Abstract
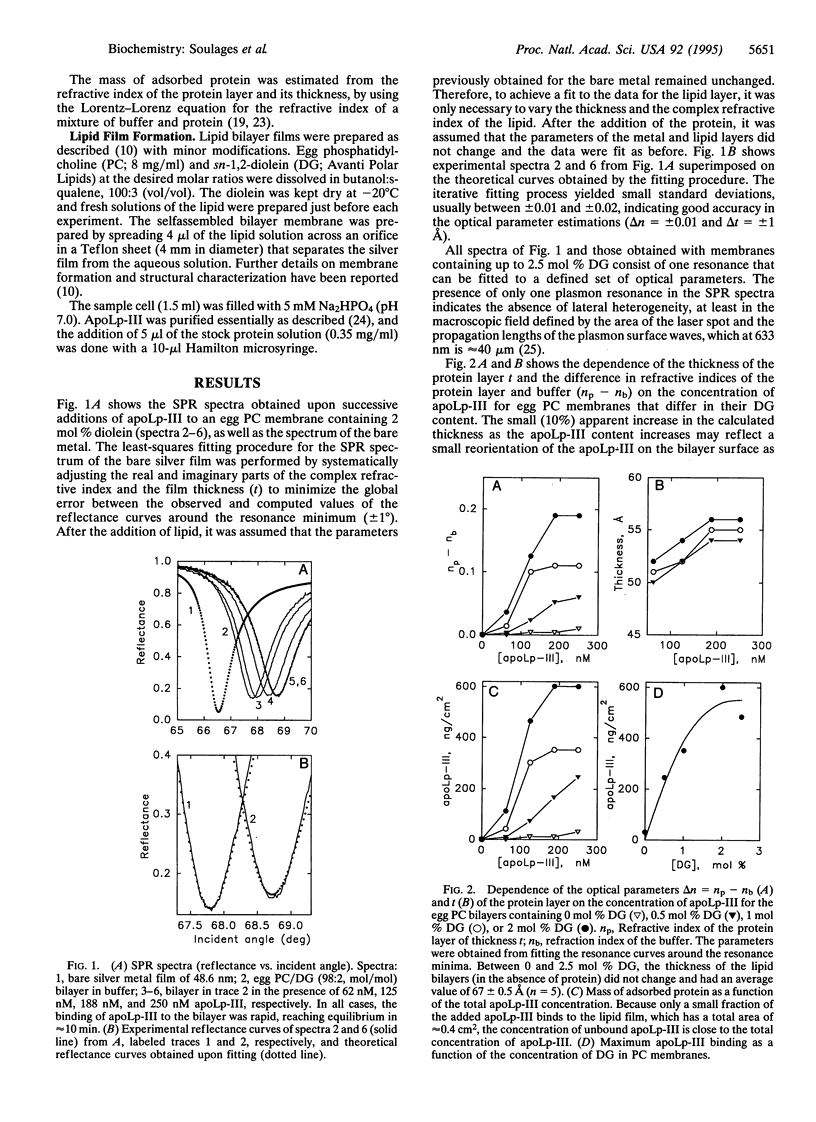
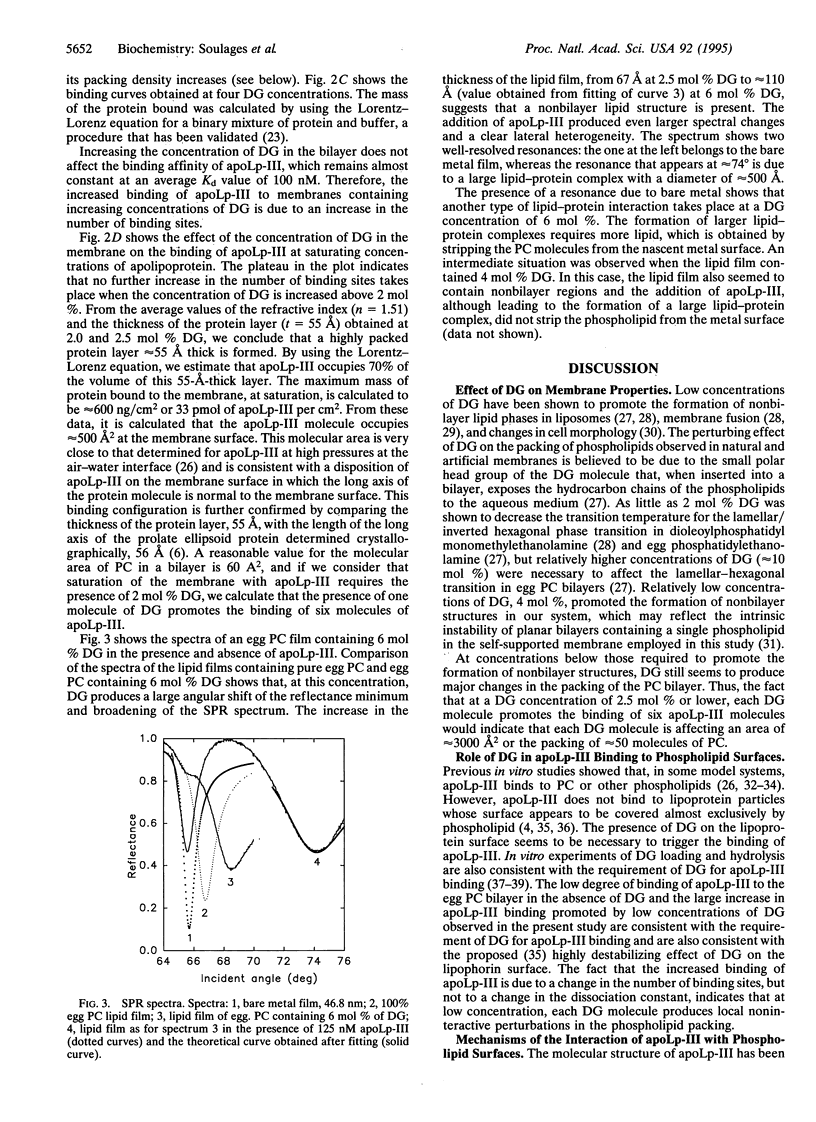
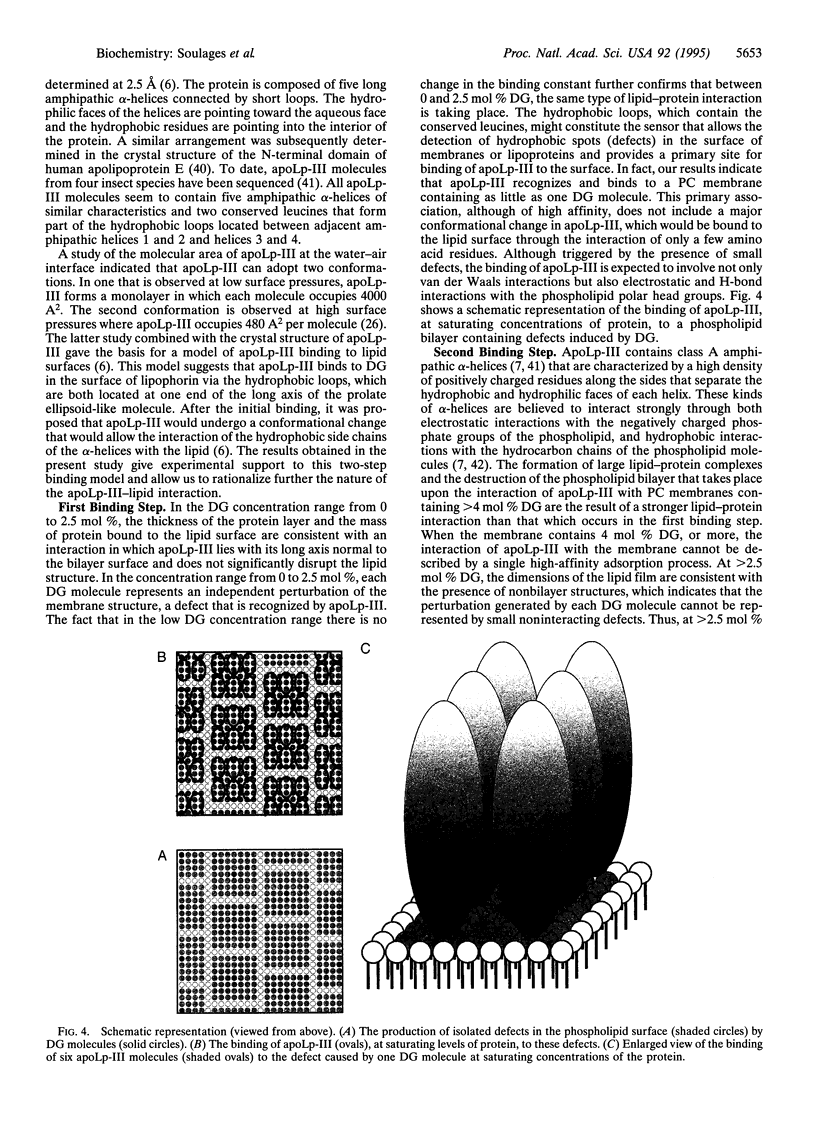
The binding of the exchangeable apolipoprotein apolipophorin III (apoLp-III) to an egg phosphatidylcholine bilayer as a function of the concentration of diacylglycerol (DG) in the bilayer was studied by surface plasmon resonance spectroscopy. At a DG concentration of 2 mol % in the bilayer, the binding of apoLp-III reached saturation. Under saturating conditions, apoLp-III forms a closely packed monolayer approximately 55 A thick, in which each molecule of protein occupies approximately 500 A2 at the membrane surface. These dimensions are consistent with the molecular size of the apoLp-III molecule determined by x-ray crystallography, if apoLp-III binds to the bilayer with the long axis of the apoLp-III normal to the membrane surface. In the absence of protein, the overall structure of the lipid bilayer was not significantly changed up to 2.5 mol% DG. However, at 4 and 6 mol % DG, the presence of nonbilayer structures was observed. The addition of apoLp-III to a membrane containing 6 mol % DG promoted the formation of large lipid-protein complexes. These data support a two-step sequential binding mechanism for binding of apoLp-III to a lipid surface. The first step is a recognition process, consisting of the adsorption of apoLp-III to a nascent hydrophobic defect in the phospholipid bilayer caused by the presence of DG. This recognition process might depend on the presence of a hydrophobic sensor located at one of the ends of the long axis of the apoLp-III molecule but would be consolidated through H-bond and electrostatic interactions. Once primary binding is achieved, subsequent enlargement of the hydrophobic defect in the lipid surface would trigger the unfolding of the apolipoprotein and binding via the amphipathic alpha-helices. This two-step sequential binding mechanism could be a general mechanism for all exchangeable apolipoproteins. A possible physiological role of the ability of apoLp-III to bind to lipid structures in two orientations is also proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Thomas P., Michell R. H. Rapid transbilayer diffusion of 1,2-diacylglycerol and its relevance to control of membrane curvature. Nature. 1978 Nov 16;276(5685):289–290. doi: 10.1038/276289a0. [DOI] [PubMed] [Google Scholar]
- Beenakkers A. M., Van der Horst D. J., Van Marrewijk W. J. Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 1985;24(1):19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Bondeson K., Frostell-Karlsson A., Fägerstam L., Magnusson G. Lactose repressor-operator DNA interactions: kinetic analysis by a surface plasmon resonance biosensor. Anal Biochem. 1993 Oct;214(1):245–251. doi: 10.1006/abio.1993.1484. [DOI] [PubMed] [Google Scholar]
- Breiter D. R., Kanost M. R., Benning M. M., Wesenberg G., Law J. H., Wells M. A., Rayment I., Holden H. M. Molecular structure of an apolipoprotein determined at 2.5-A resolution. Biochemistry. 1991 Jan 22;30(3):603–608. doi: 10.1021/bi00217a002. [DOI] [PubMed] [Google Scholar]
- Cuypers P. A., Corsel J. W., Janssen M. P., Kop J. M., Hermens W. T., Hemker H. C. The adsorption of prothrombin to phosphatidylserine multilayers quantitated by ellipsometry. J Biol Chem. 1983 Feb 25;258(4):2426–2431. [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Van Doorn J. M., Van der Horst D. J. Insect apolipophorin III: interaction of locust apolipophorin III with diacylglycerol. Biochim Biophys Acta. 1992 Mar 4;1124(2):151–158. doi: 10.1016/0005-2760(92)90091-9. [DOI] [PubMed] [Google Scholar]
- Gupta S., Morgan T. R., Gordan G. S. Calcitonin gene-related peptide in hepatorenal syndrome. A possible mediator of peripheral vasodilation? J Clin Gastroenterol. 1992 Mar;14(2):122–126. doi: 10.1097/00004836-199203000-00010. [DOI] [PubMed] [Google Scholar]
- Helm C. A., Israelachvili J. N., McGuiggan P. M. Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers. Science. 1989 Nov 17;246(4932):919–922. doi: 10.1126/science.2814514. [DOI] [PubMed] [Google Scholar]
- Kawooya J. K., Meredith S. C., Wells M. A., Kézdy F. J., Law J. H. Physical and surface properties of insect apolipophorin III. J Biol Chem. 1986 Oct 15;261(29):13588–13591. [PubMed] [Google Scholar]
- Kawooya J. K., van der Horst D. J., van Heusden M. C., Brigot B. L., van Antwerpen R., Law J. H. Lipophorin structure analyzed by in vitro treatment with lipases. J Lipid Res. 1991 Nov;32(11):1781–1788. [PubMed] [Google Scholar]
- Müller W., Ringsdorf H., Rump E., Wildburg G., Zhang X., Angermaier L., Knoll W., Liley M., Spinke J. Attempts to mimic docking processes of the immune system: recognition-induced formation of protein multilayers. Science. 1993 Dec 10;262(5140):1706–1708. doi: 10.1126/science.8259513. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Aranda F. J., Villalaín J., San Martín C., Micol V., Gómez-Fernandez J. C. 1,2-Dioleoylglycerol promotes calcium-induced fusion in phospholipid vesicles. Chem Phys Lipids. 1992 Oct;62(3):215–224. doi: 10.1016/0009-3084(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Ryan R. O. Dynamics of insect lipophorin metabolism. J Lipid Res. 1990 Oct;31(10):1725–1739. [PubMed] [Google Scholar]
- Salamon Z., Wang Y., Brown M. F., Macleod H. A., Tollin G. Conformational changes in rhodopsin probed by surface plasmon resonance spectroscopy. Biochemistry. 1994 Nov 22;33(46):13706–13711. doi: 10.1021/bi00250a022. [DOI] [PubMed] [Google Scholar]
- Salamon Z., Wang Y., Tollin G., Macleod H. A. Assembly and molecular organization of self-assembled lipid bilayers on solid substrates monitored by surface plasmon resonance spectroscopy. Biochim Biophys Acta. 1994 Nov 2;1195(2):267–275. doi: 10.1016/0005-2736(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anantharamaiah G. M. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992 Feb;33(2):141–166. [PubMed] [Google Scholar]
- Shapiro J. P., Law J. H., Wells M. A. Lipid transport in insects. Annu Rev Entomol. 1988;33:297–318. doi: 10.1146/annurev.en.33.010188.001501. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Singh T. K., Liu H., Bradley R., Scraba D. G., Ryan R. O. Effect of phospholipase C and apolipophorin III on the structure and stability of lipophorin subspecies. J Lipid Res. 1994 Sep;35(9):1561–1569. [PubMed] [Google Scholar]
- Smith A. F., Owen L. M., Strobel L. M., Chen H., Kanost M. R., Hanneman E., Wells M. A. Exchangeable apolipoproteins of insects share a common structural motif. J Lipid Res. 1994 Nov;35(11):1976–1984. [PubMed] [Google Scholar]
- Soulages J. L., Brenner R. R. Study on the composition-structure relationship of lipophorins. J Lipid Res. 1991 Mar;32(3):407–415. [PubMed] [Google Scholar]
- Soulages J. L., Rivera M., Walker F. A., Wells M. A. Hydration and localization of diacylglycerol in the insect lipoprotein lipophorin. A 13C-NMR study. Biochemistry. 1994 Mar 22;33(11):3245–3251. doi: 10.1021/bi00177a015. [DOI] [PubMed] [Google Scholar]
- Soulages J. L., Wells M. A. Effect of diacylglycerol content on some physicochemical properties of the insect lipoprotein, lipophorin. Correlation with the binding of apolipophorin-III. Biochemistry. 1994 Mar 8;33(9):2356–2362. doi: 10.1021/bi00175a002. [DOI] [PubMed] [Google Scholar]
- Soulages J. L., Wells M. A. Lipophorin: the structure of an insect lipoprotein and its role in lipid transport in insects. Adv Protein Chem. 1994;45:371–415. doi: 10.1016/s0065-3233(08)60644-0. [DOI] [PubMed] [Google Scholar]
- Van der Horst D. J. Lipid transport function of lipoproteins in flying insects. Biochim Biophys Acta. 1990 Dec 4;1047(3):195–211. doi: 10.1016/0005-2760(90)90518-3. [DOI] [PubMed] [Google Scholar]
- Wientzek M., Kay C. M., Oikawa K., Ryan R. O. Binding of insect apolipophorin III to dimyristoylphosphatidylcholine vesicles. Evidence for a conformational change. J Biol Chem. 1994 Feb 11;269(6):4605–4612. [PubMed] [Google Scholar]
- Wilson C., Wardell M. R., Weisgraber K. H., Mahley R. W., Agard D. A. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991 Jun 28;252(5014):1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- Wood S. A., Prasad K. U., Urry D. W. Cross-linked polypentapeptide of elastin as a calcifiable matrix: molecular weight dependence. Calcif Tissue Int. 1985 Sep;37(5):565–571. doi: 10.1007/BF02557843. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lewis R. N., McElhaney R. N., Ryan R. O. Calorimetric and spectroscopic studies of the interaction of Manduca sexta apolipophorin III with zwitterionic, anionic, and nonionic lipids. Biochemistry. 1993 Apr 20;32(15):3942–3952. doi: 10.1021/bi00066a014. [DOI] [PubMed] [Google Scholar]




