Abstract
Background
There has been a recent increase in availability of banked donor milk for feeding of preterm infants. This milk is pooled from donations to milk banks from carefully screened lactating women. The milk is then pasteurized by the Holder method to remove all microbes. The processed milk is frozen, banked, and sold to neonatal intensive care units (NICUs). The nutrient bioavailability of banked donor milk has been described, but little is known about preservation of immune components such as cytokines, chemokines, and growth factors (CCGF).
Objective
The objective was to compare CCGF in banked donor milk with mother's own milk (MOM).
Methods
Aliquots (0.5 mL) were collected daily from MOM pumped by 45 mothers of NICU-admitted infants weighing < 1500 grams at birth. All daily aliquots of each mother's milk were pooled each week during 6 weeks of an infant's NICU stay or for as long as the mother provided MOM. The weekly pooled milk was measured for a panel of CCGF through multiplexing using magnetic beads and a MAGPIX instrument. Banked donor milk samples (n = 25) were handled and measured in the same way as MOM.
Results
Multiplex analysis revealed that there were levels of CCGF in banked donor milk samples comparable to values obtained from MOM after 6 weeks of lactation.
Conclusion
These data suggest that many important CCGF are not destroyed by Holder pasteurization.
Keywords: breastfeeding, chemokines, cytokines, donor milk, growth factors
Well Established
The availability of banked donor milk is increasing in neonatal intensive care, to replace formula use or supplement mother's own milk (MOM). Banked donor milk is Holder pasteurized, which destroys cells and some proteins.
Newly Expressed
Cytokines, chemokines, and growth factors were present in banked donor milk. The levels of most cytokines and chemokines in preterm MOM during the early weeks of lactation were higher than in banked donor milk.
Background
The American Academy of Pediatrics Recommendations of Breastfeeding Management for Preterm Infants state that all preterm infants should receive human milk and if mother's own milk (MOM) is unavailable, pasteurized donor human milk, appropriately fortified, should be used.1 Recently, there has been an escalation in the use of banked donor milk in neonatal intensive care units (NICUs), rising from 25.1% nationally in 2007 to 45.2% in 2011 (P < .001).2 This steady increase reached an all-time high of 2.15 million ounces of human banked donor milk dispensed by the Human Milk Banking Association of North America (https://www.hmbana.org/donate-milk) in 2011.3
The literature describes benefits of banked donor milk versus formula, but there are far fewer studies comparing banked donor milk to MOM. Milk produced by donor milk banks is pasteurized by the Holder method (62.5°C for 30 minutes) to destroy harmful bacteria and viruses.3 It is then pooled, packaged, and sold to hospitals for use in their NICUs. The banked donor milk available for purchase from milk banks is pooled from several donors and is more likely to be from mothers who delivered term versus preterm infants.4 This is an important distinction as preterm MOM (milk produced by mothers delivering infants at less than 37 weeks gestation) is qualitatively different from term MOM (milk produced by mothers delivering at or after 37 weeks).
Holder pasteurization not only destroys bacteria, viruses, and cells but also destroys or significantly reduces levels of immune proteins such as secretory Immunoglobulin A (sIgA).5 Immunoglobulin A, the major antibody in human milk, showed a 45% reduction after pasteurization.6,7 Lactoferrin, lysozyme, and bile salt-stimulated lipase in milk are also significantly reduced by Holder pasteurization.8 Heat denaturation of proteins could reduce the concentrations of other immune molecules such as cytokines, chemokines, and growth factors (CCGF), but the effects of only a few of these have been measured.
There are few studies comparing immune components of banked donor milk with those of MOM.9 The available research was reviewed in 2011.5 Microbiota, cells, immunoglobulins, lysozyme, lactoferrin, and oligosaccharides in human milk were reported to be reduced after Holder pasteurization, but only 3 studies analyzing a limited number of CCGF had been done.6,10,11 Since 2011, only 3 additional studies have addressed differences in CCGF between banked donor milk and MOM.9,12,13 Cytokines, chemokines, and growth factors in milk are believed to play important roles in gastrointestinal and immune development of the recipient infant.14 They affect immune modulation, maturation, and integrity of the gastrointestinal tract as well as control of inflammation in the developing recipient infant.15 The chemokines play a role in cellular chemoattraction and activation of neutrophils, monocytes, and lymphocytes.15 Cytokines, chemokines, and growth factors probably prime intestinal immune cells, contribute to angiogenesis, help develop the intestinal epithelial barrier function, and generally suppress inflammation.16 These effects may be even more important when infants are born preterm and therefore have limited in utero development of their physiological systems.16
As banked donor milk becomes more available and is used more widely for the preterm infant, the possible effects of Holder pasteurization on a variety of immune molecules could translate into less protection for infants receiving only banked donor milk or large quantities of banked donor milk compared with MOM. Preterm infants' risks for necrotizing enterocolitis,17 sepsis,18 and adverse neurodevelopment19 are significantly reduced when infants receive human milk, but these protective effects could be affected by extensive or exclusive use of banked donor milk if there are lower levels of critical immune molecules.
The goal of this study was to compare levels of CCGF in banked donor milk versus those in MOM from mothers of preterm infants.
Methods
Data Collection
The data were collected during the course of a larger study of biological and health outcomes in the most fragile preterm infants (< 1500 grams) in relationship to volume of MOM received and exposure to selected MOM CCGF. The study was approved by the Tampa General Hospital and the University of South Florida Institutional Review Board, and all mothers who donated MOM gave informed consent. Mothers of preterm infants with birth weights less than 1500 grams were consented as soon as possible after their newborns were admitted to the hospital NICU, and the first milk samples were collected by the end of the first week of the admission. Exclusion criteria were moribund status, major congenital anomalies in the infant, and HIV positive status of mothers.
Mothers completed a demographic questionnaire and additional infant data (medical complications, weight, feeding, date of discharge) were extracted from patients' medical and nursing records during the 6 weeks of data collection. All mothers were encouraged to lactate and provide milk throughout their infants' stay in the NICU, according to the usual practice in the study NICU. The infant's nurse collected a 0.5-mL aliquot from every batch of MOM used for feeding each day before fortifier, formula, or banked donor milk was added. Milk samples were drawn into a tuberculin syringe by the nurse, labeled, and frozen at −20°C until pickup and delivery to the lab within 2 to 3 days. The syringes of milk were then frozen at −80°C until thawed in batches for analysis. This practice continued for 6 weeks, or less if infants were discharged. Some infants received only banked donor milk throughout their NICU stay. Others had banked donor milk added to MOM for some feedings. Any banked donor milk was drawn into separate syringes as described above before being mixed with MOM for that feeding so that it could be measured separately for CCGF. Daily volumes of MOM, MOM with fortifier, formula, or banked donor milk were computed.
The banked donor milk was purchased by the study hospital from 2 nonprofit milk banks in Northern Texas. This milk was shipped frozen in containers and thawed and measured as needed for infant feeds. Mothers who donate milk to these banks must be healthy, breastfeeding a baby younger than 1 year of age, nonsmoking, not using illegal drugs, and not drinking more than 2 alcoholic drinks per day, and they must have blood tests that are negative for HIV, human T-cell lymphotropic virus, hepatitis B and C, and syphilis. Although most mothers have given birth to term infants, 1 of the banks accepts milk donations from mothers of preterm infants. The milk is pooled so that the banked donor milk that is provided is a mixture of donated milks, and even more mixing could occur in the hospital when the feedings are prepared.
MOM and Banked Donor Milk
The frozen syringes containing daily samples of each mother's MOM and frozen syringes containing samples of any banked donor milk that were used in daily feedings were thawed and separately pooled at the end of each week. The pooled samples of MOM or banked donor milk were centrifuged at 1000 g at 4°C for 10 minutes and defatted by carefully removing the fat layer at the top with a Corning small spoon (Fisher Catalog No. 3004; ThermoFisher Scientific, Waltham, Massachusetts, USA.) The whey fraction was then filtered through a 0.45-μm Millipore low protein (ThermoFisher Scientific) binding PVDF filter (Fisher Catalog No. SLHVM23N S,) and stored at −80°C in 2-mL Eppendorf tubes until analysis.
This weekly pooling of milks along with weekly volume calculations provided a way to exactly calculate the weekly exposure of each infant to CCGF. Although MOM samples were frozen, thawed, and frozen again, our previous research has shown that up to 3 freeze–thaw cycles do not produce significant decreases in milk CCGF levels (Groer, unpublished data). Similar results regarding freezing were reported recently.20
Multiplexing
Cytokines, chemokines, and growth factors in the whey from each weekly, pooled sample were measured through multiplexing with the use of magnetic bead kits (EMD Millipore, Billerica, Massachusetts, USA) according to kit directions and analyzed on a MAGPIX (Luminex, Austin, Texas, USA). Multiplexing allows for quantitative measurements of multiple analytes in a small volume of fluid (25 μL) and is based on attachment of the CCGF to magnetic beads and processing using LED excitation. The MAGPIX machine is calibrated and the kits' standards and controls are used to determine the values in pg/mL of the CCGF. Before analysis, a series of experiments was done by spiking known concentrations of CCGF in different matrix solutions to optimize the multiplex assay. The matrix solution that produced the best recovery (up to 100% for some CCGF) was used for all subsequent analysis. This was a commercial serum matrix used for serum and plasma analyses and was purchased from Millipore and added to standards, controls, and samples. The CCGF analyzed were epidermal growth factor (EGF), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), macrophage inflammatory protein-α (MIP-1α), monocyte chemotactic protein (MCP), and interferon gamma inducible protein-10 (IP-10). This panel of cytokines, chemokines, and growth factors was chosen based on our preliminary data showing that the levels of these proteins are significantly different in preterm versus term milk and they are all reported to be biologically active in human milk.21
Statistics
The levels of CCGF were compared between MOM at each lactational week and the pooled banked donor milk. The data for both sample groups were not normally distributed, thus differences were analyzed by Mann–Whitney U tests. To compare changes in MOM CCGF levels from week 1 to week 6, the CCGF were log 10 transformed and t tests between weeks 1 and 6 were performed for each protein. A P value of .05 was accepted for statistical significance and SPSS, version 21, was used for analysis. Graphs of cytokines and growth factors depict the log10 on the vertical axis due to the large differences in concentrations between EGF and the chemokines.
Results
Demographic Characteristics
Demographic data are not available for the multiple mothers who donated milk to the milk banks. Forty-five MOM samples were included in the analysis, measured prospectively over the first 6 weeks of a NICU stay. The majority of mothers were enrolled at the end of their first postpartum week (n = 35), 7 were enrolled into the study after their second week, and 3 after 3 weeks. The majority of mothers were high school graduates (58%), single (49%), poor (income less than $25 000/year) (53%), and primiparous (34%). Twenty-five percent were smokers, and 4% self-reported illegal drug use. The mean maternal body mass index was 27.6 ± 6.4. The racial composition was 54% Caucasian, 41% African American, 2% Asian American, and 2% other. Twenty-four percent self-reported as Hispanic. The 2-minute APGAR (Appearance, Pulse, Grimace, Activity, Respiration) score was 5.93 and the 5-minute APGAR score was 7.9. The mean birth weight was 1107 ± 223 grams and mean gestational age was 28.3 ± 2.33 weeks gestation, and 50% of infants were male.
Samples were collected from the end of week 1 through the end of week 6 of the infant's stay in the NICU or for as long as mothers continued to provide MOM up through 6 weeks. Twenty-five weekly pooled aliquots of daily banked donor milk were analyzed and results were compared with those from 196 pooled weekly aliquots of MOM (which represented nearly 2000 samples of daily milk collections). The numbers of mothers providing MOM declined over time due to discharge before 6 weeks (N = 10), neonatal death (n = 2), and change from MOM to formula (n = 9) or to banked donor milk (n = 8) between weeks 2 and 6.
Volumes of Milk Produced over Time
The volumes of MOM produced by the mothers over the 6 weeks of data collection increased over time (< 500 mL/week at week 1 to > 1500 mL/week at week 6) (Figure 1).
Figure 1.

Mothers' Own Milk (MOM) Volumes (mL/wk) over 6 Weeks of Neonatal Intensive Care Unit (NICU) Stay.
Values shown are mL of MOM produced over 1-week time spans beginning from the end of week 1 to the end of week 6 of a NICU stay. Bars are standard errors of the mean.
CCGF in Banked Donor Milk versus MOM
In the first week, there were statistically significant differences between MOM and banked donor milk for all but 3 CCGF (IL-4, EGF, MIP-1α). By week 6, the only CCGF levels that were significantly different were MIP-1α and TNF-α. All other CCGF levels were not significantly different in MOM at 6 weeks versus banked donor milk (Table 1).
Table 1.
Comparison of CCGF in Preterm Mothers' Own Milk, Collected from Weeks 1 to 6 of a NICU Stay, to Banked Donor Milk.a
| CCGF | IL-10 | IL-4 | IL-6 | TNF-α | EGF | IP-10 | MCP-1 | MIP-1α | IL-8 |
|---|---|---|---|---|---|---|---|---|---|
| Week 1 (N = 44) | −3.02c | −0.91 | −4.3d | −5.13d | 1.13 | −3.88d | −3.32d | −1.35 | −4.21d |
| Week 2 (N = 36) | −2.47b | −1.37 | −3.17d | −4.3d | −1.26 | −3.61d | −2.4b | −0.253 | −2.08b |
| Week 3 (N = 36) | −0.575 | −1.23 | −2.33b | −3.94d | −1.07 | −3.26d | −1.67 | −1.67 | −1.8 |
| Week 4 (N = 33) | −0.628 | −0.436 | −1.84 | −3.56d | −0.36 | −3.2d | −1.37 | −1.99b | −1.12 |
| Week 5 (N = 26) | −0.134 | −0.024 | 0.801 | −2.48b | −0.27 | −1.69 | −0.452 | −1.6 | −0.63 |
| Week 6 (N = 20) | −0.012 | −0.095 | −0.549 | −2.25b | −0.289 | −0.754 | −0.297 | −2.14b | −0.89 |
Abbreviations: CCGF, cytokines, chemokines, and growth factors; EGF, epidermal growth factor; IL, interleukin; IP, interferon gamma inducible protein; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; NICU, neonatal intensive care unit; TNF, tumor necrosis factor.
Data are Mann–Whitney U Z values in preterm MOM CCGF compared to donor milk CCGF (N = 25).
P < .05.
P < .01.
P < .001.
Figure 2 depicts the mean levels of the 4 cytokines from weeks 1 to 6 for MOM and the levels in banked donor milk. The level of each cytokine generally decreased over time, but the difference between week 1 and week 6 was statistically significant only for TNF-α (t = 4.38, df = 62, P < .001) and IL-6 (t = 5.28, df = 62, P < .001). Figure 3 depicts the log10 of chemokine and growth factor means from weeks 1 to 6 for MOM and the levels in banked donor milk. There was a significant decrease from week 1 to week 6 in the levels of each chemokine except EGF (MIP-1α [t = 3.17, df = 62, P < .002], IP-10 [t = 3.309, df = 62, P < .004], IL-8 [t = 3.92, df = 62, P < .001], and MCP-1 [t = 4.07, df = 62, P < .001]).
Figure 2.

Means of Cytokines from Preterm Mothers' Milk over Time Compared to Banked Donor Milk.
Error bars represent standard errors of the means.
Figure 3.
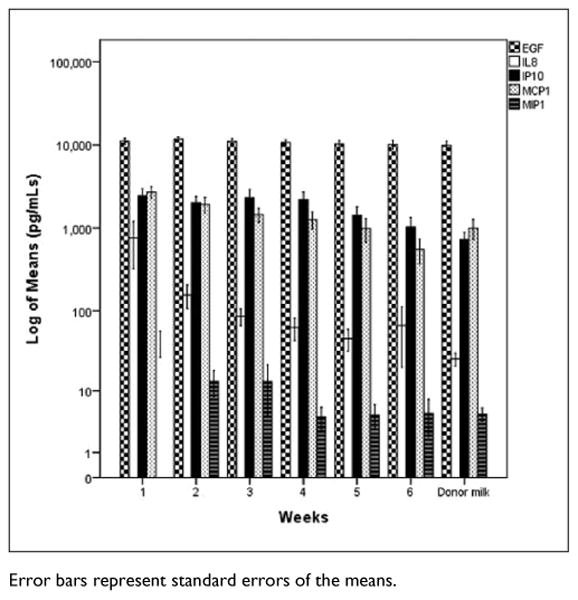
Log10 of Chemokine and Growth Factor Means in Preterm Mothers' Milk over Time Compared to Banked Donor Milk.
Error bars represent standard errors of the means.
Discussion
The data indicate that banked donor milk subjected to Holder pasteurization contains measurable levels of CCGF. Although this finding is positive, banked donor milk CCGF concentrations are more equivalent to those in MOM produced after 6 weeks of lactation than in earlier milk. Thus, preterm infants who receive banked donor milk versus MOM during their first weeks of life are receiving less of these potentially critical factors.
Holder pasteurization is the method of choice for sterilizing human milk, and the CCGF we measured appeared to have some heat resistant properties. Although they were not denatured, it is not clear if they remained functional. These data are in agreement with a recent publication of a study that used multiplexing of many of the same CCGF.22 The investigators reported measurable CCGF in both Holder pasteurized colostrum and mature milk in a smaller sample, although their reported median values of CCGF levels were different from ours, which may be related to matrix effects. We have measured analytes in many human milk samples by multiplexing and observed significant matrix effects on measurement values when using the manufacturer's (Millipore) kits and matrix solutions. Because the results can be significantly affected by the matrix solution, we carried out a series of experiments with a variety of matrix solutions and have reported on the achievement of a successful milk matrix for multiplexing.23 The successful matrix was serum matrix from Millipore added to controls, standards, and samples. The data presented here were the results of multiplexing with the matrix protocol referenced above. Matrix notwithstanding, our findings confirm those of the Espinosa-Martos et al22 study of a similar panel of CCGF in human milk after Holder pasteurization.
Banked donor milk is increasingly being used to feed pre-term infants in lieu of formula because there is good evidence that it has protective properties similar to those of MOM.24 There are, however, unknown characteristics of banked donor milk (maternal factors, heat resistant viruses, developmental stages of lactation, processing, pooling, freezing) that might influence its bioefficacy and safety.25 Neonatal intensive care units make decisions about when to institute and discontinue banked donor milk, based at least in part on the cost/benefit ratio of banked donor milk.26,27 For example, in the study NICU, banked donor milk is stopped and formula instituted around 34 weeks gestational age, a point at which the risk for necrotizing enterocolitis and infection are greatly reduced.28
Our data suggest that banked donor milk may not be developmentally appropriate in terms of CCGF during the early weeks of life in low birth weight infants, but it is superior to formula, which contains no CCGF. Although not confirmed, it is plausible that critical periods in preterm infant development are aligned with milk biology.25 We and others show a decline in cytokines over time of lactation, which may be timed to coincide with maturation of the neonatal gut and immune system.29-32
We did not measure MOM CCGF from mothers of full-term infants in the current study and do not know if the mature milk produced by mothers of preterm infants at 6 weeks postpartum differs from milk produced by mothers of term infants. It is not known if preterm milk CCGF levels differ from those in term milk over longer periods of time as most comparative studies have been done on colostrum or early milk. In addition, there are few studies that follow pre-term milk composition after discharge from the NICU and compare it to term milk. A prospective study of milk from mothers reported that protein levels were higher in preterm milk over 8 weeks of lactation.33 In a recent Korean study, the composition of milk to 3 months postpartum was compared between mothers who delivered preterm and a cohort of term mothers, and some differences in milk fatty acid composition were described.34 In another study, beta-endorphin levels at 30 days postpartum did not differ between pre-term and term milk.35
Limitations
This study was developed because of an opportunity to compare CCGF levels in banked donor milk and MOM. Other immune components and CCGF would be of interest to study in the future, as this work is really an initial exploratory effort to compare these milks. Clarifying the roles of these CCGF in normal infant development will require much more research before specific clinical recommendations could be made.
The banked donor milk was prepared at milk banks for human consumption. Although we assume that it was pasteurized using the standard Holder pasteurization, we were not involved in this process, and we also have no information about the demographic characteristics of the donors. Banked donor milk also went through more freeze–thaw cycles than MOM, which may have further reduced CCGF concentrations. Another limitation is that both banked donor milk and MOM had variability in their measures as indicated by the standard error bars in Figures 2 and 3. The source of this variability for MOM will be examined in future work.
Conclusion
Neonatal intensive care units are using banked donor milk with greater frequency,36 so it is encouraging that some essential immune components of milk, namely, cytokines, chemokines, and growth factors, remain at levels comparable to nonpasteurized mature milk from preterm mothers. Most cytokine and chemokine levels declined in MOM, whereas epidermal growth factor levels remained stable. By the end of the sixth week of lactation, most cytokine and chemokine levels were similar between MOM and banked donor milk, which is donated primarily by mothers who deliver term infants. Preterm infants fed exclusively or predominantly with banked donor milk during the first weeks of life will receive lower levels of these immune components than if fed MOM.
Acknowledgments
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Institutes of Health (R21NR3094).
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.The American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 2.Perrine CG, Scanlon KS. Prevalence of use of human milk in US advanced care neonatal units. Pediatrics. 2013;131(6):1066–1071. doi: 10.1542/peds.2012-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Updegrove K. Nonprofit human milk banking in the United States. J Midwifery Womens Health. doi: 10.1111/j.1542-2011.2012.00267.x. published online July 29, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Edwards TM, Spatz DL. Making the case for using donor human milk in vulnerable infants. Adv Neonatal Care. 2012;12(5):273–278. doi: 10.1097/ANC.0b013e31825eb094. quiz 279-280. [DOI] [PubMed] [Google Scholar]
- 5.Ewaschuk JB, Unger S, Harvey S, O'Connor DL, Field CJ. Effect of pasteurization on immune components of milk: implications for feeding preterm infants. Appl Physiol Nutr Metab. 2011;36(2):175–182. doi: 10.1139/h11-008. [DOI] [PubMed] [Google Scholar]
- 6.McPherson RJ, Wagner CL. The effect of pasteurization on transforming growth factor alpha and transforming growth factor beta 2 concentrations in human milk. Adv Exp Med Biol. 2001;501:559–566. doi: 10.1007/978-1-4615-1371-1_70. [DOI] [PubMed] [Google Scholar]
- 7.Braga LP, Palhares DB. Effect of evaporation and pasteurization in the biochemical and immunological composition of human milk. J Pediatr (Rio J) 2007;83(1):59–63. doi: 10.2223/JPED.1578. [DOI] [PubMed] [Google Scholar]
- 8.Christen L, Lai CT, Hartmann PE. Ultrasonication and the quality of human milk: variation of power and time of exposure. J Dairy Res. 2012;79(3):361–366. doi: 10.1017/S0022029912000246. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa-Martos I, Montilla A, de Segura AG, et al. Bacteriological, biochemical, and immunological modifications in human colostrum after Holder pasteurisation. J Pediatr Gastroenterol Nutr. 2013;56(5):560–568. doi: 10.1097/MPG.0b013e31828393ed. [DOI] [PubMed] [Google Scholar]
- 10.Untalan PB, Keeney SE, Palkowetz KH, Rivera A, Goldman AS. Heat susceptibility of interleukin-10 and other cytokines in donor human milk. Breastfeed Med. 2009;4(3):137–144. doi: 10.1089/bfm.2008.0145. [DOI] [PubMed] [Google Scholar]
- 11.Goelz R, Hihn E, Hamprecht K, et al. Effects of different CMV-heat-inactivation-methods on growth factors in human breast milk. Pediatr Res. 2009;65(4):458–461. doi: 10.1203/PDR.0b013e3181991f18. [DOI] [PubMed] [Google Scholar]
- 12.Reeves AA, Johnson MC, Vasquez MM, Maheshwari A, Blanco CL. TGF-β2, a protective intestinal cytokine, is abundant in maternal human milk and human-derived fortifiers but not in donor human milk. Breastfeed Med. 2013;8(6):496–502. doi: 10.1089/bfm.2013.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewaschuk JB, Unger S, O'Connor DL, et al. Effect of pasteurization on selected immune components of donated human breast milk. J Perinatol. 2011;31(9):593–598. doi: 10.1038/jp.2010.209. [DOI] [PubMed] [Google Scholar]
- 14.Oddy WH. The impact of breastmilk on infant and child health. Breastfeed Rev. 2002;10(3):5–18. [PubMed] [Google Scholar]
- 15.Garofalo R. Cytokines in human milk. J Pediatr. 2010;156(suppl 2):S36–S40. doi: 10.1016/j.jpeds.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103(6, pt 1):1150–1157. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 19.Vohr BR, Poindexter BB, Dusick AM, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120(4):e953–e959. doi: 10.1542/peds.2006-3227. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Santana C, Perez-Cano FJ, Audi C, et al. Effects of cooling and freezing storage on the stability of bioactive factors in human colostrum. J Dairy Sci. 2012;95(5):2319–2325. doi: 10.3168/jds.2011-5066. [DOI] [PubMed] [Google Scholar]
- 21.Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. 2013;45(8):1730–1747. doi: 10.1016/j.biocel.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Espinosa-Martos I, Montilla A, Segura AG, et al. Bacteriological, biochemical, and immunological modifications in human colostrum after holder pasteurisation. J Pediatr Gastroenterol Nutr. 2013;56(5):560–568. doi: 10.1097/MPG.0b013e31828393ed. [DOI] [PubMed] [Google Scholar]
- 23.Groer M, Kane B, Williams S. Multiplexing of human preterm and term cytokines. Poster presented at: Experimental Biology Conference; April 21, 2013; Boston, MA. [Google Scholar]
- 24.Heiman H, Schanler RJ. Benefits of maternal and donor human milk for premature infants. Early Hum Dev. 2006;82(12):781–787. doi: 10.1016/j.earlhumdev.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Menon G, Williams TC. Human milk for preterm infants: why, what, when and how? Arch Dis Child Fetal Neonatal. 2013;98(6):F559–F562. doi: 10.1136/archdischild-2012-303582. [DOI] [PubMed] [Google Scholar]
- 26.Arnold LD. The cost-effectiveness of using banked donor milk in the neonatal intensive care unit: prevention of necrotizing enterocolitis. J Hum Lact. 2002;18(2):172–177. doi: 10.1177/089033440201800210. [DOI] [PubMed] [Google Scholar]
- 27.Jegier BJ, Johnson TJ, Engstrom JL, Patel AL, Loera F, Meier P. The institutional cost of acquiring 100 mL of human milk for very low birth weight infants in the neonatal intensive care unit. J Hum Lact. 2013;29(3):390–399. doi: 10.1177/0890334413491629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40(1):27–51. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ustundag B, Yilmaz E, Dogan Y, et al. Levels of cytokines (IL-1β, IL-2, IL-6, IL-8, TNF-α) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators Inflamm. 2005;2005(6):331–336. doi: 10.1155/MI.2005.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kverka M, Burianova J, Lodinova-Zadnikova R, et al. Cytokine profiling in human colostrum and milk by protein array. Clin Chem. 2007;53(5):955–962. doi: 10.1373/clinchem.2006.077107. [DOI] [PubMed] [Google Scholar]
- 31.Hawkes JS, Bryan DL, James MJ, Gibson RA. Cytokines (IL-1beta, IL-6, TNF-alpha, TGF-beta1, and TGF-beta2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res. 1999;46(2):194–199. doi: 10.1203/00006450-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Castellote C, Casillas R, Ramirez-Santana C, et al. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr. 2011;141(6):1181–1187. doi: 10.3945/jn.110.133652. [DOI] [PubMed] [Google Scholar]
- 33.Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr. 2011;30(2):215–220. doi: 10.1016/j.clnu.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Jang SH, Lee BS, Park JH, et al. Serial changes of fatty acids in preterm breast milk of Korean women. J Hum Lact. 2011;27(3):279–285. doi: 10.1177/0890334411405059. [DOI] [PubMed] [Google Scholar]
- 35.Zanardo V, Nicolussi S, Carlo G, et al. Beta endorphin concentrations in human milk. J Pediatr Gastroenterol Nutr. 2001;33(2):160–164. doi: 10.1097/00005176-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Delfosse NM, Ward L, Lagomarcino AJ, et al. Donor human milk largely replaces formula-feeding of preterm infants in two urban hospitals. J Perinatol. 2013;33(6):446–451. doi: 10.1038/jp.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]


