Abstract
We examined the hypothesis that rejection automatically elicits defensive physiological reactions in people with low self-esteem (SE) but that attentional control moderates this effect. Undergraduates (N = 67) completed questionnaire measures of SE and attentional control. Their eye-blink responses to startle probes were measured while they viewed paintings related to rejection and acceptance themes. The stimuli also included positive-, negative-, and neutral-valence control paintings unrelated to rejection. As predicted, compared with people high in SE, those low in SE showed stronger startle eye-blink responses to paintings related to rejection, but not to negative paintings. Paintings related to acceptance did not attenuate their physiological reactivity. Furthermore, attentional control moderated their sensitivity to rejection, such that low SE was related to greater eye-blink responses to rejection only among individuals who were low in attentional control. Implications of the role of attentional control as a top-down process regulating emotional reactivity in people with low SE are discussed.
It has been argued that the function of the self-esteem (SE) system is to monitor the degree to which an individual is being socially rejected or excluded (Leary, Tambor, Terdal, & Downs, 1995; Nezlek, 2005). If there is a generalized pattern of social rejection, the individual experiences trait low SE, which in turn signals the person that he or she must take measures to minimize such exclusion. Hart, Shaver, and Goldenberg (2005) argued that along with attachment and terror management, SE is part of a tripartite security system that works to gain the individual’s acceptance and ensure survival.
Because trait low SE reflects a history of prior exposure to social exclusion (e.g., Harter, 1983), individuals with low SE expect rejection and allocate more attention to rejection-relevant cues than do people with high SE (e.g., Dandeneau & Baldwin, 2004; Leary et al., 1995). Furthermore, their feelings of exclusion or inclusion are contingent on the momentary cues they perceive in the environment (Campbell, Chew, & Scratchley, 1991), whereas feelings of acceptance are more stable in people with high SE and are not threatened by exclusion in any one social situation (Nezlek, Kowalski, Leary, Blevins, & Holgate, 1997).
Murray and her colleagues have argued that people with low SE show heightened sensitivity in detecting rejection and disapproval in close relationships (i.e., “self-esteem sensitivity to rejection”) and that this prevents their acceptance needs from ever being met by romantic partners (Murray, Griffin, Rose, & Bellavia, 2003). Furthermore, they respond to perceived rejection by behaving destructively in return (Murray, Bellavia, Rose, & Griffin, 2003). These negative effects of low SE on relationships accumulate over time, leading to reductions in partners’ satisfaction and in well-being of the relationship (Murray, Holmes, & Griffin, 2000).
Together, these findings suggest that although the presumed function of low SE is to motivate people to act in ways that minimize rejection and maximize acceptance, the expectation that one is likely to be rejected leads to a set of intra- and interindividual processes that undermine these higher-order goals, and maintain and perhaps even perpetuate low SE.
LOW SE AND POTENTIATION OF THE STARTLE EYE-BLINK RESPONSE BY REJECTION CUES
Despite extensive literature on SE, the impact of rejection on physiological reactivity among people with low SE remains largely unexplored. In the present study, we sought to address this gap in two ways: First, we examined the effect of rejection on accentuating physiological reactivity in low-SE individuals using the human startle-probe paradigm (Lang, Bradley, & Cuthbert, 1990). Second, we explored whether physiological reactivity to rejection could be modulated in people with low SE by individual differences in attentional control.
The startle-probe paradigm is commonly used to measure the activation of negative, defensive emotional reactions. Typically, subjects are exposed to a loud burst of white noise that elicits the startle reflex (or the eye-blink), which is part of a physiologically based, defensive-motivational-system (i.e., fight-or-flight) response. The magnitude of the eye-blink response elicited by a background auditory startle probe varies as a function of the valence of a foreground stimulus (Lang et al., 1990). For example, negative foreground stimuli, such as pictures of mutilated bodies, tend to universally amplify the eye-blink response that is elicited by the loud sound burst. In contrast, pleasant stimuli, such as photos of erotica or babies, reduce the magnitude of the eye-blink response to the startle probe. The linear pattern of affective modulation of the startle response (strongest response with negative foreground stimuli, intermediate response with neutral foreground stimuli, and smallest response with positive foreground stimuli) is best established for highly arousing slides (Cuthbert, Bradley, & Lang, 1996).
In this paradigm, activation of the defensive motivational system by foreground stimuli can also be modulated by particular sensitivity to negative foreground stimuli that represent domains of high personal concern. This effect has been demonstrated for people with specific phobias (Hamm, Cuthbert, Globisch, & Vaitl, 1997), depression (Lawson, MacLeod, & Hammond, 2002), and rejection sensitivity (Downey, Mougios, Ayduk, London, & Shoda, 2004).
Given these findings and existing evidence of low-SE individuals’ heightened sensitivity and reactivity to social rejection, we expected such individuals (compared with high-SE individuals) to show stronger eye-blink amplitude following a startle probe occurring in conjunction with rejection stimuli. Furthermore, we hypothesized that this effect would be specific to rejection, expecting that it would not generalize to negative-valence control stimuli.
Pursuing a secondary, more exploratory goal, we also assessed startle responses to stimuli related to interpersonal acceptance, as emotional responses to positive and negative stimuli tend to be orthogonal (Lang et al., 1990). We reasoned that the link between acceptance and SE might play out in one of three ways. One possibility is that low SE heightens sensitivity to the presence of acceptance cues. If so, then acceptance cues should activate approach-related, appetitive motivational tendencies more in people with low SE than in those with high SE; consequently, the former should exhibit lower eye-blink responses to startle probes presented in conjunction with stimuli related to acceptance. It is also possible, however, that people with low SE are less attuned to acceptance cues than are people with high SE (Dandeneau & Baldwin, 2004). In this case, acceptance stimuli should activate appetitive motivational tendencies, and inhibit blink responses to startle probes, more in high-SE than in low-SE individuals. Finally, it may be that the SE system is insensitive to acceptance—a possibility consistent with the finding that threat stimuli are given processing advantage over reward stimuli (Öhman, Lundqvist, & Esteves, 2001). In this case, people with low and high SE might not differ in their startle responses to noise bursts paired with acceptance.
ATTENTIONAL CONTROL AS A MODERATOR OF PHYSIOLOGICAL RESPONSES TO REJECTION
Attentional control is conceptualized as the ability to control attention in overriding, or inhibiting, a dominant, prepotent response in favor of a less accessible, subdominant response (Derryberry, Reed, & Pilkenton-Taylor, 2003; Rothbart & Bates, 1998). This ability recruits higher-order brain areas associated with executive functioning, notably the rostral area of the anterior cingulate cortex (rACC; Mathews, Yiend, & Lawrence, 2004).
Behavioral evidence suggests that high attentional control enables the modulation of reflexive emotional responses, whereas low attentional control leaves the individual vulnerable to acting on dominant emotional tendencies that might be dysfunctional (see Derryberry et al., 2003, for a review). For example, among anxious college students, higher attentional control has been linked to greater disengagement from threat-related information (Derryberry & Reed, 2002) and less negative affect after a laboratory induction of negative mood (Compton, 2000). In children prone to negative emotionality, externalizing behaviors (e.g., lying, arguing) are less common and teacher-reported social competence is higher the better the children are in regulating their attention (Eisenberg et al., 1997). Finally, in longitudinal research, greater attentional control and higher scores on related constructs in childhood predict better adjustment in adulthood (e.g., Eisenberg, Fabes, Guthrie, & Reiser, 2000; Kochanska & Knaack, 2003; see Mischel & Ayduk, 2004, for a review).
This network of evidence suggests that the degree to which rejection puts people with low SE in a state of defensive physiological arousal may depend on their ability to control their attention. Thus, this study also examined whether attentional control moderates the hypothesized link between low SE and greater startle responses to rejection. Specifically, we predicted that the main effect of low SE on startle responses would be moderated by an interaction with attentional control, such that low SE would be associated with greater potentiation of the startle reflex by rejection primarily among individuals low in attentional control.
OVERVIEW OF THE STUDY
Subjects completed questionnaire measures of SE and attentional control. Their eye-blink responses to startle probes were measured while they viewed paintings related to interpersonal rejection and acceptance, as well as negative-, positive-, and neutral-valence paintings. Nezlek et al. (1997) showed that low-SE and highly depressed individuals report similar negative emotional reactions after social rejection. Therefore, we controlled for depressive symptomatology to isolate the relation between low SE and startle responses.
Following the procedure of Downey et al. (2004), we first selected 100 paintings that we believed would elicit feelings of interpersonal rejection and acceptance, as well as neutral, positive, and negative non-rejection-related emotions.1 A group of undergraduate subjects (N = 55) then rated these paintings on the dimensions of acceptance/rejection, valence (positive-negative), and arousal (low-high). The paintings were scanned from high-quality slides and digitally presented on a computer screen. Ratings were made on scales from 1 to 9 (acceptance/rejection: 1 = acceptance, 5 = neutral, 9 = rejection; valence: 1 =very positive, 5 =neutral, 9 =very negative; arousal: 1 =very calm, 9 = very arousing). The final set included 8 paintings in each category (they can be viewed on the Web at http://socrates.berkeley.edu/~agyurak/startle/index.htm). Rejection and acceptance paintings were matched on arousal to negative and positive paintings, respectively. Rejection paintings were more rejecting, and acceptance paintings were more accepting, than neutral, negative, and positive paintings (see Table 1).
TABLE 1.
Mean Ratings of the Final Set of Paintings
| Rating | Category
|
||||
|---|---|---|---|---|---|
| Neutral | Negative | Positive | Rejection | Acceptance | |
| Arousal | 3.43a (1.09) | 5.36c (1.08) | 4.06b (1.28) | 5.19c (1.18) | 4.14b (1.22) |
| Acceptance/rejection | 4.86a (0.54) | 5.58b (0.97) | 4.39c (0.61) | 6.61d (1.07) | 3.09e (1.05) |
| Valence | 4.96a (0.53) | 6.34b (0.92) | 3.78c (0.86) | 6.63d (0.93) | 3.78c (0.79) |
Note. Ratings were made on 9-point scales. Higher ratings for valence indicate greater negativity. For acceptance/rejection, lower values indicate greater acceptance, and higher values indicate greater rejection. Within each row, means with different subscripts are significantly different, p < .05. Standard deviations are given in parentheses.
METHOD
Sample and Procedure
Undergraduates (N = 67; 38 females, 29 males) completed the study in exchange for course credit (mean age = 21.47, SD = 5.09). The ethnic composition of the sample was as follows: 53.73% Asian, 28.36% Caucasian, 5.97% Hispanic, 5.97% Black, and 5.97% other.
Subjects completed the study individually. After they gave informed consent, physiological sensors were applied. Subjects were then seated in front of a computer. Recording of a 5-min resting period was followed by 3 practice startle-probe trials to familiarize the subjects with the noises. During the experimental trials, subjects were asked to pay attention to the paintings on the screen and disregard the noises coming through the earphones. Startle responses were measured according to the procedure and guidelines described in Blumenthal et al. (2005). Upon completion of the startle-probe paradigm, subjects filled out the questionnaire measures (described next). Finally, subjects were thanked and fully debriefed.
Questionnaire Measures
Attentional Control
The ability to voluntarily control attention was measured by the 20-item Attentional Control Scale (ACS; Derryberry & Reed, 2002). This measure includes items related to attention focusing (e.g., “My concentration is good even if there is music in the room around me”), attention shifting (e.g., “After being distracted or interrupted, I can easily shift my attention back to what I was doing”), and flexible control of thought (e.g., “I can become interested in a new topic very quickly if I need to”). The scale shows good internal reliability and predictive utility. Higher scores on the ACS predict less resistance to interference in Stroop-like spatial conflict tasks, greater disengagement from threat stimuli among highly anxious people (Derryberry & Reed, 2002), and greater activation in brain areas related to top-down regulation of emotion (i.e., rACC) while looking at fear-related pictures (Mathews et al., 2004). Subjects in this sample rated the ACS items on a 4-point scale (1 = almost never, 2 = sometimes, 3 = often, 4 = always). Scores were calculated by averaging across all items (after responses were reverse-scored, when appropriate; M = 2.45, SD = 0.31, α= .71).
Self-Esteem
Rosenberg’s (1989) 10-item questionnaire (e.g., “On the whole, I am satisfied with myself”) was used to measure SE. Ratings were made on a scale from 1 (does not describe me at all) to 6 (describes me very well). Items were reverse-scored when appropriate and then averaged (M = 4.5, SD = 0.91, α= .87). SE and attentional control were positively correlated, r(65) = .41, p < .05.
Depression
Depression was measured by the 21-item Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). Subjects rated their experience of affective, cognitive, and behavioral symptoms of depression on a 4-point scale (0–3). Ratings were summed to create a composite score (M = 7.38, SD = 5.51). Depression was negatively correlated with both SE, r(65) =−.52, p < .05, and attentional control, r(65) =−.23, p = .06.
Startle-Probe Task
Eye-Blink Recording
Two 5-mm Ag/AgCl recording electrodes were placed on the lower left eyelid, over the orbicularis oculi muscle. Electrodes were filled with conductance gel and affixed with adhesive collars. The skin was prepared with an abrasive solution to keep impedance under 10 KΩ. Electromyographic (EMG) activity from the orbicularis oculi was amplified (× 5000) with a Biopac EMG100 amplifier module and then transmitted to a Biopac recording system (Biopac Systems, Inc., Santa Barbara, CA). Data were digitized and sampled at 2000 Hz.
Pictures were presented on a 16-in. monitor for 6 s with Direct RTsoftware (Empirisoft Corporation, New York, NY). The startle probe was a 50-ms burst of white noise with instantaneous rise and fall times. The noise burst was digitized and binaurally delivered at 105 dB. The timing of the startle probe was randomly determined on each trial: 3,500, 4,500, or 6,000 ms into the picture presentation. The intertrial interval varied randomly between 10 and 20 s. Seventy-five percent of the pictures within each category (six paintings per category) were probed with a startle burst. The order in which the pictures were presented was determined randomly, with the constraint that no more than two pictures from the same category appeared successively; each painting was shown once.
Data Processing
Orbicularis activity in response to the startle probes was analyzed using Mindware EMG 2.5 (Mindware Technologies, Ghanna, OH). EMG activity was digitally filtered (5- to 250-Hz band pass), rectified, and smoothed. Spectral analyses were done via fast Fourier transform. Peak EMG amplitude was determined for the 130-ms window following the startle-probe presentation (Blumenthal et al., 2005). Data for 2.1% of the blink responses were not analyzable, as no orbicularis activity was detected within the 130-ms time window. After deriving the peak amplitude of the EMG response to each probe, we created composites of startle reflex for rejection, acceptance, negative, positive, and neutral paintings. These composites were calculated for each subject by averaging the amplitude of the subject’s EMG responses for each category of paintings and served as our dependent variables.
RESULTS
We examined the relation between SE and startle eye-blink amplitude, including the moderating effect of attentional control, in multiple regression analyses using the SAS statistical package (Version 9.1). To account for the large individual differences in the strength of the eye-blink muscle activity, all analyses covaried blink responses to neutral slides, as recommended by Blumenthal et al. (2005).2 Additionally, to partial out the variance low SE shares with depression, we controlled for BDI scores. Gender and ethnicity did not moderate any of the results. Predictors were centered on their means and used as continuous variables.
Does Rejection Elicit a Stronger Startle Response in People With Lower SE?
As expected, multiple regression analysis indicated that SE was negatively related to the amplitude of the blink response to rejection paintings, F(3, 63) = 6.22, p < .05, β = −.17, Rp2 = .09. This relation remained significant when we controlled for blink amplitude for negative paintings, F(4, 62) =6.11, p < .05, β = −.14, Rp2 = .09. In contrast, SE was not significantly related to blink responses to negative slides (F < 1). This is important because it suggests that the SE system reacts specifically to rejecting stimuli, rather than overall negativity. BDI was not a significant predictor of the startle response to rejection paintings (F < 1).
Does Acceptance Potentiate or Attenuate the Startle Response in People With Low SE?
Regression analysis revealed a marginal trend for low-SE subjects to show stronger blink responses to acceptance paintings than high-SE subjects, F(3, 63) = 2.75, p = .10, β = −.10, Rp2 = .04. This trend became nonsignificant when we controlled for responses to positive stimuli, F(4, 62) = 1.9, n.s. There was no significant relation between SE and blink responses to positive paintings, F(3, 63) < 1. BDI was not a significant predictor of the startle response to acceptance stimuli (F < 1).
Does Attentional Control Interact With SE in Predicting Startle Responses?
Our next question was whether attentional control moderated the relation between SE and the potentiation of the startle response by rejection. Regression analysis conducted on the amplitude of blink responses to rejection paintings, with SE, attentional control, and their interaction as between-subjects predictors, revealed a significant interaction, F(5, 61) = 4.36, p < .05, β =.13, Rp2 = .07. In contrast, this interaction was not significant in predicting startle to negative paintings (F < 1). Furthermore, the interaction predicting startle to rejection stimuli remained significant when we controlled for blink responses to negative paintings, F(6, 60) =5.55, p < .05, β =.12, Rp2 = .08. Regression results predicting startle amplitude for rejection and negative paintings, with appropriate covariates, are presented in Table 2.
TABLE 2.
Standardized Parameter Estimates Predicting Activation of the Defensive Motivational System, for Negative and Rejection-Related Paintings
| Predictor | Blink amplitude
|
|
|---|---|---|
| Negative paintings | Rejection paintings | |
| Blink response: neutral paintings | .90 (1.03)* | .39 (0.43)* |
| Blink response: negative paintings | — | .56 (0.54)* |
| Depression | .10 (1.51) | −.05 (−1.43) |
| Self-esteem | −.06 (−8.41) | −.12 (−17.41)* |
| Attentional control | .03 (11.74) | −.02 (−9.93) |
| Self-Esteem × Attentional Control | .01 (6.45) | .11 (57.35)* |
Note. Nonstandardized parameter estimates are given in parentheses.
p ≤ 05.
We conducted simple-slope analyses (Aiken & West, 1991) at 1 standard deviation above and below the mean on each predictor to further probe the significant interaction for rejection paintings (see Fig. 1). These analyses indicated that among subjects low in attentional control, low-SE subjects showed greater startle responses to rejection than high-SE subjects did, β = −.25, t(60) = −3.26, p < .05, Rp2 = .15. In contrast, SE was not significantly related to startle response to rejection among subjects high in attentional control, β = .01, t < 1. Furthermore, low-SE subjects exhibited stronger eye-blink responses to rejection the lower their attentional control, β = −.15, t(60) = −1.96, p = .05, Rp2 = .06. Among subjects with high SE, startle response to rejection did not differ as a function of attentional control, β = .1, t = 1.4, n.s.
Fig. 1.
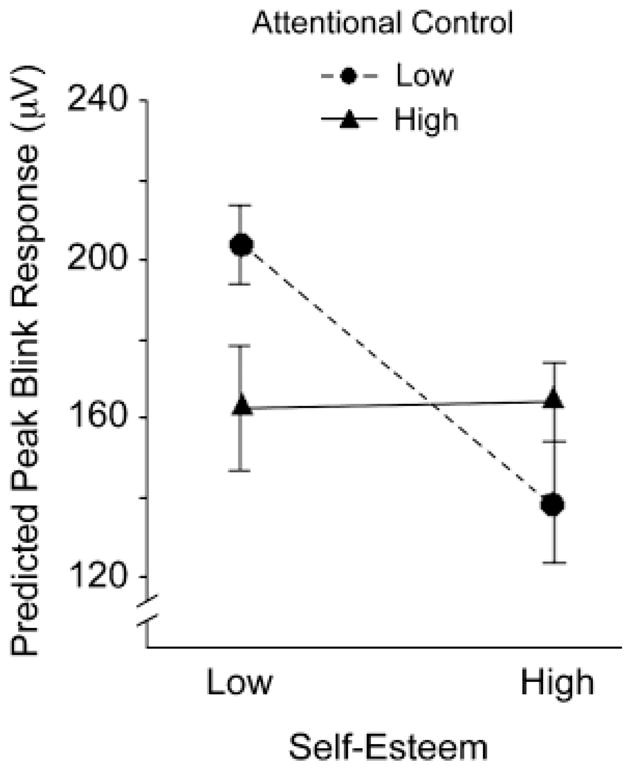
Predicted values of startle eye-blink amplitude for rejection paintings, as a function of self-esteem and attentional control (based on the parameter estimates presented in Table 2). Higher values indicate stronger muscle contraction and heightened activation of the defensive motivational system.
Attentional control did not interact with SE in predicting startle amplitude to acceptance paintings, F(5, 61) = 1.43, n.s.
DISCUSSION
The present study showed that rejection cues trigger greater startle potentiation in people with low-SE than in those with high SE. This effect held when depression scores and blink magnitude for negative paintings were partialed out. Furthermore, no significant relation between SE and startle response to negative control stimuli emerged. One conclusion this set of findings suggests is that it is specifically rejection, not general negative affective tone, that automatically activates defensive motivational states in people with low SE. In addition, because the effect of BDI was partialed out, the association between low SE and startle potentiation by rejection stimuli cannot be simply due to a propensity to experience depressive states. Instead, it is likely the network of beliefs and expectations about rejection (e.g., expectations about the partner’s regard for oneself, beliefs about one’s worthiness) that explains why low-SE individuals show greater physiological reactivity to rejection stimuli than high-SE individuals do. As a whole, these findings support the idea that people with low SE show a specific vulnerability to social rejection (Murray, Griffin, et al., 2003).
The startle reflex is generated by subcortical emotional systems such as the amygdala (Lang, Bradley, & Cuthbert, 1998). Research shows, however, that effortful top-down processes can decrease the magnitude of this mostly automatic reaction (Jackson, Malmstadt, Larson, & Davidson, 2000). For example, simply instructing subjects to suppress expressive behaviors following startle probes has been shown to attenuate the amplitude of the eye-blink response (Jackson et al., 2000).
Results from the present study are consistent with these findings. Previous research has shown that higher scores on the ACS are related to more activation in the rACC (Mathews et al., 2004), an area associated with evaluation and regulation of emotional stimuli (Bush, Luu, & Posner, 2000; Devinsky, Morrell, & Vogt, 1995). Notably, the rACC is extensively connected to the amygdala (Devinsky et al., 1995), and these direct paths allow for regulatory interplay between these regions (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006). In line with these findings, our results showed that only among subjects who were low in attentional control was the startle reflex to rejection (which is generated through the amygdala) stronger in people with low SE than in people with high SE. Among subjects high in attentional control, reactivity to rejection did not vary as a function of SE. This finding indicates that even at a relatively early and automatic stage of emotion generation, higher-order regulatory processes, possibly implemented by the rACC, can attenuate defensive physiological reactions to rejection in people with low SE.
This study also explored the relation between SE and startle responses to acceptance. We found a marginal trend for people with low SE to startle more than people with high SE while viewing acceptance paintings. This finding suggests that acceptance cues tend to have a greater calming effect on high-SE individuals. However, this effect did not hold when we controlled for blink responses to positive paintings. Therefore, a conservative interpretation of our findings is that the SE system is not sensitive to acceptance.
An important question not addressed by our data is whether attentional control’s protective effect against physiological reactivity among low-SE individuals translates into better coping in actual relationships. People with low SE feel inferior to their partners and perceive their partners to be “out of their league” (Murray et al., 2005). However, when such discrepancies are reduced experimentally by highlighting either low-SE individuals’ strengths or their partners’ weaknesses, people with low SE feel less insecure in their partners’ positive regard and report greater self-worth. We speculate that reductions in perceived discrepancies between one’s own worth and one’s partner’s worth may be one of the mechanisms through which attentional control can enhance the relationship outcomes of low-SE individuals. Many other mediating mechanisms, such as emotion regulation through reappraisal (Gross & John, 2003), inhibition of hostile attributional biases (Dodge, 1980), and being able to take one’s partner’s perspective (Arriaga & Rusbult, 1998), are likely to be at work, however, and should be examined in future research.
CAVEATS AND CONCLUSIONS
Because the data are correlational, our findings do not provide causal evidence that attentional control helps to protect against low SE. Such a causal relation should be examined in future research. It will also be important to replicate this effect with behavioral measures of executive functioning (e.g., Stroop task). Finally, the effects documented were of medium size (effect size rs between .25 and .30). However, because the startle paradigm provides an implicit measure of reactivity, the effect sizes found in the present study need to be evaluated in the context of previous research that failed to find any relation between explicit self-report and implicit measures of SE (e.g., Greenwald & Farnham, 2000). Despite these caveats, the present results suggest that researchers should further investigate the role of attentional control as a buffer against defensive physiological reactivity elicited by rejection in low-SE individuals. Such studies will have both theoretical and intervention-related implications.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (MH0697043). The authors thank Christopher J. Soto for his statistical assistance and Matthew S. Cain, Andres Martinez, and Rodolfo Mendoza-Denton for their helpful comments.
Footnotes
We developed a new, improved set of stimuli, instead of using those created by Downey et al. (2004), because we wanted to (a) include neutral paintings, (b) increase the number of stimuli in each category, (c) have negative and positive stimuli that included representational art and were parallel to the rejection and acceptance stimuli, and (d) include artwork from a variety of artists and styles (rather than one artist) in each stimulus category.
There were no significant zero-order correlations between the predictors and average blink responses for any of the picture categories. However, these relationships should not be interpreted without controlling for individual differences in muscle strength (startle responses to control stimuli), as recommended by Blumenthal et al. (2005).
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Arriaga XB, Rusbult CE. Standing in my partner’s shoes: Partner perspective taking and reactions to accommodative dilemmas. Personality and Social Psychology Bulletin. 1998;24:927–948. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campbell JD, Chew B, Scratchley LS. Cognitive and emotional reactions to daily events: The effects of self-esteem and self-complexity. Journal of Personality. 1991;59:473–505. doi: 10.1111/j.1467-6494.1991.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Compton RJ. Ability to disengage attention predicts negative affect. Cognition & Emotion. 2000;14:401–415. [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Dandeneau SD, Baldwin MW. The inhibition of socially rejecting information among people with high versus low self-esteem: The role of attentional bias and the effects of bias reduction training. Journal of Social and Clinical Psychology. 2004;23:584–602. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA, Pilkenton-Taylor C. Temperament and coping: Advantages of an individual differences perspective. Development and Psychopathology. 2003;15:1049–1066. [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain: A Journal of Neurology. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dodge KA. Social cognition and children’s aggressive behavior. Child Development. 1980;51:162–170. [PubMed] [Google Scholar]
- Downey G, Mougios V, Ayduk O, London BE, Shoda Y. Rejection sensitivity and the defensive motivational system: Insights from the startle response to rejection cues. Psychological Science. 2004;15:668–673. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: Their role in predicting quality of social functioning. Journal of Personality and Social Psychology. 2000;78:136–157. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Shepard SA, Murphy BC, Guthrie IK, Jones S, et al. Contemporaneous and longitudinal prediction of children’s social functioning from regulation and emotionality. Child Development. 1997;68:642–664. [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Farnham SD. Using the implicit association test to measure self-esteem and self-concept. Journal of Personality and Social Psychology. 2000;79:1022–1038. doi: 10.1037//0022-3514.79.6.1022. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and the startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Hart J, Shaver PR, Goldenberg JL. Attachment, self-esteem, worldviews, and terror management: Evidence for a tripartite security system. Journal of Personality and Social Psychology. 2005;88:999–1013. doi: 10.1037/0022-3514.88.6.999. [DOI] [PubMed] [Google Scholar]
- Harter S. Developmental perspectives on the self-system. In: Mussen PH, Hetherington EM, editors. Handbook of child psychology: Vol. 4. Socialization, personality and social development. New York: Wiley; 1983. pp. 275–385. [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Kochanska G, Knaack A. Effortful control as a personality characteristic of young children: Antecedents, correlates, and consequences. Journal of Personality. 2003;71:1087–1112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lawson C, MacLeod C, Hammond G. Interpretation revealed in the blink of an eye: Depressive bias in the resolution of ambiguity. Journal of Abnormal Psychology. 2002;111:321–328. doi: 10.1037//0021-843x.111.2.321. [DOI] [PubMed] [Google Scholar]
- Leary MR, Tambor ES, Terdal SK, Downs DL. Self-esteem as an interpersonal monitor: The sociometer hypothesis. Journal of Personality and Social Psychology. 1995;68:518–530. [Google Scholar]
- Mathews A, Yiend J, Lawrence AD. Individual differences in the modulation of fear-related brain activation by attentional control. Journal of Cognitive Neuroscience. 2004;16:1683–1694. doi: 10.1162/0898929042947810. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O. Willpower in a cognitive-affective processing system: The dynamics of delay of gratification. In: Baumeister R, Vohs K, editors. Handbook of self-regulation: Research, theory, and applications. New York: Guilford; 2004. pp. 99–129. [Google Scholar]
- Murray SL, Bellavia GM, Rose P, Griffin DW. Once hurt, twice hurtful: How perceived regard regulates daily marital interactions. Journal of Personality and Social Psychology. 2003;84:126–147. [PubMed] [Google Scholar]
- Murray SL, Griffin DW, Rose P, Bellavia GM. Calibrating the sociometer: The relational contingencies of self-esteem. Journal of Personality and Social Psychology. 2003;85:63–84. doi: 10.1037/0022-3514.85.1.63. [DOI] [PubMed] [Google Scholar]
- Murray SL, Holmes JG, Griffin DW. Self-esteem and the quest for felt security: How perceived regard regulates attachment processes. Journal of Personality and Social Psychology. 2000;78:478–498. doi: 10.1037//0022-3514.78.3.478. [DOI] [PubMed] [Google Scholar]
- Murray SL, Rose P, Holmes JG, Derrick J, Podchaski EJ, Bellavia G, Griffin DW. Putting the partner within reach: A dyadic perspective on felt security in close relationships. Journal of Personality and Social Psychology. 2005;88:327–347. doi: 10.1037/0022-3514.88.2.327. [DOI] [PubMed] [Google Scholar]
- Nezlek JB. Distinguishing affective and non-affective reactions to daily events. Journal of Personality. 2005;73:1539–1568. doi: 10.1111/j.1467-6494.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Kowalski RM, Leary MR, Blevins T, Holgate S. Personality moderators of reactions to interpersonal rejection: Depression and trait self-esteem. Personality and Social Psychology Bulletin. 1997;23:1235–1244. [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80:381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Middletown, CT: Wesleyan University Press; 1989. rev. ed. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. Hoboken, NJ: John Wiley & Sons; 1998. [Google Scholar]


