Background: Drug excretion into saliva has important clinical implications, but the mechanisms underlying salivary gland drug transport remain unclear.
Results: OCT3 is highly expressed in secretory epithelial cells and mediates active metformin transport in salivary glands.
Conclusion: OCT3 provides a unique pathway for metformin secretion and accumulation in salivary glands.
Significance: Carrier-mediated salivary secretion can provoke drug-induced taste disturbance.
Keywords: Diabetes, Drug Transport, Membrane Transport, Pharmacokinetics, Salivary Gland, Transporter, Metformin, Organic Cation Transporter 3
Abstract
Drug-induced taste disturbance is a common adverse drug reaction often triggered by drug secretion into saliva. Very little is known regarding the molecular mechanisms underlying salivary gland transport of xenobiotics, and most drugs are assumed to enter saliva by passive diffusion. In this study, we demonstrate that salivary glands selectively and highly express OCT3 (organic cation transporter-3), a polyspecific drug transporter in the solute carrier 22 family. OCT3 protein is localized at both basolateral (blood-facing) and apical (saliva-facing) membranes of salivary gland acinar cells, suggesting a dual role of this transporter in mediating both epithelial uptake and efflux of organic cations in the secretory cells of salivary glands. Metformin, a widely used anti-diabetic drug known to induce taste disturbance, is transported by OCT3/Oct3 in vitro. In vivo, metformin was actively transported with a high level of accumulation in the salivary glands of wild-type mice. In contrast, active uptake and accumulation of metformin in salivary glands were abolished in Oct3−/− mice. Oct3−/− mice also showed altered metformin pharmacokinetics and reduced drug exposure in the heart. These results demonstrate that OCT3 is responsible for metformin accumulation and secretion in salivary glands. Our study uncovered a novel carrier-mediated pathway for drug entry into saliva and sheds new light on the molecular mechanisms underlying drug-induced taste disorders.
Introduction
Many medications, including antihypertensive, anti-diabetic, antimicrobial, and antidepressant drugs, have the potential to adversely influence a patient's sense of taste and smell (1, 2). In some cases, medications can produce prolonged taste disturbance that cannot be quickly reversed by drug cessation (1). Taste-related adverse effects can significantly impact a patient's quality of life, dietary choices, emotional state, and compliance with medication regimens, especially in pediatric and geriatric populations (1–3). As taste receptors are located in taste buds on the tongue, the physical presence of a drug substance in saliva is a prerequisite for taste disturbance caused by many medications. Drugs are known to be excreted into saliva; however, other than passive diffusion, the molecular mechanisms by which salivary gland epithelial cells secret xenobiotics are virtually unknown (4, 5).
One drug known to be excreted into saliva and to cause taste disturbance is metformin, a first-line oral hypoglycemic agent used in the treatment of type 2 diabetes. Patients on metformin therapy frequently experience a lingering metallic taste in the mouth evidently due to a persistent presence of metformin in the saliva (6, 7). In humans after either oral or intravenous dosing, metformin is readily detectable in the saliva (8). The concentration of metformin in saliva is lower than in blood, but declines much more slowly (8). When administrated to mice, metformin was reported to accumulate in several tissues and organs, including salivary glands (9). Metformin is a hydrophilic and positively charged molecule that relies on transporters to move across cell membranes (10, 11). Metformin is a substrate of multiple polyspecific organic cation transporters, including OCT1–3 (organic cation transporter-1–3), MATE1 and MATE2-K (multidrug and toxin extrusion protein 1 and 2-K), and PMAT (plasma membrane monoamine transporter) (10–12). In humans, the liver-specific OCT1 mediates metformin uptake into the liver, which is a primary site of metformin pharmacological action (10, 11). Recent studies have shown that OCT1 is important for metformin therapeutic response and that genetic polymorphisms in OCT1 may contribute to variation in clinical response to the drug (13). Metformin undergoes negligible metabolism in vivo and is eliminated predominantly by the kidney (11). The kidney-specific OCT2 works in concert with MATE1 to mediate renal secretion of metformin (10). OCT3 and PMAT, two newer polyspecific organic cation transporters with a broad tissue distribution, also transport metformin in vitro (12, 14). However, their roles in metformin disposition and action in vivo are less clear.
In this study, we determined the expression and cellular localization of OCT3/Oct3 in the salivary glands of humans and mice. The in vivo significance of Oct3 in salivary gland drug transport was evaluated in mice with targeted deletion of the Oct3/Slc22a3 gene.
EXPERIMENTAL PROCEDURES
Animals
The Oct3 (Slc22a3) null mice of the FVB inbred strain were originally developed by Dr. Denise Barlow (15) and maintained by Dr. Alfred Schinkel (Netherlands Cancer Institute). After re-derivation at Charles River Laboratories (16), breeding pairs of Oct3+/+ and Oct3−/− mice were kindly provided to us by Dr. John Markowitz (University of Florida) with approval from Dr. Schinkel. These mice were housed in the specific pathogen-free facility at the University of Washington. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Metformin Transport Assay
The full-length mouse Oct3 (mOct3)2 cDNA was amplified from mouse brain cDNA and confirmed to fully align with the reference sequence NM_011395.2. Flp-In HEK293 cells stably expressing mOct3 was generated following a similar procedure as described previously for human OCT3 (17). Metformin uptake was determined by radiotracer uptake assay using [14C]metformin as described previously (12, 17). Cells stably transfected with pcDNA5/FRT vector were used as a control, and transporter-specific uptake was calculated by subtracting uptake in control cells from uptake in transporter-expressing cells. Metformin transport kinetic studies were carried out at varying substrate concentrations, and kinetic parameters were determined in the initial uptake phase as described previously (17).
mRNA and Protein Analysis in Mouse and Human Tissues
For mRNA determination in various mouse tissues, 10–12-week-old Oct3+/+ and Oct3−/− mice were used (n = 4). Mouse liver, kidney, small intestine, heart, salivary gland, and skeletal muscle tissues were collected, flash-frozen in liquid N2, and stored at −80 °C until analysis. For mRNA analysis in human salivary glands, total RNA (Clontech) pooled from 24 male and female Caucasians (16–60 years old) was used. For regional OCT3 mRNA and protein analysis in human parotid, submandibular, and sublingual glands, post-mortem tissues from a single female donor (30 years old) with no disease history were provided by the National Disease Resource Interchange. Total RNA was extracted, and TaqMan real-time PCR was performed on an Applied Biosystems 7900HT fast real-time PCR system as described previously (18). To determine OCT3 membrane protein levels, total membrane proteins were isolated from the human parotid, submandibular, and sublingual glands using the ProteoExtract native membrane protein extraction kit (Calbiochem). The membrane fraction was digested by trypsin, and quantification of OCT3 protein was carried out using a recently developed quantitative LC-MS/MS method on an Agilent 6460A triple-quadrupole mass spectrometer coupled to the Agilent 1290 Infinity LC system (18).
Immunofluorescence Labeling of OCT3/Oct3 in Human and Mouse Salivary Glands
Snap-frozen human submandibular gland was processed to frozen sections and fixed in ice-cold acetone. The sections were blocked in goat serum in PBS, incubated overnight at 4 °C with rabbit anti-OCT3 polyclonal antibody (1:125 dilution; Genway), and co-labeled with anti-human Na+/K+-ATPase α-subunit monoclonal antibody (1:500 dilution; Sigma). The sections were then washed with PBS, and fluorescent tag-labeled secondary antibodies (1:500 dilution; Alexa 488 for OCT3 and Alexa 568 for Na+/K+-ATPase) were applied for 1 h at room temperature. For a nonspecific control, tissue sections of human submandibular gland were incubated with blank serum, followed by secondary antibody incubation under the same conditions. For Oct3 immunolocalization in mouse salivary glands, frozen tissue sections were prepared from Oct3+/+ and Oct3−/− mice and immunostained with rabbit anti-Oct3 polyclonal antibody (1:125 dilution), followed by incubation with Alexa 488-tagged secondary antibody (1:500 dilution). After washing, ProLong Gold antifade medium with DAPI was mounted on the slides and cover-slipped. Fluorescent images were obtained with a Zeiss Axiovert 200 fluorescence microscope (19).
In Vivo Study in Mice
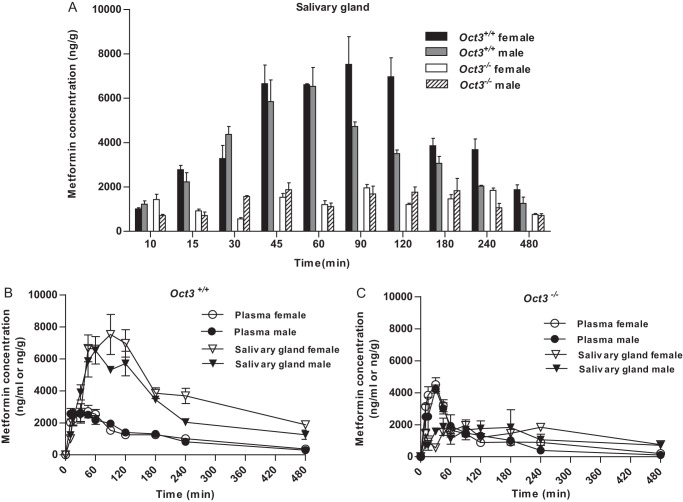
Because rodent salivary glands exhibit significant sexual dimorphism (20), in vivo studies were performed in two separate cohorts of male and female mice. Male and female mice (10–12 weeks old) were fasted for ∼10 h. Age- and gender-matched Oct3+/+ and Oct3−/− mice were administered 15 mg/kg metformin containing 0.2 mCi/kg [14C]metformin by oral gavage. At various time points (0–480 min), mice (n = three to six mice at each time point) were killed by CO2 overdose, followed by cardiac puncture. Blood was collected using a heparin-coated syringe, and plasma was separated by centrifugation at 5000 × g. Tissues, including liver, kidney, skeletal muscle, heart, salivary gland, and intestinal segments, were collected and processed at each time point using the method described by Wilcock and Bailey (9). Plasma and tissue metformin concentrations were determined by liquid scintillation counting. Metformin concentration was expressed as nanograms/g for tissues and nanograms/ml for plasma.
Pharmacokinetic Data Analysis
Metformin plasma concentration-time curves were plotted and analyzed. Because metformin concentrations in plasma and tissue were sampled in different animals at each time point (one-point sampling), a population-based bootstrap method (21, 22) was used to calculate the mean and confidence intervals of the plasma and tissue area under the concentration-time curves (AUCs) and other pharmacokinetic parameters, including oral clearance (CL/F), terminal half-life (t½,β), and volume of distribution at terminal phase (V/F), using Equations 1–4.
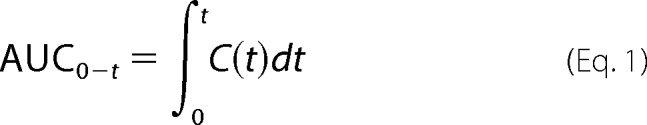 |
 |
 |
 |
The terminal slope (β) was calculated by performing a linear regression of concentrations at the last three to five time points. The 95% confidence intervals for pharmacokinetic parameters were generated using the bootstrap method with modification (21, 22). Briefly, plasma or tissue concentrations were resampled with random replacement of the total number of mice using the R program. The resampling was repeated 10,000 times to create 10,000 pseudo concentration-time profiles. For each profile, the concentrations at each nominal time point were averaged, and the pharmacokinetic parameters were calculated using Equations 1–4. The 95% confidence intervals for each parameter were calculated by taking the 2.5 and 97.5% quartiles (i.e. the 251th and 97,501th values of 10,000 bootstrap values). Peak plasma concentration (Cmax) was estimated by visual inspection of the plasma concentration-time curve.
Statistical Analysis
Quantitative assays were performed in duplicate or triplicate and repeated at least two times. Data are presented as mean ± S.D., unless specified otherwise. For pharmacokinetic data (except Cmax), 95% confidence intervals around the estimate for each pharmacokinetic parameter were determined using the non-parametric bootstrap method (21). Two-sided p values were calculated using permutation tests with 10,000 replications (23). For comparison of Cmax and non-pharmacokinetic parameters, statistical significance was determined by unpaired Student's t test. A p value <0.05 was considered statistically significant.
RESULTS
mOct3 Efficiently Transports Metformin
Metformin is known to be an excellent substrate of human OCT3 (14, 24). To confirm that mOct3 also transports metformin, we examined metformin uptake by mOct3 using Flp-In HEK293 cells stably expressing mOct3. Metformin uptake was much higher in mOct3-expressing cells, and mOct3-mediated metformin uptake exhibited typical saturable kinetics (Fig. 1, A and B). The apparent affinity (Km) was 2.65 ± 0.23 mm, and the maximal transport rate (Vmax) was 45.1 ± 1.4 nmol/mg of protein/min. These data suggest that similar to human OCT3, mOct3 also efficiently transports metformin.
FIGURE 1.
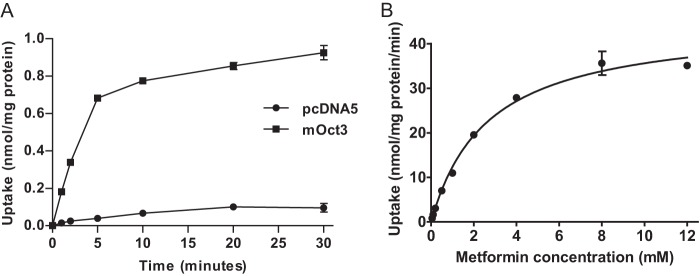
Mouse Oct3 transports metformin. A, time-dependent uptake of metformin (10 μm) in mOct3-expressing HEK293 cells and pcDNA5-transfected (control) cells. B, metformin transport kinetics by mOct3. Uptake was performed with varying metformin concentrations for 5 min in HEK293 cells stably transfected with mOct3. Transporter-specific uptake was calculated by subtracting the transport activity in control cells.
Salivary Glands Predominantly Express OCT3
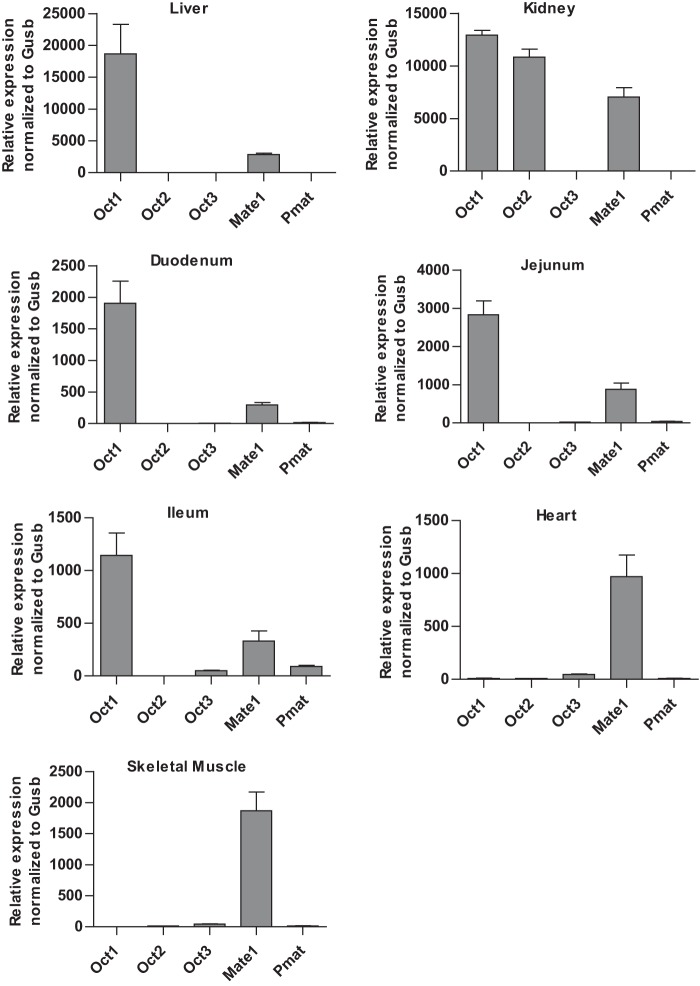
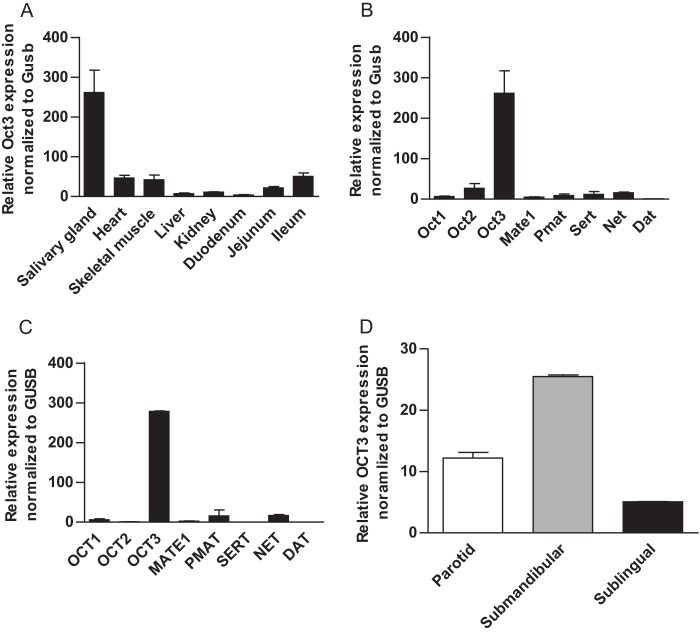
Previous gene profiling studies reported a wide distribution of OCT3 mRNA in various human tissues, with major expression in skeletal muscle, cardiac myocytes, placenta, liver, adrenal gland, prostate, and salivary glands (14, 25). As Oct3 expression was less studied in mice, we first quantified mRNA expression of Oct3 and other known metformin transporters, including Oct1, Oct2, Mate1, and Pmat, in various mouse tissues relevant to metformin disposition and response (Fig. 2). As expected, mouse liver predominantly expressed Oct1 and Mate1, and mouse kidney predominantly expressed Oct1, Oct2, and Mate1. In the small intestine, Oct1 and Mate1 prevailed in all segments, although Oct3 and Pmat were also significantly expressed in the ileum. Mouse heart and skeletal muscle had a very high expression of Mate1 together with a significant expression of Oct3. Among the mouse tissues analyzed, the highest expression of Oct3 mRNA was observed in the salivary glands (Fig. 3A), whereas the mRNA of other organic cation or biogenic amine transporters (Sert (serotonin transporter), Net (norepinephrine transporter), and Dat (dopamine transporter)) was minimally expressed (Fig. 3B). A similar predominant expression of OCT3 mRNA was observed in human salivary glands (Fig. 3C). Further analysis revealed that OCT3 mRNA was expressed in all three major types of human salivary glands, with the highest expression found in submandibular glands (Fig. 3D). Using a targeted quantitative proteomics method (18), OCT3 protein in human parotid, submandibular, and sublingual glands was quantified to be 0.42 ± 0.11, 1.53 ± 0.015, and 0.43 ± 0.29 fmol/μg of total membrane protein, respectively.
FIGURE 2.
Expression of Oct1–3, Mate1, and Pmat mRNAs in various mouse tissues determined by quantitative RT-PCR. Transporter expression was normalized to the expression of the mouse β-glucuronidase (Gusb) gene.
FIGURE 3.
Salivary glands predominantly express OCT3/Oct3. A, relative expression of mOct3 mRNA in various mouse tissues determined by quantitative RT-PCR. B and C, gene profiling of functionally related transporters in mouse (B) and human (C) salivary glands by quantitative RT-PCR. D, OCT3 mRNA expression in human parotid, submandibular, and sublingual glands. The results were normalized to the expression of the human or mouse β-glucuronidase (GUSB/Gusb) gene.
OCT3/Oct3 Protein Is Localized to Both Basolateral and Apical Membranes of Salivary Gland Epithelial Cells
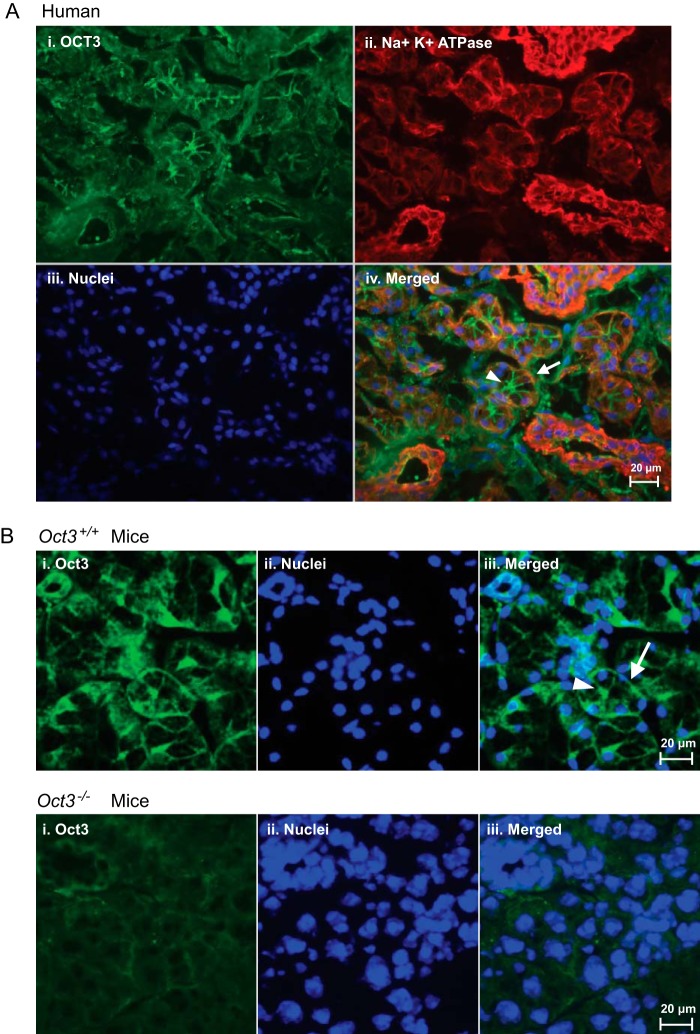
To examine the membrane localization and cell type-specific expression of OCT3/Oct3 in human and mouse salivary glands, immunohistochemistry was carried out with cryostat tissue sections using a previously validated polyclonal antibody against OCT3 (14). In human submandibular glands, robust immunoreactivity for OCT3 was observed on acini and associated ducts (Fig. 4A). There was essentially no staining when the primary anti-OCT3 antibody was substituted with blank serum (data not shown). In acinar cells (the secretory epithelial cells responsible for producing saliva), OCT3 immunostaining not only superimposed with the basolateral membrane marker Na+/K+-ATPase but also extended to the apical membranes lining the canaliculi (Fig. 4A). A similar pattern of both basolateral and apical immunostaining was observed in the salivary glands of Oct3+/+ mice (Fig. 4B). In contrast, no specific basolateral or apical staining was observed in the salivary glands of Oct3−/− mice (Fig. 4B). Together, these data revealed that the OCT3/Oct3 protein is present on both basolateral (blood-facing) and apical (saliva-facing) membranes of the secretory epithelial cells in human and mouse salivary glands, suggesting a multifaceted role of this transporter in mediating solute transport in salivary gland epithelial cells.
FIGURE 4.
Localization of OCT3/Oct3 in salivary gland epithelial cells. A, detection of OCT3 (panel i, green) in human submandibular glands. Staining of Na+/K+-ATPase (panel ii), a basolateral marker, and nuclei (panel iii) is shown in red and blue, respectively. B, detection of Oct3 (panel i, green) and nuclei (panel ii, blue) in salivary gland sections from Oct3+/+ (upper panels) and Oct3−/− (lower panels) mice. In overlays (A, panel iv, and B, panel iii), the arrow and arrowhead indicate basolateral and apical membranes of salivary gland epithelial cells, respectively.
Oct3−/− Mice Exhibit Altered Plasma Pharmacokinetics of Metformin
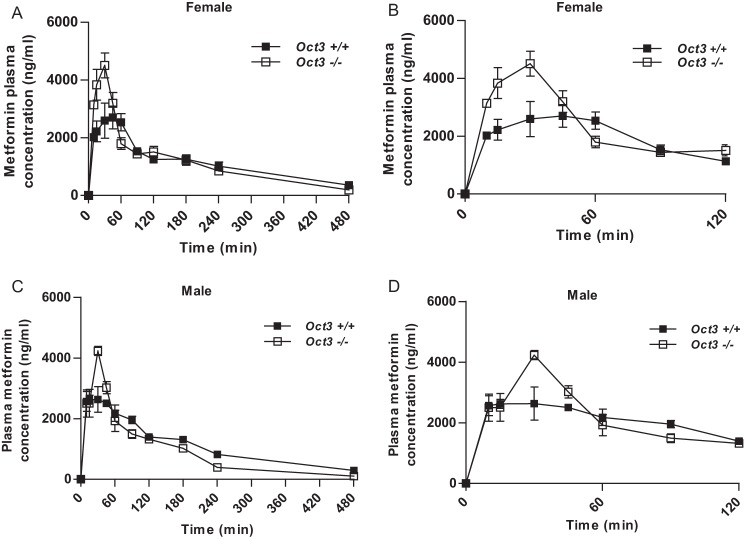
Prior to in vivo studies, we confirmed by quantitative RT-PCR that there were no significant compensatory changes in the expression of other metformin transporters (Oct1, Oct2, Mate1, Pmat) in the tissues of interest in Oct3−/− mice (data not shown). The plasma concentration-time profiles and pharmacokinetic parameters of metformin in female and male Oct3+/+ and Oct3−/− mice are shown in Fig. 5 and Tables 1 and 2. Although the overall systemic exposure (AUC0–480 min) was comparable between Oct3+/+ and Oct3−/− mice (Tables 1 and 2), a clear difference was observed in metformin plasma concentrations in the first 60 min between the two genotypes (Fig. 5). In female Oct3−/− mice, the Cmax of metformin increased by 67%, and AUC0–60 min increased by 41% (Table 1). A similar change in the metformin pharmacokinetic profile was observed in male Oct3−/− mice (Table 2). These data suggest that Oct3 influences metformin pharmacokinetics and has a significant impact on metformin plasma levels in the early phase following drug administration.
FIGURE 5.
Oct3 impacts metformin plasma kinetics. A and B, plasma metformin concentration-time profiles from 0 to 480 min (A) and from 0 to 120 min (B) in female Oct3+/+ and Oct3−/− mice. C and D, plasma metformin concentration-time profiles from 0 to 480 min (C) and from 0 to 120 min (D) in male Oct3+/+ and Oct3−/− mice. Mice were given by oral gavage a dose of 15 mg/kg metformin containing 0.2 mCi/kg [14C]metformin. Animals were killed at each time point, and the plasma and tissue concentrations of metformin were measured by liquid scintillation counting. Data represent mean ± S.E. (n = three to six mice at each time point). A total of 60 Oct3+/+ and 53 Oct3−/− female mice and a total of 48 Oct3+/+ and 40 Oct3−/− male mice were used for the pharmacokinetic studies.
TABLE 1.
Metformin pharmacokinetic parameters in female Oct3+/+ and Oct3−/− mice
Data are presented as mean ± S.D. (Cmax) or with 95% confidence interval in parentheses.
| Parameter | Oct3+/+ | Oct3−/− | Change | p value |
|---|---|---|---|---|
| % | ||||
| Cmax (μg/ml) | 2.70 ± 0.79 | 4.51 ± 0.86 | ↑67 | 0.037 |
| AUC0–60 min (μg*min/ml) | 136 (119–155) | 191 (170–211) | ↑41 | 0.002 |
| AUC0–480 min (μg*min/ml) | 546 (475–599) | 553 (494–602) | ↑1 | 0.862 |
| CL/F (ml/min/kg)a | 27.5 (25.0–31.6) | 27.1 (24.9–30.4) | ↓1 | 0.863 |
| Terminal t½ (min) | 164 (126–284) | 113 (89.4–142) | ↓31 | 0.237 |
| V/F (liters/kg) | 6.51 (4.83–11.04) | 4.42 (3.45–5.94) | ↓32 | 0.230 |
a CL/F values were calculated using dose/AUC0–480 min.
TABLE 2.
Metformin pharmacokinetic parameters in male Oct3+/+ and Oct3−/− mice
Data are presented as mean ± S.D. (Cmax) or with 95% confidence interval parentheses.
| Parameter | Oct3+/+ | Oct3−/− | Change | p value |
|---|---|---|---|---|
| % | ||||
| Cmax (μg/ml) | 2.63 ± 0.32 | 4.23 ± 0.26 | ↑61 | <0.001 |
| AUC0–60 min (μg*min/ml) | 139 (124–154) | 167 (145–181) | ↑20 | 0.063 |
| AUC0–480 min (μg*min/ml) | 529 (384–569) | 433 (373–495) | ↓18 | 0.052 |
| CL/F (ml/min/kg)a | 28.3 (26.4–39.1) | 34.7 (30.3–47.4) | ↑22 | 0.069 |
| Terminal t½ (min) | 143 (114–182) | 98.1 (71.2–124) | ↓31 | 0.308 |
| V/F (liters/kg) | 5.85 (4.68–8.06) | 4.91 (3.71–6.19) | ↓16 | 0.619 |
a CL/F value was calculated using Dose/AUC0–480min.
Metformin Accumulation Is Greatly Reduced in the Salivary Glands of Oct3−/− Mice
In Oct3+/+ mice, the highest metformin tissue exposure (defined by AUC0–480 min) was observed in the kidney and salivary glands, followed by the liver (Table 3). Metformin concentrations were much lower in the salivary glands of Oct3−/− mice at all time points after 10 min (Fig. 6A). At 60 min, the average metformin concentration in the salivary glands of Oct3−/− mice was only one-sixth of that in wild-type mice. The overall metformin exposure (AUC0–480 min) in salivary glands was reduced by 64 and 54% in female and male Oct3−/− mice, respectively (Table 3). These data strongly suggest that Oct3 actively accumulates metformin from the blood in the salivary glands. The concentrative nature of this process was evident when metformin concentration-time profiles were compared between salivary glands and plasma. In wild-type mice, metformin salivary gland tissue concentrations were 2–4-fold higher than plasma concentrations at most time points (Fig. 6B). The intracellular metformin concentrations in salivary gland epithelial cells are expected to be even greater if adjusted to intracellular water volume. In contrast, this concentrative feature was absent in Oct3−/− mice, as metformin salivary gland concentrations rarely surpassed plasma concentrations in these animals (Fig. 6C).
TABLE 3.
Tissue AUC0–480 min from female and male Oct3+/+ and Oct3−/− mice
Data are presented as the estimate, with 95% confidence interval in parentheses.
| Tissue | Oct3+/+ | Oct3−/− | Change | p value |
|---|---|---|---|---|
| μg*min/g | μg*min/g | % | ||
| Salivary gland | ||||
| Female | 1881 (1672–2087) | 677 (593–757) | ↓64 | <0.001 |
| Male | 1375 (921–1509) | 630 (422–772) | ↓54 | <0.001 |
| Heart | ||||
| Female | 389 (351–426) | 262 (229–291) | ↓33 | <0.001 |
| Male | 317 (227–340) | 208 (171–260) | ↓34 | <0.001 |
| Skeletal muscle | ||||
| Female | 318 (269–365) | 286 (235–326) | ↓10 | 0.344 |
| Male | 284 (146–318) | 188 (122–223) | ↓34 | 0.035 |
| Liver | ||||
| Female | 995 (849–1123) | 1111 (983–1242) | ↑12 | 0.263 |
| Male | 912 (730–1029) | 1242 (1045–1412) | ↑36 | 0.004 |
| Kidney | ||||
| Female | 1938 (1725–2143) | 1761 (1486–2076) | ↓9 | 0.328 |
| Male | 1770 (1215–1994) | 1723 (1417–2015) | ↓3 | 0.849 |
FIGURE 6.
Oct3 mediates metformin accumulation in salivary glands. A, metformin concentrations in salivary glands at various time points (0–480 min) in female and male Oct3+/+ and Oct3−/− mice. B and C, comparisons of plasma versus salivary gland metformin concentrations at various time points in Oct3+/+ (B) and Oct3−/− (C) mice.
Consistent with significant expression of Oct3 in the heart (Fig. 3A), metformin AUC0–480 min in this tissue was significantly reduced by 33 and 34% in female and male Oct3−/− mice, respectively (Table 3). In the skeletal muscle of Oct3−/− mice, metformin exposure appeared to be reduced in both genders, and the reduction was statistically significant in male mice. Liver metformin exposure appeared to be increased in Oct3−/− mice, and the increase in male mice was statistically significant. The reason for this observation is unclear but could be due to an increased early plasma drug exposure, which may allow more Oct1-mediated metformin uptake into the liver. No change was observed in the kidney. Tissue exposure in the gastrointestinal track could not be accurately measured due to a significant contamination of unabsorbed metformin remaining in the intestinal lumen.
DISCUSSION
Drug-induced taste disorders are common adverse effects that provoke medication compliance issues especially in children and elderly patients. Drugs are known to be excreted into saliva, which represents the first step for taste disturbance induced by many medications. Sampling drug concentrations in saliva is clinically used in the assessment of therapeutic levels of drugs and the monitoring of illicit drug use (4, 5). Nevertheless, very little is presently known regarding the molecular mechanisms underlying salivary secretion of drugs and other foreign chemicals. Most drugs have been assumed to enter saliva by passive diffusion, a process characterized by downhill, non-mediated diffusion of drug molecules across the membranes of the secretory epithelial cells in salivary glands (4). However, passive diffusion can neither explain salivary secretion of hydrophilic drugs (e.g. metformin) nor account for high drug accumulation in salivary glands. Our study revealed a critical role of OCT3/Oct3 in metformin transport in salivary glands and provided a molecular mechanism for metformin secretion into saliva and the associated long-lasting taste disturbance effect. To our knowledge, this is the first time that a carrier-mediated mechanism has been demonstrated for drug accumulation and secretion in salivary glands.
OCT proteins are electrogenic bidirectional transporters that translocate their substrates down the electrochemical gradient (26, 27). Although OCT1 and OCT2 are expressed mainly in the liver and kidney, OCT3 is known to have a broader tissue distribution. Our data clearly show that OCT3/Oct3 is highly expressed in salivary glands, and the submandibular gland, which is responsible for generating 70% of all saliva, has the highest OCT3 expression (Fig. 3). OCT3/Oct3 protein is localized to both basolateral (blood-facing) and apical (saliva-facing) membranes of salivary gland acinar cells (Fig. 4), suggesting a dual role of this transporter in metformin transport in these secretory epithelial cells. On the basis of these data, we propose a cellular model for OCT3 expression and transport function in salivary gland acinar cells (Fig. 7). The physiologic inside-negative electrical membrane potential difference (approximately −70 mV) in acinar cells provides a driving force for basolateral OCT3 to concentrate metformin from blood into acinar cells. Initial metformin concentrations in saliva are very low, however, producing an outwardly directed electrochemical gradient of metformin at the apical membrane. This would drive apical OCT3 to efflux a small amount of metformin into the saliva, leading to its low but continuous presence in the saliva. Our hypothesis is supported by the in vivo data in mice showing Oct3-mediated uptake and high accumulation of metformin in salivary glands (Fig. 6). It would be interesting to compare metformin concentrations in the salivary fluids of Oct3+/+ and Oct3−/− mice. However, we were not able to perform these experiments due to technical difficulties in collecting saliva samples in mice. Regardless, our cellular model, which indicates a low but continuous metformin secretion by apical Oct3, is in line with the clinical observation of the low but persistent presence of metformin in human saliva after drug administration (8). Although it is often anticipated that pairs of different, asymmetrically localized transporters are needed to mediate transepithelial solute transport, there are well established examples showing that a single transporter can be expressed at both membrane domains to facilitate transepithelial flux of a solute. For example, transport of glucose across the placental barrier is predominantly accomplished by a single bidirectional glucose transporter, GLUT1, which is expressed on both apical and basal membranes of placental epithelial cells to mediate maternal-to-fetal transport of glucose (28, 29). However, despite the critical role of OCT3/Oct3 in salivary gland metformin transport as revealed by our in vitro and in vivo data, low but significant expression of other metformin transporters (e.g. Oct1, Oct2, and PMAT) was observed in mouse and/or human salivary glands (Fig. 3). These transporters may also influence metformin transport in salivary glands. Further studies are needed to determine the membrane localization of these transporters and their role in drug accumulation and secretion in salivary gland epithelial cells.
FIGURE 7.
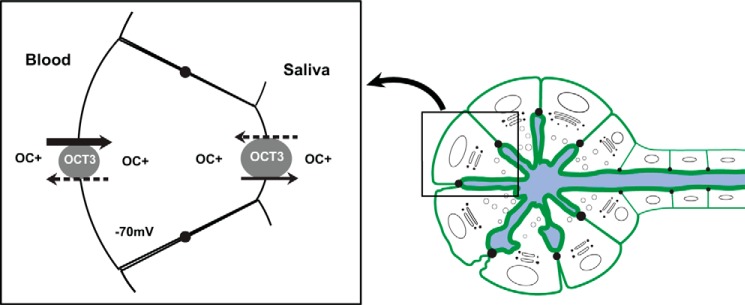
Model proposed for OCT3-mediated organic cation (OC+) transport in salivary gland epithelial cells. OCT3 on the basolateral membrane of epithelial cells mediates metformin uptake from the blood into the cells. Once metformin is highly concentrated inside the cells, OCT3 on the apical membrane facilitates efflux of metformin into the saliva. The solid arrows indicate the preferred direction of metformin transport when drug concentrations in the systemic circulation are high. The dashed arrows indicate the possible transport direction when systemic drug concentrations decline.
The primary function of salivary glands is to secrete saliva, which plays an important role in oral health, nutrient digestion, and immunity to microbial infection (30). About 0.75–1.5 liters of salivary fluid are secreted each day in healthy adults. Dysfunction of the salivary glands can lead to xerostomia, an uncomfortable and potentially harmful effect caused by medication use, radiation therapy, and autoimmune diseases (30, 31). Although xerostomia has many origins (3), excessive accumulation of foreign chemicals in salivary glands may lead to tissue toxicity and dysfunction of the salivary glands. Our in vivo studies revealed that Oct3-mediated active uptake can lead to very high metformin accumulation in salivary glands (Fig. 6). The overall tissue exposure to metformin in salivary glands is as high as that in the kidney, the major organ responsible for metformin elimination (Table 3). Oct3-mediated accumulation may thus intensify drug toxicity in salivary gland epithelial cells. Indeed, necrosis and inflammation of the salivary glands have been reported in rats chronically dosed with metformin (32). As OCT3/Oct3 is a polyspecific transporter, it can also transport other circulating drugs or toxins into salivary glands. Therefore, it is possible that Oct3-mediated or other carrier-mediated drug accumulation may interfere with the normal secretory function of salivary glands, contributing to hyposalivation and xerostomia.
In addition to salivary glands, there was a 33–34% reduction in metformin levels in the hearts of both male and female Oct3−/− mice (Table 3). Male Oct3−/− mice also showed significant changes in metformin exposure in skeletal muscle and liver. Furthermore, both male and female Oct3−/− mice exhibited altered plasma metformin kinetics, with a higher Cmax and higher drug exposure during the first 60 min after drug administration. These pharmacokinetic changes in Oct3−/− mice most likely reflect a reduced distribution of metformin to peripheral tissues due to ablation of Oct3-mediated metformin uptake in many tissues, including, but not limited to, salivary glands and heart. Metformin is a first-line type 2 diabetes drug that acts as an insulin sensitizer and lowers blood glucose levels by suppressing hepatic gluconeogenesis, reducing intestinal glucose absorption, and stimulating glucose uptake and utilization in skeletal muscle and adipose tissues (10, 11). Previous studies have suggested that hepatic and renal organic cation transporters (OCT1, OCT2, and MATE1) play an important role in influencing metformin pharmacokinetics and pharmacodynamics in vivo (10, 11). Genetic polymorphism of these transporters has been suggested to contribute to variable pharmacological response to metformin (10). Here, we showed that Oct3 impacts metformin pharmacokinetics in vivo and influences metformin distribution to tissue sites. Therefore, Oct3 could represent an additional genetic determinant of metformin disposition and action in vivo.
In summary, we demonstrated that OCT3 is responsible for metformin accumulation and secretion in salivary glands. Our study uncovered a novel carrier-mediated pathway for drug secretion into saliva and shed new light on the molecular mechanisms underlying drug-induced taste disorders. In addition, our study identified an important factor that could influence in vivo disposition of a mainstay drug used in the treatment of type 2 diabetes.
Acknowledgments
We thank Drs. John Markowitz and Haojie Zhu (University of Florida) for providing breeding pairs of Oct3+/+ and Oct3−/− mice for our study. We also thank Drs. Jashvant Unadkat and Bhagwat Prasad (University of Washington Research Affiliate Program on Transporters) for assistance in OCT3 membrane protein determination.
This work was supported, in whole or in part, by National Institutes of Health Grants GM066233, GM07750, and HD047892.
- mOct3
- mouse Oct3
- AUC
- area under the curve
- Cmax
- peak plasma concentration.
REFERENCES
- 1. Ackerman B. H., Kasbekar N. (1997) Disturbances of taste and smell induced by drugs. Pharmacotherapy 17, 482–496 [PubMed] [Google Scholar]
- 2. Doty R. L., Shah M., Bromley S. M. (2008) Drug-induced taste disorders. Drug Saf. 31, 199–215 [DOI] [PubMed] [Google Scholar]
- 3. Cassolato S. F., Turnbull R. S. (2003) Xerostomia: clinical aspects and treatment. Gerodontology 20, 64–77 [DOI] [PubMed] [Google Scholar]
- 4. Aps J. K., Martens L. C. (2005) Review: the physiology of saliva and transfer of drugs into saliva. Forensic Sci. Int. 150, 119–131 [DOI] [PubMed] [Google Scholar]
- 5. Idkaidek N., Arafat T. (2012) Saliva versus plasma pharmacokinetics: theory and application of a salivary excretion classification system. Mol. Pharm. 9, 2358–2363 [DOI] [PubMed] [Google Scholar]
- 6. Lee A. J. (1996) Metformin in noninsulin-dependent diabetes mellitus. Pharmacotherapy 16, 327–351 [PubMed] [Google Scholar]
- 7. Bristol-Myers Squibb (2009) Glucophage (metformin hydrochloride) package insert. Bristol-Myers Squibb, Princeton, NJ [Google Scholar]
- 8. Pentikäinen P. J., Neuvonen P. J., Penttilä A. (1979) Pharmacokinetics of metformin after intravenous and oral administration to man. Eur. J. Clin. Pharmacol. 16, 195–202 [DOI] [PubMed] [Google Scholar]
- 9. Wilcock C., Bailey C. J. (1994) Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 24, 49–57 [DOI] [PubMed] [Google Scholar]
- 10. Gong L., Goswami S., Giacomini K. M., Altman R. B., Klein T. E. (2012) Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet. Genomics 22, 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham G. G., Punt J., Arora M., Day R. O., Doogue M. P., Duong J. K., Furlong T. J., Greenfield J. R., Greenup L. C., Kirkpatrick C. M., Ray J. E., Timmins P., Williams K. M. (2011) Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50, 81–98 [DOI] [PubMed] [Google Scholar]
- 12. Zhou M., Xia L., Wang J. (2007) Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab. Dispos. 35, 1956–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shu Y., Sheardown S. A., Brown C., Owen R. P., Zhang S., Castro R. A., Ianculescu A. G., Yue L., Lo J. C., Burchard E. G., Brett C. M., Giacomini K. M. (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 117, 1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen L., Pawlikowski B., Schlessinger A., More S. S., Stryke D., Johns S. J., Portman M. A., Chen E., Ferrin T. E., Sali A., Giacomini K. M. (2010) Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet. Genomics 20, 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zwart R., Verhaagh S., Buitelaar M., Popp-Snijders C., Barlow D. P. (2001) Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol. Cell. Biol. 21, 4188–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu H. J., Appel D. I., Gründemann D., Markowitz J. S. (2010) Interaction of organic cation transporter 3 (SLC22A3) and amphetamine. J. Neurochem. 114, 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duan H., Wang J. (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Ther. 335, 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee N., Hebert M. F., Prasad B., Easterling T. R., Kelly E. J., Unadkat J. D., Wang J. (2013) Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab. Dispos. 41, 2225–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duan H., Wang J. (2013) Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J. Biol. Chem. 288, 3535–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinkstaff C. A. (1998) Salivary gland sexual dimorphism: a brief review. Eur. J. Morphol. 36, (suppl.) 31–34 [PubMed] [Google Scholar]
- 21. Efron B., Tibshirani R. J. (1994) An Introduction to the Bootstrap. Monographs on Statistics and Applied Probability, Book 57, Chapman & Hall, London [Google Scholar]
- 22. Mager H., Göller G. (1998) Resampling methods in sparse sampling situations in preclinical pharmacokinetic studies. J. Pharm. Sci. 87, 372–378 [DOI] [PubMed] [Google Scholar]
- 23. Westfall P. H., Young S. S. (1993) Resampling-based Multiple Testing: Examples and Methods for p-Value Adjustment, Wiley, New York [Google Scholar]
- 24. Nies A. T., Koepsell H., Winter S., Burk O., Klein K., Kerb R., Zanger U. M., Keppler D., Schwab M., Schaeffeler E. (2009) Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50, 1227–1240 [DOI] [PubMed] [Google Scholar]
- 25. Wu X., Huang W., Ganapathy M. E., Wang H., Kekuda R., Conway S. J., Leibach F. H., Ganapathy V. (2000) Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am. J. Physiol. Renal Physiol. 279, F449–F458 [DOI] [PubMed] [Google Scholar]
- 26. Cui M., Aras R., Christian W. V., Rappold P. M., Hatwar M., Panza J., Jackson-Lewis V., Javitch J. A., Ballatori N., Przedborski S., Tieu K. (2009) The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc. Natl. Acad. Sci. U.S.A. 106, 8043–8048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koepsell H., Lips K., Volk C. (2007) Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 24, 1227–1251 [DOI] [PubMed] [Google Scholar]
- 28. Illsley N. P. (2000) Glucose transporters in the human placenta. Placenta 21, 14–22 [DOI] [PubMed] [Google Scholar]
- 29. Baumann M. U., Deborde S., Illsley N. P. (2002) Placental glucose transfer and fetal growth. Endocrine 19, 13–22 [DOI] [PubMed] [Google Scholar]
- 30. Holsinger F. C., Bui B. D. (2007) Anatomy, function, and evaluation of the salivary glands. in Salivary Gland Disorders (Myers E. N., Ferris R. L., eds) pp. 1–16, Springer, Berlin [Google Scholar]
- 31. Fox P. C. (1998) Acquired salivary dysfunction. Drugs and radiation. Ann. N.Y. Acad. Sci. 842, 132–137 [DOI] [PubMed] [Google Scholar]
- 32. Quaile M. P., Melich D. H., Jordan H. L., Nold J. B., Chism J. P., Polli J. W., Smith G. A., Rhodes M. C. (2010) Toxicity and toxicokinetics of metformin in rats. Toxicol. Appl. Pharmacol. 243, 340–347 [DOI] [PubMed] [Google Scholar]