Abstract
Aims
Epidemiological studies report that individuals who exercise are less likely to abuse drugs. Preclinical studies report that exercise, in the form of treadmill or wheel running, reliably decreases the self-administration of psychomotor stimulants and opioids. To date, preclinical studies have only examined the effects of exercise on responding maintained by individual drugs and not by combinations of multiple drugs. This limits the translational appeal of these studies because polydrug abuse is common among substance abusing populations. The purpose of this study was to examine the effects of exercise on the self-administration of speedball, a combination of cocaine and heroin that is frequently encountered in intravenous drug abusing populations.
Materials and Methods
Female rats were obtained at weaning and assigned to sedentary or exercising conditions. Sedentary rats were housed in standard cages that permitted no exercise beyond normal cage ambulation; exercising rats were housed in similar cages with an activity wheel. After 6 weeks, rats were implanted with intravenous catheters and trained to self-administer cocaine, heroin, and dose combinations of cocaine and heroin (i.e., speedball) on a progressive ratio schedule of reinforcement.
Key Findings
Doses of speedball maintained greater levels of responding than corresponding doses of cocaine and heroin alone. Importantly, responding maintained by cocaine, heroin, and speedball was lower in exercising rats than sedentary rats.
Significance
These data indicate that exercise decreases the self-administration of speedball and suggest that exercise may reduce the abuse of drug combinations that have traditionally been resistant to treatment.
Keywords: cocaine, exercise, heroin, progressive ratio, rat, self-administration, speedball
1. Introduction
Epidemiological studies have consistently reported an inverse relationship between exercise and substance use (Field et al. 2001; Iannotti et al. 2009; Kirkcaldy et al. 2002; Strohle et al. 2007). Preclinical studies have supported these findings by reporting that exercise, which is typically operationalized as running on a treadmill or in an activity wheel, decreases the self-administration of many drugs of abuse. In laboratory rats, for instance, exercise reliably decreases the positive reinforcing effects of psychomotor stimulants, including cocaine and methamphetamine (Cosgrove et al. 2002; Miller et al. 2012; Smith and Witte 2012; Smith et al. 2008; 2011), and the positive reinforcing effects of opioids, including heroin and morphine (Hosseini et al. 2009; Smith and Pitts 2012). Moreover, these effects are typically observed across doses, subject populations, and schedules of reinforcement (for review, see Lynch et al. 2013; Smith and Lynch 2012).
To date, preclinical studies examining the effects of exercise on drug self-administration have measured only responding maintained by individual drugs – not responding maintained by combinations of drugs. This limits the translational appeal of these studies because polydrug use (i.e., the simultaneous use of multiple drugs) is common in clinical populations, with up to 54% of treatment-seeking drug users reporting the use of more than one substance (Substance Abuse and Mental Health Service Administration 2011). One such drug combination that is particularly resistant to treatment is the combination of cocaine and heroin, more commonly known as “speedball”. Preclinical studies examining speedball self-administration have typically reported that combined doses of cocaine and heroin have greater reinforcing strength than the same doses of cocaine and heroin administered individually (Duvauchelle et al. 1998; Ranaldi and Munn 1998; Rowlett and Woolverton 1997). This is relevant from a public health standpoint, because drug combinations that have greater reinforcing strength are also more resistant to interventions designed to decrease drug self-administration (Cox and Comiskey 2011; Grella et al. 1997; Prendergast et al. 2006).
The purpose of the present study was to examine the effects of exercise on the self-administration of cocaine, heroin, and combinations of cocaine and heroin (i.e., speedball). To this end, female rats were obtained at weaning and reared under sedentary (no activity wheel) or exercising (activity wheel) conditions for 6 weeks. Rats were then implanted with intravenous catheters and the positive reinforcing effects of cocaine, heroin, and speedball were examined on a progressive ratio (PR) schedule of reinforcement. A PR schedule was selected because responding on this schedule is characterized by a linear, monophasic dose-effect curve over a broad dose range, making changes in the reinforcing strength of a drug easily discernable by upward or downward shifts in the curve. Female rats were chosen for this study because females run more than males when given free access to activity wheels (Boakes et al. 1999; Eikelboom and Mills 1988; Smith et al. 2011; 2012) and because females are under-represented in preclinical research (Clayton and Collins 2014).
2. Method
2.1 Animals
Female, Long Evans rats (Charles River Laboratories, Raleigh, NC) were obtained at weaning (~21 days) and immediately assigned to exercising or sedentary conditions. Exercising rats were housed individually in polycarbonate cages (interior dimensions: 50 cm × 28 cm × 20 cm) fitted with an activity wheel (35 cm diameter). Mechanical switches on each wheel counted rotations and these data were recorded daily. Sedentary rats were housed in cages of identical dimensions but without an activity wheel. All rats remained in these conditions for the duration of the study. Subjects were kept on a 12-hr, light-dark cycle (lights on: 0500) in a temperature- and humidity-controlled colony room. Each rat had free access to food and drinking water except during the brief period of lever-press training. Estrous phase was not monitored and allowed to cycle normally. All animals were kept in accordance with the guidelines of the Institutional Animal Care and Use Committee of Davidson College and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 2011). Animals that lost catheter patency (4 sedentary rats, 4 exercising rats) were removed from the study and not included in the statistical analysis. A total of 14 sedentary and 9 exercising rats completed all phases of the study and were used in the data analysis.
2.2 Apparatus
Self-administration training and testing occurred in commercially available operant conditioning chambers (Med Associates, Inc., St. Albans, VT; interior dimensions: 31 cm × 24 cm × 21 cm). Chambers contained a single houselight located on the ceiling above the rear wall and two response levels positioned 10 cm above the chamber floor. A white stimulus light located above the response lever signaled drug availability. All infusions were delivered via a pump mounted outside the conditioning chamber through a Tygon tube attached to a steel swivel above the chamber. All chambers were housed in larger, sound-attenuating boxes equipped with an exhaust fan that provided air circulation and masked extraneous sounds. Data were captured and experimental events were programmed through the use of interfacing and software provided by Med Associates, Inc. For all subjects, the left lever was designated as the active lever.
2.3 Lever-press training
One week prior to surgery and five weeks after arrival, subjects were restricted to 90% of their free-feeding body weight and trained to press a response lever. During these sessions, lever pressing was reinforced on a fixed-ratio (FR1) schedule of reinforcement with a single 45 mg grain pellet. A session concluded when subjects received 40 reinforcers or 2 hr elapsed, whichever occurred first. Lever-press training was terminated when a subject obtained 40 reinforcers in three consecutive training sessions. All subjects met this criterion and returned to unrestricted feed within 7 days.
2.4 Surgery
Six weeks after arrival, rats were surgically implanted with intravenous catheters for drug-self-administration. Each subject was anesthetized with a combination of xylazine HCl (8.0 mg/kg, ip) and ketamine (100 mg/kg, ip), and an intravenous catheter was inserted into the right jugular vein that exited the body on the dorsal surface of the scapulae. Immediately after surgery, each rat was administered Ketoprofen (3.0 mg/kg, sc) as a post-operative analgesic and allowed 3 days of recovery before behavioral testing. For one week after surgery, a solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily to maintain patency and prevent infection. After one week, ticarcillin administration ended and catheter patency was maintained by daily infusions of heparinized saline.
2.5 Drug self-administration training
All drug self-administration training and testing sessions took place during the light phase of the light-dark cycle. Five training sessions were conducted over five consecutive days during which cocaine (Sessions 1–3) and heroin (Sessions 4–5) were available on an FR1 schedule of reinforcement. Each session began with a priming infusion of the dose of cocaine (0.5 mg/kg/infusion; National Institute on Drug Abuse, Research Triangle Institute, Research Triangle Park, NC) or heroin (0.005 mg/kg/infusion; National Institute on Drug Abuse, Research Triangle Institute, Research Triangle Park, NC) available during that session, illumination of the house light, and illumination of the stimulus light above the response lever. Each lever press produced a 3–4 s infusion (based on body weight) and a tone that sounded for the duration of the infusion. Coincident with each infusion, the stimulus light above the lever turned off for 20 s to signal a timeout during which the drug was not available. Each training session continued for 2 hr with no limit placed on the maximum number of infusions that could be earned.
2.6 Drug self-administration testing
Throughout self-administration testing, responding was reinforced on a PR schedule of reinforcement. On this schedule, the number of responses required to obtained a reinforcer increased through the following progression: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 346, 445, and 573 (for complete algorithm, see Suto et al. 2002). Each session continued until a breakpoint was reached, with breakpoint defined as the number of infusions obtained before one hour elapsed without an infusion. Breakpoints were obtained for saline, three doses of cocaine (0.1, 0.3, and 1.0 mg/kg/infusion), and three doses of heroin (0.001, 0.003, and 0.01 mg/kg/infusion). Breakpoints maintained by speedball were obtained at each dose combination of cocaine and heroin, for a total of nine doses (3 cocaine doses x 3 heroin doses = 9 speedball doses). Doses were tested in an irregular order that varied across rats. Similar to training, each session began with a priming infusion of the dose (or dose combination) available during that session, and each infusion was accompanied by the audiovisual stimulus (light off; tone on) and followed by a 20-s timeout during which the drug was not available.
2.7 Data Analysis
The primary outcome variable for this study was breakpoints as defined by the number of infusions per session. Secondary outcome variables included the number of active lever responses per session, session lengths, and the number of inactive lever responses per session. When cocaine and heroin were tested alone, breakpoint and session length data were analyzed via two-way, mixed-factor ANOVA, with group serving as the between-subjects factor and dose serving as the repeated measure. When speedball was tested, breakpoint and session length data were analyzed via three-way, mixed-factor ANOVA, with group serving as the between-subjects factor and dose of cocaine and dose of heroin serving as repeated measures. The number of active and inactive lever responses were compared between groups and across dose conditions via mixed-factor ANOVA using the Greenhouse-Geisser correction (the assumption of sphericity was not met; variances were not equal across dosing conditions). When relevant, post-hoc tests were used to compare breakpoints maintained by individual doses or dose-combinations after correcting for multiple pairwise comparisons. In addition, cumulative records generated by individual animals were visually inspected to determine whether individual patterns of responding were consistent with the schedule of reinforcement (FR and PR) and maintaining event (saline, cocaine, and heroin).
Pearson product-moment correlations were used to compare exercise output, defined as revolutions per day (rev/day), before surgery (weeks 1–6), after surgery (weeks 7–8), and throughout the entire study (weeks 1–8) with responding maintained by cocaine, heroin, and speedball. In these tests, responding maintained by cocaine, heroin, and speedball was quantified using the trapezoidal rule to determine area under the curve (AUC). As noted in the Results, none of these correlations were statistically significant.
3. Results
Rats assigned to the exercise condition rapidly increased their exercise output as measured by the number of wheel revolutions per day, reaching a peak of approximately 13,000 rev/day during the third week of wheel exposure (Figure 1). Exercise output plateaued at approximately 12,000 rev/day during the last several weeks of wheel exposure prior to surgery and catheter implantation. Wheel running was reduced markedly the day after surgery but recovered over the next several days, averaging 1000 revolutions on the first day after surgery but increasing to 5000 revolutions on the fifth day after surgery. Wheel running averaged between 5000 and 6000 rev/day during self-administration testing, which lasted approximately 2 weeks.
Figure 1.
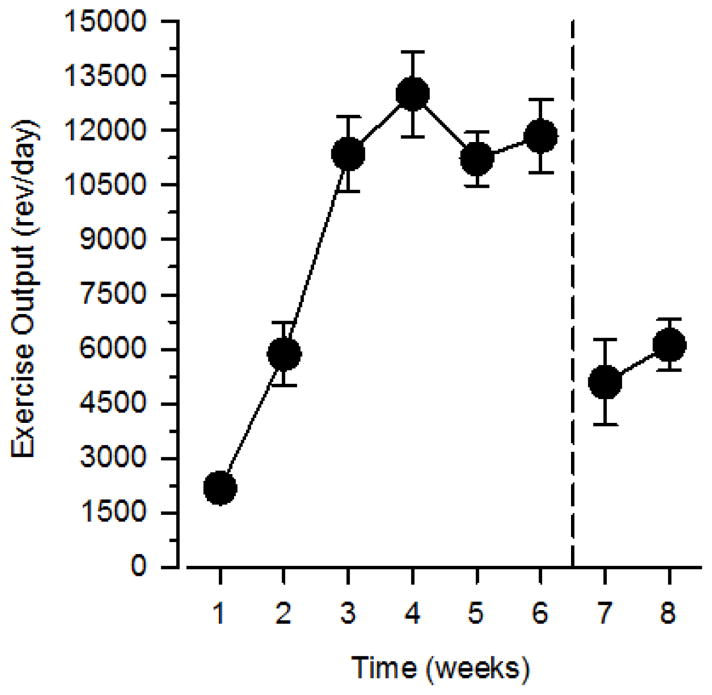
Wheel running in exercising rats. Left axis depicts exercise output expressed as the mean number of wheel revolutions per day (rev/day); horizontal axis depicts time expressed in “weeks” of 5- to 9-day intervals. Reference line after week 6 (vertical broken line extending from abscissa) indicates catheter implantation and the beginning of behavioral testing. Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point.
All rats responded on the first day of self-administration training on the FR1 schedule of reinforcement. By the third day of training, all rats showed regular patterns of responding, characterized by an initial load-up phase followed by a steady rate of responding with regular post-reinforcement pauses for the remainder of the session (Supplemental Files 1 and 2). No differences were observed between sedentary and exercising rats during these initial training sessions.
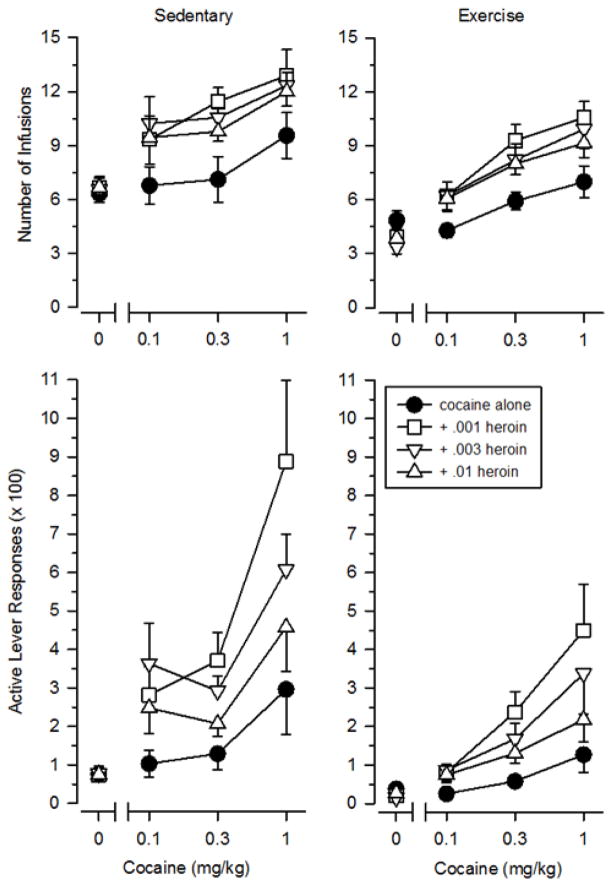
Breakpoints varied across the various dosing conditions (Figure 2, upper panels). When tested alone, breakpoints increased linearly across the three dose of cocaine [F (2, 42) = 9.041, p = .001], with the highest dose of cocaine maintaining breakpoints significantly above those obtained with saline (p = .005). Breakpoints maintained by cocaine were significantly greater in sedentary rats than exercising rats [F (1, 21) = 4.517, p = .046], and this effect was consistent across all three doses of cocaine. In contrast, breakpoints did not increase across the three doses of heroin in either group. Similar to that observed with cocaine, breakpoints maintained by heroin were greater in sedentary rats than exercising rats [F (1, 21) = 19.704, p < .001]. No dose of heroin maintained breakpoints higher than that of saline in either group (note heroin data above “0” in Figure 2). Breakpoints obtained during the saline substitution test were greater in sedentary rats than exercising rats [t (21) = 2.261, p = .036].
Figure 2.
Responding maintained by cocaine, heroin, and speedball in sedentary (left panels) and exercising (right panels) rats. Vertical axes depict breakpoints expressed as the number of infusions obtained (upper panels) or active lever responses (x 100; lower panels). Horizontal axes depict doses of cocaine. Points above “0” depict the effects of saline and individual doses of heroin when administered alone. Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point.
Similar effects were observed when responding was depicted as the number of active lever responses per session (Figure 2, lower panels), and all significant main effects regarding dose and group were identical to that observed with the breakpoint data. Specifically, active lever responses increased as a function of dose for cocaine [F (1.113, 42) = 7.827, p = .008] but not heroin, and sedentary rats responded more than exercising rats for both cocaine [F (1, 21) = 4.902, p = .038] and heroin [F (1, 21) = 16.339, p = .001]. Interestingly, unlike that obtained with the breakpoint data, responding during the saline substitution test did not differ significantly between sedentary and exercising rats when active lever responses were considered (p = .072).
Combined doses of cocaine and heroin (i.e., speedball) maintained higher breakpoints than comparable doses of cocaine and heroin administered alone. Breakpoints maintained by speedball increased linearly across the three dose of cocaine [F (2, 84) = 13.662, p < .001], with the greatest breakpoints maintained by doses that included 1.0 mg/kg/infusion cocaine. In contrast, breakpoints maintained by speedball decreased across the three doses of heroin [F (2, 84) = 4.671, p = .015]. As shown in Figure 2 (upper panels), doses of speedball that included .01 mg/kg/infusion heroin maintained lower breakpoints than doses that included .001 mg/kg/infusion heroin (p = .009). Importantly, across all dose combinations, sedentary rats responded more than exercising rats [F (1, 21) = 7.690, p = .011], indicating that exercise reduced the positive reinforcing effects of speedball under these conditions.
Similar effects were observed when responding was depicted as the number of active lever responses per session (Figure 2, lower panels). When data were depicted in this manner, steep dose-effect curves with considerable separation across the various dosing conditions were observed. Once again, all significant main effects regarding dose and group were identical to that obtained with the breakpoint data. Specifically, active lever responses increased as a function of cocaine dose [F (1.168, 84) = 9.242, p = .004], decreased as a function of heroin dose [F (1.425, 84) = 5.624, p = .015], and were greater in sedentary rats than exercising rats [F (1, 21) = 4.362, p = .049].
Differences between the various dose conditions were apparent from the cumulative records taken from individual animals. In most instances, these records revealed clear differences in the pattern of responding maintained by the schedule of reinforcement (FR and PR), the maintaining event (saline, cocaine, heroin, and speedball), and the various doses and dose combinations (Supplemental Files 1 and 2).
Session lengths varied across conditions (Figure 3), but the effects observed on this measure differed from those observed on the other outcome measures. Similar to that observed with the breakpoint data, session lengths increased as a function of dose when cocaine was tested alone [F (2, 42) = 7.416, p = .002]. In contrast to that observed with the breakpoint data, session lengths also increased as function of dose when heroin was tested alone [F (2, 42) = 9.255, p < .001]. Session lengths were greater in sedentary than exercising rats for both cocaine [F (1, 21) = 6.625, p = .018] and heroin [F (1, 21) = 15.579, p = .001]. When speedball was tested, session lengths increased as a function of both cocaine dose [F (2, 84) = 26.382, p < .001] and heroin dose [F (2, 84) = 18.985, p < .001], and were greater in sedentary than exercising rats [F (1, 21) = 17.420, p < .001]. Also unlike the breakpoint data, session lengths did not differ between sedentary and exercise rats during the saline substitution test (p = .086)
Figure 3.
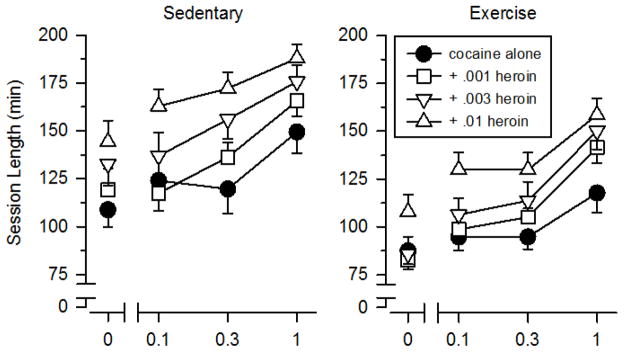
Session lengths during testing with cocaine, heroin, and speedball in sedentary (left panels) and exercising (right panels) rats. Vertical axes depict session lengths expressed in minutes (min). Horizontal axes depict doses of cocaine. Points above “0” depict the effects of saline and individual doses of heroin when administered alone. Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point.
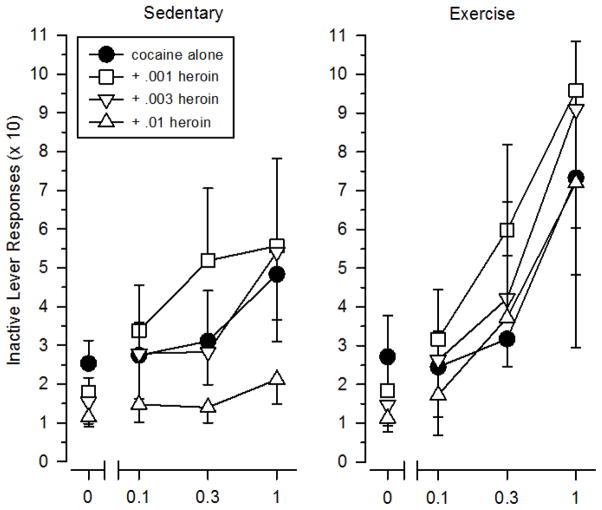
Responding on the inactive lever was generally 10-fold less than responding on the active lever (Figure 4). Inactive lever responding did not vary as a function of dose when cocaine and heroin were tested alone; however, significant differences were observed across the dosing conditions when speedball was tested. Similar to that seen with active lever responding, responses on the inactive lever increased as a function of cocaine dose [F (1.188, 84) = 4.194, p = .022] and decreased as a function of heroin dose [F (1.799, 84) = 7.460, p = .002]. Importantly, no differences in responding on the inactive lever were observed between sedentary and exercising rats during tests with cocaine, heroin, speedball, or saline. On 347 out of 368 occasions (23 rats × 16 dosing conditions = 368 occasions), rats showed a preference for the active lever over the inactive lever. Although we can’t rule out the possibility of a side bias (the left lever was the active lever for all rats), it is notable that this preference was consistent under all dosing conditions for both groups.
Figure 4.
Inactive lever responding during testing with cocaine, heroin, and speedball in sedentary (left panels) and exercising (right panels) rats. Vertical axes depict inactive lever responses (x 10). Horizontal axes depict doses of cocaine. Points above “0” depict the effects of saline and individual doses of heroin when administered alone. Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point.
Exercise output before surgery, after surgery, and throughout the entire study was not correlated with responding maintained by cocaine, heroin, or speedball (r = −.209 – .347, p = .246 – .990; data not shown).
4. Discussion
The primary goal of the present experiment was to determine if exercise altered the self-administration of cocaine, heroin, and dose combinations of cocaine and heroin (i.e., speedball) in female rats. Overall, the results indicate that wheel running reduced breakpoints across all drug and dose combinations in exercising rats compared to sedentary controls. These data are in accord with previous findings from our laboratory investigating the effects of exercise on cocaine and heroin alone (Smith and Pitts 2012; Smith et al. 2008). Importantly, this is the first experiment to examine exercise as a potential intervention for polydrug (speedball) use in an animal model.
Exercise output increased during the initial weeks of wheel access before plateauing at approximately 12,000 rev/day. Exercise output decreased markedly immediately after surgery, increased slightly in the days after surgery, and then remained stable between 5000 and 6000 rev/day for the remainder of the study. These findings are consistent with previous data from our laboratory examining the effects of exercise on cocaine and heroin self-administration (Smith and Pitts 2012; Smith et al. 2008). Importantly, no phase of running activity was correlated with breakpoints, suggesting that overall physical activity, regardless of phase or amount, reduced drug self-administration.
No differences between sedentary and exercising rats were observed in the initial training sessions on the FR1 schedule of reinforcement, which is consistent with previous studies from our laboratory when rats were trained to lever press prior to surgery (e.g., Smith et al. 2008; 2011; 2012). For both exercising and sedentary rats, breakpoints increased linearly as a function of dose when cocaine was tested alone. Cocaine-maintained breakpoints were lower in exercising rats than sedentary rats, suggesting that exercise decreased the reinforcing strength of cocaine. In contrast to that observed with cocaine, heroin-maintained breakpoints did not vary as a function of dose and did not differ from saline, indicating that heroin did not function as a reinforcer when administered alone. Nevertheless, breakpoints maintained by heroin were lower in exercising rats than sedentary rats, and this effect was apparent at all three doses of heroin tested. The findings obtained with heroin are consistent with previous studies reporting that opioids fail to maintain responding at rates significantly different from saline on PR schedules of reinforcement (Arnold and Roberts 1997; Grasing et al. 2003). One limitation of this study is that each dose (and dose combination) was tested only once, which was necessary in order to test a full dose range of cocaine and heroin, and all possible dose combinations of speedball (i.e., 16 different dose conditions including saline). We emphasize that we chose a PR schedule because it generates linear dose-response functions with a single ascending limb over a broader dose range than FR schedules, which is beneficial when measuring changes in reinforcing strength.
It is important to note that responding during the saline substitution test was also lower in exercising rats than sedentary rats (but only when the breakpoint data were compared). These data could indicate that exercise-induced reductions in reinforcing strength extend to a stimulus paired with a drug (the audiovisual stimulus accompanied each saline infusion) or that exercise decreases measures of drug-seeking behavior during extinction. Alternatively, these data could indicate that exercise non-selectively decreases operant responding, with little relation to the maintaining event; however, exercise did not alter rates of inactive lever responding, regardless of the dose or drug (or drug combination) tested. Moreover, exercise does not decrease responding maintained by food or a stimulus paired with food (Smith and Witte 2012), suggesting that exercise-induced reductions in responding are not common to all reinforcers.
In all instances, breakpoints maintained by speedball were greater than those maintained by corresponding doses of cocaine and heroin alone, and these effects were greater than what would be expected using an effect-additive model. Specifically, an effect-additive model would predict that breakpoints maintained by speedball would be no greater than those maintained by cocaine, given that heroin failed to produce reinforcing effects alone. We were unable to examine our data using a dose-additive model, which has several distinct advantages over effect-additive models (see Wessinger 1986), because heroin was not effective at any dose examined. Nevertheless, these data are consistent with previous studies reporting greater-than-additive effects of speedball when low doses of cocaine and heroin are used to comprise the dose combinations (Duvauchelle et al. 1998; Martin et al. 2006; Ranaldi and Munn 1998; Rowlett and Woolverton 1997).
As expected, speedball-maintained breakpoints increased linearly as a function of cocaine dose, indicating that increasing doses of cocaine produced corresponding increases in the reinforcing strength of speedball. In contrast, speedball-maintained breakpoints decreased linearly as a function of heroin dose, indicating that increasing doses of heroin produced corresponding decreases in the reinforcing strength of speedball. This reduction in the reinforcing strength of speedball may be due to aversive effects produced by high doses of heroin, which in turn reduced the hedonic/appetitive effects of the drug combination. This is unlikely, given that these and higher doses of heroin are self-administered on FR1 schedules of reinforcement (Smith and Pitts 2012) and are chosen on concurrent schedules of reinforcement when the response alternative is food (Lenoir et al. 2013). Alternatively, this could be due to the sedative and motor-impairing effects of heroin. Indeed, session lengths increased as a function of heroin dose when both heroin and speedball were self-administered. These effects were observed even under conditions in which breakpoints and the number of active lever responses decreased, suggesting that rats responded slower when heroin was self-administered and didn’t simply stop responding earlier. These findings are also consistent with previous studies reporting that noncontingent administration of heroin decreases schedule-controlled responding when food is the reinforcer (Galaj et al. 2013; Thornhill et al. 1975).
In one of the few studies reporting that heroin failed to increase the reinforcing strength of cocaine on a PR schedule, Ward et al. (2005) only tested doses of heroin higher than those used in the present study. We found that the greatest speedball-maintained breakpoints were obtained with the lowest doses of heroin, thus making the present data consistent with those of Ward et al. (2005) when these dose-dependent effects are considered. This finding emphasizes the need to test an extensive dose range when examining speedball self-administration, and further suggests that the addition of very low doses of opioids (or the addition of very low efficacy opioids) would be sufficient to markedly increase the abuse potential of cocaine in substance-abusing populations (for further discussion and examples using a labor-supply analysis, see Rowlett et al. 2005).
The behavioral effects observed in the present study with speedball are consistent with the neurochemical effects observed in previous studies with combinations of cocaine and heroin. For instance, in rats self-administering cocaine, heroin, or speedball on an FR schedule of reinforcement, cocaine increased extracellular dopamine in the nucleus accumbens by approximately 400%, but heroin failed to increase extracellular dopamine beyond control levels (Hemby et al. 1999). Importantly, combinations of cocaine and heroin increased extracellular dopamine by approximately 1000%, similar to the greater-than-additive effects observed in the present study. Similar neurochemical effects were observed in a follow-up study, in which doses of heroin that were ineffective when self-administered alone significantly enhanced cocaine-induced increases in dopamine concentrations during speedball self-administration (Smith et al. 2006).
From a clinical perspective, the present data indicate a protective effect of exercise on speedball self-administration. One study found that 58% of heroin users report comorbid cocaine use (Schutz et al. 1994), and some heroin-dependent individuals report using cocaine to reduce heroin withdrawal symptoms (Kosten 1989; Rosen et al. 1992). Furthermore, treatment outcomes are worse for heroin users who also use cocaine than for those who do not use cocaine (Downey et al. 2000). There are currently no approved pharmacotherapies for cocaine addiction (Dackis and O’Brien 2001). Methadone maintenance, used to treat heroin addiction, may be detrimental to treating speedball abuse because individuals report the use of methadone and cocaine as a speedball variant (Hartel et al. 1995). Given that exercise produces robust effects on both cocaine and heroin self-administration, it may represent a unique, non-pharmacological intervention to treat speedball addiction in human populations.
Supplementary Material
Acknowledgments
This study was funded by NIH Grants DA027485 and DA031725 (to MAS). The NIH had no role in the design of the study; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The authors thank the National Institute on Drug Abuse for supplying the study drugs.
Footnotes
Disclosures
The authors have no conflicts of interest to report in regard to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113:1080–1089. [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–71. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Cox GM, Comiskey CM. Does concurrent cocaine use compromise 1-year treatment outcomes for opiate users? Subst Use Misuse. 2011;46:1206–16. doi: 10.3109/10826084.2010.501649. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001;21:111–7. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent polydrug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8:176–84. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Sanders CE. Exercise is positively related to adolescents’ relationships and academics. Adolescence. 2001;36:105–10. [PubMed] [Google Scholar]
- Galaj E, Cruz I, Schachar J, Koziolek M, Ranaldi R. Differential effects on natural reward processing in rats after repeated heroin. Psychopharmacology. 2013;229:123–32. doi: 10.1007/s00213-013-3087-8. [DOI] [PubMed] [Google Scholar]
- Grasing K, Ning L, Shaunteng H, Parrish C, Delich J, Glowa J. A new progressive ratio schedule for support of morphine self-administration in opiate dependent rats. Psychopharmacology (Berl) 2003;168:387–96. doi: 10.1007/s00213-003-1442-x. [DOI] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE. Patterns and predictors of cocaine and crack use by clients in standard and enhanced methadone maintenance treatment. Am J Drug Alcohol Abuse. 1997;23:15–42. doi: 10.3109/00952999709001685. [DOI] [PubMed] [Google Scholar]
- Hartel DM, Schoenbaum EE, Selwyn PA, Kline J, Davenny K, Klein RS, Friedland GH. Heroin use during methadone maintenance treatment: the importance of methadone dose and cocaine use. Am J Public Health. 1995;85:83–8. doi: 10.2105/ajph.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288:274–80. [PubMed] [Google Scholar]
- Hosseini M, Alaei HA, Naderi A, Sharifi MR, Zahed R. Treadmill exercise reduces self-administration of morphine in male rats. Pathophysiology. 2009;16:3–7. doi: 10.1016/j.pathophys.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Iannotti RJ, Kogan MD, Janssen I, Boyce WF. Patterns of adolescent physical activity, screen-based media use, and positive and negative health indicators in the U.S. and Canada. J Adolesc Health. 2009;44:493–9. doi: 10.1016/j.jadohealth.2008.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Kirkcaldy BD, Shephard RJ, Siefen RG. The relationship between physical activity and self-image and problem behaviour among adolescents. Soc Psychiatry Psychiatr Epidemiol. 2002;37:544–50. doi: 10.1007/s00127-002-0554-7. [DOI] [PubMed] [Google Scholar]
- Kosten TA. Cocaine attenuates opiate withdrawal in human and rat. NIDA Res Monogr. 1989;95:361–2. [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kahn W, Cannon DG, Smith JE. Self-administration of heroin, cocaine and their combination under a discrete trial schedule of reinforcement in rats. Drug Alcohol Depend. 2006;82:282–6. doi: 10.1016/j.drugalcdep.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2012;121:90–6. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Munn E. Polydrug self-administration in rats: cocaine-heroin is more rewarding than cocaine-alone. Neuroreport. 1998;9:2463–6. doi: 10.1097/00001756-199808030-00007. [DOI] [PubMed] [Google Scholar]
- Rosen MI, Wallace EA, Sullivan MC, Stine S, Kosten TR. Use of cocaine to prevent opiate withdrawal. Am J Psychiatry. 1992;149:1609. doi: 10.1176/ajp.149.11.1609b. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Rodefer JS, Spealman RD. Self-administration of cocaine-opioid combinations by rhesus monkeys: evaluation of the role of mu receptor efficacy using labor supply analysis. J Pharmacol Exp Ther. 2005;312:1289–97. doi: 10.1124/jpet.104.076646. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl) 1997;133:363–71. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Schutz CG, Vlahov D, Anthony JC, Graham NM. Comparison of self-reported injection frequencies for past 30 days and 6 months among intravenous drug users. J Clin Epidemiol. 1994;47:191–5. doi: 10.1016/0895-4356(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–50. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Front Psychiatry. 2012;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Wheel running decreases the positive reinforcing effects of heroin. Pharmacol Rep. 2012;64:960–4. doi: 10.1016/s1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–35. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology (Berl) 2011;218:357–69. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20:437–46. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Hofler M, Pfister H, Muller AG, Hoyer J, Wittchen HU, Lieb R. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med. 2007;37:1657–66. doi: 10.1017/S003329170700089X. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Service Administration. Nationals admissions to substance abuse treatment services. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2011. Treatment episode data set (TEDS). 1999–2009. DASIS Series:S-56, HHS Publication No. (SMA) 11-4646. [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–9. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Thornhill JA, Hirst M, Gowdey CW. Effects of chronic administration of heroin on rats trained on two food reinforcement schedules. Arch Int Pharmacodyn Ther. 1975;218:277–89. [PubMed] [Google Scholar]
- Ward SJ, Morgan D, Roberts DC. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology. 2005;30:286–95. doi: 10.1038/sj.npp.1300560. [DOI] [PubMed] [Google Scholar]
- Wessinger WD. Approaches to the study of drug interactions in behavioral pharmacology. Neurosci Biobehav Rev. 1986;10:103–113. doi: 10.1016/0149-7634(86)90021-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.