Background: Phycobiliprotein lyases catalyze covalent attachment of chromophores to cyanobacterial antenna proteins.
Results: We present crystal structures of a T-type lyase and its complex with phycocyanobilin.
Conclusion: The proposed reaction mechanism accounts for chromophore stabilization and regio- and stereospecificity.
Significance: This work sheds light on a crucial step in phycobiliprotein maturation and provides a model for other types of phycobiliprotein lyases.
Keywords: Crystal Structure, Cyanobacteria, Photosynthetic Pigment, Stereoselectivity, X-ray Crystallography, Lyase, Phycobiliprotein
Abstract
Pigmentation of light-harvesting phycobiliproteins of cyanobacteria requires covalent attachment of open-chain tetrapyrroles, bilins, to the apoproteins. Thioether formation via addition of a cysteine residue to the 3-ethylidene substituent of bilins is mediated by lyases. T-type lyases are responsible for attachment to Cys-155 of phycobiliprotein β-subunits. We present crystal structures of CpcT (All5339) from Nostoc (Anabaena) sp. PCC 7120 and its complex with phycocyanobilin at 1.95 and 2.50 Å resolution, respectively. CpcT forms a dimer and adopts a calyx-shaped β-barrel fold. Although the overall structure of CpcT is largely retained upon chromophore binding, arginine residues at the opening of the binding pocket undergo major rotameric rearrangements anchoring the propionate groups of phycocyanobilin. Based on the structure and mutational analysis, a reaction mechanism is proposed that accounts for chromophore stabilization and regio- and stereospecificity of the addition reaction. At the dimer interface, a loop extending from one subunit partially shields the opening of the phycocyanobilin binding pocket in the other subunit. Deletion of the loop or disruptions of the dimer interface significantly reduce CpcT lyase activity, suggesting functional relevance of the dimer. Dimerization is further enhanced by chromophore binding. The chromophore is largely buried in the dimer, but in the monomer, the 3-ethylidene group is accessible for the apophycobiliprotein, preferentially from the chromophore α-side. Asp-163 and Tyr-65 at the β- and α-face near the E-configured ethylidene group, respectively, support the acid-catalyzed nucleophilic Michael addition of cysteine 155 of the apoprotein to an N-acylimmonium intermediate proposed by Grubmayr and Wagner (Grubmayr, K., and Wagner, U. G. (1988) Monatsh. Chem. 119, 965–983).
Introduction
Phycobilisomes, the light-harvesting antenna complexes in cyanobacteria and red algae, are supramolecular complexes of phycobiliproteins (PBP)4 and linkers (1–3); they are packed with hundreds of open-chain tetrapyrrole (bilin) chromophores. These antenna pigments absorb light in the wavelength range between 480 and 660 nm; and their spatial arrangement in the phycobilisome ensures directional energy transfer with high quantum efficiency to the photosynthetic reaction centers (4, 5). PBP are α,β-heterodimeric proteins, with each subunit carrying one to three bilin chromophores. Unlike chlorophylls, bilins are covalently attached to PBP regio- and stereospecifically via thioether bonds from the C-31 atoms of bilin chromophores to highly conserved cysteines. Although this attachment mode is universal to all chromophores, phycoerythrobilin and phycourobilin sometimes form an additional thioether bond via the C-181 atom of ring D (6). With a single exception of the core-membrane linker (7–9), these post-translational modifications are catalyzed by chromophore-specific lyases in a site-specific manner (10–12).
Three types of lyases have been characterized to catalyze the bilin attachment to C-31. They are thought to act like chaperones or protective carriers for labile bilin chromophores (6, 13, 14), but additional functions have been found (15–17). E/F-type lyases are specific for attaching phycocyanobilin (PCB) to a conserved cysteine (Cys-α84) in α-subunits of phycocyanin (CPC) and phycoerythrocyanin (18, 19); homologous CpeY/Z catalyze addition of phycoerythrobilin to the equivalent site in phycoerythrin (20). CpcE and CpcF are structurally related to Armadillo proteins like α-karyopherin (21), but no crystal structure has been solved. S-type lyases are more universal, among which CpcS is the best studied. Alone or in combination with CpcU (22–25), it catalyzes PCB attachment to conserved Cys-β84 sites in various biliprotein β-subunits and to the related Cys-82 sites (consensus numbering) in both subunits of allophycocyanin (22, 26). CpcS transiently binds bilins such as PCB (14). The crystal structures of a cyanobacterial CpcS (PDB code 3BDR)5 (11, 28) and a cryptophytes CPES (29) have been determined in the absence of a chromophore. They both adopt a β-barrel structure similar to those of fatty acid-binding proteins (FABP), a subfamily of the calycin superfamily (Pfam0116) that binds a variety of small, mostly lipophilic molecules with high selectivity (30–32). The name reflects their goblet (calyx)-like structures consisting of antiparallel β-sheets that in many cases is closed by an α-helix. Interestingly, the first structure reported in the calyx family was a protein from the butterfly, Pieris brassicae, which noncovalently binds a bilin chromophore, biliverdin IXγ (33). The T-type lyases constitute a third class of lyases that is distantly related to CpcS (23). They catalyze the covalent attachment of PCB or phycoerythrobilin to Cys-β155 sites (consensus numbering) in β-subunits of cyanobacterial (25, 34) and cryptophyte biliproteins (35), and with a different stereochemistry than CpcS. In Nostoc (Anabaena) sp. PCC 7120 (Nostoc), a single protein, CpcT (All5339), is responsible for β-subunit chromophorylation with PCB at Cys-β155 in CPC and phycoerythrocyanin. Sequential binding studies indicated that this step is hindered by a preceding chromophorylation at Cys-β84 by CpcS (25).
To understand the structural basis for the reaction mechanism of lyases, we have solved the crystal structure of CpcT at 1.95 Å resolution by the single-wavelength anomalous dispersion method using selenomethionine-substituted crystals. We have also determined the crystal structure of CpcT in complex with PCB at 2.5 Å resolution. Based on structural analyses and comparisons, we have further explored residues at the dimer interface and around the chromophore-binding pocket via mutagenesis. A reaction mechanism is proposed for T-type lyases that accounts for stabilizing the chromophore, as well as for the regio- and stereospecificity of the addition reaction.
EXPERIMENTAL PROCEDURES
Constructs, Expression, and Purification
CpcT was cloned according to standard procedures (36). Plasmids containing cpcT, ho1, and pcyA from Nostoc and mutated cpcB(C84S) from Mastigocladus laminosus were previously constructed (9, 22, 24, 25). The CpcT used in crystallization was obtained from expression of construct pET-cpcT; it carries a His tag at the C terminus.
CpcT was overexpressed in Escherichia coli BL21(DE3). Cells were grown at 20 °C in LB medium containing kanamycin (30 μg·ml−1), induced with isopropyl 1-thio-β-d-galactopyranoside (1 mm) at OD600 ∼0.5–0.7, and then growth was continued for an additional 12 h after induction. Cells were harvested by centrifugation, washed twice with distilled water, and stored at −20 °C until use (22, 25).
Dual plasmids were co-transformed into BL21(DE3) cells under the respective antibiotic selections (chloromycetin for pACYC derivative, streptomycin for pCDF derivative, and kanamycin for pET derivative). PCB-CpcB(C84S) was produced by co-expression of pET-cpcB(C84S), pACYC-ho1-pcyA, and pCDF-cpcT. Cells were grown at 20 °C in LB medium containing kanamycin (20 μg·ml−1), chloromycetin (17 μg·ml−1), and streptomycin (25 μg·ml−1). Cells were harvested after 12 h following induction with isopropyl 1-thio-β-d-galactopyranoside (1 mm) (22, 25).
Cell pellets were resuspended in ice-cold potassium phosphate buffer (KPB, 20 mm, pH 7.2) containing NaCl (0.5 m) and disrupted by sonication (5 min at 200 watts, JY92-II, Scientz Biotechnology, Ningbo, China). The suspension was centrifuged at 12,000 × g for 15 min at 4 °C. The supernatant containing crude proteins was purified via Ni2+-affinity chromatography on chelating-Sepharose (Amersham Biosciences) and eluted using buffer KPB (20 mm, pH 7.2) containing NaCl (0.5 m) and containing imidazole (0.2 m). The affinity-enriched proteins were further purified via gel filtration (Amersham Biosciences) and were verified by SDS-PAGE. The aggregation state of CpcB(C84S) and CpcT was assayed by gel filtration on Superdex 75 using KPB buffer (20 mm, pH 7.2) containing NaCl (150 mm) at an elution rate of 0.5 ml/min.
Protein and Activity Assays
Protein concentrations were determined by the Bradford method, using bovine serum albumin as standard (37). UV-visible absorption spectra were recorded by a DU800 spectrophotometer (Beckman-Coulter). Uncorrected fluorescence spectra were recorded with an LS55 spectrofluorimeter (PerkinElmer Life Sciences). Relative enzymatic activities of CpcT mutants compared with wild type were assayed based on the fluorescence from the chromophorylated biliprotein, similar to that of CpcS (14). PCB-CpcB(C84S) was assembled in BL21(DE3) by co-expressing plasmids carrying cpcT mutants, cpcB(C84S), and genes for producing the PCB chromophore (ho1-encoding heme oxygenase 1 and pcyA encoding PCB:ferredoxin oxidoreductase) in plasmid pACYC (25). After expression, enzymatic reactions were followed by fluorescence (emission at 630 nm and excitation at 580 nm) of the PCB-CpcB(C84S) product.
To test the PCB binding, His-tagged CpcT (37 μm) was incubated with PCB (32 μm) in KPB (0.5 m, pH 7.2) containing NaCl (0.15 m) for 1 h. Unbound PCB was removed by Ni2+-affinity chromatography with 8 volumes of the column bed of KPB buffer (0.5 m, pH 7.2) containing NaCl (0.15 m). The PCB-CpcT complex was then eluted with the same buffer containing imidazole (0.2 m). Absorption and fluorescence spectra were recorded without delay.
Crystallization, Data Collection and Structure Determination
Selenomethionine-substituted CpcT protein was prepared according to the standard protocol (38) and was crystallized under the condition containing MgCl2·6H2O (0.1 m), BisTris (0.1 m, pH 5.5), and polyethylene glycol 3350 (14% w/v). The CpcT-PCB crystals were obtained by soaking in the mother liquor with additional 0.5 mm PCB chromophore (Frontier Scientific, Inc.) for 20 h.
Both apo-CpcT and CpcT-PCB crystals were frozen with no additional cryo-protectant. All x-ray diffraction data were collected on LS-CAT beam stations at Advanced Photon Source, Argonne National Laboratory. All diffraction data were processed using HKL2000 (39). The crystal structure of apo-CpcT was determined by the single-wavelength anomalous dispersion method (Phenix (40)) using selenomethionine-substituted crystals. The initial model was built using Coot (41) following Autobuild (Phenix), and the final structure was refined at 1.95 Å resolution (Phenix). The CpcT-PCB structure was determined by molecular replacement (Phaser in CCP4 (42)) in the space group of P1 using apo-CpcT as the search model and refined at 2.5 Å resolution. Surface areas buried at the dimer interface were calculated using the PISA server (43). Statistics of data collection and structural refinement are summarized in Table 1. Both coordinates and structure factor amplitudes of apo-CpcT and CpcT-PCB structures have been deposited to Protein Data Bank under accession numbers 4O4O and 4O4S, respectively.
TABLE 1.
Statistics of data collection and structure refinement
ASU means asymmetric unit.
| Apo-CpcT | CpcT-PCB | |
|---|---|---|
| Data collection | ||
| Beamline | 21-IDG | 21-IDG |
| Resolution (Å) | 1.95 | 2.50 |
| Rmerge | 0.065 | 0.079 |
| Completeness (%) | 97.9 | 80.2 |
| No. of unique reflections | 195,467 | 76,002 |
| Redundancy | 12 | 4 |
| Space group | P3121 | P1 |
| Cell parameters (Å) | a = b = 69.3 | a = 69.7, b = 69.6 |
| c = 165.1 | c = 162.6 | |
| α = 90° | α = 90.2° | |
| β = 90° | β = 90.3° | |
| γ = 120° | γ = 60.1° | |
| Structure refinement | ||
| R-factor | 0.175 | 0.181 |
| Free R-factor | 0.206 | 0.241 |
| No. of molecules/ASU | 2 monomers | 12 monomers |
| Waters and ligands | 301 HOH | 12 PCB |
| 74 HOH | ||
| Solvent content (%) | 51 | 51 |
| PDB code | 4O4O | 4O4S |
RESULTS
Crystal Structure of CpcT
The crystal structure of apo-CpcT was determined in the space group of P3121 with two molecules in the asymmetric unit using the single-wavelength anomalous dispersion method (Fig. 1). All 199 residues of CpcT except two terminal residues (Met-1 and Glu-199) and the C-terminal tag have been accounted for by electron densities. The final structure was refined to 1.95 Å resolution with the final R-factor and free R-factor of 0.175 and 0.214, respectively (Table 1). The coordinates have been deposited to the Protein Data Bank under the accession code 4O4O.
FIGURE 1.
Crystal structure of the CpcT dimer. a, surface representation reveals a CpcT dimer with an intimate dimer interface. b, ribbon diagram shows protruding loops from one subunit extending to the other subunit like a plug. Bulky aromatic side chains at the interface are highlighted in yellow for subunit A and cyan for subunit B. c, zoom-in view of the interface reveals stacking interaction between two Tyr-59 side chains. Other Tyr residues lining at the interface are also labeled.
In the asymmetric unit, CpcT forms a dimer in which two subunits are roughly related by a noncrystallographic 2-fold symmetry and interact with each other in a surface complementary fashion (Fig. 1). A protruding loop (residues 25–32) from one subunit partially blocks the opening of the β-barrel in the other subunit like a “plug” (Fig. 1b). The dimer interface buries a surface area of ∼900 Å2 (EBI-PISA (43)) that involves direct and water-mediated interactions between two subunits. Remarkably, a number of aromatic residues (Phe-30, Tyr-31, Tyr-57, Tyr-59, Tyr-65, Trp-175, and Phe-182) lie at the dimer interface, including direct stacking interaction between two aromatic rings of Tyr-59 from both subunits (Fig. 1c).
Each CpcT subunit adopts a conical, goblet-shaped β-barrel with 10 anti-parallel β-strands; this fold is characteristic for the FABP subfamily of the calycin superfamily (32). Large insertions between β-strands are found at the opening of the β-barrel (Fig. 2a), where a disulfide bond between Cys-116 and Cys-137 brings together two adjacent strands and contributes to the overall rigidity of the β-barrel structure. A long extended structural segment (residue 87–109) folds back and docks onto the outside surface of the β-barrel largely via hydrophobic interactions (Fig. 2a). At the center of the β-barrel is a deep cleft with charged residues at the opening and hydrophobic residues at the bottom (Fig. 3b).
FIGURE 2.
CpcT structure bound with the PCB chromophore. a, PCB (in cyan) is located to a cavity enclosed by a 10-stranded β-barrel that is stabilized by a disulfide bond (yellow) between Cys-116 and Cys-137. Three large insertions between β-strands are colored in magenta. b, 2Fo − Fc map (NCS-averaged over 12 subunits; contoured at 3.5σ level) of PCB show that the chromophore adopts the ZZZsss conformation (left) with an M-configured cyclic-helical structure (right, bottom view from left). c, molecular surface of CpcT shows a deep but accessible chromophore binding pocket (marked by a red arrow), which is partially shielded by the plug structure of the partner subunit (in green). d, electrostatic potential surface representation reveals a positively charged patch (in blue) at the rim of the PCB-binding site (indicated by a red arrow).
FIGURE 3.

Protein environment in the PCB binding pocket. a, propionate side chains of PCB (in cyan) are exposed to the molecular surface. Access to the ethylidene group of ring A is hindered by bulky or hydrophobic residues from both subunits (A in green and B in cyan) at the dimer interface. The Cys-116–Cys-137 disulfide bond (yellow) is located opposite the dimer interface. b, opening of the PCB pocket is lined with positively charged residues, although nonpolar residues are clustered at the bottom of the pocket. Red dashes mark the distances between the C31 atom of PCB and two potential proton donors (Tyr-65 and Asp-163). The side view of PCB shows that ring A is located below ring D (standard orientation of formula), corresponding to M-helicity. c, top view from the pocket opening shows structural rearrangements in Arg-66, Arg-68, and Arg-141 before (yellow) and after (gray) the PCB (cyan) binds.
Superposition of two subunits (A and B) within the same CpcT dimer reveals similar structures with an overall r.m.s.d. value of 0.77 Å over 197 aligned residues (Fig. 4). More prominent differences are observed in regions at the dimer interface, in particular in a bulged segment of the β-strand (residue 177–182). Structural alignment between A/B and B/A dimers further reveals differences in relative orientations between two subunits, where the plug from subunit A is about 2.8 Å deeper into the pocket of subunit B than the other way around (Fig. 4).
FIGURE 4.

a, structural comparisons between the A/B (light blue) and B/A (green) CpcT dimers. Although subunits A and B as monomers (left) are very well superimposed (with an overall r.m.s.d. value of ∼0.77 Å), subunits on the right of the dimer are not aligned, suggesting differences in relative positioning between subunits A and B. Specifically, the plug of the subunit A is deeper into B by a distance of 2.8 Å. b, structural superposition between the dimers of apo-CpcT (light blue) and the CpcT-PCB complex (dark blue). Incorporation of the chromophore (cyan) slightly altered molecular packing in the crystal lattice, which disrupted the symmetries of space group P3121 for apo-CpcT and led to P1 for CpcT-PCB.
Crystal Structure of PCB-bound CpcT
Co-crystallization of CpcT and its substrate PCB did not generate satisfactory results. The crystal structure of CpcT bound with PCB has been obtained, however, by soaking apo-CpcT crystals with additional 0.5 mm PCB in the mother liquor for 20 h. The soaking process apparently induced structural changes in the CpcT crystal lattice, which are subtle but sufficient to disrupt crystallographic symmetries of the apo-CpcT crystals in the space group P3121. We had to solve the crystal structure of the CpcT-PCB complex in the space group P1 by molecular replacement using the apo-CpcT structure as the search model (Table 1). The P1 unit cell contains 12 CpcT molecules forming six dimers. Electron densities in the difference map with PCB omitted readily identified all 12 PCB-binding sites, indicating sufficient accessibility and occupancy of the PCB binding (Fig. 1, b and c). The CpcT-PCB structure was refined at 2.5 Å resolution with the final R-factor and free R-factor of 0.181 and 0.241, respectively. The coordinates have been deposited to the Protein Data Bank under the accession code 4O4S.
The PCB chromophore is located in a deep cleft at the center of the β-barrel (Fig. 2a) with both propionate groups of rings B and C pointing out to the molecular surface. Electron densities in both the simulated annealing omit Fo − Fc map and the 2Fo − Fc map clearly showed that PCB adopts the ZZZsss geometry in an M-helical conformation. Although the resolution is limited (2.5 Å resolution), we were able to assign the E-configuration to the 3-ethylidene side chain of ring A based on the NCS-averaged electron densities over 12 subunits (Fig. 2b). However, we were unable to resolve the stereochemistry at C2 based on the electron density of PCB bound to CpcT (see Fig. 2b for atom numbering). The chirality at C2 has been established as R both in the educt (44) and in the product (45–47), whereas the chromophore structure in the CpcT-PCB complex seems to be more compatible with an in-plane geometry (Fig. 2b), perhaps due to (partial) enolization with a C1=C2 double bond. Among the six dimers in the unit cell of P1, PCB consistently displays better resolved electron densities in one subunit (B) than in the other (A), likely due to the aforementioned observation that the plug from subunit A renders a tighter PCB binding pocket in subunit B (Fig. 4). The PCB binding pocket is occupied with water molecules in the apo-CpcT structure but slightly expands upon PCB binding. Although the CpcT structure remains largely unchanged upon PCB binding (r.m.s.d. of ∼0.33 Å in all Cα atoms between the apo-CpcT and CpcT-PCB structures), positively charged residues (Arg-66, Arg-68, and Arg-141) undergo significant rotamer rearrangements to accommodate PCB via direct interactions with the propionate side chains (Fig. 3c). The positioning of PCB is largely determined by the polarity and shape of the protein cavity such that substituents of rings A, B, and C are accessible in the CpcT monomer (Fig. 3, a and b). However, the 3-ethylidene group of ring A, which forms a covalent bond to the Cys-β155 site of the target PBP, is shielded by the plug structure in the CpcT dimer, and only becomes accessible upon dimer dissociation (Figs. 1a and 3a).
Rings A and D of PCB are largely surrounded by hydrophobic residues, including Phe-18, Phe-152, Phe-159, Phe-182, and Phe-184 (Fig. 3b). Phe-30 in the plug of the partner subunit provides additional shielding for ring A (Fig. 3a). The shielded C1=O and C19=O dipoles are nearly antiparallel (∼140°) and probably stabilize the M-helical conformation of PCB. The pyrrole nitrogens in rings A and C form hydrogen bonds with the side chain of Arg-66 and indirectly via Asp-163 and the carbonyl group of ring D (Fig. 3, b and c).
Catalytic Site
CpcT catalyzes the covalent attachment of the chromophore PCB to the Cys-β155 site of phycobiliprotein CpcB. This reaction requires protonation (see “Discussion”). Our crystal structure of CpcT-PCB reveals two candidate residues near ring A that may serve as proton donors. They are Asp-163 and Tyr-65, which approach ring A from the opposite faces of the chromophore. The carboxyl group of Asp-163 and the phenolic hydroxyl of Tyr-65 are located 3.5 and 3.2 Å from the 3-ethylidene group, respectively, and Asp-163 is also only 3.5 Å from C5 (Fig. 3b). To explore their roles in the CpcT function, we made three single mutants, Y65F, D163V, and D163A, to examine how removal of the candidate proton donor group in Y65F and D163V and/or the steric effect in D163A affect the PCB complexation with CpcT and/or its covalent attachment to the apoprotein. These mutations significantly diminish the enzymatic activities of CpcT as measured by in vivo chromophorylation of CpcB (Fig. 5). The in vitro assays show that these variants are still able to bind PCB (Fig. 6, a–c). Additional features in their absorption spectra, however, indicate chromophore heterogeneities in the CpcT complexes that have yet to be characterized. We postulate that Tyr-65 and Asp-163 are likely to play a catalytic role in the CpcT lyase function by assisting nucleophilic attack at the C31 atom that generates a covalent bond to the Cys-β155 of CpcB.
FIGURE 5.
Site-directed mutagenesis and CpcT lyase activity. a, mutation sites are highlighted in colors corresponding to their roles in CpcT lyase activity: essential (red), critical (orange), and minimal effect (green). b, CpcT lyase activities of mutants relative to the wild type (WT = 100%), as measured by reconstitution of CpcB(C84S) with PCB in E. coli. A control in the absence of CpcT did not yield chromophorylated proteins beyond the detectable limit (1%), i.e. spontaneous chromophore attachment did not occur (25, 48).
FIGURE 6.
Optical properties in solution and oligomeric state of PCB-CpcT complexes. Absorption (solid line) and fluorescence emission (dashed line, λex = 580 nm) spectra of PCB complexes with wild type CpcT (a), CpcT(Y65F) (b), and CpcT(D163A) (c). Excessive PCB was removed immediately before measurement from the CpcT-PCB complex purified by Ni2+ affinity chromatography. d, elution profile of CpcB(C84S) was obtained by gel chromatography on Superdex 75 eluted with KPB (20 mm, pH 7.2) containing NaCl (150 mm) (see “Experimental Procedures”). Two peaks correspond to CpcB oligomerized as dimer (40 kDa, calculated 47.4 kDa) and trimer (57 kDa, calculated 71.1 kDa), respectively. e, elution profiles of CpcT at different protein concentrations (2.4 and 36 mm) under the same experimental condition as in d. f, elution profiles of CpcT in the absence (solid line) and presence (dashed line) of PCB (final concentration ∼0.6 μm) in DMSO (final concentration ∼0.016% (v/v)).
Mutations That Affect PCB Attachment to Apoprotein
To explore structural elements of CpcT that may influence the PCB attachment reaction, we specifically examined how accessibility, structural rigidity, and the environment of the PCB binding pocket affect the CpcT lyase activity via site-directed mutagenesis (Fig. 5).
The CpcT dimer structure reveals that the PCB binding is partially protected by the dimer interface (Figs. 1c and 3a). Not surprisingly, deletion of the plug (spanning residues 20–32) significantly reduces the lyase activity to <10% of the wild type (WT) level, and so does removal of the aromatic side chain in either Phe-30 or Tyr-31 at the dimer interface (Figs. 3a and 5b). Y59A, designed to disrupt the Tyr-59–Tyr-59 stacking interaction, also leads to reduction in the lyase activity by at least 3-fold (Figs. 1a and 5b). Taken together, we postulate that disruption of the dimer interface leads to a less protected PCB binding pocket and thus reduced lyase activity.
Opposite to the dimer interface, the S–S bond between Cys-116 and Cys-137 structurally bridges two neighboring β-strands and presumably enhances the overall rigidity of the PCB binding pocket (Fig. 2a). Previous mutational studies suggested that this disulfide bond is critical for the CpcT lyase activity (48). Moreover, this disulfide bond may be functionally relevant. The CpcT lyase reaction is inhibited by thiols (25), although other lyases, like CpcE/F (13, 19) and CpcS (24), tolerate thiols at the concentrations used; and PecE/F (15, 21) are even activated by thiols. Activation has also been found for autocatalytic chromophorylation of ApcE (7). Products of sulfhydryl of lyase attaching to PCB have been reported at very low levels, which may result from reversible addition of thiols to bilin under similar experimental conditions (17).
We have also explored the role of other residues surrounding the bound PCB, including Arg-68 and Arg-141, which directly interact with the propionate side chains (Fig. 3, b and c). Substitution with alanine at either site significantly lowers the lyase activity (<10% of the WT level) (Fig. 5b). Other modifications to the PCB environment, for example Q55A, D63N, R66A, N83A, and M118A, affect the CpcT function to various extents (Fig. 5b).
Before the crystal structure of CpcT was solved, Trp-13, His-33, Arg-66, Arg-97, Cys-116, Cys-137, and Trp-175 of CpcT had been identified as amino acids that are critical for the CpcT activity. Among these, mutations at His-33, Cys-116, Cys-137, and Trp-175 rendered CpcT inactive, whereas mutations at Trp-13, Arg-66, and Arg-97 resulted in moderately reduced activities (48). We speculate that His-33 and Trp-175 at the dimer interface are required for proper positioning of the plug relative to the other subunit, thereby modulating the CpcT dimerization and PCB binding.
PCB Binding and Oligomeric State
Complex formation of PCB with CpcT was shown to be transient and weak at low concentration conditions, based on fluorescence and gel filtration experiments (48). In the crystal lattice where CpcT and PCB were present at high concentrations, PCB was incorporated with sufficient occupancies that allowed assignment of pyrrole rings based on the electron density map (Fig. 2b). To characterize the spectroscopic properties of the CpcT-PCB complex in solution, His-tagged CpcT was incubated with PCB at high concentrations (32 μm) in a buffer of high ionic strength (650 mm). After removal of excessive unbound PCB with an affinity column, optical spectra of the CpcT-PCB complex were measured (Fig. 6a). Both the peak positions (λmax = 620 and 370 nm) and their absorption ratio (QUV-Vis = 0.48) are very similar to those of the free, unprotonated PCB chromophore that exists in a cyclic-helical conformation. This state is consistent with the crystal structure, in which no acidic residues are found near the pyrrole nitrogens of PCB. Protonation of dihydrobiliverdins causes a ∼30-nm red shift of the 620-nm band and a doubling of its extinction coefficient (QUV-Vis ∼1). Deprotonation leads to a much larger red shift (>100 nm), although the extinction coefficient changes very little (49). Protonation also increases the fluorescence yield, but other factors, including, in particular, the rigidity of the chromophore, also contribute to enhanced fluorescence (50, 51). The yield of ΦF = 0.023 measured from CpcT-PCB is larger than that of the free chromophore, which is consistent with PCB with moderately increased rigidity in the CpcT pocket.
Although the CpcT dimers are important to protect the PCB during the relatively slow lyase-catalyzed reaction, dimer dissociation is equally important for releasing PCB when CpcT is docked onto its target sites. In other words, reversible dimerization of CpcT would be crucial for its lyase function. In solution, CpcT appears to dimerize in a concentration-dependent manner, and its dimerization is further enhanced in the presence of PCB (Fig. 6, e and f). At the concentration of 3 μm, CpcT is mostly monomeric in the absence of PCB, but it tends to dimerize in the presence of 0.6 μm PCB (shoulder in elution profile; Fig. 6f). For a CpcT-catalyzed reaction in E. coli (see “Experimental Procedures”), the concentration of CpcT is estimated to be around 150 μm. Given the cellular concentration of PCB (∼3 μm in E. coli and ∼2 μm in mammalian cells (52), CpcT dimerization is favorable.
DISCUSSION
Structure
CpcT is a β-barrel protein with an FABP fold that is characterized by a 10-stranded structure with an N-terminal helix and a loop between the first two strands. FABPs belong to the calycin superfamily (32), in which members share low sequence homology, but they exhibit conserved structures and short motifs. Seven crystal structures of bilin-binding calycins, including CpcT, are known, four of which contain a bound chromophore (Fig. 7). Although all bound chromophores are open-chain tetrapyrroles, they differ considerably in both structure and function.
FIGURE 7.
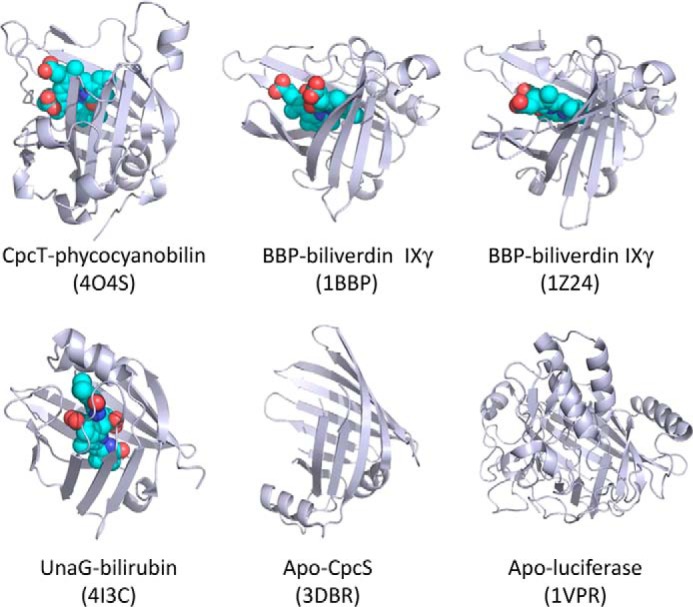
Structural comparisons among six bilin-binding members in the FABP protein family. All ribbon diagrams are shown in the structurally aligned orientation. Four of the six structures are bound with bilin chromophores (shown as CPK models with C in cyan, O in red, and N in blue). The PDB accession numbers are shown in parentheses, and the chemical nature of the chromophore is indicated following a dash.
Two insect biliproteins from P. brassicae (PDB code 1BBP) (33) and Manduca sexta (PDB code 1Z24) (53) carry biliverdin IXγ. This unusual biliverdin isomer is derived from heme cleaved at the C-15 methine bridge between rings C and D, such that the propionic acid side chains are located at the ends of the open-chain tetrapyrrole. Both biliproteins exhibit narrow pockets facilitating tight chromophore binding such that biliverdin IXγ is retained during protein purification. The opening of the chromophore pocket in CpcT is somewhat wider. This may contribute to relatively weak binding of PCB that is only retained during chromatography under special conditions (such as high ionic strength and rapid work). The bound chromophore, however, adopts a similar cyclic-helical conformation in all three proteins, except that the chromophore helicity in CpcT is opposite those in the insect biliproteins. PCB forms complexes with several CpcT variants that are catalytically inactive, but unlike the WT complex, their structured spectra indicate chromophore heterogeneity (Fig. 6), which may result from the mixture of conformers, protonation, or tautomerization states; chemical reactions are unlikely to occur on the time scale of the experiment. It is possible that such heterogeneities contribute to the reduced CpcT activity, because protonation and/or tautomerization are key steps for the proposed CpcT-catalyzed chromophorylation via an acylimmonium cation at ring A (see below and Ref. 54).
Biliverdins and PCB assume a helical geometry due to steric hindrance between the carbonyl oxygens at C-1 and C-19 in a cyclical planar geometry. In solution, P- and M-configured conformers are in equilibrium (55, 56). Although a helical geometry is retained, CpcT selectively binds the chromophore in the M-helical configuration (Fig. 3), whereas the biliverdin IXγ chromophores are P-configured in insect biliproteins (Fig. 8). In all three structures, the propionic acid side chains are exposed, whereas hydrophobic rings A and D are deeply buried in the amphipathic pocket. Interestingly, the overall orientation of the chromophore largely remains the same despite phycobilins, and insect bilins are derived from the ring opening at different methine bridges, i.e. between rings A and D in PCB, and between rings C and D in biliverdin IXγ (Fig. 8).
FIGURE 8.
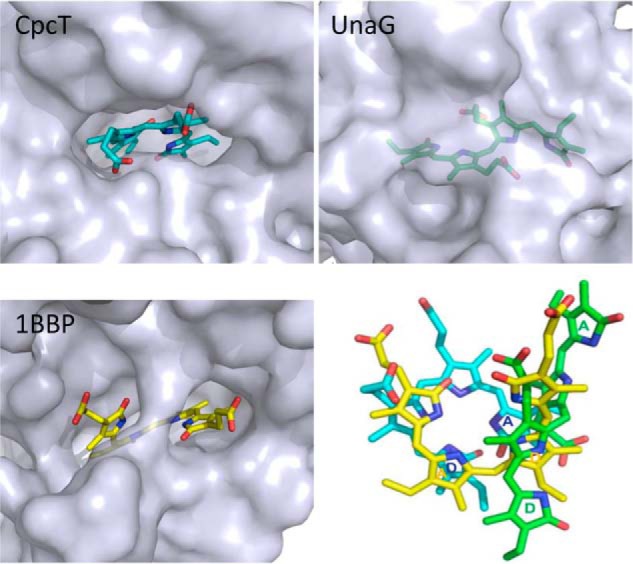
Conformations, accessibility, and alignment of the bilin chromophores bound to three representative FABP proteins. In both CpcT and 1BBP, bilins are exposed to the molecular surface via their propionate side chains, although their chromophores open up at different methine bridges as follows: PCB in CpcT (cyan) opens between rings A and D, and biliverdin IXγ (yellow) opens between rings B and C. The bilirubin chromophore in UnaG (green) adopts an extended conformation and is completely buried (visible only with semi-transparent surface). The chromophore alignment (right/bottom panel) is based on the least squares fittings of their protein scaffolds. For clarity, rings A and D are labeled for three chromophores in corresponding colors; in BV IXγ, the central rings correspond to the outer rings in PCB and are therefore labeled the same way.
The recently published bilirubin protein, UnaG from eel, is also an FABP protein (PDB code 4I3B (57)), in which the bound chromophore adopts a structure considerably different from that of CpcT. In UnaG, the bilirubin chromophore has an extended conformation, which is completely buried and shielded by two helices at the opening of the goblet (Figs. 7 and 8). Unlike biliverdins and PCB, isolated bilirubin is very hydrophobic, and strong internal H-bonds (58, 59) must be disrupted inside a chromoprotein and stabilized by hydrogen bonds to buried residues (57).
Three other bilin-binding calycins have been crystallized in the absence of any chromophore. The dinoflagellate luciferase (PDB code 1VPR) contains a β-barrel domain that is decorated with several α-helices; it binds a chlorophyll-derived bilin cleaved at the C20 methine bridge (Fig. 7) (60). CpcS from Thermosynechococcus elongatus (PDB code 3BDR5 (28)) is another PBP lyase that shares 12% sequence identity with CpcT. CpcS exhibits a β-barrel structure very similar to CpcT with r.m.s.d. of 2.7 Å over 116 aligned residues (12, 23). Residues 19–38 of CpcS adopt an extended anti-parallel β-strand conformation in contrast to the equivalent plug structure of CpcT that contains two helical turns (Fig. 7). CPES from the cryptophyte, Gaillardia theta, has a very similar structure but differs from CpcT in the chromophore specificity (29). The putative chromophore binding pocket in CpcS would be sufficiently spacious for PCB binding without significant changes in the protein structure (Fig. 7) (28),5 but the absorption spectrum of PCB-loaded CpcS indicates an extended chromophore geometry (61). Ligand binding did not require dramatic changes in the protein structure of a lipocalin engineered to bind digitonin (62). CPES shows a tighter cavity that may be relevant to its specificity for chromophores with a reduced Δ15,16 double bond (29). Unlike CpcT, CpcS is less selective in its target site to which the chromophore is transferred. In vitro experiments indicated that chromophorylation of Cys-β155 by CpcT is slower than that of Cys-β84 by CpcS, although the presence of a Cys-β84 chromophore seems to hinder subsequent chromophorylation at Cys-β155 (25). Formation of the relatively stable complex of CpcT and CpcS may play a role in coordinating the functions of CpcT and CpcS (25). It is noteworthy that CpcS exhibits a very different dimeric arrangement in the crystal lattice, in which two CpcS monomers pack against each other via close interactions between the outer surfaces of the β-barrels, and buries a surface area of 703 Å2 (28). This type of dimerization is also found in CPES, where it is strengthened by an intermolecular disulfide bond (29). The crystal structure of CPES, however, also reveals interactions involving the rim of a putative chromophore binding pocket.
Among all lyases, only CpcT is inhibited markedly by thiols (25). We speculate that this property is pertinent to the disulfide bond between Cys-116 and Cys-137 in CpcT and provides another level of regulation for chromophorylation (63), because the higher reduction state in a photosynthetic organism, the less demand for light harvesting.
Site-specific Interaction with CpcB
As a chromophore chaperone, CpcT must balance two aspects of its lyase function. On the one hand, tight chromophore binding would be favorable given the low concentration of free PCB in cyanobacteria, the chemical reactivity of PCB, and the slow reaction rates of CpcT (25) in solution. On the other hand, CpcT as lyase must present and release the chromophore for covalent attachment to Cys-β155. We postulate that the observed CpcT dimerization plays an important role for its chaperone function. In particular, the plug-like structures at the dimer interface presumably shield and protect the reactive and labile chromophore from unwanted reactions with, for example, other thiols or with oxygen. The CpcT dimer has to dissociate upon docking onto the bilin-accepting apoprotein, CpcB.
In the absence of a crystal structure for any apo-PBP, we used a fully assembled PBP structure (PDB code 1CPC (45)) to examine structural features surrounding the target site. Cys-β155 is located at the outer rim of the (αβ)3 trimer with the propionate side chains of the bound chromophore exposed (Fig. 9). A few highly conserved structural features are found in the vicinity of Cys-β155. These include the charged face of a long, bent helix (spanning residues 20–62 in the β-subunit of 1CPC) that crosses over the PCB in an extended ZZZasa configuration, and the anchor cysteine containing a GDC sequence motif in a helical hairpin loop (Figs. 9 and 10). Surface analysis suggests that both surface complementarity and electrostatic interactions between charged patches are responsible for guiding specific docking of CpcT onto Cys-β155 (Fig. 9). Specifically, two large loop insertions of CpcT (residues 87–109 and 56–65) (Fig. 1a) may be directly involved in docking onto the concave surface near Cys-155 (Fig. 9). Furthermore, interactions between positively charged residues at the opening of the PCB binding pocket in CpcT (Arg-66, Arg-68, and Arg-141) and the negative patch of CpcB (Asp-33, Asp-39, and Asp-154) might contribute to precise positioning between the donor and target sites. Such docking via loop insertions is expected to disrupt the dimer interface of CpcT, thereby allowing access to the chromophore. Kronfel et al. (28) pointed out the difference in charges between the two binding sites of the β-subunit and suggested its role in assisting docking of CpcS to the Cys-β84-binding site. Overkamp et al. (29) also concluded preferential docking of CPES to the β-subunit of the cryptophyte biliprotein, PE545, which is structurally and phylogenetically related to cyanobacterial biliprotein β-subunits. By the same token, docking of the positively charged rim of the CpcT pocket will preferentially dock to the negatively charged Cys-155-binding site of C-PC β-subunits.
FIGURE 9.

Proposed docking mode of CpcT (green, this work) to the target site Cys-β155 of CpcB (gray, PDB code 1CPC). The locations of PCB are also shown at the docking interface between CpcT and CpcB before and after PCB is transferred from CpcT to CpcB for covalent attachment. Upon docking, a segment of CpcB containing Cys-β155 (in gold) may reach into the cleft of the CpcT monomer to bind the chromophore in the ZZZsss geometry (cyan) and then undergoes structural transformation as PCB attached to CpcB adopts the ZZZasa geometry (gray).
FIGURE 10.

Sequence alignment among β-subunits of CPC and PEC using pairwise HMM logos (27). A signature motif (E/D)RD (residues 33–37-39) and a highly conservative Asp-152 in CpcB/PecB are shown, both of which may play a role in guiding the docking of CpcT. The protein sequences of CPC and PEC were taken from Anabaena sp. 90 (NC_019427), Cyanothece sp. ATCC 51142 (NC_010546), Nostoc sp. PCC 7120 (NC_003272.1), Rivularia sp. PCC 7116 (NC_019678), Synechococcus elongatus PCC 6301 (NC_006576.1), Synechococcus sp. JA-2–3B'a(2–13) (NC_007776), Synechococcus sp. PCC 7002 (NC_010475.1), Synechocystis sp. PCC 6803 (NC_000911.1), T. elongatus BP-1 (NC_004113.1), and Thermosynechococcus sp. NK55a (NC_023033.1).
Catalytic Action
In the fully chromophorylated PBP trimer, the binding Cys-β155 is located in a loop that interacts intimately with rings A and B of the bound chromophore (Fig. 9). We speculate that this loop is less structured in the nonchromophorylated PBP and is thus flexible enough to act as a “fishing line.” Similar to the plug in the dimer, it reaches into the cleft of CpcT and allows the covalent attachment of PCB. Such specificity may be guided by electrostatic interactions between charged surface patches of CpcB and CpcT (Figs. 1d, 9, and 10). Approach of the cysteine sulfhydryl probably occurs for steric reasons from the more open α-face of the chromophore. The subsequent binding reaction (Fig. 11) is rationalized on the basis of the mechanism involving an acyl-immonium intermediate proposed by Grubmayr and Walter (54). It starts by protonation of the chromophore at C5 by the Cys-155-sulfhydryl of the approaching apoprotein, assisted by hydrogen bonding to Tyr-65, possibly via transient protonation of a Tyr-65 anion, which is also located on the more open α-face of the chromophore. The resulting N21-acylimmonium cation is stabilized by the negatively charged carboxylate group of Asp-163 on the sterically hindered β-face of the chromophore. The C–S bond is then formed by nucleophilic attack of the thiolate at C31 of the chromophore from the less hindered α-side, resulting in the 31S configuration that distinguishes the β155 chromophore from those attached to other sites (46, 47, 64). Protonation at C3 from the α-face would then result in the correct 3R-configuration, with the C2 and C3 substituents in trans-position. This protonation is either intramolecular (as shown in Fig. 11), but possibly assisted again by Tyr-65 that is located on the proper face. Consistent with this proposal, single mutations at either position (D163A, D163V, and Y65F) largely abolish CpcT activity (Fig. 5b).
FIGURE 11.
Proposed reaction mechanism of CpcT. CpcT carries and protects an unprotonated PCB chromophore in the ZZZsss geometry. CpcB, the accepting apophycobiliprotein, presents an unstructured segment containing the attachment site Cys-β155 as a “fishing rod.” CpcB approaches the Δ3,31 double bond of PCB from the sterically unhindered α-side and results in the 31S configuration. During or after its release from the CpcT pocket, the chromophore bound to CpcB assumes the ZZZasa geometry. The acylimmonium ion is formed only transiently after docking of CpcB. See the lower right structure for atom and ring labeling. In the mechanism shown, the critical Tyr-65 and Asp-163 play somewhat passive roles in polarizing the double bond and offering H-bond partners. However, more active roles are also possible, in particular transient protonation of a tyrosinate anion in the first steps of the reaction.
The proposed catalytic mechanism rationalizes current knowledge about the CpcT action. The CpcT dimer protects the labile chromophore. By docking of CpcB, the CpcT dimer dissociates. The anchor cysteine of CpcB is then allowed to access C31 of the chromophore for proper chromophore attachment. The mechanism implies that the attachment occurs, although the chromophore is still in the CpcT pocket in the ZZZsss geometry. Structural transformation to the final ZZZasa geometry, together with local rearrangements in the binding site of CpcB, is expected to occur during or after release from CpcT following the covalent bond formation. This sequence of events does not contradict spontaneous additions, in which PBPs bind chromophores in an extended conformation, although with low stereochemical fidelity and side reactions due to lack of protection (10, 13, 14, 21, 61, 65). It is not clear if this reaction mechanism also applies to CpcS. A chromophore has been modeled into the structure of the empty lyase, CpcS, in an extended, bilirubin-like ridge-tile conformation that retains the Zs geometry at both the C5 and C15 methine bridges (28). Similarly, phycoerythrobilin in an extended geometry fits in the cavity of an eukaryotic CPES (29). This would also require subsequent rotation at both bridges. A more extended chromophore conformation in CpcS than in CpcT would be in line with the red shift and increase of the long wavelength band in the absorption spectra of PCB bound to CpcS (61) and of phycoerythrobilin bound to CPES (29). A twisting of the two halves by 90° at the central methine bridge, as in bilirubin (57, 66), however, would interrupt the conjugation and result in a strong blue shift (50).
Although S- and T-type bilin lyases are distantly related, they may differ in mechanistic details, particularly the conformation and protonation state in which the chromophore is presented. This would account for their differences in stereochemistry of the respective binding sites as well as in chromophore selectivity. Nonetheless, both lyases carry out three main functions as follows: 1) assisting site selectivity in the apophycobiliprotein; 2) protecting the chromophore; and 3) ensuring the regio- and stereoselectivity of the addition, because both do not seem to present the chromophore in the final ZZZasa geometry. The reaction of the phylogenetically unrelated E/F-type lyases may still be different. Last, but not least, it should be noted that deprotonation of the cationic acylimmonium intermediate (Fig. 11) at C2 rather than C5 would result in the conversion of PCB to a phycoviolobilin chromophore; such isomerization occurs in E/F-type lyases (15, 16, 67) as well as in several cyanobacteriochromes during autocatalytic chromophore attachment (68–70).
Acknowledgments
Use of the Advanced Photon Source was supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02–06CH11357.
This work was supported, in whole or in part, by National Natural Science Foundation of China Grants 31110103912 and 31270893 (to K. H. Z.) and 31370777 (to M. Z.) and State Key Laboratory of Agricultural Microbiology. This work was also supported by National Institutes of Health Grant R01EY024363 (to X. Y.).
This article was selected as a Paper of the Week.
The atomic coordinates and structure factors (codes 4O4O and 4O4S) have been deposited in the Protein Data Bank (http://wwpdb.org/).
A. P. Kuzin, M. Su, J. Seetharaman, F. Forouhar, D. Wang, H. Janjua, K. Cunningham, L.-C. Ma, R. Xiao, J. Liu, M. C. Baran, T. B. Acton, B. Rost, G. T. Montelione, L. Tong, and J. F. Hunt, unpublished data.
- PBP
- phycobiliprotein
- PCB
- phycocyanobilin
- CPC
- cyanobacterial phycocyanin
- CpcB
- apoprotein of CPC β-subunit
- FABP
- fatty acid-binding protein
- KPB
- potassium phosphate buffer
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- PDB
- Protein Data Bank
- r.m.s.d.
- root mean square deviation.
REFERENCES
- 1. Glazer A. N. (1984) Phycobilisome–a macromolecular complex optimized for light energy transfer. Biochim. Biophys. Acta 768, 29–51 [Google Scholar]
- 2. Scheer H. (1982) in Light Reaction Path of Photosynthesis (Fong F. K., ed) pp. 7–45, Springer-Verlag, Berlin [Google Scholar]
- 3. Sidler W. A. (1994) in The Molecular Biology of Cyanobacteria (Bryant D. A., ed) pp. 139–216, Kluwer Academic Publishers Group, Dordrecht, Netherlands [Google Scholar]
- 4. Grossman A. R., Bhaya D., Apt K. E., Kehoe D. M. (1995) Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Annu. Rev. Genet. 29, 231–288 [DOI] [PubMed] [Google Scholar]
- 5. Gantt B., Grabowski B., Cunningham F. X. (2003) in Light-harvesting Antennas in Photosynthesis (Green B., Parson W., eds) pp. 307–322, Kluwer Academic Publishers Group, Dordrecht, Netherlands [Google Scholar]
- 6. Schluchter W. M., Glazer A. N. (1999) in The Phototrophic Prokaryotes (Peschek G. A., Löffelhardt W., Schmetterer G., eds) pp. 83–95, Kluwer/Plenum Press, New York [Google Scholar]
- 7. Zhao K. H., Su P., Böhm S., Song B., Zhou M., Bubenzer C., Scheer H. (2005) Reconstitution of phycobilisome core-membrane linker, LCM, by autocatalytic chromophore binding to ApcE. Biochim. Biophys. Acta 1706, 81–87 [DOI] [PubMed] [Google Scholar]
- 8. Alvey R. M., Biswas A., Schluchter W. M., Bryant D. A. (2011) Attachment of noncognate chromophores to CpcA of Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 by heterologous expression in Escherichia coli. Biochemistry 50, 4890–4902 [DOI] [PubMed] [Google Scholar]
- 9. Tang K., Zeng X. L., Yang Y., Wang Z. B., Wu X. J., Zhou M., Noy D., Scheer H., Zhao K. H. (2012) A minimal phycobilisome: fusion and chromophorylation of the truncated core-membrane linker and phycocyanin. Biochim. Biophys. Acta 1817, 1030–1036 [DOI] [PubMed] [Google Scholar]
- 10. Scheer H., Zhao K. H. (2008) Biliprotein maturation: the chromophore attachment. Mol. Microbiol. 68, 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schluchter W. M., Shen G., Alvey R. M., Biswas A., Saunée N. A., Williams S. R., Mille C. A., Bryant D. A. (2010) Phycobiliprotein biosynthesis in cyanobacteria: structure and function of enzymes involved in post-translational modification. Adv. Exp. Med. Biol. 675, 211–228 [DOI] [PubMed] [Google Scholar]
- 12. Bretaudeau A., Coste F., Humily F., Garczarek L., Le Corguillé G., Six C., Ratin M., Collin O., Schluchter W. M., Partensky F. (2013) CyanoLyase: a database of phycobilin lyase sequences, motifs and functions. Nucleic Acids Res. 41, D396–D401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao K. H., Wu D., Zhang L., Zhou M., Böhm S., Bubenzer C., Scheer H. (2006) Chromophore attachment in phycocyanin. Functional amino acids of phycocyanobilin-α-phycocyanin lyase and evidence for chromophore binding. FEBS J. 273, 1262–1274 [DOI] [PubMed] [Google Scholar]
- 14. Kupka M., Zhang J., Fu W. L., Tu J. M., Böhm S., Su P., Chen Y., Zhou M., Scheer H., Zhao K. H. (2009) Catalytic mechanism of S-type phycobiliprotein lyase: chaperone-like action and functional amino acid residues. J. Biol. Chem. 284, 36405–36414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao K. H., Deng M. G., Zheng M., Zhou M., Parbel A., Storf M., Meyer M., Strohmann B., Scheer H. (2000) Novel activity of a phycobiliprotein lyase: both the attachment of phycocyanobilin and the isomerization to phycoviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon. FEBS Lett. 469, 9–13 [DOI] [PubMed] [Google Scholar]
- 16. Shukla A., Biswas A., Blot N., Partensky F., Karty J. A., Hammad L. A., Garczarek L., Gutu A., Schluchter W. M., Kehoe D. M. (2012) Phycoerythrin-specific bilin lyase-isomerase controls blue-green chromatic acclimation in marine Synechococcus. Proc. Natl. Acad. Sci. U.S.A. 109, 20136–20141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tu J. M., Zhou M., Haessner R., Plöscher M., Eichacker L., Scheer H., Zhao K. H. (2009) Toward a mechanism for biliprotein lyases: revisiting nucleophilic addition to phycocyanobilin. J. Am. Chem. Soc. 131, 5399–5401 [DOI] [PubMed] [Google Scholar]
- 18. Fairchild C. D., Zhao J., Zhou J., Colson S. E., Bryant D. A., Glazer A. N. (1992) Phycocyanin α-subunit phycocyanobilin lyase. Proc. Natl. Acad. Sci. U.S.A. 89, 7017–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fairchild C. D., Glazer A. N. (1994) Oligomeric structure, enzyme kinetics, and substrate specificity of the phycocyanin α subunit phycocyanobilin lyase. J. Biol. Chem. 269, 8686–8694 [PubMed] [Google Scholar]
- 20. Biswas A., Boutaghou M. N., Alvey R. M., Kronfel C. M., Cole R. B., Bryant D. A., Schluchter W. M. (2011) Characterization of the activities of the CpeY, CpeZ, and CpeS bilin lyases in phycoerythrin biosynthesis in Fremyella diplosiphon strain UTEX 481. J. Biol. Chem. 286, 35509–35521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao K. H., Wu D., Zhou M., Zhang L., Böhm S., Bubenzer C., Scheer H. (2005) Amino acid residues associated with enzymatic activities of the isomerizing phycoviolobilin-lyase PecE/F. Biochemistry 44, 8126–8137 [DOI] [PubMed] [Google Scholar]
- 22. Zhao K. H., Su P., Tu J. M., Wang X., Liu H., Plöscher M., Eichacker L., Yang B., Zhou M., Scheer H. (2007) Phycobilin:cystein-84 biliprotein lyase, a near-universal lyase for cysteine-84-binding sites in cyanobacterial phycobiliproteins. Proc. Natl. Acad. Sci. U.S.A. 104, 14300–14305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saunée N. A., Williams S. R., Bryant D. A., Schluchter W. M. (2008) Biogenesis of phycobiliproteins: II. CpcS-I and CpcU comprise the heterodimeric bilin lyase that attaches phycocyanobilin to CYS-82 of β-phycocyanin and CYS-81 of allophycocyanin subunits in Synechococcus sp. PCC 7002. J. Biol. Chem. 283, 7513–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao K. H., Su P., Li J., Tu J. M., Zhou M., Bubenzer C., Scheer H. (2006) Chromophore attachment to phycobiliprotein β-subunits: phycocyanobilin:cysteine-β84 phycobiliprotein lyase activity of CpeS-like protein from Anabaena sp. PCC7120. J. Biol. Chem. 281, 8573–8581 [DOI] [PubMed] [Google Scholar]
- 25. Zhao K. H., Zhang J., Tu J. M., Böhm S., Plöscher M., Eichacker L., Bubenzer C., Scheer H., Wang X., Zhou M. (2007) Lyase activities of CpcS- and CpcT-like proteins from Nostoc PCC7120 and sequential reconstitution of binding sites of phycoerythrocyanin and phycocyanin β-subunits. J. Biol. Chem. 282, 34093–34103 [DOI] [PubMed] [Google Scholar]
- 26. Biswas A., Vasquez Y. M., Dragomani T. M., Kronfel M. L., Williams S. R., Alvey R. M., Bryant D. A., Schluchter W. M. (2010) Biosynthesis of cyanobacterial phycobiliproteins in Escherichia coli: chromophorylation efficiency and specificity of all bilin lyases from Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 76, 2729–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schuster-Böckler B., Bateman A. (2005) Visualizing profile-profile alignment: pairwise HMM Logos. Bioinformatics 21, 2912–2913 [DOI] [PubMed] [Google Scholar]
- 28. Kronfel C. M., Kuzin A. P., Forouhar F., Biswas A., Su M., Lew S., Seetharaman J., Xiao R., Everett J. K., Ma L. C., Acton T. B., Montelione G. T., Hunt J. F., Paul C. E., Dragomani T. M., Boutaghou M. N., Cole R. B., Riml C., Alvey R. M., Bryant D. A., Schluchter W. M. (2013) Structural and biochemical characterization of the bilin lyase CpcS from Thermosynechococcus elongatus. Biochemistry 52, 8663–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Overkamp K. E., Gasper R., Kock K., Herrmann C., Hofmann E., Frankenberg-Dinkel N. (2014) Insights into the biosynthesis and assembly of eukaryotic phycobiliproteins. J. Biol. Chem. 289, 26691–26707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skerra A. (2008) Alternative binding proteins: anticalins-harnessing the structural plasticity of the lipocalin ligand pocket to engineer novel binding activities. FEBS J. 275, 2677–2683 [DOI] [PubMed] [Google Scholar]
- 31. Vopel S., Mühlbach H., Skerra A. (2005) Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. Biol. Chem. 386, 1097–1104 [DOI] [PubMed] [Google Scholar]
- 32. Flower D. R., North A. C., Sansom C. E. (2000) The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta 1482, 9–24 [DOI] [PubMed] [Google Scholar]
- 33. Huber R., Schneider M., Mayr I., Müller R., Deutzmann R., Suter F., Zuber H., Falk H., Kayser H. (1987) Molecular structure of the bilin binding protein (BBP) from Pieris brassicae after refinement at 2.0 Å resolution. J. Mol. Biol. 198, 499–513 [DOI] [PubMed] [Google Scholar]
- 34. Shen G., Saunée N. A., Williams S. R., Gallo E. F., Schluchter W. M., Bryant D. A. (2006) Identification and characterization of a new class of bilin lyase: the cpcT gene encodes a bilin lyase responsible for attachment of phycocyanobilin to Cys-153 on the β-subunit of phycocyanin in Synechococcus sp. PCC 7002. J. Biol. Chem. 281, 17768–17778 [DOI] [PubMed] [Google Scholar]
- 35. Bolte K., Kawach O., Prechtl J., Gruenheit N., Nyalwidhe J., Maier U. G. (2008) Complementation of a phycocyanin-bilin lyase from Synechocystis sp. PCC 6803 with a nucleomorph-encoded open reading frame from the cryptophyte Guillardia theta. BMC Plant Biol. 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sambrook J., Fritsch E., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Bradford M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 38. Doublié S. (1997) in Methods in Enzymology (Charles W., Carter J., eds) pp. 523–530, Academic Press, New York [Google Scholar]
- 39. Otwinowski Z., Minor W. (1997) in Methods in Enzymology (Charles W., Carter J., eds) pp. 307–326, Academic Press, New York: [DOI] [PubMed] [Google Scholar]
- 40. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 42. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., Read R. J., Vagin A., Wilson K. S. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krissinel E., Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 44. Brockmann H. (1978) in The Porphyrins (Dolphin D., ed) pp. 288–321, Academic Press, New York [Google Scholar]
- 45. Duerring M., Schmidt G. B., Huber R. (1991) Isolation, crystallization, crystal structure analysis and refinement of constitutive C-phycocyanin from the chromatically adapting cyanobacterium Fremyella diplosiphon at 1.66 Å resolution. J. Mol. Biol. 217, 577–592 [DOI] [PubMed] [Google Scholar]
- 46. Adir N., Vainer R., Lerner N. (2002) Refined structure of C-phycocyanin from the cyanobacterium Synechococcus vulcanus at 1.6 Å: insights into the role of solvent molecules in thermal stability and co-factor structure. Biochim. Biophys. Acta 1556, 168–174 [DOI] [PubMed] [Google Scholar]
- 47. David L., Marx A., Adir N. (2011) High-resolution crystal structures of trimeric and rod phycocyanin. J. Mol. Biol. 405, 201–213 [DOI] [PubMed] [Google Scholar]
- 48. Zhang J., Sun Y. F., Zhao K. H., Zhou M. (2012) Identification of amino acid residues essential to the activity of lyase CpcT1 from Nostoc sp. PCC7120. Gene 511, 88–95 [DOI] [PubMed] [Google Scholar]
- 49. Scheer H. (1976) Studies on plant bile pigments: Characterization of a model for the phytochrome Pr chromophore. Z. Naturforsch. 31c, 413–417 [Google Scholar]
- 50. Falk H. (1989) The Chemistry of Linear Oligopyrroles and Bile Pigments, Springer, Wien [Google Scholar]
- 51. Braslavsky S. E., Holzwarth A. R., Schaffner K. (1983) Solution conformations, photophysics, and photochemistry of bili-pigments-bilirubin and biliverdin dimethylesters and related linear tetrapyrroles. Angew. Chem. Int. Ed. Engl. 22, 656–674 [Google Scholar]
- 52. Müller K., Engesser R., Timmer J., Nagy F., Zurbriggen M. D., Weber W. (2013) Synthesis of phycocyanobilin in mammalian cells. Chem. Commun. 49, 8970–8972 [DOI] [PubMed] [Google Scholar]
- 53. Holden H. M., Rypniewski W. R., Law J. H. (1987) The molecular structure of insecticyanin from the tobacco hornworm Manduca sexta L. at 2.6 Å resolution. EMBO J. 6, 1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grubmayr K., Wagner U. G. (1988) Zur Chemie der Thioladdition an 2,3-Dihydro-3-ethylidendipyrrin-l(10H)-one–eine modellstudie zur kovalenten chromophor-protein-bindung in biliproteiden. Monatsh. Chem. 119, 965–983 [Google Scholar]
- 55. Lehner H., Riemer W., Schaffner K. (1979) Die Interkonversionsbarriere der Bilatrien-Helix. Liebig's Ann. Chem. 1979, 1798–1801 [Google Scholar]
- 56. Haidl E., Krois D., Lehner H. (1985) Chiral induction in biliverdin covalently bound to amino acids. J. Chem. Soc. Perkin Trans. II 3, 421–425 [Google Scholar]
- 57. Kumagai A., Ando R., Miyatake H., Greimel P., Kobayashi T., Hirabayashi Y., Shimogori T., Miyawaki A. (2013) A bilirubin-inducible fluorescent protein from eel muscle. Cell 153, 1602–1611 [DOI] [PubMed] [Google Scholar]
- 58. Person R. V., Peterson B. R., Lightner D. A. (1994) Bilirubin conformational analysis and circular dichroism. J. Am. Chem. Soc. 116, 42–59 [Google Scholar]
- 59. Bonnett R., Davies J. E., Hursthouse M. B., Sheldrick G. M. (1978) The structure of bilirubin. Proc. R. Soc. Lond. B Biol. Sci. 202, 249–268 [DOI] [PubMed] [Google Scholar]
- 60. Schultz L. W., Liu L., Cegielski M., Hastings J. W. (2005) Crystal structure of a pH-regulated luciferase catalyzing the bioluminescent oxidation of an open tetrapyrrole. Proc. Natl. Acad. Sci. U.S.A. 102, 1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tu J. M., Kupka M., Böhm S., Plöscher M., Eichacker L., Zhao K. H., Scheer H. (2008) Intermediate binding of phycocyanobilin to the lyase, CpeS1, and transfer to apoprotein. Photosynth. Res. 95, 163–168 [DOI] [PubMed] [Google Scholar]
- 62. Korndörfer I. P., Schlehuber S., Skerra A. (2003) Structural mechanism of specific ligand recognition by a lipocalin tailored for the complexation of digoxigenin. J. Mol. Biol. 330, 385–396 [DOI] [PubMed] [Google Scholar]
- 63. Go Y. M., Jones D. P. (2013) Thiol/disulfide redox states in signaling and sensing. Crit. Rev. Biochem. Mol. Biol. 48, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schirmer T., Bode W., Huber R. (1987) Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 Å resolution. A common principle of phycobilin-protein interaction. J. Mol. Biol. 196, 677–695 [DOI] [PubMed] [Google Scholar]
- 65. Böhm S., Endres S., Scheer H., Zhao K. H. (2007) Biliprotein chromophore attachment: chaperone-like function of the PecE subunit of α-phycoerythrocyanin lyase. J. Biol. Chem. 282, 25357–25366 [DOI] [PubMed] [Google Scholar]
- 66. Bonnett R., Davies J. E., Hursthouse M. B. (1976) Structure of bilirubin. Nature 262, 327–328 [DOI] [PubMed] [Google Scholar]
- 67. Storf M., Parbel A., Meyer M., Strohmann B., Scheer H., Deng M. G., Zheng M., Zhou M., Zhao K. H. (2001) Chromophore attachment to biliproteins: specificity of PecE/PecF, a lyase-isomerase for the photoactive 31-Cys-α84-phycoviolobilin chromophore of phycoerythrocyanin. Biochemistry 40, 12444–12456 [DOI] [PubMed] [Google Scholar]
- 68. Ishizuka T., Narikawa R., Kohchi T., Katayama M., Ikeuchi M. (2007) Cyanobacteriochrome TePixJ of Thermosynechococcus elongatus harbors phycoviolobilin as a chromophore. Plant. Cell Physiol. 48, 1385–1390 [DOI] [PubMed] [Google Scholar]
- 69. Ishizuka T., Kamiya A., Suzuki H., Narikawa R., Noguchi T., Kohchi T., Inomata K., Ikeuchi M. (2011) The cyanobacteriochrome, TePixJ, isomerizes its own chromophore by converting phycocyanobilin to phycoviolobilin. Biochemistry 50, 953–961 [DOI] [PubMed] [Google Scholar]
- 70. Rockwell N. C., Martin S. S., Gulevich A. G., Lagarias J. C. (2012) Phycoviolobilin formation and spectral tuning in the DXCF cyanobacteriochrome subfamily. Biochemistry 51, 1449–1463 [DOI] [PubMed] [Google Scholar]







