Background: DPP-4 inhibitors exert pleiotropic effects that modulate cardiovascular disease.
Results: The DPP-4 inhibitor vildagliptin stimulates ischemia-induced revascularization through eNOS signaling. The angiogenic actions of vildagliptin are mediated by both GLP-1-dependent and -independent mechanisms.
Conclusion: DPP-4 inhibitor promotes endothelial cell function via eNOS signaling.
Significance: DPP-4 inhibitor could be beneficial in patients with diabetes-related vascular complications.
Keywords: Adiponectin, Angiogenesis, Endothelial Cell, Nitric-oxide Synthase, Signaling
Abstract
Dipeptidyl peptidase-4 inhibitors are known to lower glucose levels and are also beneficial in the management of cardiovascular disease. Here, we investigated whether a dipeptidyl peptidase-4 inhibitor, vildagliptin, modulates endothelial cell network formation and revascularization processes in vitro and in vivo. Treatment with vildagliptin enhanced blood flow recovery and capillary density in the ischemic limbs of wild-type mice, with accompanying increases in phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). In contrast to wild-type mice, treatment with vildagliptin did not improve blood flow in ischemic muscles of eNOS-deficient mice. Treatment with vildagliptin increased the levels of glucagon-like peptide-1 (GLP-1) and adiponectin, which have protective effects on the vasculature. Both vildagliptin and GLP-1 increased the differentiation of cultured human umbilical vein endothelial cells (HUVECs) into vascular-like structures, although vildagliptin was less effective than GLP-1. GLP-1 and vildagliptin also stimulated the phosphorylation of Akt and eNOS in HUVECs. Pretreatment with a PI3 kinase or NOS inhibitor blocked the stimulatory effects of both vildagliptin and GLP-1 on HUVEC differentiation. Furthermore, treatment with vildagliptin only partially increased the limb flow of ischemic muscle in adiponectin-deficient mice in vivo. GLP-1, but not vildagliptin, significantly increased adiponectin expression in differentiated 3T3-L1 adipocytes in vitro. These data indicate that vildagliptin promotes endothelial cell function via eNOS signaling, an effect that may be mediated by both GLP-1-dependent and GLP-1-independent mechanisms. The beneficial activity of GLP-1 for revascularization may also be partially mediated by its ability to increase adiponectin production.
Introduction
Diabetes is closely associated with microvascular rarefaction and reduced collateralization in ischemic tissues. These circulatory changes can accelerate vulnerability to ischemic injury and impair wound healing, ultimately leading to lower-limb amputation. In fact, the incidence of diabetes is high in patients with peripheral arterial disease (1). Promoting the formation of collateral vessels with therapeutic intervention has potential clinical benefits in these patients (2).
Dipeptidyl peptidase-4 (DPP-4),3 also known as CD26, is a membrane-bound exopeptidase that cleaves dipeptides from the N terminus of proline- or alanine-containing polypeptides such as glucagon-like peptide-1 (GLP-1). GLP-1 is a potent incretin hormone primarily secreted into the gut by intestinal L cells in response to the presence of nutrients (3). DPP-4 inhibitors are widely used as novel anti-hyperglycemic drugs for the treatment of type 2 diabetes. These drugs inhibit DPP-4, thus increasing concentrations of GLP-1.
DPP-4 inhibitors have a glucose-lowering effect, and recent experimental and clinical studies have also shown that DPP-4 inhibitors such as sitagliptin and alogliptin exert pleiotropic effects that modulate cardiovascular disorders (4–8). Treatment with sitagliptin attenuated intimal hyperplasia in response to vascular injury in rats (9), and both sitagliptin and alogliptin reduced atherosclerotic lesions in a mouse model (10, 11). Alogliptin treatment was also found to modulate endothelium-dependent vasodilation in mouse aortic segments (12), and sitagliptin augments neovascularization by increasing circulating endothelial progenitor cells in vivo (13). Treatment with sitagliptin in diabetic patients reverses vascular endothelial dysfunction as assessed by increased flow-mediated dilation and also increases circulating adiponectin levels (14). This drug has also been shown to increase circulating endothelial progenitor cells in type 2 diabetic patients (15).
In this study, we investigated the effects of the DPP-4 inhibitor vildagliptin on ischemia-induced revascularization processes in vivo. We also examined the potential involvement of GLP-1 and adiponectin, which have vasculature protective actions, in the regulation of the vildagliptin-mediated revascularization.
EXPERIMENTAL PROCEDURES
Materials
The following primary antibodies were purchased from Cell Signaling Technology: phospho-Akt (Ser-473) antibody, Akt antibody, phospho-eNOS (Ser-1177) antibody, eNOS antibody, and β-actin antibody. Human DPP-4 antibody was purchased from Abcam (Cambridge, UK). LY294002 was purchased from Calbiochem. Recombinant human GLP-1 and l-NAME were purchased from Sigma. PP2 was purchased from Merck Millipore (Darmstadt, Germany). Recombinant human adiponectin protein generated by a mammalian cell expression system was purchased from BioVendor (Asheville, NC). Vildagliptin was provided as a generous gift by Novartis Pharm AG (Basel, Switzerland).
Animals and Experimental Protocol
Wild-type C57BL6/J mice at 8–10 weeks of age were obtained from SLC Co., Ltd. (Nagoya, Japan). Age-matched eNOS-deficient (eNOS-KO) and adiponectin-deficient (APN-KO) mice on a C57BL/6J background obtained from the same source were also used in this study. All study protocols were approved by the Institutional Animal Care and Use Committee of the Nagoya University School of Medicine. All mice were housed on a 12-h light/dark cycle with food and water ad libitum. Mice were given vildagliptin in the drinking water (0.6 mg/ml·day−1·mouse−1) 3 days before the hind limb ischemia operation, and vildagliptin administration was continued for up to 3 weeks following surgery. To create hind limb ischemia, mice were anesthetized with sodium pentobarbital (30 mg/kg intraperitoneally), and the proximal portion of the femoral artery and the distal portion of the saphenous artery were ligated and excised. At five time points (before surgery and postoperative days 3, 7, 14, and 21), we measured the ratio of the ischemic/normal hind limb blood flow using a laser Doppler blood flowmetry (LDBF) (Moor LDI, Moor Instruments, Inc.) as described previously. To minimize variations due to ambient light, blood flow was expressed as the ischemic/normal hind limb LDBF ratio.
Histological Analysis
At day 21 after surgery, mice were killed by overdose with anesthetics, and adductor muscle tissues were excised. Frozen sections of 5-μm thickness were prepared and stained with an anti-CD31 mAb (BD Biosciences) to detect capillary endothelial cells. Capillary endothelial cells were counted using fluorescence microscopy (×200). Five fields from five different muscle samples of each animal were randomly selected for the capillary density analysis.
Cell Culture and Differentiation of 3T3-L1 Fibroblasts into Adipocytes
Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD). HUVECs were cultured in endothelial cell basal medium-2; Clonetics) supplemented with EGM-2 MV at 37 °C, 5% CO2. Mouse 3T3-L1 fibroblasts (American Type Culture Collection) were cultured in DMEM containing 10% fetal bovine serum and then differentiated into adipocytes by DMEM supplemented with 5 μg/ml insulin, 0.5 mm 1-methyl-3-isobutylxanthine, and 1 μm dexamethasone as described previously (16).
Tube Formation Assay
The formation of vascular-like structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was assessed according to the manufacturer's instructions as described previously (17, 18). Before the experiment, HUVECs were placed in endothelial cell basal medium-2 for 15 h for serum starvation, and then cells were pretreated with LY294002 (50 μm), l-NAME (1 mmol/liter) for 60 min. HUVECs were seeded on Matrigel-coated plates at 5 × 104 cells/cm2 in endothelial cell basal medium and incubated with either vildagliptin (1 nm, 5 nm or 10 nm) or GLP-1 (1 nm, 5 nm or 10 nm) at 37 °C for 6 h. In some experiments, HUVECs were treated with combination of vildagliptin (5 nm) and GLP-1 (5 nm). For gene ablation studies, HUVECs were transfected with small interfering RNAs (siRNAs) targeting human DPP-4 (CD26) or unrelated siRNAs (Santa Cruz Biotechnology) by Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. After transfection, tube formation assay was performed. Network formation was assessed using an inverted phase contrast microscope (Olympus, Tokyo, Japan), and photomicrographs were taken at ×40 magnification. The degree of tube formation was quantified by measuring the length of tubes in five randomly chosen fields from each well using the ImageJ program. Each experiment was repeated three times.
Plasma Glucose Levels and ELISA Analysis
Plasma glucose levels on postoperative days 7 and 21 were measured using a NIPRO FreeStyle Blood Glucose Monitoring System (NIPRO, Osaka, Japan). Plasma GLP-1, stromal cell-derived factor-1α (SDF-1α), and adiponectin levels on days 7 and 21 after hind limb ischemia were measured by GLP-1 (Active) ELISA kit (Millipore, Billerica, MA), mouse SDF-1α ELISA kit (R&D Systems), and mouse adiponectin ELISA kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) according to the manufacturer's instructions (19, 20).
Real-time RT-PCR Analysis
Differentiated 3T3-L1 adipocytes were incubated with GLP-1 or vildagliptin (1, 10, and 100 nm) for 24 h, and then total RNA was isolated from cells. Reverse transcription was performed using 1 μg of RNA, random primers, and Moloney murine leukemia virus reverse transcriptase (ReverTraAce-α TOYOBO, Osaka, Japan). Quantitative real-time PCR was performed with the Stratagene Mx3000P System (Agilent Technologies) and Power SYBR Green PCR kit (Applied biosystems). Primers used in this study were as follows: adiponectin, 5′-GTTGCAAGCTCTCCTGTTCC-3′ (sense) and 5′-TCTCCAGGAGTGCCATCTCT-3′ (antisense); GAPDH, 5′-ACCCAGAAGACTGTGGATGG-3′ (sense) and 5′-CACATTGGGGGTAGGAACAC-3′ (antisense).
Immunoblotting Analysis
At day 7 after surgery, ischemic adductor muscle tissues were excised and lysed in radioimmune precipitation assay lysis buffer (Thermo Scientific), and the lysate was used for immunoblotting analysis as described previously (21). HUVECs were incubated with 5 nm GLP-1 (Sigma) or 5 nm vildagliptin for various times, and then cells were lysed in radioimmune precipitation assay lysis buffer, and cell lysates were subjected to immunoblotting. The following antibodies were used: anti-Akt, anti-phospho-Akt (Ser-473), anti-eNOS, anti-phospho-eNOS (Ser-1177), and anti-β-actin (Cell Signaling).
Statistical Analysis
The data are presented as mean ± S.D. values. Comparisons between two groups were made using Student's t test. Statistical analysis for multiple comparisons among the groups was performed using one-way analysis of variance. p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Vildagliptin Enhances Ischemia-induced Revascularization in Vivo
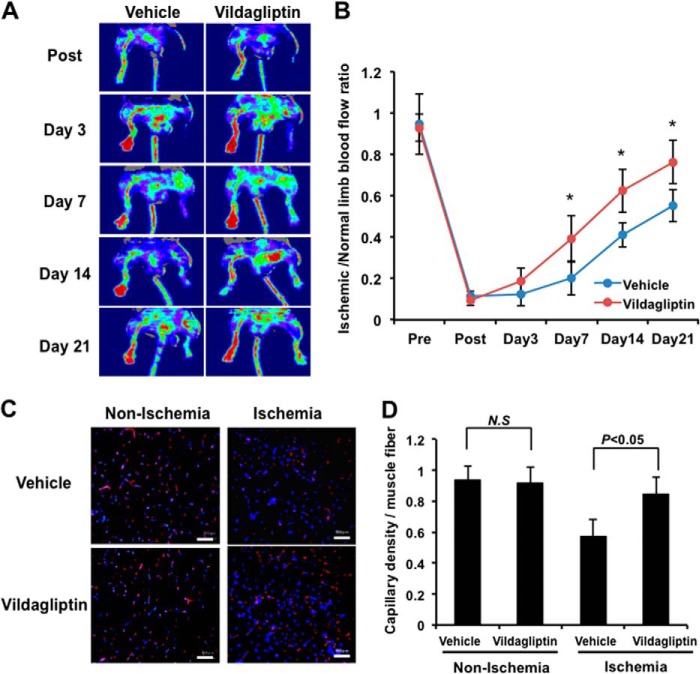
We first examined the effect of vildagliptin on revascularization processes in response to ischemia. WT mice were subjected to unilateral surgery-induced hind limb ischemia and then treated with either vildagliptin or the vehicle control. All mice survived surgery and appeared healthy during the follow-up period. Fig. 1A shows representative LDBF images of hind limb blood flow at different time points after surgery in vildagliptin-treated mice and controls. Blood flow recovery in the ischemic hindlimb appeared to be accelerated in mice treated with vildagliptin compared with mice treated with vehicle alone. Quantitative analysis of hindlimb perfusion showed that treatment with vildagliptin significantly increased blood flow in the ischemic limbs compared with controls on postoperative days 7, 14, and 21 (Fig. 1B).
FIGURE 1.
Vildagliptin promotes revascularization following hindlimb ischemia in mice. A, representative images of LDBF for wild-type (WT) mice subjected to surgery-induced hind limb ischemia and treated with vildagliptin or vehicle. B, quantitative analysis of the ischemic/normal LDBF ratio in the vildagliptin or vehicle-treated mice on postoperative days 3, 7, 14, and 21 (n = 10 in each group). *, p < 0.05 versus vehicle control. C, microscopic photographs of capillary density in ischemic adductor muscles 21 days after surgery. Capillaries were immunostained with anti-CD31 antibody. Red, CD31; blue, DAPI. Scale bar, 50 μm. D, quantitative analysis of the capillary density of ischemic muscles in vildagliptin or vehicle-treated mice (n = 5). Results are presented as mean ± S.D. N.S, not significant.
To investigate the extent of revascularization at the microcirculatory level, we also measured capillary density in histological sections harvested from ischemic adductor muscles. Fig. 1C shows representative photomicrographs of muscle tissue stained with the endothelial cell marker CD31. Quantitative analysis of CD31-positive cells revealed that on postoperative day 21, the capillary density in the ischemic adductor muscle was significantly higher in vildagliptin-treated mice compared with vehicle-treated controls (Fig. 1D). Vildagliptin treatment did not affect capillary density in the non-ischemic adductor muscle (Fig. 1, C and D).
eNOS Activation Is Essential for Vildagliptin-induced Revascularization in Vivo
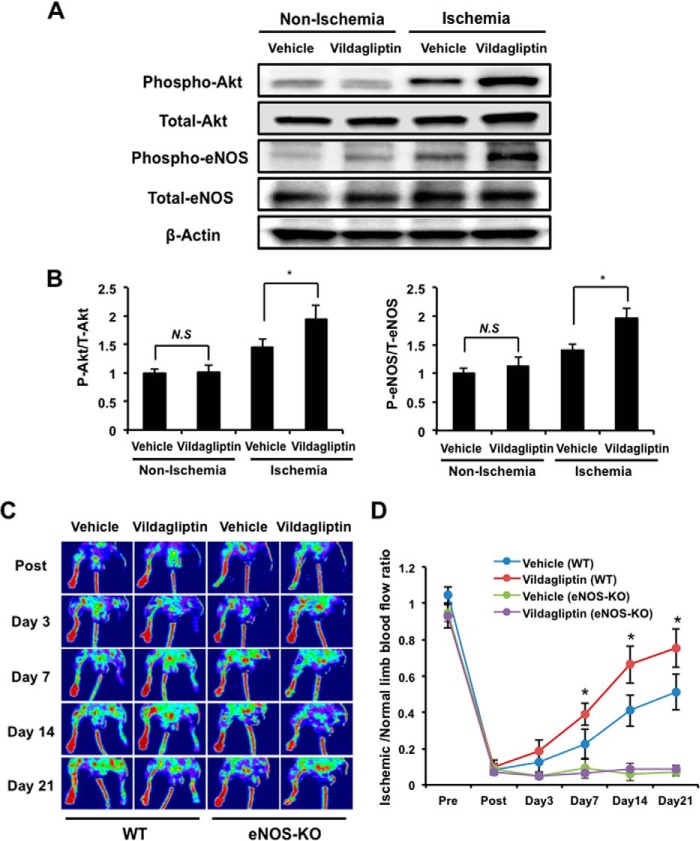
Akt-eNOS signaling plays an important role in revascularization following hind limb ischemia (22, 23). To examine the participation of Akt-eNOS signaling in the observed improvements in revascularization following vildagliptin treatment, the expression and phosphorylation of Akt at Ser-473 and eNOS at Ser-1179 in the ischemic adductor muscle were assessed by Western blot analysis on day 3 after surgery. The expression of total Akt and eNOS protein in ischemic muscles were similar between vildagliptin- and vehicle-treated mice. The extent of phosphorylation of both Akt and eNOS in the ischemic muscle, however, was significantly higher in vildagliptin-treated mice compared with those treated with vehicle control (Fig. 2, A and B). Vildagliptin treatment did not affect the phosphorylation of Akt and eNOS in the non-ischemic adductor muscle (Fig. 2, A and B).
FIGURE 2.
eNOS signaling is essential for vildagliptin-induced revascularization. A, immunoblotting with the indicated antibodies was performed on the ischemic adductor muscle of mice treated with vildagliptin or vehicle 3 days after surgery. Representative blots are shown. B, quantitative analysis of relative changes in phosphorylated Akt (left) and eNOS (right). Relative phosphorylation levels were normalized to total protein signal (n = 4). Results are presented as mean ± S.D. C, representative images of LDBF for WT and eNOS-KO mice treated with vildagliptin or vehicle control. D, quantitative analysis of the ischemic/normal LDBF ratio in vildagliptin or vehicle-treated WT and eNOS-KO mice on postoperative days 3, 7, 14, and 21 (n = 5 in each group). *, p < 0.05 versus vehicle controls (WT). Results are presented as mean ± S.D. N.S, not significant.
To further analyze the potential involvement of eNOS, we also examined the impact of vildagliptin treatment on the revascularization of ischemic muscles in eNOS-KO mice. Treatment with vildagliptin did not improve blood flow recovery in the ischemic limbs of eNOS-KO mice throughout the experimental period (Fig. 2, C and D). As expected from previous studies, vascularization was also significantly greater in WT versus eNOS-KO mice treated only with vehicle controls. These data reinforce the importance of eNOS signaling for revascularization after ischemia and suggest that the positive effect of vildagliptin on revascularization following surgery-induced ischemia is dependent on eNOS activity.
Effect of Vildagliptin on Plasma Levels of GLP-1 and Adiponectin
Treatment of mice with vildagliptin has been reported to increase circulating GLP-1, SDF-1α, and adiponectin (24–26), which have protective effects on vasculature. We therefore assessed plasma levels of active GLP-1, SDF-1α, and adiponectin in WT mice treated with or without vildagliptin for 7 and 21 days after induced hind limb ischemia. Vildagliptin treatment did not affect blood glucose levels on days 7 and 21 following surgery in non-diabetic WT mice, as described in previous reports (27). There were significant increases in active GLP-1 and adiponectin, but not SDF-1α in the plasma of WT mice following treatment with vildagliptin compared with treatment with vehicle control on postoperative days 7 and 21 (Table 1).
TABLE 1.
Effect of vildagliptin on plasma levels of GLP-1 and adiponectin
Plasma glucose and GLP-1 (active), adiponectin, and SDF-1α levels were measured in mice treated with vildagliptin or vehicle on postoperative days 7 and 21 (n = 10 in each). Results are presented as mean ± S.D. *, p < 0.05 vs. vehicle control at the same time point.
| Day 7 |
Day 21 |
|||
|---|---|---|---|---|
| Vehicle | Vildagliptin | Vehicle | Vildagliptin | |
| Glucose (mg/dl) | 117.1 ± 20.6 | 124.3 ± 21.1 | 123.6 ± 31.8 | 127.9 ± 23.7 |
| GLP-1 (pm) | 2.70 ± 0.46 | 3.82 ± 0.42* | 2.81 ± 0.33 | 3.68 ± 0.37* |
| Adiponectin (μg/ml) | 14.7 ± 1.32 | 19.4 ± 1.57* | 15.8 ± 2.10 | 22.9 ± 1.86* |
| SDF-1α (ng/ml) | 2.19 ± 0.19 | 1.91 ± 0.18 | 2.38 ± 0.21 | 2.20 ± 0.13 |
Vildagliptin Promotes Endothelial Cell Differentiation in Vitro
First, we assessed whether GLP-1 is produced by HUVECs or contaminated in the culture medium. After HUVECs were cultured for 24 h, we collected the conditioned medium and assessed active GLP-1 levels in the medium. However, active GLP-1 could not be detected in the medium.
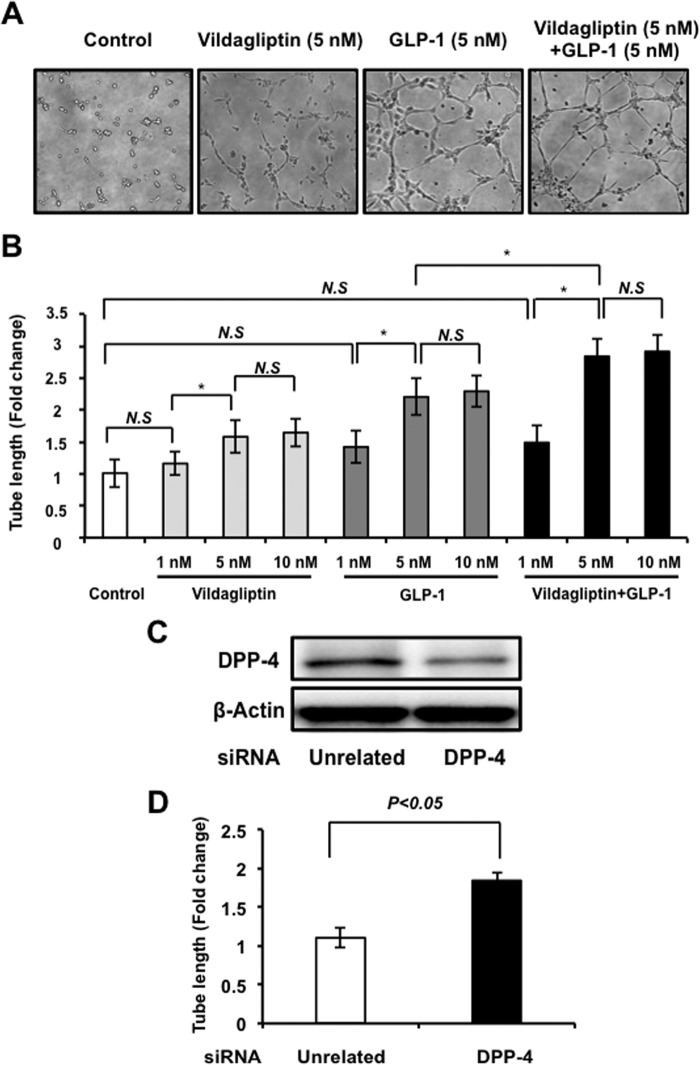
We next examined whether vildagliptin and/or GLP-1 modulates endothelial cell differentiation into vascular-like structures using HUVECs plated on a Matrigel matrix. HUVECs were treated with vildagliptin, recombinant human GLP-1, or vehicle control. Treatment with vildagliptin or GLP-1 promoted the formation of vascular-like tubes (Fig. 3A). Quantitative analysis of endothelial cell networks revealed that treatment with vildagliptin or GLP-1 significantly increased the tube length compared with control cultures. The fold change in tube length resulting from GLP-1 treatment was also significantly greater than the change resulting from treatment with vildagliptin in a dose-dependent manner (Fig. 3B).
FIGURE 3.
Vildagliptin promotes endothelial cell differentiation. A, representative photomicrographs of network formation of HUVECs treated with 5 nm vildagliptin and/or 5 nm GLP-1. B, quantitative analyses of network tube length are shown (n = 3). *, p < 0.05. HUVECs were seeded on Matrigel-coated plates incubated with vildagliptin (1, 5, or 10 nm) and/or GLP-1 (1, 5, or 10 nm) at 37 °C for 6 h (n = 3). *, p < 0.05. C, the representative immunoblots in DPP-4 in the presence of siRNA targeting DPP-4 or unrelated siRNA in HUVECs. D, effect of knockdown of DPP-4 on the network tube length. Results are shown as the mean ± S.D. (n = 3). N.S, not significant.
We also assessed whether vildagliptin enhances GLP-1-stimulated vascular-like structure. Combination treatment with vildagliptin (5 nm) and GLP-1 (5 nm) significantly increased the tube length relative to GLP-1 treatment (5 nm) (Fig. 3, A and B).
Furthermore, to corroborate these data, knockdown experiments were performed with siRNA against DPP4. Transfection with siRNA targeting DPP4 resulted in 78% reduction of DPP4 expression by Western blot analysis (Fig. 3C). Knockdown of DPP4 significantly increased endothelial cell differentiation into vascular-like structure (Fig. 3D).
eNOS Signaling in Vildagliptin-stimulated Endothelial Cell Differentiation in Vitro
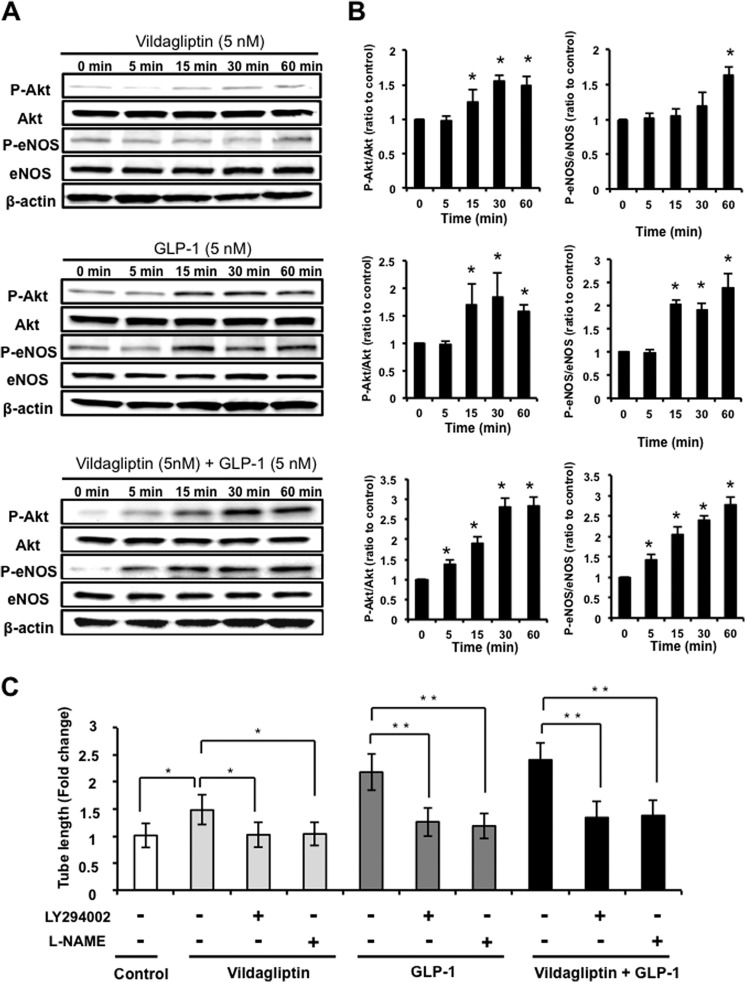
It has been reported that GLP-1 can activate Akt-eNOS signaling (28, 29), so we also assessed the effects of vildagliptin or GLP-1 on the phosphorylation of Akt and eNOS in HUVECs by Western blot analysis. Treatment with vildagliptin slightly increased the phosphorylation of Akt and eNOS (Fig. 4A). In contrast, GLP-1 treatment strongly stimulated the phosphorylation of Akt and eNOS in a time-dependent manner (Fig. 4B). Furthermore, combination treatment with vildagliptin and GLP-1 enhanced the phosphorylation of Akt and eNOS compared with GLP-1 treatment (Fig. 4, A and B). Neither vildagliptin or GLP-1 had any effect on Akt and eNOS protein levels.
FIGURE 4.
eNOS signaling participates in endothelial cell responses to vildagliptin. A and B, time-dependent changes in the phosphorylation of Akt and eNOS signaling in HUVECs treated with 5 nm vildagliptin and/or 5 nm GLP-1. Shown are quantitative analysis of relative changes in phosphorylated Akt and eNOS. Relative phosphorylation levels were normalized to each total protein signal (n = 3). *, p < 0.05 versus control (0 min). C, contribution of PI3K and eNOS to vildagliptin-mediated endothelial cell differentiation. HUVECs were pretreated with LY294002 (50 μmol/liter), l-NAME (1 mmol/liter), or vehicle (dimethyl sulfoxide), and treated with 5 nm vildagliptin and/or 5 nm GLP-1 at 37 °C for 6 h (n = 3). The Matrigel assay was performed. Quantitative analyses of the network tube length are shown. Results are shown as the mean ± S.D. (n = 5). *, p < 0.05; **, p < 0.01.
To investigate whether Akt-eNOS signaling participates in vildagliptin- or GLP-1-induced endothelial cell tube formation, HUVECs were treated with the PI3K inhibitor, LY294002, or the NOS inhibitor, l-NAME, and endothelial cell differentiation was assessed. Treatment with LY294002 or l-NAME abolished both vildagliptin- and GLP-1-mediated HUVEC differentiation into vascular-like structures (Fig. 4C).
Involvement of Src Kinase in Endothelial Cell Response to Vildagliptin
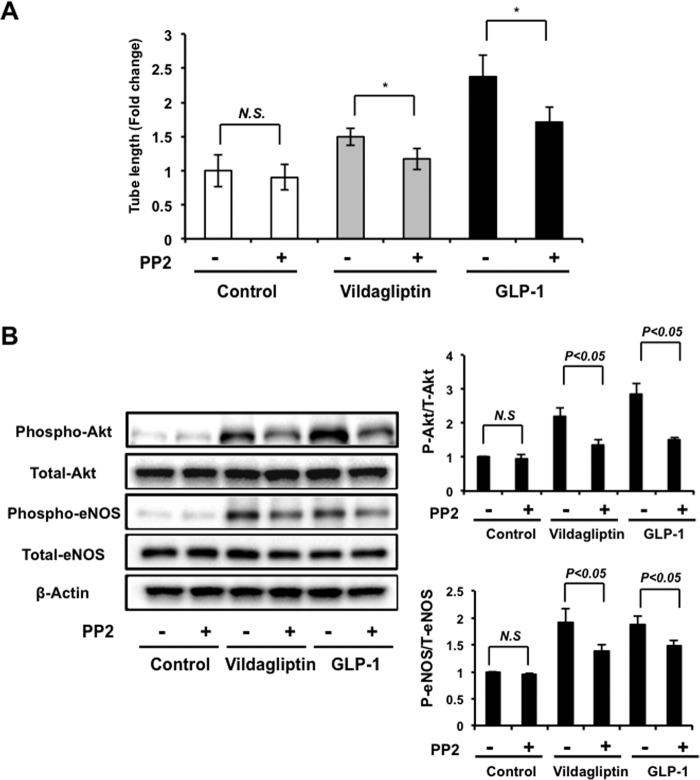
It has been reported that DPP-4 inhibition mediates rapid vascular relaxation through activation of Src-Akt-eNOS signaling that is independent of GLP-1(12). To determine whether Src kinase is involved in vildagliptin-stimulated endothelial cell network formation, HUVECs were pretreated with a Src kinase inhibitor, PP2, followed by treatment with vildagliptin or GLP-1. Treatment with PP2 abolished the stimulatory actions of vildagliptin or GLP-1 on HUVEC differentiation into vascular-like structure (Fig. 5A). Treatment with PP2 also reduced vildagliptin- or GLP-1-stimulated phosphorylation of Akt and eNOS (Fig. 5B). Thus, Src kinase contributes in both vildagliptin- and GLP-1-mediated endothelial cell network formation.
FIGURE 5.
Src kinase is involved in vildagliptin-stimulated endothelial cell differentiation. A, quantitative analyses of network tube length are shown. HUVECs were pretreated with Src kinase inhibitor PP2 (10 μm) for 30 min and treated with vildagliptin (5 nm) or GLP-1 (5 nm) at 37 °C for 6 h (n = 3). The Matrigel assay was performed. * p < 0.05. B, effects of PP2 on vildagliptin or GLP-1 stimulated phosphorylation of Akt and eNOS. HUVECs were pretreated with PP2 (10 μm) for 30 min and treated with vildagliptin (5 nm) or GLP-1 (5 nm) at 37 °C for 60 min. Relative phosphorylation levels were normalized to total protein signal (n = 4). Results are presented as mean ± S.D. N.S., not significant.
Role of Adiponectin in Vildagliptin-mediated Revascularization
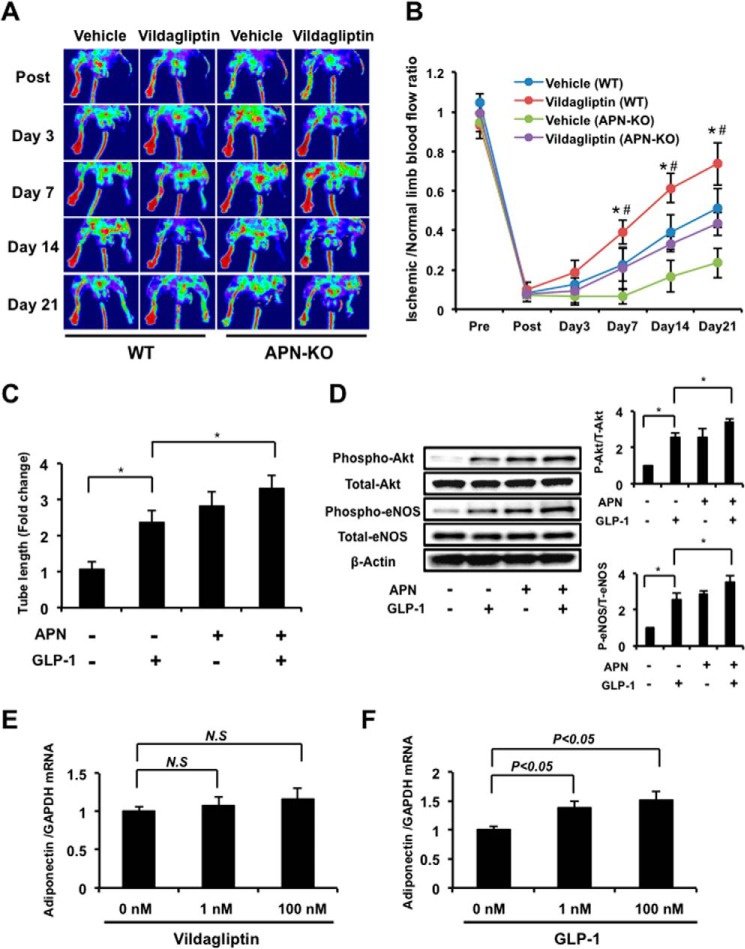
In addition to GLP-1, we showed that treatment with vildagliptin enhanced circulating adiponectin levels in limb ischemic mice (Table 1), so we examined the role of adiponectin in the vildagliptin-mediated improvement of ischemia-induced revascularization. APN-KO mice treated with or without vildagliptin were subjected to hind limb ischemia. Recovery of blood flow in the ischemic limbs of mice treated with vehicle control was greatly reduced in APN-KO mice compared with WT. Treatment with vildagliptin only partially increased the limb flow of ischemic muscle in APN-KO mice (Fig. 6, A and B).
FIGURE 6.
Role of adiponectin in the vildagliptin-induced angiogenic response. A, representative images of LDBF in WT and adiponectin-KO (APN-KO) mice treated with vildagliptin or vehicle control. B, quantitative analysis of the ischemic/normal LDBF ratio in vildagliptin or vehicle-treated WT and APN-KO mice on postoperative days 3, 7, 14, and 21 (n = 5 in WT, n = 4 in APN-KO). *, p < 0.05 versus vehicle (WT); #, p < 0.05 versus vildagliptin treatment (APN-KO). Results are presented as mean ± S.D. C, quantitative analyses of network tube length are shown. HUVECs were treated with adiponectin (30 μg/ml) and/or GLP-1 (5 nm) at 37 °C for 6 h (n = 3). The Matrigel assay was performed. Results were expressed relative to the values compared with control. *, p < 0.05. D, effects of adiponectin on phosphorylation of Akt and eNOS. HUVECs were treated with adiponectin (30 μg/ml) and/or GLP-1 (5 nm) at 37 °C for 60 min. Relative phosphorylation levels were normalized to total protein signal (n = 4). Results are presented as mean ± S.D. *, p < 0.05. E and F, expression of adiponectin in differentiated 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were incubated with vildagliptin (0, 1, or 100 nm) or GLP-1 (0, 1, or 100 nm) for 24 h, then adiponectin mRNA expression was determined by real-time RT-PCR (n = 3 in each group). Results are presented as mean ± S.D. N.S, not significant.
We next examined the effect of adiponectin on endothelial cell tube formation and phosphorylation of Akt and eNOS. Treatment with a physiological concentration of adiponectin promoted the capillary-like tube formation of endothelial cells, consistent with previous reports (30). The effect of adiponectin on enhancement of endothelial cell tube formation was similar to that of recombinant human GLP-1 treatment. Combination of adiponectin and GLP-1 significantly promoted the vascular-like tube formation compared with GLP-1 alone (Fig. 6C).
Treatment of HUVECs with adiponectin also led to an increase in Akt and eNOS phosphorylation consistent with previous reports (30). The phosphorylation level of Akt and eNOS in response to adiponectin stimulation was similar to that seen with GLP-1 treatment. GLP-1-induced phosphorylation of Akt and eNOS was further enhanced by combination treatment with adiponectin and GLP-1 (Fig. 6D). Thus, co-treatment with adiponectin and GLP-1 led to an additive increase in endothelial cell tube formation and phosphorylation of Akt and eNOS.
Finally, we investigated whether vildagliptin and GLP-1 directly induced adiponectin expression. Cultured 3T3-L1 adipocytes were treated with vildagliptin or GLP-1, and adiponectin mRNA expression was assessed. Treatment with 1 nm or 100 nm vildagliptin for 24 h did not significantly affect adiponectin mRNA expression in 3T3-L1 adipocytes (Fig. 6E). In contrast, GLP-1 treatment increased adiponectin mRNA expression in a dose-dependent manner in these cells (Fig. 6F). The ability of vildagliptin to increase adiponectin levels in mice may therefore be dependent on GLP-1.
DISCUSSION
The present study shows that the DPP-4 inhibitor vildagliptin promotes endothelial cell network formation and revascularization under conditions of ischemic stress. WT mice treated with vildagliptin had greater recovery of limb perfusion and enhanced capillary density compared with untreated mice, and this improvement was accompanied by increased levels of circulating GLP-1. Treatment with vildagliptin or GLP-1 also led to enhanced endothelial cell differentiation. DPP-4 inhibitors therefore improve revascularization under ischemic conditions.
It is well established that eNOS is beneficial for various types of vascular disease (22, 23). We showed that vildagliptin increased phosphorylated eNOS levels in ischemic muscles and that the ability of vildagliptin to enhance revascularization was abrogated in eNOS-KO mice. Consistent with these observations, previous studies have shown that phosphorylation of eNOS in heart tissue was increased in linagliptin-treated Zucker obese rats (31) and that sitagliptin increased eNOS activation in ischemic muscle (13). It was also reported that treatment of GLP-1 regulated eNOS activity in endothelial cells in vitro (28). Collectively, these data suggest that vildagliptin promotes revascularization in the ischemic state by activating eNOS signaling.
A number of studies have shown that GLP-1 attenuates ischemia-induced myocardial damage and atherosclerotic lesion formation (3) and promotes angiogenesis in endothelial cells through the Akt, PKC, and Src pathways (29). Previous reports also indicate that inhibition of DPP-4 directly regulates the endothelial cell growth and migration induced by inflammatory cytokines (32). In the present study, vildagliptin increased circulating GLP-1 levels 1.8-fold in WT mice, in agreement with previous reports (27, 33). In HUVECs, GLP-1 enhanced Akt-eNOS signaling and promoted the formation of capillary-like structures to a greater extent than vildagliptin, but the ability of vildagliptin to mediate these effects with no GLP-1 in HUVECs suggests that the vasculo-protective effects of vildagliptin occur at least partially through a mechanism that is independent of GLP-1. Inhibition of Akt-eNOS signaling effectively abrogated both vildagliptin- and GLP-1-mediated endothelial cell differentiation, indicating that the activity of both these agents is dependent on this signaling pathway.
The ability of GLP-1 to increase adiponectin levels is likely to contribute to revascularization in our experimental design. Adiponectin is an adipocytokine that is down-regulated in obesity-linked diseases and exerts protective actions on a variety of metabolic and cardiovascular disorders (30, 34–36). Experimental studies have shown that adiponectin has favorable effects on vascular function by direct action on vascular component cells. Vildagliptin-induced revascularization was associated with increased levels of GLP-1 and adiponectin, and APN-KO mice exhibited impaired revascularization in response to hind limb ischemia. Adiponectin also stimulates phosphorylation of eNOS, which is associated with enhanced endothelial cell migration and differentiation into capillary-like structures (30). In the present study, treatment with GLP-1, but not vildagliptin, significantly increased adiponectin expression in differentiated 3T3-L1 adipocytes. Furthermore, treatment with vildagliptin in APN-KO mice did not restore overall ischemic limb perfusion. Consistent with these findings, the GLP-1 receptor agonist exendin-4 was found to directly induce the expression of adiponectin in differentiated 3T3-L1 adipocytes (37). Clinically, sitagliptin treatment has been shown to improve vascular endothelial function, which was accompanied by increase in circulating adiponectin levels in diabetic patients (14). The beneficial actions of GLP-1 on the protection of cardiovascular tissues to stress might therefore be mediated, at least in part, by its ability to increase adiponectin production.
In conclusion, our data demonstrated that the DPP-4 inhibitor vildagliptin stimulates ischemia-induced revascularization via an eNOS-dependent mechanism. The vasculo-protective activities of vildagliptin in vivo are mediated by both GLP-1-dependent effects and the direct actions of vildagliptin. The beneficial actions of GLP-1 on revascularization in vivo are mediated, partly through its ability to increase adiponectin production (Fig. 7). In addition to its glucose lowering effect, DPP-4 inhibitors could be beneficial in patients with diabetes-related vascular complications.
FIGURE 7.
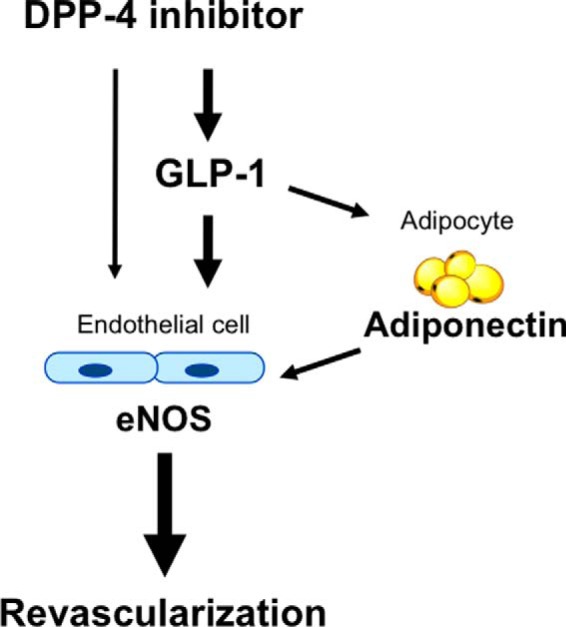
Proposed scheme for the angiogenic actions of vildagliptin. The DPP-4 inhibitor, vildagliptin, stimulates revascularization through an eNOS-dependent mechanism. The angiogenic actions of vildagliptin are mediated by both the GLP-1-dependent effects and the direct actions of vildagliptin. The beneficial actions of GLP-1 on revascularization are mediated partly through its ability to increase adiponectin production.
Acknowledgment
We gratefully acknowledge the technical assistance of Yoko Inoue.
This work was supported by a grant-in-aid for Young Scientists B, Banyu Life Science Foundation International, the Kanae Foundation, and the Kowa Life Science Foundation (to R. S.).
- DPP-4
- dipeptidyl peptidase-4
- eNOS
- endothelial nitric-oxide synthase
- GLP-1
- glucagon-like peptide-1
- LDBF
- laser Doppler blood flowmetry
- HUVEC
- human umbilical vein endothelial cell
- APN-KO
- adiponectin-deficient
- l-NAME
- Nω-nitro-l-arginine methyl ester
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine.
REFERENCES
- 1. Wang C. C., Reusch J. E. (2012) Diabetes and cardiovascular disease: changing the focus from glycemic control to improving long-term survival. Am. J. Cardiol. 110, 58B–68B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weitz J. I., Byrne J., Clagett G. P., Farkouh M. E., Porter J. M., Sackett D. L., Strandness D. E., Jr., Taylor L. M. (1996) Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation 94, 3026–3049 [DOI] [PubMed] [Google Scholar]
- 3. Sheikh A. (2013) Direct cardiovascular effects of glucagon like peptide-1. Diabetol. Metab. Syndr. 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X. M., Yang Y. J., Wu Y. J. (2013) The emerging role of dipeptidyl peptidase-4 inhibitors in cardiovascular protection: current position and perspectives. Cardiovasc. Drugs Ther. 27, 297–307 [DOI] [PubMed] [Google Scholar]
- 5. Murohara T. (2012) Dipeptidyl peptidase-4 inhibitor: another player for cardiovascular protection. J. Am. Coll. Cardiol. 59, 277–279 [DOI] [PubMed] [Google Scholar]
- 6. Chrysant S. G., Chrysant G. S. (2012) Clinical implications of cardiovascular preventing pleiotropic effects of dipeptidyl peptidase-4 inhibitors. Am. J. Cardiol. 109, 1681–1685 [DOI] [PubMed] [Google Scholar]
- 7. Dai Y., Dai D., Mercanti F., Ding Z., Wang X., Mehta J. L. (2013) Dipeptidyl peptidase-4 inhibitors in cardioprotection: a promising therapeutic approach. Acta Diabetol. 50, 827–835 [DOI] [PubMed] [Google Scholar]
- 8. Shigeta T., Aoyama M., Bando Y. K., Monji A., Mitsui T., Takatsu M., Cheng X. W., Okumura T., Hirashiki A., Nagata K., Murohara T. (2012) Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation 126, 1838–1851 [DOI] [PubMed] [Google Scholar]
- 9. Lim S., Choi S. H., Shin H., Cho B. J., Park H. S., Ahn B. Y., Kang S. M., Yoon J. W., Jang H. C., Kim Y. B., Park K. S. (2012) Effect of a dipeptidyl peptidase-IV inhibitor, des-fluoro-sitagliptin, on neointimal formation after balloon injury in rats. PLoS One 7, e35007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsubara J., Sugiyama S., Sugamura K., Nakamura T., Fujiwara Y., Akiyama E., Kurokawa H., Nozaki T., Ohba K., Konishi M., Maeda H., Izumiya Y., Kaikita K., Sumida H., Jinnouchi H., Matsui K., Kim-Mitsuyama S., Takeya M., Ogawa H. (2012) A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J. Am. Coll. Cardiol. 59, 265–276 [DOI] [PubMed] [Google Scholar]
- 11. Shah Z., Kampfrath T., Deiuliis J. A., Zhong J., Pineda C., Ying Z., Xu X., Lu B., Moffatt-Bruce S., Durairaj R., Sun Q., Mihai G., Maiseyeu A., Rajagopalan S. (2011) Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124, 2338–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah Z., Pineda C., Kampfrath T., Maiseyeu A., Ying Z., Racoma I., Deiuliis J., Xu X., Sun Q., Moffatt-Bruce S., Villamena F., Rajagopalan S. (2011) Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul. Pharmacol. 55, 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C. Y., Shih C. M., Tsao N. W., Lin Y. W., Huang P. H., Wu S. C., Lee A. W., Kao Y. T., Chang N. C., Nakagami H., Morishita R., Ou K. L., Hou W. C., Lin C. Y., Shyu K. G., Lin F. Y. (2012) Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br. J. Pharmacol. 167, 1506–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kubota Y., Miyamoto M., Takagi G., Ikeda T., Kirinoki-Ichikawa S., Tanaka K., Mizuno K. (2012) The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J. Korean Med. Sci. 27, 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fadini G. P., Avogaro A. (2013) Dipeptidyl peptidase-4 inhibition and vascular repair by mobilization of endogenous stem cells in diabetes and beyond. Atherosclerosis 229, 23–29 [DOI] [PubMed] [Google Scholar]
- 16. Kambara T., Ohashi K., Shibata R., Ogura Y., Maruyama S., Enomoto T., Uemura Y., Shimizu Y., Yuasa D., Matsuo K., Miyabe M., Kataoka Y., Murohara T., Ouchi N. (2012) CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J. Biol. Chem. 287, 18965–18973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maruyama S., Shibata R., Kikuchi R., Izumiya Y., Rokutanda T., Araki S., Kataoka Y., Ohashi K., Daida H., Kihara S., Ogawa H., Murohara T., Ouchi N. (2012) Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J. Biol. Chem. 287, 408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimizu Y., Shibata R., Ishii M., Ohashi K., Kambara T., Uemura Y., Yuasa D., Kataoka Y., Kihara S., Murohara T., Ouchi N. (2013) Adiponectin-mediated modulation of lymphatic vessel formation and lymphedema. J. Am. Heart Assoc. 2, e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kondo K., Shintani S., Shibata R., Murakami H., Murakami R., Imaizumi M., Kitagawa Y., Murohara T. (2009) Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 61–66 [DOI] [PubMed] [Google Scholar]
- 20. Kondo M., Shibata R., Miura R., Shimano M., Kondo K., Li P., Ohashi T., Kihara S., Maeda N., Walsh K., Ouchi N., Murohara T. (2009) Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J. Biol. Chem. 284, 1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishii M., Shibata R., Numaguchi Y., Kito T., Suzuki H., Shimizu K., Ito A., Honda H., Murohara T. (2011) Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 31, 2210–2215 [DOI] [PubMed] [Google Scholar]
- 22. Murohara T., Asahara T., Silver M., Bauters C., Masuda H., Kalka C., Kearney M., Chen D., Symes J. F., Fishman M. C., Huang P. L., Isner J. M. (1998) Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Invest. 101, 2567–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H., Wallerath T., Münzel T., Förstermann U. (2002) Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide 7, 149–164 [DOI] [PubMed] [Google Scholar]
- 24. Jungraithmayr W., De Meester I., Matheeussen V., Baerts L., Arni S., Weder W. (2012) CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur. J. Cardiothorac. Surg. 41, 1166–1173 [DOI] [PubMed] [Google Scholar]
- 25. Terasaki M., Nagashima M., Nohtomi K., Kohashi K., Tomoyasu M., Sinmura K., Nogi Y., Katayama Y., Sato K., Itoh F., Watanabe T., Hirano T. (2013) Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin's actions in nondiabetic and diabetic apolipoprotein E-null mice. PLoS One 8, e70933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyagawa K., Kondo T., Goto R., Matsuyama R., Ono K., Kitano S., Kawasaki S., Igata M., Kawashima J., Matsumura T., Motoshima H., Araki E. (2013) Effects of combination therapy with vildagliptin and valsartan in a mouse model of type 2 diabetes. Cardiovasc. Diabetol. 12, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimizu S., Hosooka T., Matsuda T., Asahara S., Koyanagi-Kimura M., Kanno A., Bartolome A., Etoh H., Fuchita M., Teruyama K., Takahashi H., Inoue H., Mieda Y., Hashimoto N., Seino S., Kido Y. (2012) DPP4 inhibitor vildagliptin preserves beta-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J. Mol. Endocrinol. 49, 125–135 [DOI] [PubMed] [Google Scholar]
- 28. Ding L., Zhang J. (2012) Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol. Sin. 33, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aronis K. N., Chamberland J. P., Mantzoros C. S. (2013) GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism 62, 1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aroor A. R., Sowers J. R., Bender S. B., Nistala R., Garro M., Mugerfeld I., Hayden M. R., Johnson M. S., Salam M., Whaley-Connell A., Demarco V. G. (2013) Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology 154, 2501–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takasawa W., Ohnuma K., Hatano R., Endo Y., Dang N. H., Morimoto C. (2010) Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem. Biophys. Res. Commun. 401, 7–12 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi A., Asakura M., Ito S., Min K. D., Shindo K., Yan Y., Liao Y., Yamazaki S., Sanada S., Asano Y., Ishibashi-Ueda H., Takashima S., Minamino T., Asanuma H., Mochizuki N., Kitakaze M. (2013) Dipeptidyl-peptidase IV inhibition improves pathophysiology of heart failure and increases survival rate in pressure-overloaded mice. Am. J. Physiol. Heart Circ. Physiol. 304, H1361–H1369 [DOI] [PubMed] [Google Scholar]
- 34. Ouchi N., Kihara S., Funahashi T., Matsuzawa Y., Walsh K. (2003) Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 14, 561–566 [DOI] [PubMed] [Google Scholar]
- 35. Shibata R., Ouchi N., Murohara T. (2009) Adiponectin and cardiovascular disease. Circ. J. 73, 608–614 [DOI] [PubMed] [Google Scholar]
- 36. Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 37. Kim Chung le T., Hosaka T., Yoshida M., Harada N., Sakaue H., Sakai T., Nakaya Y. (2009) Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 390, 613–618 [DOI] [PubMed] [Google Scholar]