 Conversely, adipose specific VEGF-A knockouts display capillary rarefaction, inflammation, and metabolic collapse. 10 These data collectively provide strong evidence that qualitative features of fat and altered tissue homeostasis as a function of impaired vascular support play a central role in shaping systemic phenotypes.
Clinical studies demonstrate that subcutaneous adipose tissue exhibits higher capillary density and angiogenic capacity compared to the visceral depot,7 despite consistent published data demonstrating higher VEGF-A expression in visceral fat.11–13 This paradoxical finding remains unexplained in human studies of obesity. While initially described in the oncologic literature,14, 15 it is now recognized that alternative VEGF-A gene splicing may generate a number of VEGF-A isoforms which differ in their biological action. As such, proximal splicing that includes an exon 8a sequence results in the pro-angiogenic VEGF-A165a , while distal splicing inclusive of exon 8b yields the anti-angiogenic isoform, VEGF-A165b.16 VEGF-A165b exhibits similar binding affinity as VEGF-A165a to vascular endothelial growth factor receptor-2 (VEGFR-2), but fails to activate receptor phosphorylation due to lack of binding to the neuropilin-1 co-receptor, consequently impairing angiogenesis.17-19 To date, essentially nothing is known about the biological relevance of VEGF-A165b in obesity-related cardiometabolic disease.
In this study, we aimed to characterize the role of two major VEGF-A splice variants with opposing actions: pro-angiogenic, VEGF-A165a, and anti-angiogenic, VEGF-A165b in mediating angiogenic responses in subcutaneous and visceral human adipose tissue. Additionally, we sought to gain evidence that anti-angiogenic VEGF-A165b is over-expressed systemically and favorably modified following bariatric surgical weight loss in obese subjects.
METHODS
Study subjects
Consecutive obese men and women (BMI 30 kg/m2, age 18 years), with severe longstanding obesity enrolled in the Boston Medical Center Bariatric Surgery Program were recruited into the study. Samples of subcutaneous and visceral adipose tissue were both collected intraoperatively from the lower abdominal wall and greater omentum, respectively, during planned bariatric surgery, as previously described. 13, 20 Each subject provided one biopsy specimen from the subcutaneous depot and one specimen from the visceral depot (paired samples). No subject provided more than one fat sample per depot. A subset of obese individuals were followed prospectively and serum analyses performed before and 10 ± 2 months after bariatric surgery, and compared to lean controls (BMI 18 to <25 kg/m2) recruited through general public advertisement. Subjects with unstable medical conditions or pregnancy were not eligible for gastric bypass surgery and thus excluded. The study was approved by the Boston University Medical Center Institutional Review Board, and written consent was obtained from all participants.
Anthropometric and biochemical measures
During a single outpatient visit before planned bariatric surgery, clinical characteristics including blood pressure, height, weight, and waist circumference were measured, and cardiovascular risk factors recorded. Fasting blood was taken via an antecubital vein for biochemical parameters including lipids, glucose, insulin, glycosylated hemoglobin (HgA1c), high-sensitivity CRP (hs-CRP), and serum analyses of VEGF-A165a, and VEGF-A165b isoforms. Homeostasis model assessment (HOMA) was used as the index of insulin sensitivity. All biochemical analyses were performed by the Boston Medical Center clinical chemistry laboratory.
Angiogenesis assay of adipose tissue
Freshly collected fat biopsy samples during bariatric surgery were immediately placed in sterile endothelial cell basal medium-2 EBM-2 media (Lonza), and angiogenesis studies performed by method as previously published.7 Briefly, fat pads were finely minced with scissors, and enzymatically digested with 1mg/ml Collagenase type I (Worthington Biochemical) for 30 min at 37 C. Digested pieces of adipose tissue was passed through 100μm nylon filter (BD Falcon), and washed in EBM-2. Pieces of about 1mm2 were embedded on ice in 250μL/well of growth factor depleted Matrigel (BD Discovery) on a 24-well plate, and incubated for 30 minutes at 37 C. After polymerization of Matrigel, wells were covered with 500μL of EBM-2 media supplemented with growth factors (EGM-MV) (Lonza), and cultured at 37 C for 7 days. Half of the media was removed and replaced with fresh media every other day. Sprouts growing from the adipose tissue explant were counted along the perimeter of each sample under x100 magnification as previously described 7 by a blinded investigator.
Immunofluorescence staining
Adipose tissue embedded in Matrigel was fixed in 4% paraformaldehyde (Sigma Aldrich) and stained with endothelial specific markers: anti-mouse CD31 (1:100) (Pierce Thermo Scientific), anti-rabbit VEGFR-2 (1:50) (Cell Signaling), and anti-rabbit von Willebrand factor (vWF) (1:50) (Dako) as previously published.21 Diamidino-2-phenylindole (DAPI) stain (Molecular Probes, Invitrogen) was utilized for cell nucleus identification. Fat tissue was also stained for CD90 (BD Pharmingen), and CD73 (Invitrogen) as markers for mesenchymal stem cells.22 Immunopositive depots were imaged with a fluorescence microscope (Nikon Eclipse Ti, NY, USA) at x10 magnification for CD31, VEGFR-2, and x20 magnification for vWF. Digital images of the cells were captured using an Andor Clara E Camera (NY, USA).
Neutralizing antibody experiments
To examine the effects of neutralizing VEGF-A165b antibody (R&D systems) on the angiogenic capacity of visceral adipose tissue, fat pads were processed as described above, and EGM-MV media not supplemented with VEGF was used. Nonspecific mouse IgG or VEGF-A165b neutralizing antibody (R&D systems clone 56-1 cat. No.MAB3045) antibody was added to media at concentrations of 10ug/mL. Recombinant VEGF-A165b protein (R&D systems, cat. No. 3045-VE) was used at concentration of 500ng/mL. Half of the media and treatment was removed and replaced every other day for sprout analyses. Angiogenesis was quantified from the tissue explant by a blinded investigator.
For whole adipose tissue incubation with neutralizing antibody, fat tissue was minced and placed in 1mL of EGM-MV media (not supplemented with VEGF) containing either mouse IgG control or VEGF-A165b antibody at 10ug/mL for 24 hours. Tissue was collected and snapped frozen in liquid nitrogen, stored at -80C for analysis of protein expression levels of VEGF-A165b, VEGF-A165a, VEGFR-2 p(Y951), VEGFR-2, phosphorylated ERK1/2 (Y204/197), and total ERK1/2 using western blot.
Flow cytometry
Adipose tissue macrophages were isolated as previously described.13 Briefly, whole adipose tissue samples were cut into small pieces, gently homogenized in RPMI using disposable tissue grinders (Fisher Brand), and filtered through 70μm cell strainers (BD Flacon, Bedford, MA) to obtain single cell suspension. Macrophages were isolated by dual density gradient (Histopaque -1077 and Histopaque-1119, Sigma Aldrich). Isolated cells were incubated with FACS permeabilization solution 2 (BD Biosciences) for 30 min at 37 C, then measured by flow cytometry for cells positive for both CD14 (BD Pharmingen), and hVEGF-A total (which does not distinguish isoforms) (R&D systems).
Western immunoblot analyses
VEGF-A165b antibody for western blot was purchased from Abcam (cat. No 14994). The polyclonal antibody against human VEGF-A165a, C-terminal (Exon 8a) specific antibody (VEGF-A165a) was generated by immunization of a rabbit with a KLH conjugated peptide TCRCDKPRR, corresponding to the last 9 amino acids of VEGF-A165a using standard immunization techniques. Proteins were extracted from adipose tissue by homogenization in liquid nitrogen followed by the addition of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.25% sodium dodecyl sulfate [SDS]) supplemented with 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, protease inhibitor cocktail, phosphatase inhibitor I and II (Sigma Aldrich). Samples were assayed for protein content using Bradford’s method. Twenty to thirty micrograms of protein was subjected to electrophoresis in 12% SDS-polyacrylamide gel under reducing conditions and then blotted to a PVDF membrane using the Bio-Rad Transblot Turbo Transfer system, blocked with Odyssey Blocking Buffer (Licor Biosciences), and incubated overnight with the respective antibodies at 4°C with primary mouse or rabbit anti-human antibodies (1:500–1000). The amount of protein loaded was the same for both depots for each specific subject. Immunostained membranes for VEGF-A165b (Abcam), phosphorylated ERK1/2 (Y204/197) (Abcam, MitoSciences), and total ERK1/2 (BD Biosciences) were incubated with IR dye-conjugated secondary antibodies and visualized and quantified using the LI-COR Odyssey system. Membranes probed for VEGF-A165a (from Dr David Bates), VEGFR-2 phosphorylated (Y951) (Cell Signaling), and VEGFR-2 (Cell Signaling) were visualized and quantified using horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (R&D systems) for 1 hour at room temperature. Immune complexes were detected with the enhanced chemiluminescence ECL detection system (Bio-Rad). Densitometric analysis of the bands was performed using ImageQuant™ LAS 4000 biomolecular imaging system (GE Healthcare). Immunoblots were normalized to β-actin (Cell Signaling).
Immunoprecipitation
Immunoprecipitation was performed using G-Sepharose coupled beads. Beads were incubated with mouse anti human VEGFR-2 (Cell Signaling) antibody (1:100) for 1 hour, followed by overnight incubation at 4°C with 300μg protein in the presence of protease and phosphatase inhibitor cocktail (Sigma Aldrich). The eluate was subjected to SDS/PAGE. Mouse anti-human VEGF-A165b at 1:500 dilution was blotted using standard western blot protocols.
Serum VEGF-A165a and VEGF-A165b protein
Serum protein samples were resolved on SDS/PAGE for western blot analyses of VEGF-A165a and VEGF-A165b protein expression in both lean and obese subjects. Additionally, serum VEGF-A165a and VEGF-A165b were also measured in obese patients before and after bariatric surgical weight loss. Immunopositive blots were normalized to total protein stained using MemCode TM Reversible Protein stain (Thermo Scientific).
Statistics
Group differences in clinical characteristics of subjects were analyzed using t-tests or chi-square tests with SPSS 20.0. Other analyses were performed using GraphPad Prism 6.0 software. Area under the curve (AUC) of the plot for cumulative angiogenic growth (quantified as capillary sprout count) over the time period of 7 days was examined using GraphPad Prism 6.0. Differences between subcutaneous and visceral tissue in angiogenic studies, VEGF-A protein isoform expression and receptor signaling, and neutralizing antibody experiments were analyzed using paired t-tests. Serum measures of VEFG-A isoforms before and after weight loss were examined by paired t-tests. All other analyses were performed using unpaired t-tests with correction for non-equal variances using Welch’s approximation, as indicated in figure legends. A value of p<0.05 was accepted as statistically significant. Data are presented as mean ± SD unless otherwise indicated.
RESULTS
Quantification of angiogenesis in subcutaneous and visceral adipose tissue
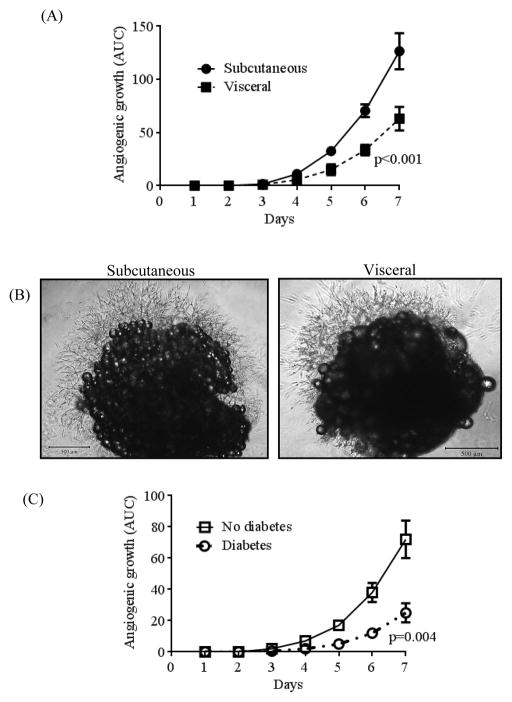
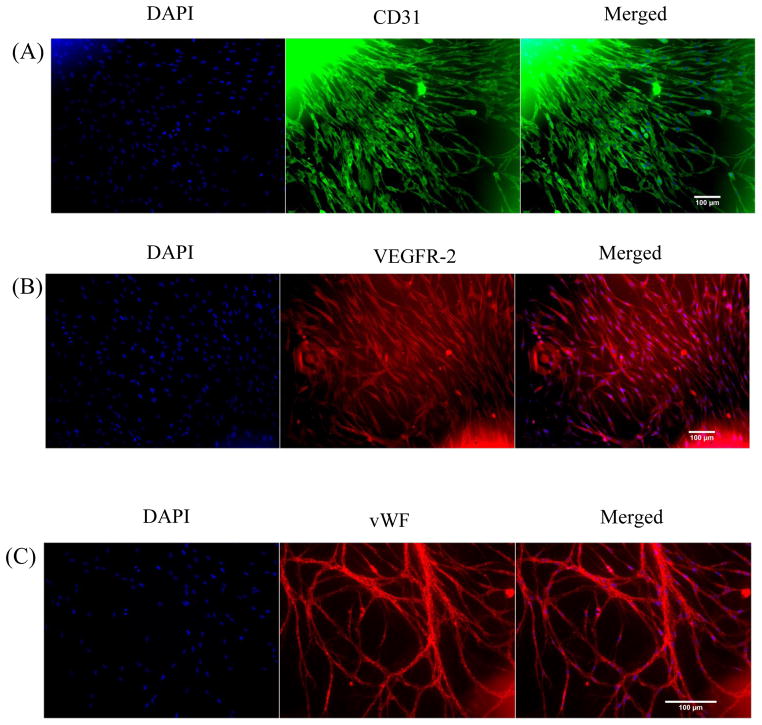
A total of 40 subjects were recruited who provided subcutaneous and visceral adipose tissue samples. Clinical characteristics of subjects are displayed in Table 1, and are consistent with the bariatric population at our medical center. In agreement with prior data7, angiogenic capacity was significantly impaired in visceral compared to subcutaneous fat pads quantified by either area under the curve plot of capillary growth (AUC: 63 ± 11 visceral vs. 127 ± 17 subcutaneous, p<0.001) over 7 days (figure 1A), or total capillary branch counts at 7 days of culture (34 ± 25, visceral vs. 63 ± 30, subcutaneous, p<0.001) (Supplemental figure 1A). Illustrations of preserved (subcutaneous) and blunted (visceral) angiogenic capacity are displayed in figure 1B. Subjects with diabetes mellitus exhibited markedly reduced capillary growth in the visceral depot compared to non-diabetics (Figure 1C), while no difference was noted in the subcutaneous depot. In line with prior published data, we confirmed that sprout extensions from central fat pad represent burgeoning capillary networks which immunostained positively for endothelium-specific markers including CD 31, VEGFR-2 and von Willebrand factor (Figure 2A, 2B, and 2C) respectively.7 Lastly, stains for CD73 and CD90 as mesenchymal stem cell markers were negative (Supplemental figure 2).
Table 1.
Clinical characteristics of study population.
| Clinical parameter | N=40 |
|---|---|
| Age (yrs) | 45 ± 11 |
| Female (%) | 78% |
| BMI (kg/m2) | 45 ± 8 |
| Waist circumference (cm) | 127 ± 15 |
| Weight (kg) | 124 ± 24 |
| Insulin (mIU/mL) | 16 ± 17 |
| Glucose (mg/dL) | 125 ± 70 |
| HbA1C (%) | 6.7 ± 1.9 |
| HOMA-IR | 5.5 ± 9.2 |
| hsCRP (mg/dL) | 8.3 ± 6.7 |
| Triglycerides (mg/dL) | 124 ± 72 |
| Total cholesterol (mg/dL) | 187 ± 35 |
| HDL-C (mg/dL) | 46 ± 11 |
| LDL-C (mg/dL) | 117 ± 34 |
| Systolic BP (mmHg) | 132 ± 13 |
| Diastolic BP (mmHg) | 77 ± 8 |
| Diabetes mellitus (%) | 35.9% |
| Hypertension (%) | 59.0% |
| Hypercholesterolemia (%) | 18.4% |
| Smokers (%) | 28.2% |
Figure 1.
Angiogenesis comparison between subcutaneous and visceral adipose tissue. A) Quantitative area under the curve (AUC) of the plot for cumulative angiogenic growth in subcutaneous (n=25) vs. visceral (n=25) fat pads over 7 days of culture (p<0.001) by paired t-test. B) Representative images of subcutaneous vs. visceral adipose tissue fat pad explants after 7 days of culture taken with the Nikon Eclipse TS100, at magnification of x40. C) Subjects with diabetes (n=10), (AUC 25 ± 6) had significantly reduced angiogenic capacity in the visceral depot compared to non-diabetics (n=15), (AUC 72 ± 13), p=0.004 by unpaired t-test with Welch’s correction. Data are presented as mean ± SEM.
Figure 2.
Immunofluorescence staining of adipose tissue sprouts. Representative images of adipose tissue capillaries stained for A) CD31, B) VEGFR-2, and C) von Willebrand factor (vWF). Left panels (DAPI: nuclear stain), middle panels (endothelium-specific marker), and right panels (merged images). Sprouts emanating from adipose tissue fat pads stained positive for endothelial cell-specific markers CD31, VEGFR-2, and vWF.
Depot-specific VEGFR-2 signaling in adipose tissue
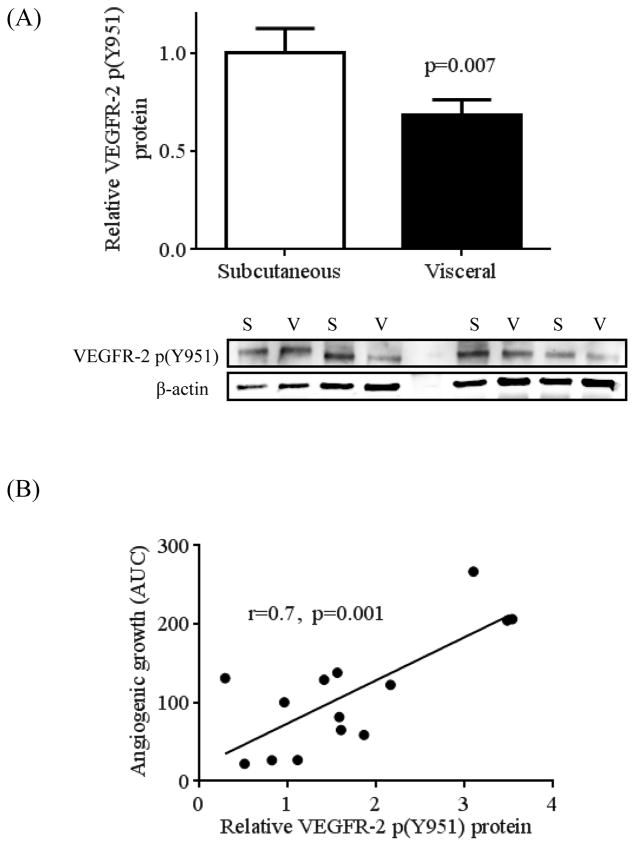
We and others have demonstrated a “paradoxical” increase of VEGF-A total mRNA and protein expression in whole visceral fat despite impaired angiogenic potential. 6, 13 Using flow cytometry, we similarly observed up-regulation of VEGF-A total expression in adipose macrophages which correlated negatively with capillary growth. (Supplemental figures 3A and 3B, respectively). Furthermore, we demonstrated that pro-angiogenic activation of VEGFR-2 through phosphorylation of its tyrosine kinase receptor VEGFR-2 at Y951 (kinase insert domain) (VEGFR-2 p(Y951)22 was significantly impaired in the visceral compared to subcutaneous depot by western blot analysis (p=0.007, n=20 per group) (figure 3A). However, total VEGFR-2 protein expression was not different between depots (Supplemental figure 4). As shown in figure 3B, extent of VEGFR-2 phosphorylation correlated positively with angiogenic capacity (r=0.7, p=0.001).
Figure 3.
Protein levels in adipose tissue of phosphorylated VEGF-receptor 2 (VEGFR-2 p(Y951) using western blot analysis. A) Visceral adipose tissue (n=20) exhibited significantly reduced protein expression of VEGFR-2 p(Y951) vs. the subcutaneous depot (n=20), p=0.007. B) Higher protein expression of VEGFR-2 p(Y951) was strongly correlated with increased angiogenic capacity, (r=0.7, p=0.001). Data are presented as mean ± SEM.
Depot-specific characterization of VEGF-A165a and VEGF-A165b in adipose tissue
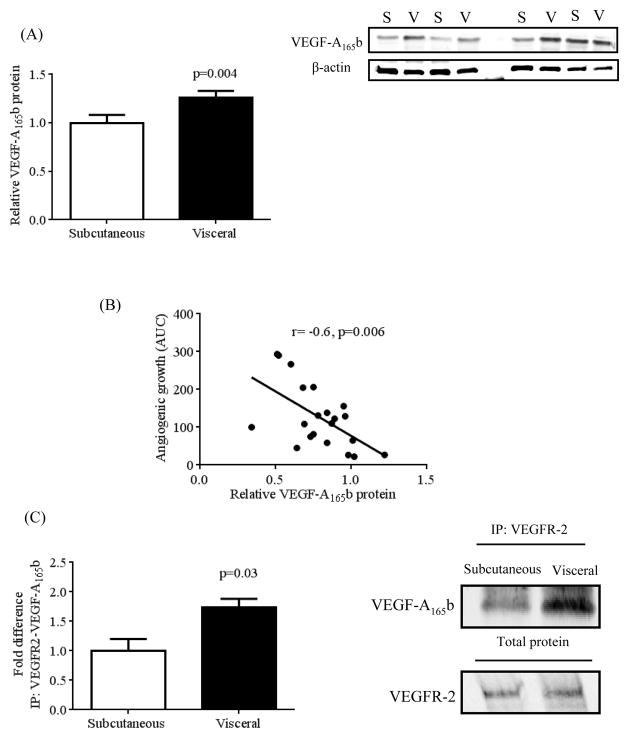
Altered VEGFR-2 signaling in visceral adipose tissue led us to characterize protein expression patterns of the major pro-angiogenic (VEGF-A165a) and the anti-angiogenic (VEGF-A165b) isoforms using isoform-specific antibodies. VEGF-A165a protein was not different between subcutaneous vs. visceral depots (p=0.8) (Supplemental figure 5), while VEGF-A165b expression was 1.3 ± 0.2 fold higher in visceral fat (p=0.004, n=23 per group) (Figure 4A). Furthermore, visceral adipose tissue VEGF-A165b expression was significantly higher in subjects with diabetes vs. non-diabetics (Supplemental figure 6). There was no correlation between VEGF-A165a expression and capillary growth, however adipose VEGF-A165b correlated significantly with lower angiogenic capacity (r= - 0.6, p=0.006) (Figure 4B), providing support that it negatively regulates vascular proliferation. Moreover, co-immunoprecipitation experiments demonstrated greater VEGF-A165b coupling with its receptor VEGFR-2 in visceral fat (Figure 4C), indicating higher VEGF-A165b occupancy of VEGFR-2 (p=0.03). These data led us to hypothesize that the anti-angiogenic action of VEGF-A165b predominated despite a seemingly “VEGF-A total abundant” adipose milieu.
Figure 4.
Differential protein expression of VEGF-A165b in subcutaneous vs. visceral adipose tissue. A) Visceral adipose tissue (n=23) exhibited significant up-regulation of VEGF-A165b protein expression vs. subcutaneous depot (n=23), p=0.004. B) Increased adipose tissue protein expression of VEGF-A165b correlated with reduced angiogenic capacity, (r= −0.6, p=0.006). C) Co-immunoprecipitation of VEGF-A165b and its receptor VEGFR-2 was significantly higher in visceral vs. subcutaneous adipose tissue (n=3 in each group, p=0.03). Data are presented as mean ± SEM.
Effect of neutralizing VEGF-A165b antibody on angiogenic responses
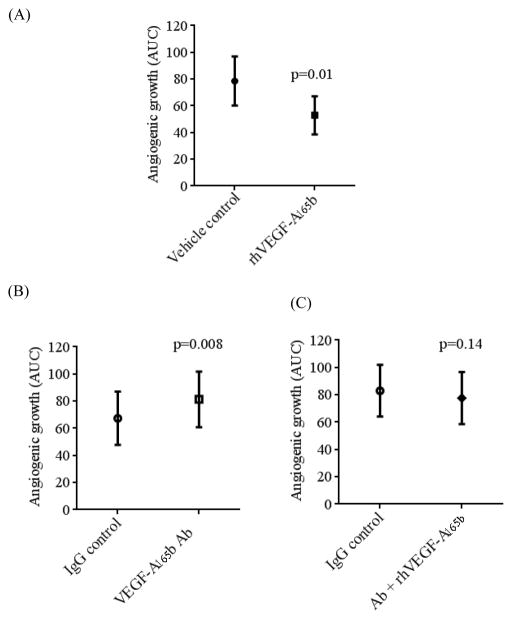
To confirm that VEGF-A165b is involved in angiogenic impairment, addition of human recombinant VEGF-A165b protein (rhVEGF-A165b) to culture media significantly reduced angiogenic growth of visceral adipose tissue (p=0.01, n=6) (Figure 5A). In contrast, treatment with a neutralizing VEGF-A165b antibody significantly bolstered angiogenesis in fat by 56 ± 29% vs. IgG control (p=0.008, n=10) (Figure 5B). Combining rhVEGF-A165b with VEGF-A165b antibody abolished the positive angiogenic effects of the neutralizing antibody (Figure 5C). These data provide strong support for the functional significance of VEGF-A165b as a negative modulator of angiogenesis in human fat.
Figure 5.
Effects of recombinant VEGF-A165b (rhVEGF-A165b) and VEGF-A165b neutralizing antibody (VEGF-A165b Ab) on angiogenesis in adipose tissue. A) Addition of human recombinant VEGF-A165b (rhVEGF-A165b) protein (500ng/mL) significantly reduced sprouting capacity of visceral adipose tissue explants vs. vehicle control (AUC: 79 ± 18 vs. 53 ± 14, n=6, respectively, p=0.01). B) VEGF-A165b neutralizing antibody (10μg/mL) (VEGF-A165b antibody) increased angiogenic capacity vs. IgG control (10μg/mL) (AUC: 67 ± 20 vs. 81 ± 20, n=10, respectively, p=0.008). C) Combination of VEGF-A165b Ab (10μg/mL) + rhVEGF-A165b (500ng/mL) reversed angiogenic potentiation of VEGF-A165b antibody vs. IgG control; (AUC: 83 ± 19 vs 78 ± 19, n=9, respectively, p=0.14). Data are presented as mean ± SEM.
Effect of VEGF-A165b neutralizing antibody on VEGFR-2 phosphorylation and ERK activation
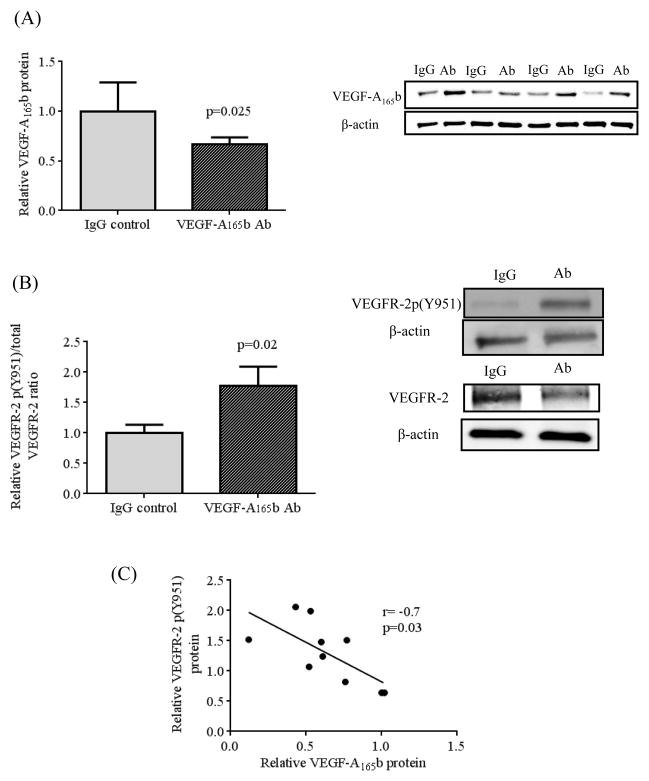
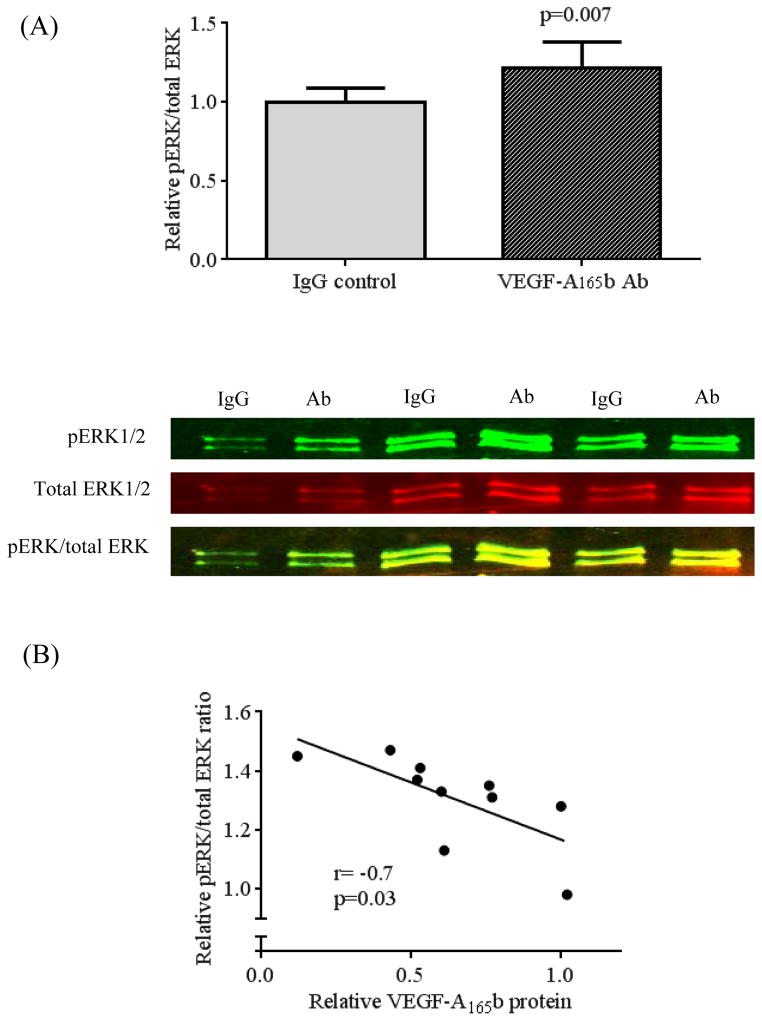
Incubation of whole visceral adipose tissue with VEGF-A165b neutralizing antibody significantly suppressed VEGF-A165b protein expression (p=0.025, n=10) (Figure 6A), without affecting VEGF-A165a protein (Supplemental Figure 7). Importantly, VEGF-A165b suppression via incubation with the neutralizing antibody increased VEGFR-2 p(Y951)/VEGFR-2 ratio (p=0.02) (Figure 6B). Furthermore, reduced VEGF-A165b expression correlated negatively with up-regulation of VEGFR-2 p(Y951) (r= −0.7, p=0.03) (Figure 6C). Restoration of phospho-VEGFR-2 and suppression of VEGF-A165b led to significant up-regulation of phosphor-ERK1/2 to total ERK1/2 ratio (p=0.007) (Figure 7A). Additionally, VEGF-A165b inhibition correlated significantly with activation of ERK1/2 phosphorylation (r= −0.7, p=0.03) (Figure 7B). These combined findings suggest a key role of VEGF-A165b in anti-angiogenic signaling in adipose tissue.
Figure 6.
Effects of VEGF-A165b neutralizing antibody on phosphorylation of VEGFR-2. A) Addition of VEGF-A165b neutralizing antibody significantly suppressed VEGF-A165b protein expression (n=10, p=0.025). B) Suppression of VEGF-A165b protein expression with VEGF- A165b neutralizing antibody increased VEGFR-2 p(y951) / VEGFR-2 (n=10, p=0.02) (B). Degree of VEGF-A165b suppression correlated significantly with higher protein expressions of VEGFR-2 p(Y951) (R=-0.7, p=0.03, n=10). Data are presented as mean ± SEM.
Figure 7.
Effects of VEGF-A165b neutralizing antibody on phosphorylation of ERK1/2. A) Suppression of VEGF-A165b protein expression with VEGF-A165b neutralizing antibody increased phospho-ERK1/2 (Y204/197) / total ERK1/2 ratio (n=10, p=0.007) (B). Degree of VEGF-A165b suppression correlated significantly with higher phospho ERK1/2 / total ERK1/2 ratio (R= −0.7, p=0.03, n=10). Data are presented as mean ± SEM.
Serum levels of VEGF isoforms in obesity and effect of weight loss
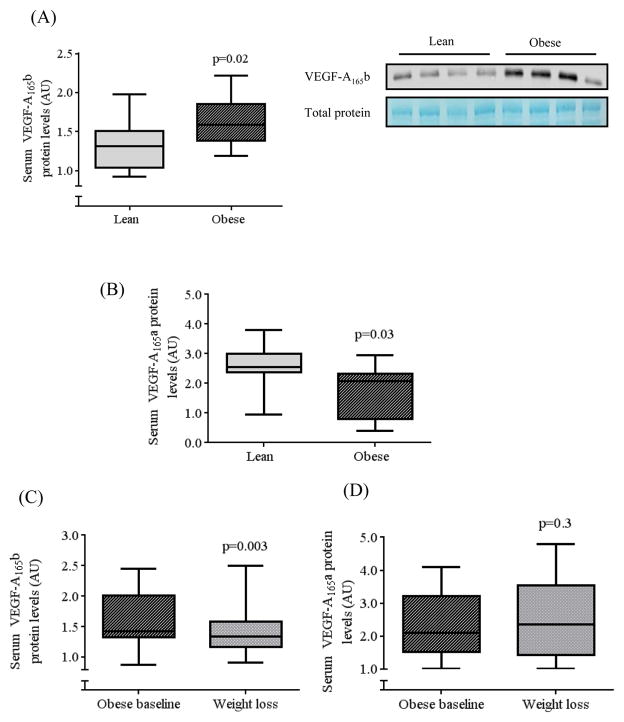
We compared serum levels of VEGF-A165b protein by western immunoblotting and demonstrated significantly higher anti-angiogenic VEGF-A165b protein levels in obese (n=19) compared to lean (n=15) subjects (p=0.02) (Figure 8A). In contrast, pro-angiogenic VEGF-A165a was significantly lower in obese (n=14) compared to lean subjects (n=11) (p=0.03) (Figure 8B). In a cohort of obese individuals that were followed prospectively for 10 ± 2 months following bariatric surgery, serum VEGF-A165b declined significantly (p=0.003, n=14) following a 30 ± 9 % weight reduction (Figure 8C) without significant change in VEGF-A165a (Figure 8D). In obese subjects, there was no significant difference in VEGF-A165b protein levels in diabetic (1.5 ± 0.2, n=10) vs. non-diabetics (1.4 ± 0.2 au, n=9). Clinical characteristics of lean subjects and obese individuals before and after weight loss are displayed in supplemental tables 1 and 2.
Figure 8.
Circulating levels of A) VEGF-A165b and B) VEGF-A165a in lean vs. obese patients, C/D) VEGF-A165b/a in obese subjects before vs. after weight loss. Lean subjects had significantly lower serum content of A) VEGF-A165b (p=0.02), and higher B) VEGF-A165a compared to obese subjects (p=0.03) by unpaired t-tests with equal variance. C) In obese patients, weight loss significantly reduced serum VEGF-A165b (p=0.003) (n=14), D) while there was no change in VEGF-A165a by paired t-tests.
DISCUSSION
We describe, for the first time, an endogenous anti-angiogenic isoform of VEGF-A, VEGF-A165b, that is over-expressed in human visceral fat and associated with impaired angiogenesis in adipose tissue. Targeted VEGF-A165b inhibition restored pro-angiogenic receptor VEGFR-2 phosphorylation and ERK activation, and stimulated angiogenesis in visceral fat pads, suggesting that this approach may promote vasculogenesis in fat and potentially influence metabolism. Importantly, we observed markedly elevated blood levels of VEGF-A165b in obese subjects which decreased significantly following bariatric surgical weight loss. This latter finding has potentially important clinical implications, as systemic up-regulation of a circulating anti-angiogenic factor in obesity may have wider ranging implications beyond the adipose milieu that could support pathological changes in other organ systems including coronary and peripheral circulations.23
Adequate vascularization and expansion of adipose tissue is critically important for whole body metabolic homeostasis.3 Strategies to limit adipose tissue growth and attempt to combat obesity with anti-angiogenic interventions have actually failed to confer any metabolic benefit.3,24 Rather, angiogenic insufficiency has been implicated in the development of adipose tissue dysfunction, hypoxia, capillary rarefaction, and inflammation that trigger metabolic dysregulation and insulin resistance.4–6 Experimental studies show that stimulating adipose vascularization improves insulin sensitivity, while VEGF-A deficiency promotes capillary dropout and metabolic dysfunction.10 As such, enhanced adipose tissue vascularization may serve as a therapeutic approach for the generation of healthy adipocytes 25 and preserved metabolism in the face of obesogenic stress, strengthening the growing paradigm that “quality” in addition to quantity of fat plays a significant role in shaping metabolic and cardiovascular phenotypes in human obesity.13, 20, 26
A major novel finding in our study is our identification of significant up-regulation of anti-angiogenic VEGF-A165b in the visceral fat of obese subjects. We performed a number of experiments confirming the pathological actions of VEGF-A165b in human tissue. We demonstrated that its up-regulation correlates negatively with capillary growth and recombinant VEGF-A165b significantly impairs angiogenesis. Conversely, targeted inhibition of VEGF-A165b with neutralizing antibody stimulated fat pad angiogenesis by activating VEGFR-2 phosphorylation without altering VEGF-A165a. Furthermore, we showed that suppression of VEGF-A165b correlated significantly with increased ERK phosphorylation, which has been shown to confer cellular proliferation and survival in endothelial cells.27 Taken together, these findings suggest that reduced VEGF-A165b may promote VEGF-A165a-VEGFR-2 interaction and activate pro-angiogenic signaling in fat, as previously demonstrated in non-adipose tissue. 14, 28 Our results therefore provide a potential mechanistic explanation for the seemingly paradoxical clinical observation of increased VEGF-A expression in the face of angiogenic impairment by demonstrating that the anti-angiogenic isoform VEGF-A165b may predominate in disease states. Compared with VEGF-A165a, very little is known about VEGF-A165b or its potential pathophysiological role in human disease, but it is expressed in a range of tissues including human microvascular endothelial cells,15, 29 kidney5, skeletal muscle5, pituitary15, and eyes.14 Its pathogenic role has been described in systemic sclerosis where VEGF-A165b neutralizing antibody significantly improved angiogenesis in human skin microvascular endothelial cells via activation of VEGFR-2 phosphorylation.29 Conversely, a potential therapeutic use has been explored in the treatment of proliferative diabetic retinopathy where VEGF-A165b significantly inhibited retinal neovascularization.30, 31 As alternative VEGF splicing is increasingly being linked to the etiology of specific cancers, clinical applications have also been considered for the prevention of tumor growth. 32
Relatively little is known about the regulation of VEGF-A alternative splicing. Insulin-growth factor 1 (IGF-1) and TNF-α promote formation of VEGF-A165a, whereas transforming growth factor-β (TGF-β) favors VEGF-A165b expression.33 IGF-1 modulates splicing of VEGF isoforms by preferential use of the proximal splice site to up-regulate pro-angiogenic expression 34 that may be modulated by via protein kinase C and SR protein kinases 1 and 2 (SRPK1/2) 33. On the other hand, TGF-β is a potent inducer of the splice isoform VEGF-A165b in both retinal epithelial and endothelial cells by increasing the splicing factor SRp55.29, 33 It remains unknown whether VEGF-A splicing is under metabolic control or whether metabolic dysregulation itself is a consequence of vascular dysfunction in human disease. In our study, we observed that the diabetic state was associated with up-regulated VEGF-A165b expression and blunted angiogenic capacity in visceral fat. While the “chicken or egg” dilemma remains, pharmacological treatment with rosiglitazone has been shown to simultaneously improve adipose tissue angiogenesis and insulin sensitivity.35 Furthermore, clinical imaging studies with computed tomography have linked qualitative measures of adipose tissue lipid and vascular density to adverse cardiometabolic profiles such as insulin resistance, hypertension, and dyslipidemia .36 It is thus becoming increasingly clear that qualitative features of adipose tissue including its vascularity could play an important role in the pathogenesis of obesity-induced cardiometabolic complications.
Another major finding in our study was our demonstration of markedly increased serum levels of anti-angiogenic VEGF-A165b in obese compared to lean subjects. Detection of VEGF-A165b in the systemic circulation raises the possibility that elevated VEGF-A165b may contribute to vascular disease and ischemia beyond the adipose environment. Impaired angiogenic regulation in key vascular territories including coronary, renal, cerebral, and peripheral beds have the potential to contribute to the clinical expression of coronary and peripheral arterial disease, renal dysfunction, and stroke. Importantly, we showed that marked weight loss induced by bariatric surgery, the only weight reduction intervention shown to reduce cardiovascular risk, 37 significantly lowers VEGF-A165b levels, demonstrating the feasibility of modulating anti-angiogenic isoforms with clinical intervention.
It remains unclear whether up-regulation of VEGF-A165b in visceral adipose tissue is pathogenic or a compensatory mechanism that regulates adipose remodeling. It is possible that analogous to a neoplastic process, growing adipose tissue is highly dependent on its vascular support, and VEGF-A165b over-expression may represent an endogenous mechanism to attempt to limit ectopic adipose expansion by depriving it of oxygen and nutrients to “the point of death”.38 As such, it is also possible that VEGF-A165b levels decrease with weight loss since the stimulus for angiogenesis is reduced with weight reduction as there is no longer a physiologic demand for adipose expansion. Lastly, VEGF-A165b may play a stabilizing role in the innate vasculature of other organs including coronary and peripheral circulation by curbing uncontrolled or immature angiogenesis, although essentially nothing is known about these processes in the context of human obesity.
Our study had several limitations. We did not have access to adipose tissue, particularly the visceral depot, from lean subjects thus we did not study angiogenesis or VEGF-A isoform expression in leans, which will be the topic of future investigation. Additionally, we were not able to re-biopsy visceral fat tissue in obese subjects after weight loss, since this would require a repeat invasive abdominal operation that cannot be justified without clinical indication. Although we have shown that VEGF-A165b exhibits anti-angiogenic actions in human adipose tissue, compensatory changes or isoform regulation of other angiogenic mediators in fat such as angiopoietin 47 and thrombospondin-139 were not examined. Lastly, we did not comprehensively investigate all VEGF-A isoforms and acknowledge that other anti-angiogenic isoforms may exist that modulate adipose tissue angiogenesis.
In conclusion, we demonstrate over-expression of a novel anti-angiogenic factor VEGF- A165b that may play a key pathogenic role in obesity-related cardiovascular and metabolic disease. VEGF-A165b may represent a novel area of investigation and potential intervention to modulate angiogenesis in human adipose tissue and possibly cardiometabolic consequences of obesity.
Supplementary Material
Acknowledgments
We would like to acknowledge assistance with microscopy from Dr Michael Kirber and the Cellular Imaging Core of Boston University.
Funding Sources: Dr. Gokce is supported by National Institutes of Health (NIH) grants HL081587, HL1145675, and HL084213. Dr. Farb is supported by an American Heart Association Postdoctoral Fellowship grant 12POST11780028. Dr. Karki is supported by NIH grant T32 HL07224. Dr. Hamburg is supported by NIH grants HL109790 and HL102299. Dr. Vita is supported by NIH grants HL081587, HL083801, HL083269, HL75795, and K12 HL083781. Dr. Walsh is supported by NIH grants P01 HL081587, HL068758, and AG034972.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 4.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 5.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–4055. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of vegf-a on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: Mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 12.Villaret A, Galitzky J, Decaunes P, Esteve D, Marques MA, Sengenes C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumie A. Adipose tissue endothelial cells from obese human subjects: Differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol. 2012;32:467–473. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO. Vegf165b, an inhibitory vascular endothelial growth factor splice variant: Mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 15.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. Vegf165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 16.Harper SJ, Bates DO. Vegf-a splicing: The key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rennel ES, Harper SJ, Bates DO. Therapeutic potential of manipulating vegf splice isoforms in oncology. Future Oncol. 2009;5:703–712. doi: 10.2217/fon.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, Sugiono M, Gillatt D, Kleinerman E, Bates D, Harper S. The endogenous anti-angiogenic vegf isoform, vegf165b inhibits human tumour growth in mice. Brit J Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO. Expression of vegf(xxx)b, the inhibitory isoforms of vegf, in malignant melanoma. Brit J Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, Gokce N. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D'Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protocols. 2012;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Mugishima H. Signal transduction via vascular endothelial growth factor (vegf) receptors and their roles in atherogenesis. J Atheroscler Thromb. 2006;13:130–135. doi: 10.5551/jat.13.130. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz MB, Biyikoglu SF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27:1541–1545. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 24.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, tnp-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 25.Corvera S, Gealekman O. Adipose tissue angiogenesis: Impact on obesity and type-2 diabetes. Biochimica et Biophysica acta. 2014;1842:463–72. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roskoski R., Jr Erk1/2 map kinases: Structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Delcombel R, Janssen L, Vassy R, Gammons M, Haddad O, Richard B, Letourneur D, Bates D, Hendricks C, Waltenberger J, Starzec A, Sounni NE, Noel A, Deroanne C, Lambert C, Colige A. New prospects in the roles of the c-terminal domains of vegf-a and their cooperation for ligand binding, cellular signaling and vessels formation. Angiogenesis. 2013;16:353–371. doi: 10.1007/s10456-012-9320-y. [DOI] [PubMed] [Google Scholar]
- 29.Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Impaired angiogenesis in systemic sclerosis: The emerging role of the antiangiogenic vegf(165)b splice variant. Trends Cardiovasc Med. 2011;21:204–210. doi: 10.1016/j.tcm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. Vegf165b, an endogenous c-terminal splice variant of vegf, inhibits retinal neovascularization in mice. Mol Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- 31.Magnussen AL, Rennel ES, Hua J, Bevan HS, Beazley Long N, Lehrling C, Gammons M, Floege J, Harper SJ, Agostini HT, Bates DO, Churchill AJ. Vegf-a165b is cytoprotective and antiangiogenic in the retina. Invest Ophthalmol Vis Sci. 2010;51:4273–4281. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates DO, Harper SJ. Therapeutic potential of inhibitory vegf splice variants. Future Oncol. 2005;1:467–473. doi: 10.2217/14796694.1.4.467. [DOI] [PubMed] [Google Scholar]
- 33.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, Bates DO. Regulation of vascular endothelial growth factor (vegf) splicing from pro-angiogenic to anti-angiogenic isoforms: A novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of vegf is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gealekman O, Guseva N, Gurav K, Gusev A, Hartigan C, Thompson M, Malkani S, Corvera S. Effect of rosiglitazone on capillary density and angiogenesis in adipose tissue of normoglycaemic humans in a randomised controlled trial. Diabetologia. 2012;55:2794–2799. doi: 10.1007/s00125-012-2658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging. 2013;6:762–771. doi: 10.1016/j.jcmg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz M, Hotamisligil GS. Damned if you do, damned if you don't: The conundrum of adipose tissue vascularization. Cell Metab. 2013;17:7–9. doi: 10.1016/j.cmet.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Tan BK, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, Lehnert H, Randeva HS. Metformin decreases angiogenesis via nf-kappab and erk1/2/erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res. 2009;83:566–574. doi: 10.1093/cvr/cvp131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.