Abstract
Two fundamental issues in emotion theory and research concern: (a) the role of emotion in promoting response coherence across different emotion systems; and (b) the role of awareness of bodily sensations in the experience of emotion. The present study poses a question bridging the two domains; namely, whether training in Vipassana meditation or dance, both of which promote attention to certain kinds of bodily sensations, is associated with greater coherence between the subjective and physiological aspects of emotion. We used lag correlations to examine second-by-second coherence between subjective emotional experience and heart period within individuals across four different films. Participants were either: (a) experienced Vipassana meditators (attention to visceral sensations), (b) experienced dancers (attention to somatic sensations), and (c) controls with no meditation or dance experience. Results indicated a linear relationship in coherence, with meditators having highest levels, dancers having intermediary levels, and controls having lowest levels. We conclude that the coherence between subjective and cardiac aspects of emotion is greater in those who have specialized training that promotes greater body awareness.
Keywords: body awareness, coherence, emotional responses, meditation, physiological responses
The concepts of response coherence (Darwin, 1872; Ekman, 1992; Lazarus, 1991; Levenson, 1994; Scherer, 1984; Tomkins, 1962) and awareness of bodily sensations (Damasio, 2000; James, 1884; Levenson, 2003a) are central to many emotion theories. Response coherence implies that emotions organize and synchronize different response systems (Darwin, 1872; Ekman, 1992; Lazarus, 1991; Levenson, 1994; Tomkins, 1962) such that when we are in the throes of a strong emotion, our subjective, behavioral, and physiological responses should track each other more closely than when we are at rest. Awareness of bodily sensations implies that information that is visceral (e.g., heart pounding) or somatic (e.g., muscle tension) in origin is critical for determining subjective emotional experience (Lakoff, 1987; Levenson, 1999) and may even be a proximate cause of emotional experience (James, 1884). Although many contemporary emotion theories espouse both of these concepts, little is known about how response coherence and awareness of bodily sensations interact in the experience of emotion. The present study poses a question bridging these two domains, asking whether individuals who have specialized training in body awareness will have greater coherence between subjective and physiological aspects of emotion. We reason that if sensations emanating from bodily responses are primary constituents of subjective emotional experience, then subjective emotional experience should more closely track and be coherent with physiological responding for those who are more aware of their bodily responses.
Coherence Across Emotion Response Systems
Emotion theorists since Darwin (1872) have argued that one of the functions of emotion is to impose coherence among a person’s subjective, behavioral, and physiological responses. In fact, many theorists describe coherence as one of the defining features of emotion, variously referring to it as response system coherence (Ekman, 1992; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005), concordance (Nesse et al., 1985; Wilhelm & Roth, 2001), synchronization (Scherer, 1984), and organization of response tendencies (Lazarus, 1991; Levenson, 1994). The construct of coherence is typically associated with a functionalist perspective: namely, that coherence across response systems is adaptive, creating optimal conditions for the organism to cope successfully with significant challenges and opportunities. For instance, in proposing the concept of “affect programs,” Tomkins (1984) argues that certain circuits in the brain are responsible for orchestrating cascades of associated responses specific to each basic emotion. These affect programs are thought to promote coherence across multiple response systems, thereby initiating a unified response to the immediate demand at hand (e.g., fight or flight in response to threat). We (Levenson, 2003b) have likened this coordination to a “just in time” factory model. In this account, the autonomic nervous system is responsible not only for delivering the components needed to construct an appropriate emotional response, but also for the precise timing of this delivery, providing sufficient quantities when needed and quickly removing that which is no longer needed. In these ways, response coherence helps the individual respond in an organized, timely, and optimal fashion to changing environmental demands.
Empirical Findings and Measurement Issues
While there are strong theoretical arguments for the existence of response coherence, empirical support has been inconsistent and inconclusive (Barrett, 2006). Some studies have found coherence among systems (Ekman, Davidson, & Friesen, 1990; Ekman, Friesen, & Ancoli, 1980; Mauss et al., 2005; Rosenberg & Ekman, 1997), while others have found none (Buck, 1977; Fernández-Dols, Sánchez, Carrera, & Ruiz-Belda, 1997; Fridlund, 1991; Jakobs, Mansteaed, & Fischer, 2001; Mauss, Wilhelm, & Gross, 2004).
In examining these studies, two very different paradigms have been used to assess coherence (Buck, 1980; Mauss et al., 2005). In the most common approach, the “between-individual” paradigm (Mauss et al., 2005), the question is typically whether individuals who report greater levels of subjective emotional experience also exhibit greater levels of behavioral and physiological responses. Such studies have yielded disparate findings, with some finding positive relationships among systems (e.g., individuals instructed to diminish facial expression during shock delivery had smaller physiological reactions; Lanzetta, Cartwright-Smith, and Kleck, 1976), others finding no relationships (e.g., the magnitude of individuals’ reports of anxiety are not associated with the magnitude of their physiological reaction; Mauss et al., 2004), and yet others finding inverse relationships (e.g., individuals exhibiting greater spontaneous facial expression have smaller physiological reactions; Buck, Miller, & Caul, 1974; Lanzetta & Kleck, 1970; Notarius & Levenson, 1979).
Another approach to studying response coherence, which arguably is much closer to the theoretical accounts, takes a “within-individual” approach, conceptualizing coherence as the extent to which responses become coordinated with each other while the person is having an emotion. To evaluate coherence in this way, a study must minimally meet three criteria. First, it should measure response systems continuously over time. This is fairly easy to achieve for physiological and behavioral aspects of emotional response, which are typically measured in this way. However, subjective experience is often measured using a single retrospective rating. This approach fails the requirement for continuous measurement and introduces retrospective memory biases (Barrett, 1997; Kahneman & Tversky, 2003). In our work studying the emotions that occur during marital interactions, we developed a rating dial methodology for obtaining continuous ratings of subjective emotional experience (Gottman & Levenson, 1985; Levenson & Gottman, 1983) that we subsequently adopted to obtain continuous ratings of subjective emotional experience (Mauss et al., 2005). Second, it should take into account the different temporal characteristics of various systems (e.g., facial muscles respond more quickly than sweat glands). If lead and lag relationships across response systems are not considered, the degree of coherence can easily be underestimated. Third, it should measure coherence during periods when participants are actually in the throes of an emotion. Theoretical accounts of emotion that posit coherence predict close coordination among response systems during emotional episodes (Levenson, 1994); low levels of coherence would be expected when individuals are at rest (e.g., Lacey, 1967; Lazarus, Speisman, & Mordkoff, 1963).
To our knowledge, only one prior study has assessed within-individual coherence in a manner that meets all three of these criteria. We (Mauss et al., 2005) found moderate time-lagged correlations among subjective emotional experience, facial behavior, and physiology during film-induced emotions, thus providing empirical support for theoretical accounts of coherence. However, even under these arguably ideal methodological conditions, we noted a high degree of individual variability in the extent of coherence, thus raising the question of the sources of this variability. As explained below, we believe that individual differences in body awareness may constitute one source of this variability.
Body Awareness and Emotion
The notion that bodily sensations contribute importantly to our subjective emotional experience appears in the literature in a number of forms. James (1884) famously asserted that emotions occur when the perception of an exciting fact causes a collection of bodily changes and that “our feeling of the same changes as they occur is the emotion” (p. 189). Lakoff (1987) noted the prominence of bodily metaphors when we describe the experience of certain emotions (e.g., heat and pressure metaphors when describing anger). Consistent with these views, we (Levenson, 1999) postulated that the “subjective experience of a given emotion derives largely from the sensations that are generated by the activation of the associated response package” (p. 496). Similarly, theories of embodied cognition suggest that there is a reciprocal relationship between bodily activity and the way emotional information is processed (for a review, see Niedenthal, 2007). These notions are also found in contemporary neuroscience, with Damasio (2000) postulating that one of the main roles of emotion is to bring the autonomic processes of our bodies into awareness, providing “somatic markers” that guide our choices and actions.
Empirical Findings and Methodological Issues
Research from a wide variety of sources supports the notion that body awareness plays a central role in emotion. Activity in brain regions related to interoceptive and somatosensory processing (e.g., the insular and somatosensory cortices) has been shown to be present during emotions (Craig, 2009; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Damasio, 2003). Individuals with spinal cord injuries at locations that would disrupt visceral feedback show attenuation in emotional experience, with this attenuation increasing with the height of the injury (Hohmann, 1966). Additionally, a number of studies have shown that experimental manipulation of bodily posture or facial expression (e.g., sitting slouched or upright, holding a pen in one’s mouth to inhibit or facilitate smiling) affects the subjective experience of emotion (Duckworth, Bargh, Garcia, & Chaiken, 2002; Larsen, Kasimatis, & Frey, 1992; Stepper & Strack, 1993; Strack, Martin, & Stepper, 1988; Tom, Pettersen, Lau, Burton, & Cook, 1991). Linguistic (Heelas, 1996; Lakoff, 1987; Pennebaker, 1982) and psychophysiological research (Marchitelli & Levenson, 1992) suggests that the language we use to describe our emotions is associated with the underlying physiology. As Heelas (1996) has noted, virtually every culture uses bodily expressions to describe their emotional states, from “broken hearts” to “bad intestines,” with evidence from cross-national studies that there are consistencies in these bodily representations across cultures (Wallbott & Scherer, 1988).
Individuals differ greatly in the extent to which they are focused on and aware of their bodies. These kinds of individual differences have a long history in research in psychophysiology and psychosomatic disorders (Mandler, Mandler, Kremen, & Sholiton, 1961; Mandler, Mandler, & Uviller, 1958; Puente, Beiman, Doom, & Young, 1980) as well as in psychopathologies (e.g., excessive levels of body focus are seen in eating disorders, Mangweth et al., 2005; hypochondriasis, Olatunji, Deacon, Abramowitz, & Valentiner, 2007; and body dysmorphic disorder, Gupta, 2006). Attempts to quantify these individual differences in the empirical literature have focused on two main methodologies: (a) self-report questionnaires and (b) heartbeat tracking and detection tasks. Self-report questionnaires consist of items that ask about perceptions of bodily responses (Mandler et al., 1958; Miller, Murphy, & Buss, 1981; Shields, Mallory, & Simon, 1989). In heartbeat tracking tasks, participants are typically asked to count their heartbeats during fixed, brief time periods (Dale & Anderson, 1978; Schandry, 1981). In heartbeat detection tasks, participants are typically asked to indicate whether their heartbeats match the rates of external stimuli (typically, auditory tones) that are either synchronous or asynchronous with each heartbeat (Brener, Liu, & Ring, 1993; Davis, Langer, Sutterer, Gelling, & Marlin, 1986; Katkin, Morell, Goldband, Bernstein, & Wise, 1982).
Unfortunately, both reliability and validity of these measures remains controversial and, thus, none has emerged as the “gold standard” for measuring body awareness (Knapp-Kline & Kline, 2005; Pennebaker & Hoover, 1984). Moreover, estimates of body awareness derived from these various approaches have been shown to be virtually unrelated (Critchley, 2004; Pennebaker & Hoover, 1984; Whitehead, Drescher, Heiman, & Blackwell, 1977). Finally, the ecological validity of these measures is questionable. Namely, these approaches involve instructing people to self-rate their accuracy in attending to particular bodily functions or to monitor and detect a particular bodily function in a nonemotional state. The relationship between performance on these tasks and awareness of bodily sensations during actual emotion states has not been established.
The Present Study
The present study tests the hypothesis that individuals who are high in body awareness will show greater coherence between subjective emotional experience and physiological responding during emotion (see Figure 1). Testing this hypothesis requires deciding: (a) how best to measure coherence and (b) how best to measure body awareness. In terms of response coherence, we believe the case is strong for using the kind of within-individual approach we have used previously (Mauss et al., 2005) in which time-lagged correlations between continuous measures of subjective emotional experience and physiology are assessed. In terms of body awareness, the shortcomings in self-report measures and heartbeat tracking/detection tasks motivated us to try a different approach, seeking out individuals, who by virtue of specialized body-focused training, would likely have different levels of body awareness.
Figure 1.
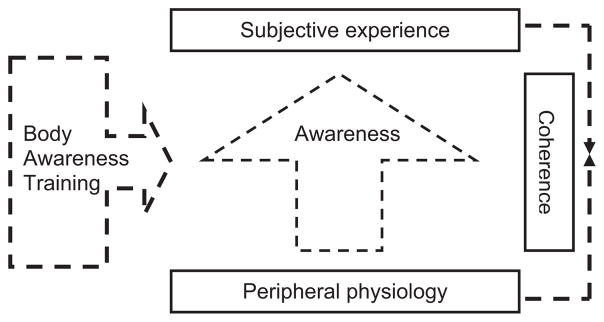
Model for the relationship between awareness of bodily sensations and coherence between subjective emotional experience and physiology.
We studied three groups of participants. To represent individuals high in body awareness with an emphasis on visceral response, we recruited Vipassana meditators. To represent individuals high in body awareness with an emphasis on somatic response, we recruited modern and ballet dancers. Finally, we also recruited a demographically matched control group with neither meditation, dance, nor other body-focused experience. Our primary hypothesis was that coherence between subjective experience and visceral activity (i.e., heart period) would be highest among Vipassana meditators (because of their training in attending to visceral responses), next highest among dancers (body focus, but somatic, not visceral), and lowest among controls.
Method
Participants
General demographics
Twenty-one Vipassana meditators (61.9% female), 21 dancers (61.9% female), and 21 controls (66.7% female) participated in the study. Participants’ ages ranged from 18 to 40 years. Participants were recruited by posting flyers at various locations in the San Francisco Bay Area, such as meditation centers, dance centers, and general stores, as well as by posting announcements online in meditation, dance, and community forums. The majority of the participants in this study were Caucasian (77.8%), followed by Asian (12.7%), Latino (3.2%), African American (1.6%), and other ethnicities (4.8%). While the three study groups were statistically comparable in terms of gender and ethnicity, Fs < 1, there was a group difference in age, F(2, 60) = 3.74, p < .05. Post hoc analyses revealed that meditators’ mean age in years (M = 31.1, SD = 5.22) was significantly higher than that of dancers (M = 26.6, SD = 6.14), t(40) = 2.6, p < .05. However, neither meditators nor dancers differed significantly in age from the control group (M = 27.6, SD = 5.68), ps > .05. Because of the age difference between meditators and dancers, age was included as a covariate in all subsequent analyses.
Vipassana meditators
To be included in this group, participants had to meet the following criteria: (a) minimum of two years of formal Vipassana meditation practice; (b) engage in daily practice regimen; and (c) attended at least one meditation retreat during the previous two years. On average, members of this group reported meditating for 7.1 years (SD = 3.75).
Vipassana meditation is a form of mindfulness-based meditation in which continuous, sustained observation of bodily sensations (particularly visceral sensations like breathing and heartbeats) is thought to result in greater levels of body awareness (Hart, 1987; Kabat-Zinn, 1990). Vipassana meditation has been found to have greater gray matter concentration in the right anterior insula, a region associated with interoceptive awareness (Hölzel et al., 2008). Furthermore, mindfulness-based meditation has been associated with improved performance on attentional measures, such as endogenous orienting and internally induced shifts in attention (Jha, Krompinger, & Baime, 2007), skills thought to be critically involved in body awareness (Craig, 2009; Vaitl, 1996).
Dancers
To be included in this group, participants had to meet the following criteria: (a) a minimum of two years of formal modern and/or ballet dance practice; (b) daily practice regimen; and (c) professional dance experience as a performer or teacher. On average, members of this group reported dancing for 15.9 years (SD = 7.44).1
Modern and ballet dancers are taught to cultivate heightened awareness of proprioceptive sensations from muscles, balance, and posture to guide and coordinate complex movements as they move through space (Aalten, 2004). Thus, their training is similar to meditation in that it emphasizes body awareness but differs in the focus of that awareness (more somatic in dancers, more visceral in meditators) and in its attentional quality (dancers must switch focus between time, music, space, and the body; meditators try for continuous attention to the body).
Controls
To be included in this group, participants had to meet the following criteria: (a) no formal meditation or dance training, and (b) no participation in other body-focused activities, such as yoga, Pilates, or professional sports.
Apparatus and Measures
Subjective emotional experience
Similar to our previous work on response coherence (Mauss et al., 2005), we used an affect rating dial (Levenson & Gottman, 1983) to obtain continuous ratings of subjective emotional experience. The dial had a pointer that moved over a 180-degree scale divided into nine divisions ranging from very negative (−4) to neutral (0) to very positive (+4). A computer sampled the dial position every 5 ms and averaged these readings into 1-s measurement periods using a program written by one of the authors (R.W.L.). Intensity of emotional experience was calculated as the absolute difference of the rating dial position from the 0 midpoint. This intensity measure was chosen on the basis of what is known about the relationship between cardiac activity and emotion (i.e., that heart period is affected by the intensity of both positive and negative emotion; Bradley & Lang, 1997) and given the nature of our stimuli (i.e., films that induced both positive and negative emotion).
Heart period
Heart period was measured continuously using a system consisting of a Grass Model 7 polygraph connected to a computer with analog signal processing capability. EKG electrodes were placed in a bipolar configuration on the participant’s torso. The same computer program that processed the rating dial data also calculated heart period (i.e., the time interval between successive R-waves on the EKG), with both measures averaged into 1-s measurement periods. As is typical in our laboratory, we monitored a broad range of physiological responses (skin conductance, finger pulse transmission time, ear pulse transmission time, respiration period, respiration depth, blood pressure, and general somatic activity) using standard recording procedures (see Shiota & Levenson, 2009, for a full description). For the present purposes, we focused our analyses on heart period because: (a) the heart is a powerful source of visceral sensations, (b) heart sensations are commonly reflected in emotion metaphors (e.g., pounding, broken, heavy), and (c) there is an extensive literature using heart rate and heart period in research on visceral awareness. For these reasons, we believed heart period was the physiological measure for which we had the strongest theoretical basis to expect differences in coherence as a function of body awareness group. The remaining physiological measures were examined in follow-up exploratory analyses.
Self-reported visceral awareness
Three different scales were used. The Private Body Consciousness subscale of the Body Consciousness Questionnaire (Miller et al., 1981) measures awareness of internal body sensations (e.g., “I am sensitive to internal bodily tensions”). The scale was administered using a 5-point Likert scale (1 = not true of me, 5 = very true of me). The Body Awareness Questionnaire (BAQ, Shields et al., 1989) measures awareness of body processes (e.g., “I notice distinct body reactions when I am fatigued”). A subset of this scale (8 of the 18 items) was administered using a 5-point Likert scale (1 = not true of me, 5 = very true of me).2 The Autonomic Perception Questionnaire—Revised (Mandler et al., 1958; Shields, 1984) measures perceptions of autonomic nervous system activity (e.g., “When I feel angry, I am aware of increased muscle tension in my body”). The scale was administered using a 7-point Likert scale (1 = not true of me, 7 = very true of me).
The scores on the three scales were significantly correlated (see Table 1) and thus, the items were combined into a single scale. The reliability of the resulting 43-item scale was high for the entire sample (α = .85), and for meditators (α = .79), dancers (α = .84), and controls (α = .88) analyzed separately.
Table 1.
Correlations Among Self-Report Visceral Awareness Measures
p < .05.
p < .01.
Other questionnaires
To determine whether the three groups differed in other ways, we administered: (a) Big Five Inventory-44 (BFI-44; Benet-Martinez & John, 1998), which assesses five broad personality dimensions (openness, conscientiousness, extraversion, agreeableness, neuroticism); (b) Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988), which assesses trait positive and negative affect; (c) Mindful Attention Awareness Scale (MAAS; Brown & Ryan, 2003), which assesses dispositional mindfulness; and (d) Wahler Physical Health Symptoms Inventory (WPHS; Wahler, 1968), which assesses physical symptoms such as headaches and heart trouble. All four of these measures have demonstrated adequate reliability and validity in prior studies.
Procedure
Participants completed the questionnaires online three to five days prior to the laboratory session. On arrival at the Berkeley Psychophysiology Laboratory, participants were seated in a chair in a well-lit 3 × 6 m room, and physiological recording devices were attached. The experimenters informed participants that we were interested in “learning about different aspects of people’s emotional experiences.” Emotional stimuli were shown on a 27-inch color TV monitor at a distance of 5.75 feet from the participant. During the 90-min experimental session, participants watched four films designed to induce alternating positive and negative emotional states. All films were preceded by a 1-min baseline, in which participants were asked to clear their minds and try to relax while focusing on a blank screen with a fixation X in the center. Participants received $50 for participating in the study.
Emotion-inducting films
Reflecting our definition of coherence as emotion-driven coordinated changes in response systems over time, films were designed to induce dynamic changes in emotional state, ranging from neutral to intensely positive and intensely negative. Each film consisted of two or three emotion-inducing scenes (length ranged from 55 s to 65 s) with 15 seconds of blank screen in between scenes. The emotional scenes were as follows: Film 1 (comedians doing a humorous improvisational skit, an underwater scene with sea creatures interacting, depiction of atrocities in Darfur); Film 2 (a man chewing cow intestines, an underwater scene with sea creatures interacting, comedian Bill Cosby doing stand-up); Film 3 (a woman reacting to news that her family members have died, an underwater scene with sea creatures interacting, a man injecting himself with a needle); and Film 4 (a violent scene in which a man crushes his victim’s head, an underwater scene with sea creatures interacting). Total lengths of the films were 3.75 min, 3.63 min, 3.70 min, and 2.58 min, respectively. Films 1 through 3 were presented in counterbalanced orders. Due to its violent nature, Film 4 was always shown last to avoid potential carry-over effects into the other trials.
Affect rating instructions
While watching each film, participants were instructed to rate their emotional experience continuously by adjusting the affect rating dial position as often as necessary so that it always reflected how positively or negatively they were feeling at the moment. In previous work, we have demonstrated that this kind of online rating can be done without unduly disrupting emotional responding (Mauss et al., 2005).
Data Analysis: Coherence Score Calculation
Second-by-second averages of rating dial position and heart period were determined for each film. Coherence between these two time series was calculated using a lagged cross-correlational analysis following procedures we have used previously (Mauss et al., 2005). For each participant during each film, we calculated the absolute value of the maximum cross-correlation between the rating dial and heart period data within lags of −10 to +10 s as a measure of the coherence between the intensity of subjective emotional experience and the duration of heart period. The absolute value was chosen because previous research has shown that changes in subjective emotional experience can be associated with either acceleration or deceleration in heart rate (Bradley & Lang, 1997). The time window of −10 to +10 s was chosen because we have used it in our past research on coherence (Mauss et al., 2005) and because it conforms to theoretical notions about the duration and temporal characteristics of emotional and physiological responses (Gratton, 2000; Kettunen, Ravaja, Näätänen, Keskivaara, & Keltikangas-Järvinen, 1998; Levenson, 2003a).3
Results
Overall Analysis Strategy
The overall design of the study was 3 × 2 × 3 × 4 (Group X Sex X Order X Film), with group, sex, and presentation order treated as between subject factors and film treated as a within subject factor. To control for group differences in age, age was centered on its grand mean (Aiken & West, 1991) and entered as a covariate in all analyses. ANCOVAs examining coherence estimates as the dependent variable were conducted on r (Pearson correlation) values, consistent with previous research analyzing coherence at a within-individual level of analysis (Mauss et al., 2005). When a significant main effect was found for body awareness group, we conducted a polynomial trend analysis and tested whether a linear or quadratic pattern best captured the effect of age. Because polynomial trend analyses capture overall patterns of difference across groups and not differences between groups, we also conducted Bonferroni-adjusted post hoc tests to identify differences between groups. For ease of interpretation, estimated marginal means (corrected for all covariates) are reported for all analyses of covariance (ANCOVAs). The p < .05 rejection level was used for all statistical tests and partial eta squares (representing the portion of explained variance in the dependent variable) are reported for each significant effect.
There were no significant main effects or interactions involving sex or presentation order; thus, in all reported analyses, we collapsed across both of these factors.
Manipulation Checks and Differences Among Meditators, Dancers, and Controls
Did the films induce variability in subjective emotional experience and heart period?
To determine whether the four films induced significant variability in subjective emotional experience and heart period, we first computed the standard deviations for the second-by-second subjective emotional experience and heart period data for each individual during the prefilm baseline and during the film. Three × 4 × 2 (Group X Film X Segment: prefilm, film) ANCOVAs were then conducted for both dependent variables.
For subjective experience, there was a significant interaction for Film X Segment, F(3, 177) = 7.43, p < .01, . Follow-up t tests revealed significantly greater variability during the film segment than during the prefilm segment for all four films, t(62) = 6.27, 6.89, 5.89, 6.66, respectively, ps < .01. For heart period, there was an overall main effect of segment, F(1, 177) = 98.50, p < .01, , revealing significantly greater variability during the film segment than during the prefilm segment across all four films. The interaction of Film X Segment, F(3, 177) = 1.72, p = .17, was not significant for heart period. Means and SDs for these analyses are presented in Table 2.
Table 2.
Means and Standard Deviations for Variability in Subjective Experience and Heart Period Before and During the Films
| Pre-film
|
Film
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean1 (SD)
|
Mean2 (SD)
|
Mean1 (SD)
|
Mean2 (SD)
|
|||||
| All | M | D | C | All | M | D | C | |
| Subjective experience | ||||||||
| Film 1 | .09 (.19) | .12 (.09) | .07 (.07) | .08 (.20) | 1.66 (.49) | 1.46 (.43) | 1.71 (.37) | 1.58 (.42) |
| Film 2 | .09 (.17) | 1.60 (.59) | ||||||
| Film 3 | .11 (.21) | 1.40 (.45) | ||||||
| Film 4 | .06 (.11) | 1.68 (.64) | ||||||
| Heart period | ||||||||
| Film 1 | 46.23 (23.71) | 43.58 (18.08) | 51.73 (19.90) | 42.67 (14.29) | 64.83 (26.07) | 62.64 (18.54) | 69.60 (28.02) | 58.83 (13.02) |
| Film 2 | 48.60 (23.49) | 64.79 (20.14) | ||||||
| Film 3 | 41.72 (21.37) | 56.04 (22.45) | ||||||
| Film 4 | 47.42 (24.09) | 69.09 (26.67) | ||||||
Note. M = Meditators; D = Dancers; C = Controls.
Means are presented collapsed across groups.
Means are presented collapsed across films.
To summarize, these analyses indicate that the films were successful in producing significant variability in self-reported emotion and in cardiac activity.
Did the groups differ in variability and reactivity in subjective emotional experience and heart period?
As shown in Table 2, results of the ANCOVAs described above indicated no main effect of group on variability for subjective emotional experience, F(2, 59) = 1.03, p = .36, ; or for heart period, F(2, 59) = 1.99, p = .15, . Furthermore, there were no significant interactions with group for subjective emotional experience, Group X Segment, F(2, 177) = 2.23, p =.12, , Group X Segment X Film, F(6, 177) = 1.20, p =.31, ; and for heart period, Group X Segment, F(2, 177) < 1, , and Group X Film X Segment, F(6, 177) = 2.12, p = .06, .
To determine whether the groups differed in subjective emotional reactivity and cardiac reactivity to the films, we computed reactivity scores (film average minus prefilm average) for subjective experience and heart period. Using 3 × 4 (Group X Film) ANCOVAs, there was no evidence found for group differences in reactivity for subjective experience, main effect of group: F(2, 59) = 1.43, p = .25, , Group X Film interaction: F(6, 177) = 1.67, p = .13, ; or for heart period, main effect of group: F(2, 59) = 2.24, p =.12, , Group X Film interaction, F(6, 177) < 1, . Means and SDs by group are found in Table 3.
Table 3.
Means and SDs for Reactivity in Subjective Experience and Heart Period to the Films
| Mean1 (SD)
|
|||
|---|---|---|---|
| Control | Dancers | Meditators | |
| Subjective experience | −.29 (.39) | −.36 (.43) | −.14 (.40) |
| Heart period | 3.19 (20.91) | .01 (20.31) | −11.34 (20.80) |
Means are presented collapsed across films.
To summarize, these analyses indicate that the three groups were equivalent in both variability and reactivity in subjective emotional experience and in cardiac reactivity.
Did the groups differ in self-reported visceral awareness?
To evaluate the efficacy of our strategy of using specialized training experience as a proxy for visceral awareness, we used a univariate ANCOVA to analyze group differences in self-reported visceral awareness on our composite measure. This revealed that the groups did differ in self-reported visceral awareness, F(2, 59) = 3.33, p < .05, . There was a linear relationship in visceral awareness, with meditators reporting highest levels, dancers reporting intermediary levels, and controls reporting the lowest self-reported visceral awareness, contrast estimate = .23, p < .05. The quadratic relationship was not significant, contrast estimate = .10, p = .28. Means and SDs for these analyses are presented in Table 4. Thus, the three groups showed the expected pattern of differences in visceral awareness as measured via questionnaires.
Table 4.
Means, Standard Deviations, and ANCOVA Analyses Comparing the Three Groups Across Trait and Health Factors
| Mean (SD)
|
F | p | |||
|---|---|---|---|---|---|
| Controls | Dancers | Meditators | |||
| Visceral awareness | −.12a (.46) | −.08a (.44) | .20b (.35) | 3.33 | 0.04 |
| Neuroticism | 2.60 (.66) | 2.77 (.74) | 2.61 (.80) | <1 | 0.58 |
| Extraversion | 3.26 (.76) | 3.55 (.73) | 3.33 (.75) | 1.34 | 0.27 |
| Openness to experience | 3.98 (.44) | 4.12 (.32) | 3.98 (.51) | 1.34 | 0.26 |
| Conscientiousness | 3.59 (.52) | 3.82 (.60) | 3.56 (.60) | 1.42 | 0.25 |
| Agreeableness | 3.99 (.45) | 3.94 (.63) | 3.88 (.38) | <1 | 0.87 |
| Trait positive affect | 3.33 (.49) | 3.44 (.43) | 3.23 (.48) | 1.2 | 0.31 |
| Trait negative affect | 1.86 (.51) | 1.94 (.65) | 1.77 (.35) | <1 | 0.61 |
| Trait mindfulness | 4.22 (.68) | 4.25 (.64) | 4.06 (.37) | <1 | 0.66 |
| Physical symptoms | 71.5 (19.2) | 79.7 (20.7) | 76.5 (22.8) | 1.3 | 0.29 |
Note. Age was controlled for in all analyses. Within each row, different subscripts denote significantly different means at p < .05. Bonferroni adjustments on pairwise comparisons served to protect an alpha level of .05.
Did the groups differ in trait measures and health?
Univariate ANCOVAs revealed that the three groups did not differ in personality (as assessed by the five subscales of the BFI-44), trait affect (as assessed by the PANAS), trait mindfulness (as assessed by the MAAS),4 or physical health (as assessed by the WPHS). Means, SDs, and statistical tests are presented in Table 4. Thus, the three groups did not differ in trait and health variables that could have obscured our primary interest in group differences in visceral awareness.
Testing the Primary Hypothesis: Groups With Greater Visceral Awareness Will Have Greater Coherence Between Subjective Experience and Heart Period
We examined group differences in our index of coherence between subjective experience and heart period using a 3 × 4 (Group X Film) ANCOVA. This analysis revealed that the main effect of group was significant, F(2, 59) = 3.42, p < .05, . As hypothesized, there was a significant linear relationship for group, contrast estimate = .05, p = .01, with meditators having the largest coherence, dancers have intermediate levels of coherence, and controls having the smallest coherence. The quadratic term for group was not significant, contrast estimate = .01, p = .59. Moreover, the Group X Film interaction was not significant, F(6, 177) = 1.26, p = .28, . Thus, there were no indications that the group differences in coherence differed across the four films. Although the groups differed in levels of coherence, it is important to note that all three groups demonstrated significant associations between subjective experience and heart period across the films (average rs for meditators = .35, average rs for dancers = .29, and average rs for controls = .27, ps < .05). Means, SDs, and pairwise comparisons among groups for coherence are presented in Table 5.5,6
Table 5.
Means and Standard Deviations for Maximum Cross-Correlations at Lags of +/− 10 S for Subjective Experience and Heart Period
| Mean (SD)
|
Mean1 (SD)
|
Mean2 (SD)
|
|||||
|---|---|---|---|---|---|---|---|
| C | D | M | All | C | D | M | |
| Film 1 | .24 (.12) | .23 (.11) | .28 (.19) | .25a (.14) | .27a (.05) | .29a (.09) | .35b (.12) |
| Film 2 | .26 (.15) | .27 (.16) | .35 (.19) | .29a (.17) | |||
| Film 3 | .24 (.12) | .29 (.14) | .26 (.17) | .26a (.14) | |||
| Film 4 | .34 (.13) | .37 (.24) | .50 (.17) | .40b (.20) | |||
Note. Within each row, different subscripts denote significantly different means at p < .05. Bonferroni adjustments on pairwise comparisons served to protect an alpha level of .05. C = Controls; D = Dancers; M = Meditators.
Means are presented collapsed across groups.
Means are presented collapsed across films.
Exploratory Analyses
Self-reported visceral awareness
Because the three groups differed in self-reported visceral awareness, we conducted several additional analyses using these data. First, we found that self-reported visceral awareness, by itself, was not a significant predictor of coherence, F(1, 61) < 1, . Additionally, there was no self-reported visceral awareness X Film interaction, F(1, 61) = 1.28, p = .28, . Second, examining this relationship by group, we found that self-reported visceral awareness was not significantly correlated with coherence (averaged across the four films) for any of the three groups, meditators, r(21) = .04, p = .89; dancers, r(21) = −.13, p = .69; controls, .r(21) = .29, p = .21. Third, we asked whether self-reported visceral awareness added significantly to the variance in coherence that was accounted for by training group. A stepwise analysis, in which age was entered in the first step, group in the second step, and self-reported visceral awareness in the third step revealed that self-reported visceral awareness did not account for additional variance beyond age and group membership, R2 increment = .002, F increment = .16, df = 59, p = .69.
Years of training
To determine whether differences in duration of training within our groups contributed to coherence, we conducted exploratory analyses separately for meditators and dancers. For both groups, coherence scores (averaged across the four films) were correlated positively but not significantly with years of training (for meditation training in the meditation group, r(21) = .21, p = .37; for dance training in the dance group, r(21) = .12, p = .61). Of course, this is not a definite evaluation of training effects, given our relatively small sample sizes and the restriction of range associated with our inclusion criteria.
Coherence between subjective emotional experience and other physiological measures
As noted earlier, we chose heart period as our physiological response for historical reasons and because the heart is a rich source of visceral information. To explore the relationships between subjective emotional experience and the other physiological measures, we calculated coherence scores for the other physiological measures collected in this study (skin conductance, finger pulse transmission time, ear pulse transmission time, respiration period, respiration depth, systolic blood pressure, diastolic blood pressure, and general somatic activity) using the same method described earlier.7 We then examined group differences in the coherence values averaged across measures. The group means were consistent with those we found for heart period, with meditators having the highest coherence (M = .32, SD = .06), dancers intermediary (M = .30, SD = .04), and controls lowest (M = .28, SD = .03). Consistent with this pattern, the linear relationship for group was significant, contrast estimate = .02, p < .05. However, the overall main effect of group was not significant, F(2, 59) = 2.23, p = .11, . We interpret this as providing some support for the conclusion that the differences in coherence we found between groups might extend to physiological measures other than heart period.
Discussion
The present study determined whether groups thought to differ in visceral awareness by virtue of specialized training would differ in the extent of emotion-related coherence between subjective emotional experience and heart period. In addition to using training as a proxy for body awareness, the study’s design (a) used film stimuli that proved to be effective in producing subjective emotional experience and physiological responding; (b) measured coherence at the within-individual level, which has the advantage over more common between-subjects approaches in that it assesses the extent that responses in multiple systems are associated within the same individual across time (Buck, 1980; Mauss et al., 2005; Stemmler, 1992); (c) used our rating dial methodology (Ruef & Levenson, 2007) to measure subjective emotional experience with the same temporal resolution as physiological responses; and (d) used time-lagged correlations (Mauss et al., 2005) to assess coherence, which accounts for different temporal characteristics of response systems.
Results indicated a linear relationship among the groups such that those with training in Vipassana meditation (which emphasizes attention to visceral activity) had the greatest coherence, followed by those with dance training (which emphasizes attention to somatic activity), followed by those with neither kind of training. Additional analyses revealed that the three groups did not differ in potentially confounding ways including reactivity to the films (heart period and subjective emotional experience) and self-reported personality, trait affect, trait mindfulness, and health. These findings lend support to our primary hypothesis that body awareness training thought to increase visceral awareness is associated with greater emotional response coherence.
A compelling alternative hypothesis for our findings posits that it is not the kind of training that our participants received that led to greater coherence but rather that it is the type of people who seek out these kinds of training. To evaluate this possibility, we compared group members in terms of personality characteristics, trait affect, trait mindfulness, health, and emotional reactivity. The fact that we found no group differences in these measures makes this alternative hypothesis less viable. Nonetheless, the possibility still remains that the groups differed in some yet unmeasured way that is associated with differences in coherence. Another alternative hypothesis is that training is associated with differences in level of emotional responding and that these create differences in the availability of visceral information. However, our training groups did not differ in the magnitude or variability of physiological or subjective emotional responding.
One way that our groups did differ was in terms of self-reported visceral awareness, with meditators reporting the highest levels, followed by dancers, and then by controls. These differences lend support to our rationale for designating the three groups on the basis of training, providing a “manipulation check” of sorts. Analyses using self-reported visceral awareness were also quite informative. Notably, considered by itself, self-reported visceral awareness was not significantly related to coherence. In addition, self-reported visceral awareness did not account for significant variance in coherence beyond that accounted for by training group. This pattern of findings raises the issue of whether the basis for our findings of higher coherence among meditators and dancers results from something other than increased visceral awareness or whether the self-report inventory we used is not a very accurate measure of this kind of visceral awareness. We think the latter is most likely. Based on the literature, self-report measures of visceral awareness seem most closely related to general concerns with bodily functioning and unrelated to objective criteria of body awareness (e.g., heartbeat detection tasks; for a review, see Mehling et al., 2009). In a broader sense, our estimates of visceral awareness, unlike those of other abilities (e.g., cognitive or physical abilities), are not subject to objective sources of feedback that would help us finely hone their accuracy against a standardized norm. That is not to say that those who meditate and dance do not have any basis for rating themselves as having greater visceral awareness than controls. What seems to be lacking is the kind of fine-grained accuracy that would produce within-group correlations with coherence.
Why Is Body Awareness Training Associated With Greater Coherence?
The linear pattern found between our groups in the extent of coherence is intriguing. Vipassana meditators, who had the highest coherence, receive training emphasizing attending to visceral responses, such as cardiac activity and respiration. Modern and ballet dancers, who had intermediate level of coherence, receive training that emphasizes attending to somatic cues (e.g., muscle tone, alignment, body position in space), rather than visceral activity. Normal controls, who had the lowest coherence, were selected so as to have neither meditation or dance training and to not have participated in other forms of body awareness training such as yoga or Pilates. We believe the linear relationship reflects the important role that organs controlled by the autonomic nervous system play in emotion and the critical contribution that afferent feedback from these organs plays in the construction of subjective emotional experience (Cacioppo, Berntson, & Klein, 1992; Damasio, 2000; James, 1884; Lakoff, 1987; Levenson, 1999, 2003a). Among autonomic organ systems, the heart plays a major role in providing metabolic support for emotion-related behavioral adaptations (e.g., for fighting and fleeing in anger and fear) and is a very rich source of visceral information.
Individuals who are trained to develop increased attention to and awareness of their visceral sensations should have access to more accurate information about the state of their viscera. Thus, when these individuals construct their subjective emotional experience, it should be more closely linked to and coherent with their actual physiological responding. The linear relationship found between body awareness training and coherence is consistent with this model.
Implications
Our findings lend support to the intuitive (but not well established) idea that body-related practices, such as meditation and dance, are associated with heightened body awareness and other important consequences. Most centrally, our findings bridge two important themes in emotion theory: response coherence and body awareness.
In terms of coherence, theorists have proposed that emotion imposes coherence across disparate response systems, thereby helping individuals respond to changing environmental demands in an organized, timely fashion (Ekman, 1992; Lazarus, 1991; Levenson, 1994; Tomkins, 1962). The present findings are consistent with this notion in that meditators, dancers, and controls all demonstrated significant associations between subjective experience and physiology. Although certainly not definitive, the low-moderate to moderate effect sizes (Cohen, 1988) associated with these correlations and the differences between groups is consistent with a view that there is a certain amount of coherence built into our emotion system but that this may also be increased by specialized body-focused training.
Furthermore, in terms of body awareness, theorists propose that information from our viscera shapes our subjective emotional experience (Damasio, 2000; James, 1884; Lakoff, 1987; Levenson, 1999, 2003a). It is interesting to note that body awareness training was not associated with differences in emotional reactivity (i.e., levels of variability and reactivity in subjective and heart period response induced by emotional films) but only with the relationship between these two systems.
In these ways, our results help inform the role of both coherence and body awareness in emotional experience, and they lend further support to emotion theories that emphasize the interaction between these processes. In the present study, our findings connect the two literatures by demonstrating that those who have been trained to attend to bodily information (especially visceral information) have greater coherence between subjective experience and visceral responses during emotional episodes.
In our earlier work, we noted that there was a high degree of individual variability in the extent of coherence (Mauss et al., 2005). The present study suggests one possible source of this variation but does not address the larger question of the implications that these individual differences have for adaptive functioning. We are not aware of any published research that has examined the consequences and/or correlates of individual differences in coherence, measured in the within-individual manner we used here. However, lack of coherence between emotion response systems assessed using the between-subjects approach has been associated with a number of negative outcomes, including schizophrenia (lack of coherence between self-reported emotion and behavioral expression; Kring & Neale, 1996), alexithymia (lack of coherence between self-reported emotion and physiological response; Stone & Nielson, 2001), and a repressive coping style (lack of coherence between self-reported anxiety and physiological and behavioral responses; Weinberger, Schwartz, & Davidson, 1979). Consistent with this theme, in an unpublished study using the within-subject approach, greater coherence was associated with increased positive affect and well-being (Mauss et al., in preparation). Thus, the implications of individual differences in coherence (hopefully including assessment of coherence in its within-subject form) are clearly an intriguing area for future investigation.
Limitations
There were three main limitations of this study that will be important to address in future research. First, because individuals were not randomly assigned to training groups, the question remains whether heightened coherence resulted from the body awareness training or whether people who are drawn to such training already had heightened coherence. Random assignment and longitudinal designs that assess coherence before and after training would be most helpful in future studies. Second, because we wanted to induce a broad range of emotions to ensure variability across time, our design did not enable us to focus on the question of how specific emotions might affect coherence and how these might interact with body awareness training. For example, it is quite possible that for low-arousal emotions, such as sadness and contentment, we would find lower levels of coherence and smaller differences among groups. In fact, we had some indications of this from our own data, in which one film (Film 4) that included a particularly violent, high-arousal segment was associated with higher levels of coherence than the other films. In future studies, it would be useful to examine systematically emotions that differ in kind and arousal level. Third, our results focused on coherence between subjective emotional experience and one particular aspect of physiological response (i.e., heart period). Our finding of a consistent pattern (albeit marginally significant) across a number of other physiological measures is encouraging, but this question is far from settled. In future studies, it will be important to assess the role of other autonomic systems that are important sources of visceral information (e.g., gastrointestinal) as well as considering somatic systems and associated proprioceptive feedback.
Conclusion
This study bridges two important areas of emotion research, response coherence and body awareness. Using a within-individual approach for assessing coherence, the hypothesis was supported that body awareness training is associated with greater coherence between subjective emotional experience and heart period during film-induced emotional episodes. Moreover, this association was strongest in those whose training emphasizes visceral awareness (Vipassana meditation), intermediate in those whose training emphasizes somatic awareness (modern dance and ballet), and weakest in those who have neither kind of training. These findings also provide more nuanced support for theories of emotion that posit that emotions help organize disparate response systems and for those that posit an important role for body sensations in the construction of emotional experience.
Footnotes
In recruiting meditators and dancers, a primary goal was to find participants with as much training experience as possible in order to illuminate differences among groups along our variable of interest (i.e., body awareness training). However, because most experienced dancers begin dance training as children, and most experienced meditators begin meditation training as adolescents or adults, balancing groups in terms of both years of training and age (i.e., finding experienced meditators with as many years of training but as young as dancers) was a challenge. Thus, unsurprisingly, in the present sample, dancers had more years of training than meditators, t(40) = 4.85, p < .01. It is possible that if we were to examine meditators with as many years of training as our dancers, the group differences in coherence would have been even greater than those found in the present sample.
Items that included content about food or exercise (e.g., “I notice differences in the way my body reacts to various foods,” and “When my exercise habits change, I can predict very accurately how that will affect my energy level”) were removed in keeping with an a priori decision that these items might be confounded by group differences in attitudes toward food and exercise.
As we have noted (Mauss et al., 2005), there are other ways of assessing coherence using time series methods (e.g., autoregression, ARIMA). However, such techniques typically require stationarity of the data. Given that one of the primary goals of this study was to induce a range of emotional experiences across time (i.e., nonstationarity), cross-correlations were deemed the optimal analysis technique because they are least affected by nonstationarity of the data (Mauss et al., 2005).
While some research has found mindfulness meditation to be associated with increased mindfulness scores on the MAAS (Brown & Ryan, 2003), other research has found no association (MacKillop & Anderson, 2007). Recently, the face validity of this measure in assessing mindfulness from a Buddhist meditation perspective has been called into question (Rosch, 2007). Therefore, it is possible that the lack of group differences in trait mindfulness may be explained by inadequate measurement or lack of power, rather than an actual lack of group differences in trait mindfulness.
Lag times for which the maximum cross-correlation between subjective experience and heart period occurred were not a primary focus of the present study; however, these measures are helpful in characterizing the data. Therefore, we conducted a 3 × 4 (Group X Film) ANCOVA on the absolute value of lag times for each participant during each film. Results indicated no group differences in lags, Mmeditators = 4.33, Mdancers = 4.88, Mcontrols = 5.41, SDmeditators = 1.79, SDdancers = 1.55, SDcontrols = 1.56, main effect of group: F(2, 59) = 2.16, p =.13, ; Group X Film interaction: F(6, 177) < 1, . Furthermore, lags did not differ by film, F(3, 177) < 1, .
To examine whether other variables could account for the main effect of group, we entered the variability, reactivity, and individual difference variables into the analysis separately as covariates (in addition to including age as a covariate, consistent with all group analyses). None significantly altered the main effect of group (Fs ranged between 3.09 and 4.39, p values between .01 and .05).
Reliabilities for this composite across physiological measures were adequate for each film, alphas =.78, .75, .60, .62, for Films 1 through 4, respectively.
References
- Aalten A. “The Moment When it All Comes Together”: Embodied experiences in ballet. European Journal of Women’s Studies. 2004;11(3):263–276. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Barrett LF. The relationships among momentary emotion experiences, personality descriptions, and retrospective ratings of emotion. Personality and Social Psychology Bulletin. 1997;23(10):1100–1110. [Google Scholar]
- Barrett LF. Are emotions natural kinds? Perspectives on Psychological Science. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Benet-Martinez V, John OP. Los Cinco Grandes across cultures and ethnic groups: Multitrait multimethod analyses of the Big Five in Spanish and English. Journal of Personality and Social Psychology. 1998;75(3):729–750. doi: 10.1037//0022-3514.75.3.729. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge, England: Cambridge University Press; 1997. pp. 581–607. [Google Scholar]
- Brener J, Liu X, Ring C. A method of constant stimuli for examining heartbeat detection: Comparison with the Brener-Kluvitse and Whitehead methods. Psychophysiology. 1993;30(6):657–665. doi: 10.1111/j.1469-8986.1993.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: The role of mindfulness in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Buck R. Nonverbal communication of affect in preschool in children: Relationships with personality and skin conductance. Journal of Personality and Social Psychology. 1977;35(4):225–236. doi: 10.1037//0022-3514.35.4.225. [DOI] [PubMed] [Google Scholar]
- Buck R. Nonverbal behavior and the theory of emotion: The facial feedback hypothesis. Journal of Personality and Social Psychology. 1980;38(5):811–824. doi: 10.1037//0022-3514.38.5.811. [DOI] [PubMed] [Google Scholar]
- Buck R, Miller RE, Caul WF. Sex, personality, and physiological variables in the communication of affect via facial expression. Journal of Personality and Social Psychology. 1974;30(4):587–596. doi: 10.1037/h0037041. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Klein DJ. What is an emotion? The role of somatovisceral afference, with special emphasis on somatovisceral “illusions. Review of Personality and Social Psychology. 1992;14:63–98. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Craig AD. How do you feel – now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proceedings of the National Academy of Sciences, USA of the United States of America. 2004;101(17):6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dale A, Anderson D. Information variables in voluntary control and classical conditioning of heart rate: Field dependence and heart-rate perception. Perceptual and Motor Skills. 1978;47(1):79–85. doi: 10.2466/pms.1978.47.1.79. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: Emotion, reason, and the human brain. New York, NY: Quill; 2000. [Google Scholar]
- Damasio AR. Looking for Spinoza: Joy, sorrow, and the feeling brain. New York, NY: Harcourt; 2003. [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. London, England: Murray; 1872. [Google Scholar]
- Davis MR, Langer AW, Sutterer JR, Gelling PD, Marlin M. Relative discriminability of heartbeat-contingent stimuli under three procedures for assessing cardiac perception. Psychophysiology. 1986;23(1):76–81. doi: 10.1111/j.1469-8986.1986.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Duckworth KL, Bargh JA, Garcia M, Chaiken S. The automatic evaluation of novel stimuli. Psychological Science. 2002:513–519. doi: 10.1111/1467-9280.00490. [DOI] [PubMed] [Google Scholar]
- Ekman P. Are there basic emotions? Psychological Review. 1992;99(3):550–553. doi: 10.1037/0033-295x.99.3.550. [DOI] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: Emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;58:342–353. [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Ancoli S. Facial signs of emotional experience. Journal of Personality and Social Psychology. 1980;39(6):1125–1134. [Google Scholar]
- Fernández-Dols JM, Sánchez F, Carrera P, Ruiz-Belda MA. Are spontaneous expressions and emotions linked? An experimental test of coherence. Journal of Nonverbal Behavior. 1997;21(3):163–177. [Google Scholar]
- Fridlund AJ. Sociality of solitary smiling: Potentiation by an implicit audience. Journal of Personality and Social Psychology. 1991;60(2):229–240. [Google Scholar]
- Gottman JM, Levenson RW. A valid procedure for obtaining self-report of affect in marital interaction. Journal of Consulting and Clinical Psychology. 1985;53(2):151–160. doi: 10.1037//0022-006x.53.2.151. [DOI] [PubMed] [Google Scholar]
- Gratton G. Biosignal processing. Handbook of Psychophysiology. 2000;2:900–923. [Google Scholar]
- Gupta MA. Somatization disorders in dermatology. International Review of Psychiatry Special Issue: Somatoform Disorders. 2006;18(1):41–47. doi: 10.1080/09540260500466832. [DOI] [PubMed] [Google Scholar]
- Hart W. The art of living: Vipassana meditation as taught by SN Goenka. San Francisco, CA: HarperOne; 1987. [Google Scholar]
- Heelas P. Emotion talk across cultures. In: Parrott GW, Harré R, editors. The emotions: Social, cultural and biological dimensions. London, England: Sage Publications; 1996. pp. 171–199. [Google Scholar]
- Hohmann GW. Some effects of spinal cord lesions on experienced emotional feelings. Psychophysiology. 1966;3(2):143–156. doi: 10.1111/j.1469-8986.1966.tb02690.x. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs E, Mansteaed A, Fischer A. Social context effects on facial activity in a negative emotional setting. Emotion. 2001;1(1):51–69. doi: 10.1037/1528-3542.1.1.51. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective & Behavioral Neuroscience. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living. New York, NY: Delacorte Press; 1990. [Google Scholar]
- Kahneman D, Tversky A. Experienced utility and objective happiness: A moment-based approach. The Psychology of Economic Decisions. 2003;1:187–208. [Google Scholar]
- Katkin ES, Morell MA, Goldband S, Bernstein GL, Wise JA. Individual differences in heartbeat discrimination. Psychophysiology. 1982;19(2):160–166. doi: 10.1111/j.1469-8986.1982.tb02538.x. [DOI] [PubMed] [Google Scholar]
- Kettunen J, Ravaja N, Näätänen P, Keskivaara P, Keltikangas-Järvinen L. The synchronization of electrodermal activity and heart rate and its relationship to energetic arousal: A time series approach. Biological Psychology. 1998;48(3):209–225. doi: 10.1016/s0301-0511(98)00017-9. [DOI] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: Slow and steady wins the race. Biological Psychology. 2005;69(3):387–396. doi: 10.1016/j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Kring A, Neale J. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? Journal of Abnormal Psychology. 1996;105(2):249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- Lacey JI. Somatic response patterning and stress: Some revisions of activation theory. In: Appley MH, Trumbull R, editors. Psychological Stress: Issues in Research. New York, NY: Appleton Century Crofts; 1967. pp. 14–37. [Google Scholar]
- Lakoff G. Women, fire, and dangerous things: What categories reveal about the mind. Chicago, IL: University of Chicago Press; 1987. [Google Scholar]
- Lanzetta JT, Cartwright-Smith J, Kleck RE. Effects of nonverbal dissimulation on emotional experience and autonomic arousal. Journal of Personality and Social Psychology. 1976;33(3):354–370. doi: 10.1037//0022-3514.33.3.354. [DOI] [PubMed] [Google Scholar]
- Lanzetta JT, Kleck RE. Encoding and decoding of nonverbal affect in humans. Journal of Personality and Social Psychology. 1970;16(1):12–19. doi: 10.1037/h0029850. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: An unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cognition & Emotion. 1992;6(5):321–338. doi: 10.1080/02699939208409689. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Emotion and adaptation. New York, NY: Oxford University Press; 1991. [Google Scholar]
- Lazarus RS, Speisman J, Mordkoff A. The relationship between autonomic indicators of psychological stress: Heart rate and skin conductance. Psychosomatic Medicine. 1963;25(1):19–30. [Google Scholar]
- Levenson RW. Human emotion: A functional view. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. Oxford, England: Oxford University Press; 1994. pp. 123–126. [Google Scholar]
- Levenson RW. The intrapersonal functions of emotion. Cognition & Emotion. 1999;13(5):481–504. [Google Scholar]
- Levenson RW. Blood, sweat, and fears: The autonomic architecture of emotion. Annals of the New York Academy of Sciences. 2003a;1000:348–366. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Autonomic specificity and emotion. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. New York, NY: Oxford University Press; 2003b. pp. 212–224. [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45(3):587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Anderson EJ. Further psychometric validation of the Mindful Attention Awareness Scale (MAAS) Journal of Psychopathology and Behavioral Assessment. 2007;29(4):289–293. [Google Scholar]
- Mandler G, Mandler JM, Kremen I, Sholiton RD. The response to threat: Relations among verbal and physiological indices. Psychological Monographs. 1961;75:22. [Google Scholar]
- Mandler G, Mandler JM, Uviller E. Autonomic feedback: The perception of autonomic activity. Journal of Abnormal Psychology. 1958;56(3):367–373. doi: 10.1037/h0048083. [DOI] [PubMed] [Google Scholar]
- Mangweth B, Hausmann A, Danzl C, Walch T, Rupp CI, Biebl W, Pope HG., Jr Childhood body-focused behaviors and social behaviors as risk factors of eating disorders. Psychotherapy and Psychosomatics. 2005;74(4):247–253. doi: 10.1159/000085149. [DOI] [PubMed] [Google Scholar]
- Marchitelli L, Levenson RW. When couples converse: The language and physiology of emotion. Presented at the Society for Psychophysiological Research; San Diego, CA. 1992. [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotional experience, behavior, and autonomic physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Shallcross AJ, Troy AS, Ferrer E, John OP, Wilhelm FH, Gross JJ. Don’t hide your happiness! Positive emotion dissociation, social connectedness, and psychological functioning. doi: 10.1037/a0022410. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Wilhelm FH, Gross JJ. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition & Emotion. 2004;18(5):631–642. [Google Scholar]
- Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, Stewart A. Body awareness: Construct and self-report measures. PLoS One. 2009;4(5) doi: 10.1371/journal.pone.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, Murphy R, Buss AH. Consciousness of body: Private and public. Journal of Personality and Social Psychology. 1981;41(2):397–406. [Google Scholar]
- Nesse RM, Curtis GC, Thyer BA, McCann DS, Huber-Smith MJ, Knopf RF. Endocrine and cardiovascular responses during phobic anxiety. Psychosomatic Medicine. 1985;47(4):320–332. doi: 10.1097/00006842-198507000-00002. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316(5827):1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Notarius CI, Levenson RW. Expressive tendencies and physiological response to stress. Journal of Personality and Social Psychology. 1979;37(7):1204–1210. doi: 10.1037//0022-3514.37.7.1204. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Deacon BJ, Abramowitz JS, Valentiner DP. Body vigilance in nonclinical and anxiety disorder samples: Structure, correlates, and prediction of health concerns. Behavior Therapy. 2007;38(4):392–401. doi: 10.1016/j.beth.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. The psychology of physical symptoms. New York, NY: Springer-Verlag; 1982. [Google Scholar]
- Pennebaker JW, Hoover CW. Visceral perception versus visceral detection: Disentangling methods and assumptions. Applied Psychophysiology and Biofeedback. 1984;9(3):339–352. doi: 10.1007/BF00998977. [DOI] [PubMed] [Google Scholar]
- Puente AE, Beiman IH, Doom W, Young C. Relationship between physiological and self-reported stressful response and psychosomatic disorders. Perceptual and Motor Skills. 1980;50(2):463–466. doi: 10.1177/003151258005000216. [DOI] [PubMed] [Google Scholar]
- Rosch E. More than mindfulness: When you have a tiger by the tail, let it eat you. Psychological Inquiry. 2007;18(4):258–264. [Google Scholar]
- Rosenberg EL, Ekman P. Coherence between expressive and experiential systems in emotion. In: Ekman P, Rosenberg EL, editors. What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System (FACS. New York, NY: Oxford University Press; 1997. pp. 63–88. [Google Scholar]
- Ruef AM, Levenson RW. Continuous measurement of emotion. In: Coan J, Allen J, editors. Handbook of emotion elicitation and assessment. New York, NY: Oxford University Press; 2007. pp. 286–297. [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Scherer KR. On the nature and function of emotion: A component process approach. In: Scherer Klaus R, Ekman Paul., editors. Approaches to emotion. Hillsdale, NJ: Erlbaum; 1984. pp. 293–317. [Google Scholar]
- Shields SA. Reports of bodily change in anxiety, sadness, and anger. Motivation and Emotion. 1984;8(1):1–21. [Google Scholar]
- Shields SA, Mallory ME, Simon A. The Body Awareness Questionnaire: Reliability and validity. Journal of Personality Assessment. 1989;53(4):802–815. [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24(4):890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmler G. The vagueness of specificity: Models of peripheral physiological emotion specificity in emotion theories and their experimental discriminability. Journal of Psychophysiology. 1992;6(1):17–28. [Google Scholar]
- Stepper S, Strack F. Proprioceptive determinants of emotional and nonemotional feelings. Journal of Personality and Social Psychology. 1993;64(2):211–220. [Google Scholar]
- Stone LA, Nielson KA. Intact physiological responses to arousal with impaired emotional recognition in alexithymia. Psychotherapy and Psychosomatics. 2001;70(2):92–102. doi: 10.1159/000056232. [DOI] [PubMed] [Google Scholar]
- Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: A nonobtrusive test of the facial feedback hypothesis. Journal of Personality and Social Psychology. 1988;54(5):768–777. doi: 10.1037//0022-3514.54.5.768. [DOI] [PubMed] [Google Scholar]
- Tom G, Pettersen P, Lau T, Burton T, Cook J. The role of overt head movement in the formation of affect. Basic and Applied Social Psychology. 1991;12(3):280–289. [Google Scholar]
- Tomkins SS. Affect, imagery, consciousness: I. The positive affects. Oxford, England: Springer; 1962. [Google Scholar]
- Tomkins SS. Affect theory. In: Scherer KR, Ekman P, editors. Approaches to emotion. Hillsdale, NJ: Erlbaum; 1984. pp. 163–196. [Google Scholar]
- Vaitl D. Interoception. Biological Psychology. 1996;42(1–2):1–27. doi: 10.1016/0301-0511(95)05144-9. [DOI] [PubMed] [Google Scholar]
- Wahler HJ. The Physical Symptoms Inventory: Measuring levels of somatic complaining behavior. Journal of Clinical Psychology. 1968;24(2):207–211. doi: 10.1002/1097-4679(196804)24:2<207::aid-jclp2270240223>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wallbott HG, Scherer KR. How universal and specific is emotional experience? Evidence from 27 countries on five continents. In: Scherer KR, editor. Facets of emotion: Recent research. Vol. 25. Hillsdale, NJ: Erlbaum, Inc; 1988. pp. 31–56. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberger DA, Schwartz GE, Davidson RJ. Low-anxious, high-anxious, and repressive coping styles: Psychometric patterns and behavioral and physiological responses to stress. Journal of Abnormal Psychology. 1979;88(4):369–380. doi: 10.1037//0021-843x.88.4.369. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P, Blackwell B. Relation of heart rate control to heartbeat perception. Applied Psychophysiology and Biofeedback. 1977;2(4):371–392. [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT. The somatic symptom paradox in DSM–IV anxiety disorders: Suggestions for a clinical focus in psychophysiology. Biological Psychology. 2001;57(1–3):105–140. doi: 10.1016/s0301-0511(01)00091-6. [DOI] [PubMed] [Google Scholar]


