Abstract
BACKGROUND & AIMS
The final step in bile acid synthesis involves conjugation with glycine and taurine, which promotes a high intraluminal micellar concentration to facilitate lipid absorption. We investigated the clinical, biochemical, molecular, and morphologic features of a genetic defect in bile acid conjugation in 10 pediatric patients with fat-soluble vitamin deficiency, some with growth failure or transient neonatal cholestatic hepatitis.
METHODS
We identified the genetic defect that causes this disorder using mass spectrometry analysis of urine, bile, and serum samples, and sequence analysis of the genes encoding bile acid-CoA:amino acid N-acyltransferase (BAAT) and bile acid-Co A ligase (SLC27A5).
RESULTS
Levels of urinary bile acids were increased (432±248 μmol/L) and predominantly excreted in unconjugated forms (79.4%±3.9%), and as sulfates and glucuronides. Glycine or taurine conjugates were absent in the urine, bile and serum. Unconjugated bile acids accounted for 95.7%±5.8% of the bile acids in duodenal bile, with cholic acid accounting for 82.4%±5.5% of total. Duodenal bile acid concentrations were 12.1±5.9 mmol/L—a concentration too low for efficient lipid absorption. The biochemical profile was consistent with defective bile acid amidation. Molecular analysis of BAAT confirmed 4 different homozygous mutations in 8 patients tested.
CONCLUSIONS
Based on a study of 10 pediatric patients, genetic defects that disrupt bile acid amidation cause fat-soluble vitamin deficiency and growth failure, indicating the importance of bile acid conjugation in lipid absorption. Some patients developed liver disease with features of a cholangiopathy. These findings indicate that patients with idiopathic neonatal cholestasis or later onset of unexplained fat-soluble vitamin deficiency should be screened for defects in bile acid conjugation.
Keywords: Chronic Liver Disease, Hepatic, Inherited, Nutrient, Bile acid conjugation, Bile acid-CoA amino acid N-acyltransferase, BAAT, Glycine, Taurine, Mass Spectrometry, Cholestasis, Fat-soluble vitamin deficiency
INTRODUCTION
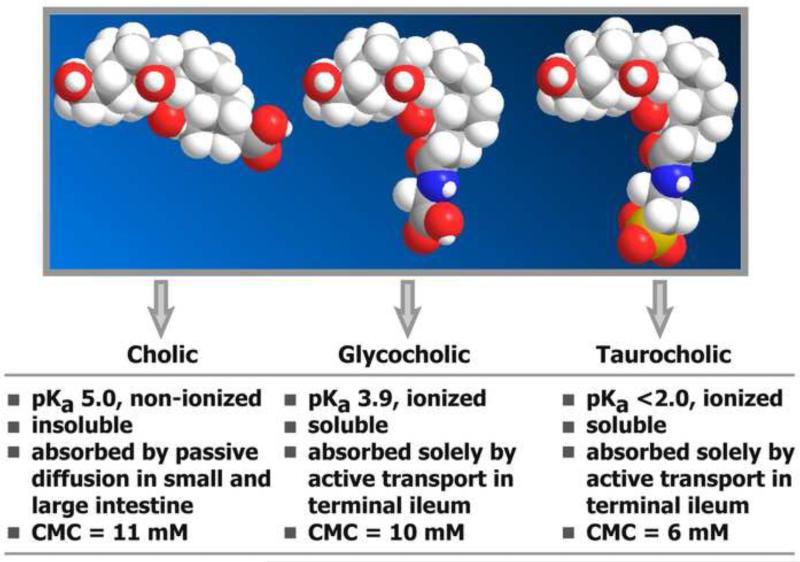
Hepatic bile acid conjugation with the amino acids glycine and taurine represents the final step in primary bile acid synthesis in humans1. The liver has a high capacity for conjugation and as a result negligible amounts of unconjugated bile acids (<2%) typically appear in bile under normal or cholestatic conditions2. Conjugation significantly alters the physicochemical characteristics of an unconjugated bile acid, by increasing the molecular size (Fig. 1) and lowering the pKa, thus enhancing aqueous solubility at the pH of the proximal intestine and preventing non-ionic passive absorption3. Conjugation thus promotes a high intraluminal bile acid concentration and therefore efficient solubilization of lipids with low aqueous solubility such as saturated fatty acids and fat-soluble vitamins. Two enzymes catalyze the reactions leading to bile acid amidation. A CoA thioester is first formed by the rate-limiting bile acid-CoA ligase enzyme (BACL; encoded by SLC27A5)4, 5, and then the amino acids, glycine or taurine, are coupled to the carboxyl group of the bile acid in a reaction catalyzed by a cytosolic bile acid-CoA:amino acid N-acyltransferase (BAAT; encoded by BAAT)6.
Figure 1.
Space-filling models showing the influence of bile acid conjugation with glycine and taurine on the size of the molecule, its physicochemical properties, and physiological features.
In 1994 we first described in a preliminary report a defect in bile acid amidation in a 14-year-old boy with fat malabsorption and fat-soluble vitamin deficiency7. This child presented in the first 3 months of life with conjugated hyperbilirubinemia, elevated serum transaminases, and a normal gamma-glutamyl transpeptidase (GGT). Two other patients, a 5-year-old Saudi Arabian boy and his 8-year-old sister, the products of a consanguineous marriage, were later identified with the same bile acid defect. Remarkably, the boy had undergone a portoenterostomy for a diagnosis of “extrahepatic biliary hypoplasia”, while his sister was reportedly asymptomatic. We have now identified a bile acid conjugation defect in 10 patients with clinical histories of normal or mildly elevated liver chemistries, but with a severe fat-soluble vitamin deficiency, often resulting in coagulopathy and rickets. The main feature, fat-soluble vitamin deficiency, occurs because of reduced biliary secretion of conjugated bile acids and an inability to form mixed micelles because of rapid passive absorption of unconjugated cholic acid in the proximal small intestine. The recognition that genetic defects in bile acid synthesis are associated with unexplained fat-soluble vitamin deficiency warrants a concerted effort to explore this patient population for these disorders. This report describes the clinical, biochemical and molecular features of defective bile acid conjugation in the largest cohort of patients thus far reported.
EXPERIMENTAL
Clinical descriptions of patients
Demographics and presentations of 10 patients from 7 families are summarized below and in Table 1, with more detail in Supplemental online data:
Table 1.
Summary of the demographics, main features, and clinical findings of 10 patients with a confirmed bile acid conjugation defect and features of their urine and duodenal bile acids
| Patient # | Gender | Age at diagnosis | Consanguinity | Origin/Ethnicity | Liver | Serum AST /ALT | Serum direct bilirubin | Serum fat-soluble vitamin levels | Body weight (Percentile) | Bone |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | M | 14 y | Not known | Laotian | Hepatomegaly | Normal | Elevated | Low | <5th | Rickets |
| #2 | M | 4y | Yes | Saudi Arabia/Asian |
Hepatomegaly - portoenterostomy |
Elevated | Elevated | Low | normal | Rickets with bone fracture |
| #3 | F | 8 y | Yes | Saudi Arabia/Asian |
- | Normal | - | Low | <50th | Rickets with fractures |
| #4 | F | 1 y | No | USA/Hispanic | Normal | Elevated | Normal | Low | <3rd | Rickets |
| #5 | M | 3 months | Yes | USA/Hispanic | Liver failure/OLT | Elevated | Elevated | - | 75th ^ | - |
| #6 | F | 11 y | Yes | USA/Hispanic | Normal | Normal | Normal | Low | 50th | - |
| #7 | F | 10 y | Yes | USA/Hispanic | Normal | Normal | Normal | Low | 10th | - |
| #8 | F | 3 months | Not known | USA/Amish | Normal | Normal | Normal | Low | 25th | - |
| #9 | M | 6 months | No | USA/Hispanic | Hepatomegaly | Elevated | Elevated | Low | <3rd | Rickets |
| #10 | F | 6.5 y | Not known | USA/Caucasian | - | - | - | <5th | - |
| Patient # | Urine FAB-MS analysis: Amidated bile acids (Present/Absent) | Total urinary bile acid concentration (μmol/L) | % Unconjugated bile acids in urine | % Cholic acid In urine | Total Duodenal bile acid concentration (μmol/L) | % Unconjugated biliary acids | % Cholic acid in bile |
|---|---|---|---|---|---|---|---|
| #1 | Absent | 217 | 80.8% | 65.5% | 5,528 | 99.7% | 95.1% |
| #2 | Absent | 1,111 | 64.9% | 59.2% | 35,806 | 99.9% | 58.7% |
| #3 | Absent | - | - | - | - | - | - |
| #4 | Absent | 173 | 80.6% | 56.5% | 76 | 92.0% | 76.9% |
| #5 | Absent | 153 | 78.8% | 50.7% | 472 | 82.6% | 85.8% |
| #6 | Absent | 135 | 72.4% | 49.6% | 3,023 | 97.1% | 92.0% |
| #7 | Absent | - | - | - | 24.083 | 98.2% | 93.3% |
| #8 | Absent | 82.5 | 95.7% | 79.0% | 23,509 | 99.4% | 94.0% |
| #9 | Absent | - | - | - | - | - | - |
| #10 | Absent | 1156 | 82.7% | 23.7% | 3,997 | 96.8% | 63.3% |
Footnote
Body weight at birth and 3½ months age
Evaluation of jaundice and anemia at age 40 days in Patient #1, male, born at term (2.6 kg) to parents not identifying as consanguine, found alpha-thalassemia. A prolonged prothrombin time (PT) at age 5 months responded to fresh-frozen plasma and vitamin K. Severe anemia, congestive heart failure, pulmonary edema, and rickets with a proximal fibula fracture were recorded at 1 year. Marked growth retardation and hepatosplenomegaly at age 14 years prompted re-evaluation, with bile acid analysis by mass-spectrometry.
On evaluation of jaundice with acholic stools at age 28 days in Patient #2, male, born at term (3.3 kg) to first-cousin parents with two nominally well children, sonography found no gallbladder and cholescintigraphy no contrast excretion; an intraoperative cholangiogram was interpreted as consonant with biliary atresia. Jaundice did not resolve with portoenterostomy. At 7 months rickets was diagnosed, with a right humerus fracture and low serum vitamins D and E. Bile acid analysis of urine was performed at 4 years, with follow-up liver biopsy.
Urine and serum from Patient #3, the 8 year-old full sister of Patient #2, were screened for a bile acid synthetic defect by mass spectrometry although family members considered her healthy; she was later found to have had rickets at age 6 months and fractures aged 3 years. At 12 years, serum vitamin E was low without hypocoagulability, other hypovitaminosis, or other clinical-biochemistry test-result abnormality.
Immunizations at age 4 months in Patient #4, female, born at 36 weeks’ gestation (2.3 kg) to parents not identifying as consanguine and with one well child, immediately produced large ecchymoses. A prolonged PT responded to fresh-frozen plasma and vitamin K. Serum vitamins D and E were low, with rickets on imaging; serum vitamin A was normal, without other clinical/biochemistry test result abnormalities. Liver biopsy was performed at 15 months, with mass-spectrometry screening for a bile acid synthetic defect.
Hydrocephalus ascribed to aqueductal stenosis, respiratory distress, and hypoglycemia suspect for sepsis led to hospital admission aged 3 days for Patient #5, male, born at term (4.58 kg) to consanguine parents. Liver failure developed and was successfully treated by liver transplantation. Evaluation before transplant included liver biopsy and screening for a bile acid synthesis defect.
Patient #6, the full sister of Patient #5, had never been ill and was clinically well when, aged 9 years, her urine was screened by mass spectrometry for abnormal bile acids. The only clinical-laboratory test-result abnormality identified was a low total serum tocopherol concentration; hypocoagulability, other hypovitaminoses, or other clinical-biochemistry test-result abnormalities were not found.
Patient #7, the full sister of Patients #5 and #6, had severe jaundice as a neonate. Hepatitis was diagnosed. Ursodeoxycholic acid and vitamin A were given for several years. When at 10 years her urine was screened for a bile acid synthesis defect by mass spectroscopy, serum vitamins A and D and total tocopherols levels were low, without hypocoagulability or other clinical-biochemistry test-result abnormality.
Growth failure aged 4 months prompted evaluation of Patient #8, female, born at term (3.63 kg) to parents not identifying as consanguine and with one well child. A prolonged PT responded to parenteral vitamin K; serum vitamins A, D, and E were low and serum alkaline-phosphatase activity was high, without other clinical-biochemistry test-result abnormality. Urine was screened by mass spectroscopy for a bile acid synthesis defect.
On evaluation at age 5 months of growth retardation, jaundice, and rickets, Patient #9, male, born at term (2.5 kg), exhibited mild hepatomegaly without splenomegaly. A prolonged PT responded to parenteral vitamin K; serum vitamins D and E were low, without hypovitaminosis A. Conjugated and non-conjugated hyperbilirubinemia accompanied elevations in serum transaminase and alkaline-phosphatase activities. Liver biopsy was done, as was bile acid analysis by mass-spectroscopy.
Poor weight gain led to evaluation of Patient 10, female; urine was screened by mass spectroscopy at age 8 years, when duodenal stenosis was surgically palliated, and earlier clinical details are lacking. Urine was again screened at age 10 years.
Analytical techniques
The bile acid composition of urine, serum, bile and feces was examined in detail using a combination of methodologies previously published, including liquid-solid extraction, lipophilic anion exchange chromatography to isolate bile acids based on conjugate classes and analysis of these fractions by gas chromatography-mass spectrometry (GC-MS) after derivatization to methyl ester-trimethylsilyl (Me-TMS) ethers 8. The initial screening procedure for diagnosis of a bile acid synthetic defect was performed by direct analysis of the urine using fast atom bombardment ionization-mass spectrometry (FAB-MS), and GC-MS8, 9.
Molecular Genetic Analysis of BAAT and SLC27A5
Human genomic DNA was isolated from white blood cells using Puregene DNA isolation kits (Qiagen, Valencia, CA). The 3 coding exons of BAAT and the 10 coding exons of SLC27A5 were amplified by PCR. The PCR products were purified and sequenced using standard approaches. Sequences were aligned to a reference gene sequence. Absence of candidate mutations from publically (dbSNP) and locally available control sequence data was confirmed. Predicted functional consequences of missense changes were evaluated using Polyphen2 (Polymorphism Phenotyping v2; http://genetics.bwh.harvard.edu/pph2/).
Control samples: For the mutation in patients 2 and 3, 80 control chromosomes from individuals of Arab ancestry were assayed. For the other mutations, 113 control chromosomes from HAPMAP families of Northern and Western European ancestry were assayed10.
Histological Analysis
Sections of formalin-fixed paraffin embedded liver tissue from patients #1, 2, #4, and #5 were stained with hematoxylin and eosin, PAS-diastase, reticulin, and Masson trichrome methods. Patients #1, #2, and #5 had second liver samples obtained at ages 14 years, 4.5 years, and 6 months respectively. Tissue samples from the second biopsy specimen in Patient #2, the only specimen from patient #4 and the first specimen in Patient #5 were processed for ultrastructural study (glutaraldehyde-fixed, osmium-tetroxide post-fixed, resin-embedded). Ultrathin sections of resin-embedded liver were stained with uranyl oxide / lead citrate and examined using a transmission electron microscope. In patients #2, #4, and #5, expression of BACL and BAAT was assessed immunohistochemically using antibodies against BACL (HPA007292, Sigma) and BAAT (ab97455;Abcam, Cambridge, UK) with EnVision reaction development (DAKO UK, Ely, UK) and hematoxylin counterstaining as described elsewhere11.
RESULTS
Urinary bile acid analysis
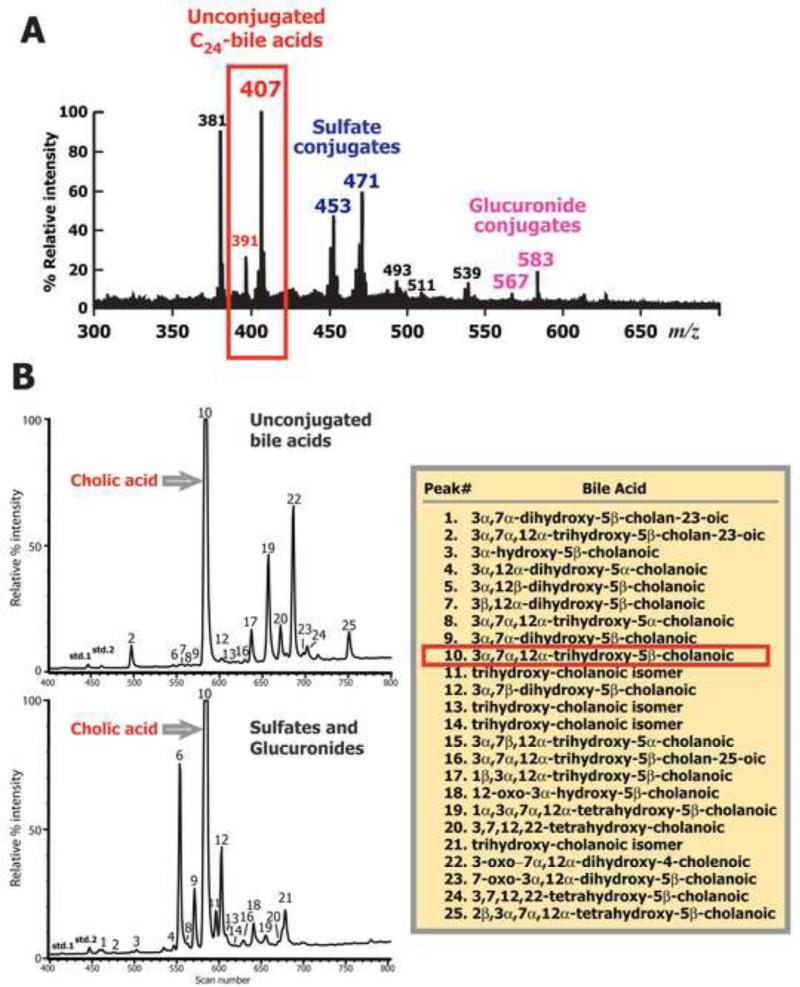
The negative ion FAB-MS spectra of urines from the 10 patients were qualitatively similar to the index case (patient #1) shown in Fig. 2. While the relative intensity of individual ions in the mass spectra varied among patients, remarkable and consistent throughout the spectra was the complete absence of glycine (m/z 464/448) and taurine (m/z 514/498) conjugated bile acids, the usual products of hepatic primary bile acid synthesis, and a dominance of unconjugated and sulfated bile acids (see Supplemental Table 1). A conspicuous feature of all spectra was an intense ion at m/z 407 consistent with the deprotonated molecular ion of an unconjugated trihydroxy-cholanoic (C24) bile acid, and prominent ions for sulfate conjugates of monohydroxy-cholenoates (m/z 453) and dihydroxy cholanoates (m/z 471) were observed. Ions of lower abundance were usually present, in particular at m/z 391 for unconjugated dihydroxy-cholanoic (C24) acids, and m/z 567 and 583 corresponding to glucuronide conjugates of dihydroxy- and trihydroxy-cholanoic acids, respectively. When the urine extracts were fractionated on the lipophilic anion exchanger Lipidex-DEAP to separate bile acids based on mode of conjugation, FAB-MS of the fractions confirmed these structural assignments and further established an absence of any glycine or taurine conjugated bile acids.
Figure 2.
Panel A: Typical negative ion FAB-MS spectrum of the urine from a patient with a defect in bile acid amidation. Panel B: GC- MS total ion current profiles of the methyl ester-trimethylsilyl ether derivatives of urinary bile acids excreted in unconjugated form and as glucuronide and sulfate conjugates combined. No glycine or taurine conjugates were found. Bile acids were identified from their mass spectra and retention indices. Peak numbers correspond to bile acids listed in Supplemental Online data Table 1.
GC-MS analysis of the Me-TMS ether derivatives of urinary bile acids isolated in these conjugate fractions confirmed the majority of bile acids to be unconjugated in agreement with the findings from FAB-MS analysis. At the time of diagnosis the mean (±SEM) total urinary unconjugated bile acid concentration for the 7 patients for which there was sufficient urine for analysis was 327 ± 195 μmol/L (see Supplementary Data - Table 2) representing 79.4 ± 3.9% of the total bile acids excreted. Cholic acid was the predominant urinary bile acid accounting for 55.8 ± 8.1% of the bile acids in the unconjugated fraction. Low proportions and concentrations of deoxycholic, chenodeoxycholic, and lithocholic acids were found. The mean (±SEM) concentration of bile acids excreted in urine as glucuronide and sulfate conjugates was 106 ± 53 μmol/L, and cholic acid accounted for 50.0 ± 7.0% of the total bile acids. Qualitatively the bile acid composition of this conjugate fraction differed from that of the unconjugated fraction (Fig. 2) by the presence of a more diverse array of bile acids, notably 1β-, 2β, and 22-hydroxylated metabolites (Fig. 2 and Supplemental data Table 2). Overall, the mean total urinary bile acid concentration of these patients was 432 ± 248 μmol/L, which was markedly elevated (normal <20 μmol/L) and cholic acid accounted for 54.9 ± 6.9% of all bile acids excreted.
Biliary bile acid analysis
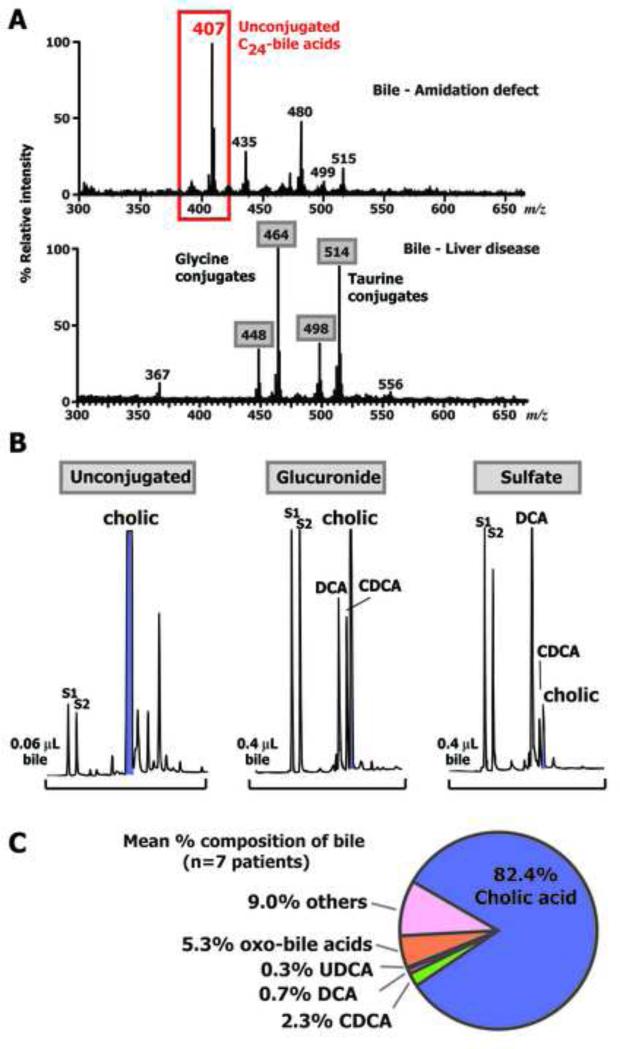
Duodenal bile was available from only 8 of the patients (#1, 2, 4, 5, 6, 7, 8, and 10) and the FAB-MS mass spectra were all similar to that of the index case (Fig. 3). Consistent with urine, the striking and significant feature of the mass spectra of the duodenal bile extracts was the absence of ions corresponding to glycine and taurine conjugated primary bile acids, typically present when bile acid synthesis is intact. For comparison the mass spectrum of a patient with liver disease but normal primary bile acid synthesis is shown in Fig. 3. The major ion in the spectra of the bile from these patients was at m/z 407, corresponding to unconjugated trihydroxy-cholanoic acid, and other ions of variable intensity at m/z 391 (unconjugated dihydroxy-cholanoic), m/z 471 (sulfated dihydroxy-cholanoic), m/z 567 (dihydroxy-cholanoic glucuronide) and m/z 583 (trihydroxy-cholanoic glucuronide) were present. Ions at m/z 499 and 515 represent bile alcohol sulfates.
Figure 3.
Panel A: Typical negative ion FAB-MS spectra comparing bile from a patient with a defect in bile acid amidation (top spectrum) with bile from a patient with liver disease and intact bile acid synthesis (Ions at m/z 448, 464, 498, and 514 represent the glycine and taurine conjugated primary bile acids, chenodeoxycholic and cholic acids. Panel B: Typical GC-MS profiles of methyl ester-trimethylsilyl ether derivatives of biliary bile acids of a patient with a defect in bile acid amidation. Bile acids were fractionated according to conjugate class on Lipidex-DEAP. No bile acids were found in the glycine or taurine fractions. S1 and S2 represent internal standards, (coprostanol and nordeoxycholic acid, respectively) and indicated is the relative volumes (μL) of bile on-column. Panel C: Venn diagram showing the mean (n=8) relative proportion of the principal bile acids in duodenal bile. Oxo-bile acids refers to all hydroxylated bile acids with a oxo group.
After fractionation of the bile into conjugate classes using Lipidex-DEAP, hydrolysis/solvolysis of the conjugates, and derivatization, GC-MS analysis (Fig. 3) established the identity and distribution of the individual bile acids observed in the FAB-MS spectra. No bile acids were found in the glycine and taurine fractions. GC profiles of the unconjugated, glucuronide and sulfate conjugated bile acid fractions of the bile from the index case confirmed the majority of biliary bile acids to be unconjugated. The major peak in the chromatogram was definitively confirmed from its electron ionization mass spectrum and retention index to be cholic acid. There were traces of other bile acids in this fraction, including deoxycholic acid, and there was a notable lack of unconjugated chenodeoxycholic acid, which was nevertheless present in low concentrations in the glucuronide and sulfate fractions together with cholic and deoxycholic acids. The biliary bile acid profiles of the 8 patients were qualitatively similar although quantitatively there was considerable variation in concentrations due to sampling differences during intubation. The total biliary unconjugated bile acid concentration of the bile from the 8 patients was 12.06 ± 5.95 mmol/L, which was significantly greater than the concentration of biliary bile acid glucuronides and sulfates combined (mean, 112 ± 62 μmol/L). Unconjugated bile acids in duodenal bile therefore accounted for 95.7 ± 5.8% of the total bile acids, with cholic acid accounting for 82.4 ± 5.5% of all bile acids secreted (Supplemental data - Table 3).
Serum bile acid analysis
Negative ion FAB-MS analysis of the serum from the index patient (#1) yielded a similar mass spectrum to that obtained for the patient's urine and bile. The major ion and base peak was m/z 407, representing unconjugated trihydroxy-cholanoic acid. There was an absence of taurine and glycine conjugated bile acids. Ions at m/z 453 and 471 were accounted for by sulfate conjugates of monohydroxy-cholenoates and dihydroxy-cholanoates, respectively, while the ions at m/z 567 and 583 were consistent with glucuronides of dihydroxy- and trihydroxy-cholanoates, respectively. The mean serum total bile acid concentration of 5 of the patients determined by GC-MS was markedly elevated, being 257 ± 157 μmol/L (normal <3.5μmol/L). GC-MS analysis of the serum revealed cholic acid as the major serum bile acid, accounting 64.0 ± 6.8% of the total.
Fecal bile acid analysis
The GC profile of the Me-TMS ethers of bile acids isolated from the feces from patient #1 is shown in the Supplemental data Fig. 1. Mass spectrometry confirmed the major fecal bile acid to be deoxycholic acid, accounting for 47.9% of the total bile acids, and there were several stereoisomers of deoxycholic acid, including the 3β-hydroxy-, and 12β-hydroxy- forms of both the 5β-H and 5α-H(allo-) cholanoic acids. Cholic acid was identified as were several epimers and oxo-derived metabolites of cholic acid The total bile acid concentration in the feces from this patient was 8.85 mg/g. Notable was the absence of lithocholic acid, normally one of the major bile acids in feces12, indicating a relatively low level of chenodeoxycholic acid synthesis and consistent with the relative absence of chenodeoxycholic in other fluids analyzed.
Molecular analysis
Molecular analysis of the 3 coding exons of BAAT in the 8 patients from whom DNA was available resulted in identification of 4 different mutations, each present in homozygous form in one of the families tested (Table 2). In one patient (#9), no mutation was identified despite the finding of a urinary profile consistent with defective bile acid conjugation; this patient was also screened for mutation in SLC27A5, and no mutation was identified. Parents of all patients homozygous for a mutation in BAAT were confirmed to be heterozygous carriers of the mutations present in their children; results of genotyping in unaffected siblings are shown (Table 2). None of the 4 mutations detected were found in assayed control chromosomes, nor were these alterations present in dbSNP, consistent with these being disease-causing mutations. Furthermore, all 3 missense mutations are predicted to damage protein structure and/or function; the 4th mutation introduces a premature stop codon early in the gene's coding sequence, and is therefore expected to result in lack of functional protein.
Table 2.
Summary of molecular genetic analyses identifying defects in bile acid CoA:amino acid N-acyltransferase (BAAT)
| Family | Patient | DNA available and screened | Nucleotide change | Predicted effect on protein | Nature of mutation | Homozygous | Prediction of functional effect† | Number of unaffected siblings genotyped, and results** |
|---|---|---|---|---|---|---|---|---|
| 1 | #1 | No | - | - | - | - | - | - |
| 2 | #2 | Yes | c1156G→A | p.G386R | Missense | Yes* | Probably damaging | 1: +/+ 1: +/− |
| #3 | Yes | c1156G→A | p.G386R | Yes* | ||||
| 3 | #4 | Yes | c.206A→T | p.D69V | Missense | Yes* | Probably damaging | 1: +/− |
| 4 | #5 | Yes | c.58C→T | p.R20X | Premature stop codon | Yes* | - | 1: +/− |
| #6 | Yes | c.58C→T | p.R20X | Yes* | ||||
| #7 | Yes | c.58C→T | p.R20X | Yes* | ||||
| 5 | #8 | Yes | c.250C→A | p.P84T | Missense | Yes* | Probably damaging | 1: +/− |
| 6 | #9 | Yes | No mutation identified | - | - | - | - | - |
| 7 | #10 | No | - | - | - | - | - | - |
Both parents confirmed heterozygous.
Using Polyphen 2; to note, ‘probably damaging’ is the worst functional category reported by this software.
‘+’ =normal allele; ‘−’ = mutation present in the family
Morphological Findings
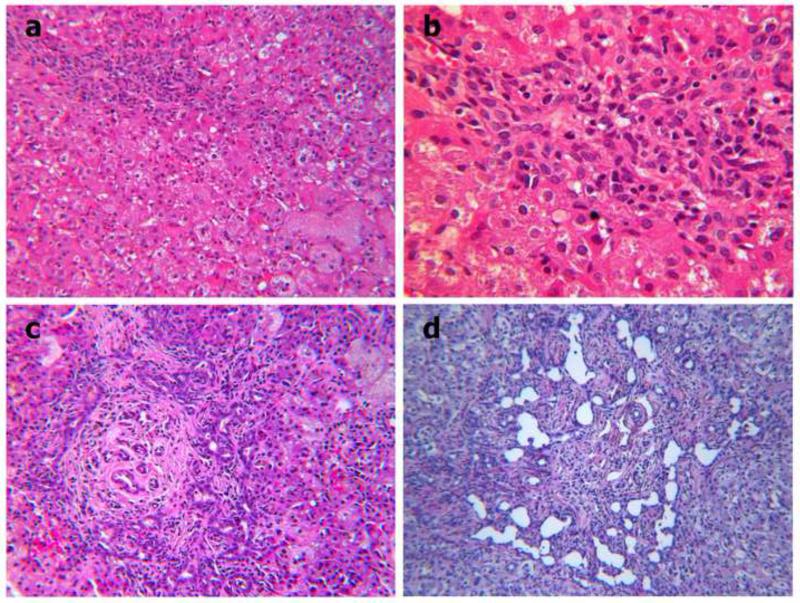
Four of the 10 patients underwent liver biopsy. The livers of 3 patients, #1, #2, and #5, were biopsied in early infancy: Patients #1 and #5 came to biopsy to investigate unexplained direct hyperbilirubinemia. Patient #2 had liver biopsy performed at a hepatic portoenterostomy at age 40 days (Figure 4a). Patient #5 had a small-duct cholangiopathy of unusual severity at age 11 weeks (Figure 4b - d) that progressed to cirrhosis, liver failure, and need for transplantation at age 6 months. The explanted liver showed persistent severe small-duct injury (Figure 4e), severe intralobular cholestasis, and periportal fibrosis with bridging. In many respects the findings in the 2 (of 3) early biopsy specimens from Patients #2 and #5 resemble those in idiopathic neonatal hepatitis, as do those described in the report of initial findings in Patient #1. Prominent, even severe, ductular reaction in d, however, is a point of difference.
Figure 4.
Panel a. Patient #2. Open liver biopsy performed at hepatic portoenterostomy (“Kasai”): Mild portal mononuclear cell infiltration, absent bile plugs in interlobular bile ducts, lobular cholestasis with spotty zone 1 hepatocyte necrosis, and prominent zone 3 giant cell transformation. Hematoxylin / eosin (H&E) stain, x 10. Panel b. Patient #2. Mild proliferation of small bile ducts and ductular reaction at the limiting plate are highlighted. H&E stain. x 25. Panel c. Patient #5. Open liver biopsy performed at age 10 weeks: Severe periportal fibrosis with bridging and lobular cholestasis with prominent giant cell transformation in zones 2 and 3. Giant cells have slightly foamy cytoplasm. Periportal fibrosis accompanies florid ductular and mild small duct proliferation. Lumina of ductules and ducts contain wispy bile residue and degenerate cholangiocytes but no bile plugs. Focally a brisk pericholangitis is associated. H&E stain. x 10. Panel d. Patient #5. At age 6 months, the explanted liver demonstrated a severe cholangiopathy with florid ductular proliferation and focally extreme dilatation without bile plugs. Periportal fibrosis had progressed to cirrhosis since the previous biopsy. H&E stain, x 10.
Samples of liver tissue were obtained beyond infancy in 3 patients. Two of the 3 patients who had come to liver biopsy during infancy had follow-up liver biopsies at ages 4.5 years and 14 years. In Patient #1 cholestasis and ductular proliferation had resolved although he had, during the intervening years, acquired transfusion-related hemosiderosis and mild portal fibrosis. In Patient #2 the liver at age 4.5 years showed mild persistent ductular reaction and focal periportal fibrosis. Signs of obstructive cholangiopathy and lobular cholestasis were absent. Light microscopy of a single liver biopsy specimen obtained from Patient #4 at age 15 months showed mild steatosis and rare necrotic hepatocytes but no changes in bile ducts or ductules and no fibrosis.
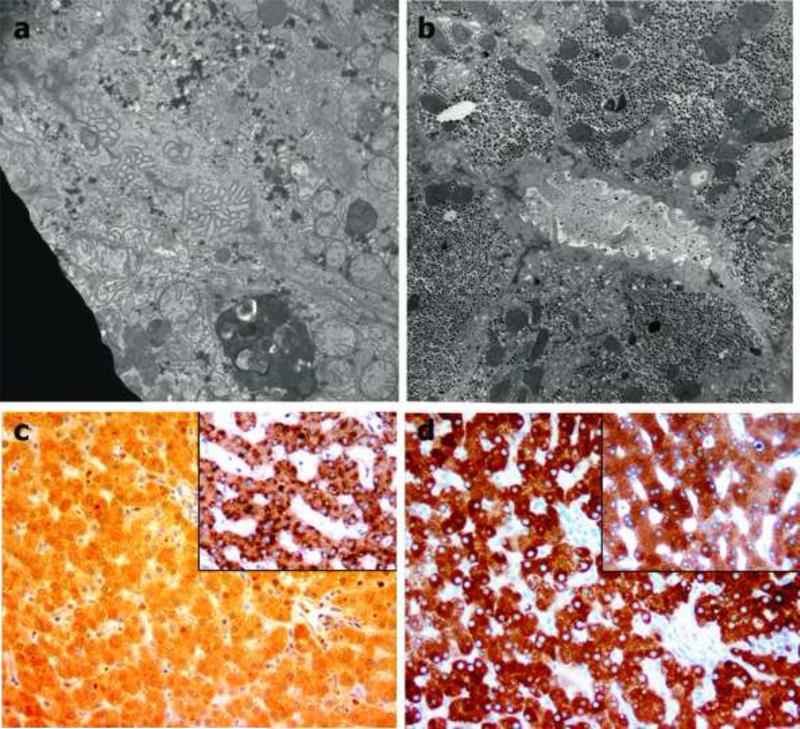
Liver ultrastructure at age 10 weeks in Patient #5 was of note for extremely prominent autophagy, diffuse disorganization of mitochondrial cristae, and a severe but non-specific pattern of injury to cholangiocytes of small ducts and ductules with substantial accumulation of bulky residual bodies in cholangiocyte cytoplasm. In addition, architectural distortion of canaliculi was unexpectedly severe and unusual, similar to that reported in another bile acid synthesis defect, 5-beta reductase deficiency13 (Figure 5a). The ultrastructure of canaliculi and cholangiocytes at age 15 months in Patient #4 was minimally altered. However, prominently dilated endoplasmic reticulum was universally present, as was mild mitochondrial pleomorphism with occasional matrix crystalloids. Canaliculi at age 4.5 years in patient 2 were normal or were dilated with accumulation of pericanalicular filaments (Figure 5b).
Figure 5.
Electron microscopy. Patient #5. Biopsy #1. Panel a. Canaliculus exhibits unusual tortuous folding of microvilli. x 5000. Panel b. Patient #2 at age 4.5 years. A dilated canaliculus is surrounded by a prominent circumferential band of thin filaments, a sign of chronic injury. x 5000. Both panels: Osmium tetroxide post-fixation, uranyl acetate and lead citrate stain. Panel c. Patient #4. All hepatocytes exhibit uniform cytoplasmic background stain without the strong punctate granular reaction product present in cytoplasm of normal hepatocytes (positive control, inset). Anti-BAAT antibody, hematoxylin counterstain. Original magnification x200. Panel d. Patient #4. Diffuse cytoplasmic reaction of variable intensity is observed in hepatocytes of both patient and normal control (inset). Anti-BACL antibody, hematoxylin counterstain. Original magnification x200.
Immunostaining for BAAT demonstrated strong punctate diffuse cytoplasmic localization in normal hepatocytes that was uniformly depleted in liver biopsy tissue from patients #2, #4, and #5 (Figure 5c). Immunostaining for BACL, also involved in amidation, was normal in these 3 patients (Figure 5d), with non-uniform intensity ascribed to lobular unrest.
DISCUSSION
We describe the clinical, biochemical and molecular characterization of 10 patients with a defect in bile acid conjugation. These cases illustrate the essential role that bile acids play in facilitating the absorption of fat-soluble vitamins and dietary fatty acids, while conversely highlighting serum fat-soluble vitamin status as a sensitive marker for disturbances in hepatic bile acid synthesis and intraluminal bile acid composition. Our findings indicate that bile acid conjugation is essential for the normal enterohepatic circulation of bile acids and suggest that patients with unexplained fat-soluble vitamin deficiency should be investigated for the possibility of defects in bile acid conjugation.
Bile acids are synthesized in the liver from cholesterol by a complex series of chemical reactions catalyzed by 17 different hepatic enzymes located in different subcellular fractions. The enzymes and their genes are well characterized and cDNAs described14. There are multiple pathways in bile acid synthesis15, but irrespective of the pathway by which unconjugated cholic and chenodeoxycholic acids are formed, the final step leads to the formation of the glycine and taurine conjugates1, and these account for >95% of the bile acids secreted in bile and are responsible for driving bile flow.
While inborn errors in bile acid synthesis involving impaired synthesis of cholic and chenodeoxycholic acids usually present as well defined progressive familial cholestatic liver disease9, by contrast, cholestasis, is generally not the primary manifestation of a bile acid conjugation defect. The variable degree of cholestasis is difficult to explain. We speculate that in some patients high levels of unconjugated cholic acid maintain bile flow and do not accumulate to toxic levels in hepatocytes. Alternatively, unconjugated bile acids are not well transported by canalicular transporters and in some patients may accumulate in hepatocytes causing direct injury and/or recruitment of inflammatory factors. In liver biopsies that we were able to obtain there was evidence of an interface inflammation, which would support the latter.
The phenotype of defective bile acid conjugation is quite variable with patients having little, or mild to severe liver disease, presumably because cholic acid is synthesized at a normal rate and its efficient intestinal absorption leads to a recycling pool of bile acids that can generate bile flow. In one patient (#5), severe cholestasis and liver failure required liver transplantation; however, all the patients we describe shared the common feature of severe fat-soluble vitamin deficiency with subnormal levels of retinol, vitamin E, 25-hydroxy-vitamin D and prolonged prothrombin time. Chronically, these led to rickets in 4 of the 10 patients described, and in 2, fractures resulted. Poor growth is variable and largely limited to infants and young children. While a low serum GGT is a characteristic feature of patients with PFIC1 and PFIC216 this is also the case for most patients with bile acid synthetic defects9, including the 4 patients with this amidation defect in which serum GGT was measured at baseline. Differential diagnosis of PFIC1 and 2 from bile acid synthetic defects can be established from the presence, in the case of PFIC, or absence in the case of bile acid synthetic defects, of primary bile acids. The clinical presentation and biochemical features of defective amidation closely parallel the predicted features hypothesized by Hofmann & Strandvik some 6 years prior to this first discovery17. Their hypothesis was based on studies of C23 nor-bile acids, bile acids that are poorly conjugated with glycine or taurine enter the smooth endoplasmic reticulum, undergo glucuronidation or sulfation followed by secretion into bile and/or urine but do not undergo an enterohepatic circulation18. In our patients, newly synthesized chenodeoxycholic and deoxycholic acids (formed by bacterial 7-dehydroxylation of cholic acid) should, in the absence of amidation, undergo such glucuronidation (and possibly some sulfation) and be rapidly eliminated from the body, explaining the low proportions in bile.
Definitive diagnosis of a defect in bile acid amidation in all 10 patients was accomplished by mass spectrometry using FAB-MS analysis of the urine8, 9, the same approach used to identify other bile acid synthetic defects. ESI-MS can also be used to make this diagnosis19, as was recently reported for a patient with defective amidation due to a bile acid-CoA ligase deficiency20. The striking feature of the mass spectra of the urine, bile and serum of patients with defective amidation is the complete absence of ions corresponding to glycine- and taurineconjugated bile acids, and the presence of a dominant ion at m/z 407 representing unconjugated cholic acid; this conclusion was confirmed by GC-MS analysis. Although these patients conjugate bile acids with glucuronic and sulfuric acids, these conjugates collectively accounted for on average only <5% of the bile acids secreted in bile and in 3 patients <0.2%, and are apparently of little help in promoting intestinal lipid absorption. Unconjugated bile acids in duodenal bile accounted for 95.7±5.8% of the bile acids. Quantitatively, duodenal bile obtained after induced gallbladder concentration by cholecystokinin administration had relatively high concentrations of unconjugated bile acids (mean±SEM, 12.06±5.95 mM) of which cholic acid accounted for 82.4±5.5% of the bile acids secreted. Cholic acid was likewise quantitatively the major bile acid in serum and urine, and concentrations were markedly elevated. The duodenal bile acid concentrations were on average close to the CMC for unconjugated cholic acid, which is approximately 11 mM3, meaning that the concentration of bile acids in micelles is quite low. It is likely that the postprandial intraluminal bile acid concentrations would be even lower after a meal, as has been reported previously21. Conjugation of cholic acid with glycine and taurine has only a small effect on CMC. The reduced fat-soluble vitamin concentrations and prolonged prothrombin time in these patients is explained by the rapid non-ionic passive diffusion of unconjugated cholic acid from the proximal intestine, which reduces its intraluminal effectiveness for absorption of lipophilic compounds. Amidation of bile acids is an important final step in bile acid synthesis because this modification serves to lower the pKa of the unconjugated bile acid and promotes ionization at intestinal pH, thus preventing absorption from the proximal small bowel. The secondary bile acid, deoxycholic acid was quantitatively the second most abundant bile acid in duodenal bile, albeit in low concentrations, and interestingly chenodeoxycholic acid was only found in traces in all biological fluids. The marked reduction in chenodeoxycholic acid was supported by the finding of negligible amounts of its secondary bile acid metabolite, lithocholic acid in the feces of the index case, the only patient whose feces were available for analysis. It is probable that the reduced synthesis of chenodeoxycholic acid is caused by the excessive production of unconjugated cholic acid because cholic acid down-regulates chenodeoxycholic acid synthesis. Diarrhea, previously hypothesized as a possible feature of an amidation defect17 was not seen in any patient. This is perhaps explained by a rapid recycling of unconjugated bile acids in the proximal small bowel thus preventing excessive loss into the colon where they would be cathartic. Furthermore, it could be speculated that release of FGF19 might downregulate bile acid synthesis, or that liver disease in some patients resulted in a failure of a compensatory increase in bile acid synthesis.
Discerning whether an amidation defect resides in the bile acid CoA ligase (encoded by SLC27A5) or in the bile acid-CoA:amino acid N-acyltransferase (encoded by BAAT), requires the use of molecular techniques to sequence these 2 genes for mutations, or immunostaining of a liver tissue to detect absence of one enzyme, because both defects yield seemingly indistinguishable negative ion mass spectra of the urine. Screening of SLC27A5 and BAAT for mutations can be performed in suspected cases of defects in bile acid conjugation. DNA was obtained from 8 of the 10 patients with a biochemically confirmed diagnosis and homozygous mutations (Table 2) were identified in all but one patient. Since we did not detect mutation in BAAT in Patient #9, we sequenced the coding exons of SLC27A5 in his DNA; however, we also found no mutations were found in this gene. In each family in which a BAAT mutation was detected, the affected children were found to be homozygous for the familial mutation, and other unaffected family members were heterozygous, or did not carry the mutation. These results indicate that this amidation defect behaves as an autosomal recessive trait. Of interest is that BAAT mutation in Patient #8, who is Amish, is different from the BAAT mutation previously reported in individuals with Lancaster County Old Order Amish ancestry22, consistent with the finding of genetic heterogeneity for some other rare genetic disorders amongst the Amish.
Liver biopsy findings in 4 of 10 patients suggest that transient and potentially severe cholestatic liver disease may be associated with BAAT deficiency only during infancy. On the other hand, the findings in the late liver biopsies in Patients #1 and #2, and clinical evidence in the other 8 patients, indicate that BAAT deficiency does not regularly produce cholestasis in infancy or serious chronic liver disease. Most unusual in symptomatic infants was excessive proliferation of bile ductules that exceeds what is usual for idiopathic neonatal hepatitis or in other genetic defects in bile acid synthesis. This overlaps with findings in both biliary atresia and severe cholestasis related to parenteral alimentation. Also of interest is that periportal and pericellular fibrosis was already established in patient #5 at age 10 weeks a feature generally considered a hallmark of an underlying metabolic disease. These findings permit postulation that transient hepatocyte injury with small duct cholangiopathy occurs in BAAT deficiency; that it may have a biochemical basis and, when severe, may produce direct hyperbilirubinemia with potential to progress to liver failure in infants. The common lesion in those infants who came to liver biopsy suggests biliary obstruction (as seen with biliary atresia). Of importance is that no obstruction of large bile ducts was demonstrated, although a cholangiogram reportedly was abnormal in Patient #2. The cause of the ductular injury pattern is not apparent. That non-amidated bile acids or salts themselves are not strongly irritant to mature hepatocytes or cholangiocytes can be inferred from the absence of clinical hepatobiliary disease in most patients with BAAT deficiency.
Defective bile acid conjugation associated with mutations in BAAT has been described in a number of patients from an Amish kindred; hypercholanemia in Amish patients carrying a homozygous mutation in TJP2 and heterozygous mutation in BAAT occurred more often than expected by chance, suggesting that heterozygosity for BAAT mutation may increase penetrance of disease associated with TJP2 mutation22. Recently, the first confirmed defect associated with a mutation in SLC27A5 was reported20. The patient, of Pakistani origin and born to consanguineous parents, presented with cholestasis, elevated serum bilirubin and transaminases, normal serum γ-GT concentrations and low serum fat-soluble vitamins - a similar presentation to that of the patients with BAAT deficiency described here. A liver biopsy from this child showed extensive fibrosis. The patient was homozygous for a missense mutation C.1012C>T in SLC27A5. No mutations were found in BAAT but interestingly a second mutation was found in ABCB11, encoding the bile salt export pump (BSEP).
UDCA therapy was been reportedly beneficial in a single patient with defective bile acid amidation caused by a mutation in SLC27A520. However, oral administration of primary conjugated bile acids should provide a better and rational therapeutic approach to correcting the fat-soluble vitamin malabsorption in patients with amidation defects23. A trial of oral glycocholic acid is currently ongoing in 5 of the patients described here.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health through Grants 8 UL1 TR000077-04 to JEH, NIH UO1 DK62497 to KDRS, JEH, PR, KEB and LNB, and R01 DK58214 to LNB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank U. Sanford (UCSF) for technical support with the molecular genetic studies.
Abbreviations used in this paper
- FAB-MS
fast atom bombardment ionization-mass spectrometry
- GC-MS
gas chromatography-mass spectrometry
- Me-TMS
methyl ester-trimethylsilyl ether
- MU
methylene unit (retention time relative to a homologous series of n-alkanes)
- cholic acid
3α,7α,12α-trihydroxy-5β-cholan-24-oic acid
- chenodeoxycholic acid
3α,7α-dihydroxy-5β-cholan-24-oic acid
- deoxycholic acid
3α,12α-dihydroxy-5β-cholan-24-oic acid
- ursodeoxycholic acid (UDCA)
3α,7β-dihydroxy-5β-cholan-24-oic acid
- nordeoxycholic acid
3α,12α-dihydroxy-5β-norcholan-23-oic acid
- BAAT
bile acid-CoA:amino acid N-acyltransferase
- CMC
critical micellar concentration
Footnotes
* Preliminary details of the index case were presented at the Falk Symposium No. 93 (XIV International Bile Acid Meeting), Freiberg, Germany, October 22-24, 1996.
Conflicts of Interest:
There are no conflicts of interest to declare.
Author Contributions:
KDRS and JEH, the Principal Investigators acquired, analyzed and interpreted data and prepared the manuscript. LNB and DW performed the molecular genetic studies and interpreted the findings, BW and WZ provided technical support on the mass spectrometry, NOC and PJ provided technical support on the preparation of samples for bile acid analysis, JEL, DS, MAL, CP, JEH and PR were responsible for the primary care of the patients and for providing clinical samples and information, KEB and ASK evaluated the histology, immunohistochemistry and ultrastructure of liver biopsies. AFH provided intellectual and critical review of the manuscript. All of the authors contributed to, read and edited the manuscript.
REFERENCES
- 1.Sjövall J. Dietary glycine and taurine conjugation in man. Proc Soc Exp Biol Med. 1959;100:676–8. doi: 10.3181/00379727-100-24741. [DOI] [PubMed] [Google Scholar]
- 2.Matoba N, Une M, Hoshita T. Identification of unconjugated bile acids in human bile. J Lipid Res. 1986;27:1154–62. [PubMed] [Google Scholar]
- 3.Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25:1477–89. [PubMed] [Google Scholar]
- 4.Vessey DA, Zakim D. Characterization of microsomal choloyl-coenzyme A synthetase. Biochem J. 1977;163:357–62. doi: 10.1042/bj1630357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killenberg PG. Measurement and subcellular distribution of choloyl-CoA synthetase and bile acid-CoA:amino acid N-acyltransferase activities in rat liver. J Lipid Res. 1978;19:24–31. [PubMed] [Google Scholar]
- 6.Johnson MR, Barnes S, Kwakye JB, et al. Purification and characterization of bile acid-CoA:amino acid N-acyltransferase from human liver. J Biol Chem. 1991;266:10227–33. [PubMed] [Google Scholar]
- 7.Setchell KDR, Heubi JE, O'Connell NC, et al. Identification of a unique inborn error in bile acid conjugation involving a deficiency in amidation. In: Paumgartner G, Stiehl A, Gerok W, editors. Bile Acids in Hepatobiliary Diseases: Basic Research and Clinical Application. Kluwer Academic Publishers; Dordrecht/Boston/London: 1997. pp. 43–47. [Google Scholar]
- 8.Sjövall J, Griffiths WJ, Setchell KDR, et al. Gower DB. Analysis of bile acids. In: Makin HLJ, editor. Steroid Analysis (ISBN 978-1-4020-9774-4) Springer; Dordrecht Heidleberg London New York: 2010. pp. 837–966. [Google Scholar]
- 9.Setchell KDR, Heubi JE. Defects in bile acid biosynthesis--diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S17–22. doi: 10.1097/01.mpg.0000226386.79483.7b. [DOI] [PubMed] [Google Scholar]
- 10.Consortium TIH. A second generation human haplotype map of over 3.1 million SNP's. Nature Genetics. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadzic N, Bull LN, Clayton PT, et al. Diagnosis in bile acid-CoA: amino acid N-acyltransferase deficiency. World J Gastroenterol. 2012;18:3322–6. doi: 10.3748/wjg.v18.i25.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setchell KDR, Street JM, Sjövall J. Fecal bile acids. In: Setchell KDR, Kritchevsky D, Nair PP, editors. The Bile Acids: Methods and Applications. Plenum Press; New York: 1988. pp. 441–570. [Google Scholar]
- 13.Daugherty CC, Setchell KDR, Heubi JE, et al. Resolution of liver biopsy alterations in three siblings with bile acid treatment of an inborn error of bile acid metabolism (delta 4-3-oxosteroid 5 beta-reductase deficiency). Hepatology. 1993;18:1096–101. [PubMed] [Google Scholar]
- 14.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 15.Axelson M, Sjövall J. Potential bile acid precursors in plasma--possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem. 1990;36:631–40. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- 16.Bezerra JA, Balistreri WF. Intrahepatic cholestasis: order out of chaos. Gastroenterology. 1999;117:1496–8. doi: 10.1016/s0016-5085(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann AF, Strandvik B. Defective bile acid amidation: predicted features of a new inborn error of metabolism. Lancet. 1988;2:311–3. doi: 10.1016/s0140-6736(88)92359-8. [DOI] [PubMed] [Google Scholar]
- 18.Yoon YB, Hagey LR, Hofmann AF, et al. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837–52. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 19.Haas D, Gan-Schreier H, Langhans CD, et al. Differential diagnosis in patients with suspected bile acid synthesis defects. World J Gastroenterol. 2012;18:1067–76. doi: 10.3748/wjg.v18.i10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong CP, Mills PB, McClean P, et al. Bile acid-CoA ligase deficiency - a new inborn error of bile acid metabolism. J Inherit Metab Dis. 2011;35:521–530. doi: 10.1007/s10545-011-9416-3. [DOI] [PubMed] [Google Scholar]
- 21.Porter HP, Saunders DR. Isolation of the aqueous phase of human intestinal contents during the digestion of a fatty meal. Gastroenterology. 1971;60:997–1007. [PubMed] [Google Scholar]
- 22.Carlton VEH, Harris BZ, Puffenberger EG, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nature Genetics. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 23.Heubi JE, Setchell KDR, Rosenthal P, et al. Oral glycocholic acid treatment of patients with bile acid amidation defects improves growth and fat soluble vitamin absorption. Hepatology. 2009;50:895A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.