Abstract
The nucleus basalis is located at the confluence of the limbic and reticular activating systems. It receives dopaminergic input from the ventral tegmental area/substantia nigra, serotonergic input from the raphe nuclei, and noradrenergic input from the nucleus locus coeruleus. Its cholinergic contingent, known as Ch4, provides the principal source of acetylcholine for the cerebral cortex and amygdala. More than half of presynaptic varicosities along its cholinergic axons make traditional synaptic contacts with cortical neurons. Limbic and paralimbic cortices of the brain receive the heaviest cholinergic input from Ch4 and are also the principal sources of reciprocal cortical projections back to the nucleus basalis. This limbic affiliation explains the role of the nucleus basalis in modulating the impact and memorability of incoming sensory information. The anatomical continuity of the nucleus basalis with other basomedial limbic structures may underlie its early and high vulnerability to the tauopathy and neurofibrillary degeneration of Alzheimer's disease. The tauopathy in Ch4 eventually leads to the degeneration of the cholinergic axons that it sends to the cerebral cortex. The early involvement of Ch4 has a magnifying effect on Alzheimer's pathology, because neurofibrillary degeneration in a small number of neurons can perturb neurotransmission in all cortical areas. Although the exact contribution of the Ch4 lesion to the cognitive changes of Alzheimer's disease remains poorly understood, the cholinergic circuitry of the nucleus basalis is emerging as one of the most strategically positioned and behaviorally consequential modulatory systems of the human cerebral cortex.
Indexing Terms: cholinergic circuitry, nucleus basalis, Alzheimer's disease
The human cerebral cortex contains at least 20 billion neurons spread over 2.5 m2 of surface area (Pakkenberg and Gundersen, 1997; Tramo et al., 1995). These neurons and the trillions of synaptic contacts through which they communicate are ultimately responsible for transforming simple sensations and muscle twitches into experiences, memories, and actions. Although most of the underlying detail remains to be clarified, it has become axiomatic that different parts of the cerebral cortex display different functional specializations and that these differences reflect regional variations of afferent and efferent connectivity. The afferent connections of each cortical area can be divided into two interacting superclasses: a set of corticocortical and specific thalamocortical inputs that conveys the factual content of incoming information (e.g., auditory vs. visual, face vs. word, visceral vs. exteroceptive), and a set of extrathalamic and nonspecific thalamic inputs that modulates the collative properties of these inputs. The extrathalamic contingent of this second set includes noradrenergic axons from the nucleus locus coeruleus, serotonergic axons from the midbrain raphe, dopaminergic axons from the ventral tegmental area, and cholinergic axons from the nucleus basalis. Each of these extrathalamic projections arises from a relatively small nucleus and becomes widely distributed throughout the cortical mantle, where it influences properties such as the synaptic impact, valence, fidelity, resonance, and perhaps even transcortical routing of the incoming information. The cholinergic component of this extrathalamic subset is by far the most extensive and has been implicated in the modulation of an ever-expanding set of behavioral states, including attention, arousal, memory, learning, and sleep (Berger-Sweeney et al., 1994; Croxson et al., 2011; Karczmar, 1975, 2007; Krnjevic, 1981; Sarter et al., 2009; Thiel et al., 2002). This component of cortical innervation attracted a great deal of research attention, especially in the 1980s and 1990s, a period that witnessed the rise and fall of the cholinergic era in Alzheimer's disease studies (AD; Mesulam, 2004).
On the occasion of this special issue celebrating the contributions of Gary Van Hoesen to neuroanatomy, I thought it would be fitting to prepare a review of this pathway, based predominantly on neuroanatomical experiments that started in collaboration with Gary and that continued for more than 2 decades in my Boston and Chicago laboratories. A comprehensive coverage of the rich literature on cholinergic systems is beyond the scope of this review, so notable limitations had to be imposed. First, the synopsis is heavily focused on the work of a single laboratory. Second, it is heavily focused on the human brain. Results in the monkey are reported only when analogous information is not available for the human brain, and nonprimate species are mentioned only if relevant data are not available for the monkey. Third, the review is heavily anatomical and provides a cursory coverage of the immensely complicated neurophysiology of cholinergic pathways. These important limitations notwithstanding, the facts that have been chosen for inclusion offer a unitary neuroanatomical overview of cortically projecting cholinergic pathways in the human brain and their response to AD.
Searching for the Source of Cortical Cholinergic Innervation
In the 1920s, Otto Loewi identified acetylcholine (ACh) as the cardioactive substance released by the vagus nerve (Loewi, 1921). It took many years of additional work to establish that ACh was also a neurotransmitter of the central nervous system. Even 50 years after Loewi's discovery, the cholinergic innervation of the cerebral cortex was poorly understood and even questioned (Silver, 1974). During that period cases of delirium in response to pilocarpine, amnesia in response to scopolamine, and electroencephalographic (EEG) slowing in response to atropine had strengthened the belief in the presence of cortical cholinergic neurotransmission just as histochemical observations in the rat and cat had started to point to the basal forebrain as its likely source (Jacobowitz and Palkovits, 1974; Krnjevic and Silver, 1965). The advent of axonally transported tracers in the early 1970s did, in fact, demonstrate monosynaptic projections from basal forebrain neurons to the cerebral cortex but without offering additional characterization of their transmitter characteristics (Divac, 1975; Kievit and Kuypers, 1975). The specific localization of cholinergic cell bodies that innervate the cerebral cortex was made possible by the combined visualization of the hydrolytic enzyme acetylcholinesterase (AChE) with retrogradely transported horseradish peroxidase (HRP; Fig. 1). Experiments based on this methodology in the macaque monkey showed that cortically injected HRP led to the retrograde labeling of AChE-rich basal forebrain neurons (Mesulam and Van Hoesen, 1976). In time, the sensitivity of the HRP method was improved, and AChE histochemistry was replaced by immunolabeling with the synthetic enzyme of ACh, choline acetyltrasferase (ChAT), for the more definitive identification of cholinergic neurons (Mesulam et al., 1986). These developments helped to clarify the anatomical organization of cortically projecting cholinergic pathways in the primate brain and to localize their neurons of origin within a family of basal forebrain cell groups that came to be known as the Chi-4 complex (Mesulam et al., 1983a, 1986).
Figure 1.
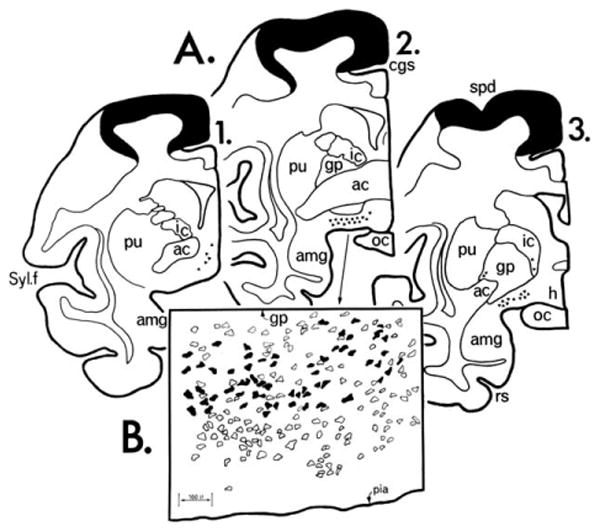
Three coronal sections from a rhesus monkey with HRP injected into Brodmann areas 4 and 6. A: The cortical areas within the injection site are blackened. Triangles represent nucleus basalis neurons. B: Camera lucida drawing from section 2 in A. Open profiles represent AChE-rich perikarya, blackened ones indicate double labeling of AChE-rich perikarya with retrogradely transported HRP. The original version of this figure was hand drawn in India ink by Gary Van Hoesen. ac, Anterior commissure; amg, amygdala; cgs, cingulate sulcus; gp, globus palidus; h, hypothalamus; ic, internal capsule; oc, optic chiasm; pu, putamen; rs, rhinal sulcus; spd, superior precentral dimple; Syl. f., Sylvian fissure. From Mesulam and Van Hoesen (1976) with permission.
Cytology of the Ch1-4 Complex in the Monkey and Human Brains
The Ch1-4 nomenclature was introduced to designate cholinergic (i.e., ChAT-containing) neurons within four overlapping cell groups of the basal forebrain (Mesulam and Geula, 1988; Mesulam et al., 1983a,b). In this nomenclature, Ch 1 designates the cholinergic cells associated predominantly with the medial septal nucleus, Ch2 those associated with the vertical limb of the diagonal band, Ch3 those associated with the horizontal limb of the diagonal band, and Ch4 those associated with the nucleus basalis of Meynert. In the macaque monkey, the Ch4 group is by far the largest of the basal forebrain cholinergic cell groups by volume and number of neurons and has been subdivided into several distinct sectors (Fig. 2).
Figure 2.
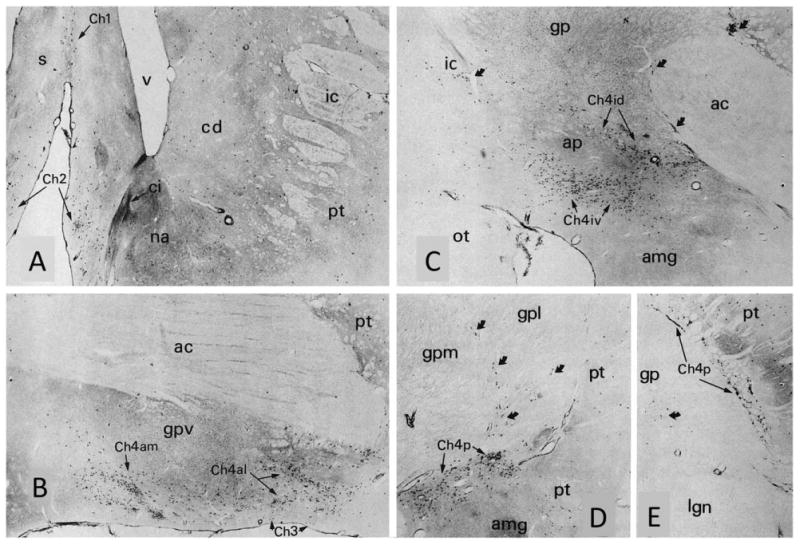
Choline acetyltransferase immunohistochemistry in the macaque monkey showing the Ch1-4 cell groups and anteromedial (am), anterolateral (al), intermediodorsal (id), intermedioventral (iv), and posterior (p) sectors of Ch4. Black dot-like profiles represent ChAT-positive neurons. The coronal sections (A-E) are from progressively more caudal levels of the brain. Medial is to the left, dorsal toward the top. Curved arrows point to interstitial components of Ch4 embedded in the internal capsule, anterior commissure, and borders of the globus pallidus. Note that the caudate, nucleus accumbens, and putamen also contain ChAT-positive cells. These project internally within the striatum and are not part of Ch1-4. ×24. ac, Anterior commissure; amg, amygdala; ap, ansa peduncularis; cd, caudate; ci, islands of Calleja; gp, gpl, gpm, gpv, globus pallidus and its lateral, medial, and ventral segments; ic, internal capsule; iml, internal medullary lamina of the globus pallidus; lg, lateral geniculate nucleus; ot, optic tract; pt, putamen. From Mesulam et al. (1984) with permission.
The human nucleus basalis is even more differentiated than that of the monkey (Mesulam and Geula, 1988). It displays the greatest concentration of neurons under the anterior commissure, in a region known as the substantia innominata, and can be subdivided into neuronal clusters that form anteromedial (am), anterolateral (al), anterointermediate (ai), intermediodorsal (id), intermedioventral (iv), and posterior (p) sectors (Figs. 3, 4). It extends from the level of the olfactory tubercle to that of the posterior amygdala, spanning a distance of 13-16 mm in the anteroposterior axis and attaining a mediolateral width of 18 mm within the substantia innominata. Arendt et al. (1985) have estimated that the human nucleus basalis contains 200,000 neurons in each hemisphere. This number is at least 10 times higher that the number of neurons in the human nucleus locus coeruleus (Vijayashankar and Brody, 1979). Cholinergic Ch4 neurons are intermingled with a heterogeneous population of noncholinergic neurons (Geula et al., 1993). The terms Ch4 and nucleus basalis are therefore not synonymous. The term nucleus basalis is used to designate all neuronal components of this nucleus, whereas the more restrictive Ch4 designation is reserved for the contingent of cholinergic neurons identified by ChAT immunohistochemistry (Fig. 5A). The proportion of cholinergic to noncholinergic neurons varies within the regions that contain the Ch1-4 cell groups. Nearly 90% of nucleus basalis neurons are cholinergic, whereas this ratio is much lower in the nuclei within which Ch1-3 are embedded.
Figure 3.
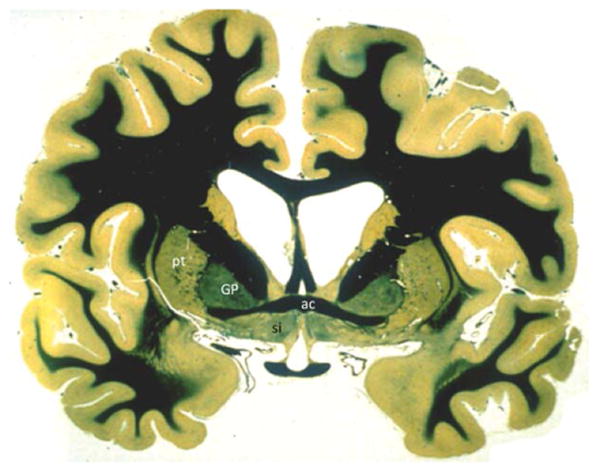
Bielschowsky myelin staining of a coronal section of the human brain. The region under the anterior commissure (ac) is also known as the substantia innominata (si) and contains the anterior sector of Ch4. GP, globus pallidus; pt, putamen.
Figure 4.
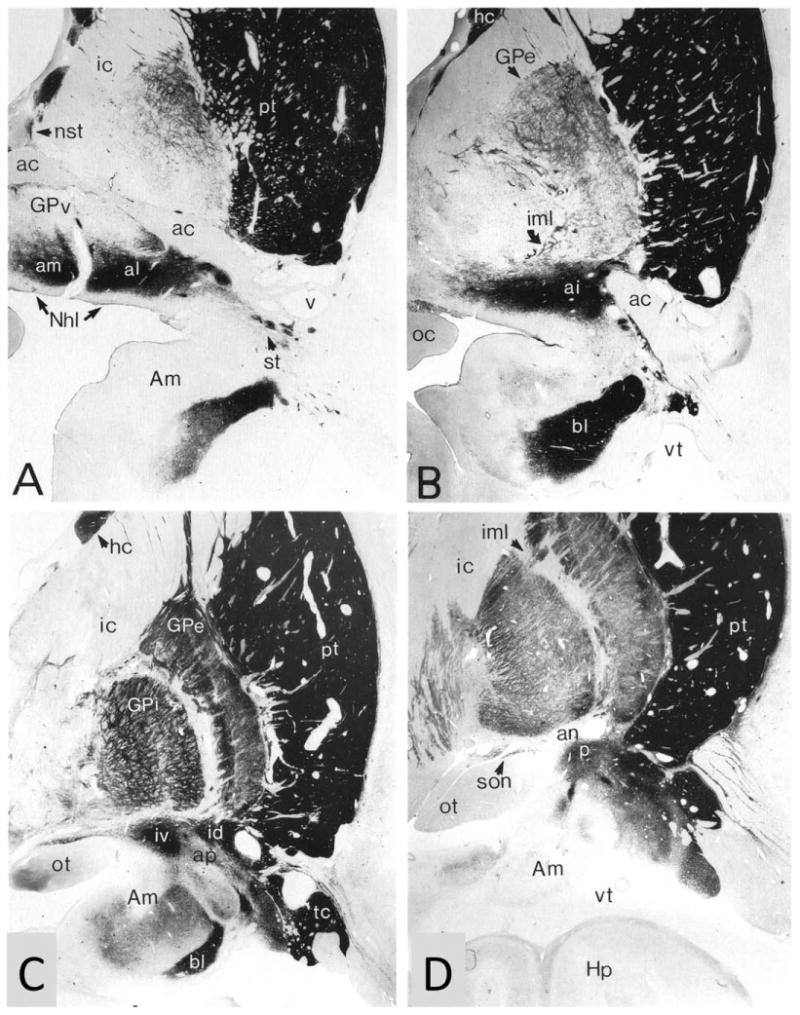
Acetylcholinesterase histochemistry was used in a 91-year-old control brain to delineate Ch4 from other components of the fore-brain. A-D represent increasingly more caudal coronal sections and contain the anteromedial (am), anterolateral (al), anterointermediate (ai), intermediodorsal (id), intermedioventral (iv), and posterior (p) sectors of Ch4. Medial is to the left, dorsal toward the top. Section A is at approximately the same level as Figure 3. ×5. ac, Anterior commissure; Am, amygdala; an, ansa lenticularis; ap, ansa peduncularis; bl, basolateral nucleus of the amygdala; GPe, GPi, GPv, external, internal, and medial sectors of the globus pallidus; he, head of the caudate; Hp, hippocampal formation; ic, internal capsule; iml, internal medullary lamina of the globus pallidus; Nhl, nucleus of the horizontal limb; nst, nucleus of the stria terminalis; ot, optic tract; pt, putamen; son, supraoptic nucleus; st, striatal islands; tc, tail of caudate; vt, temporal horn of lateral ventricle. From Mesulam and Geula (1988) with permission.
Figure 5.
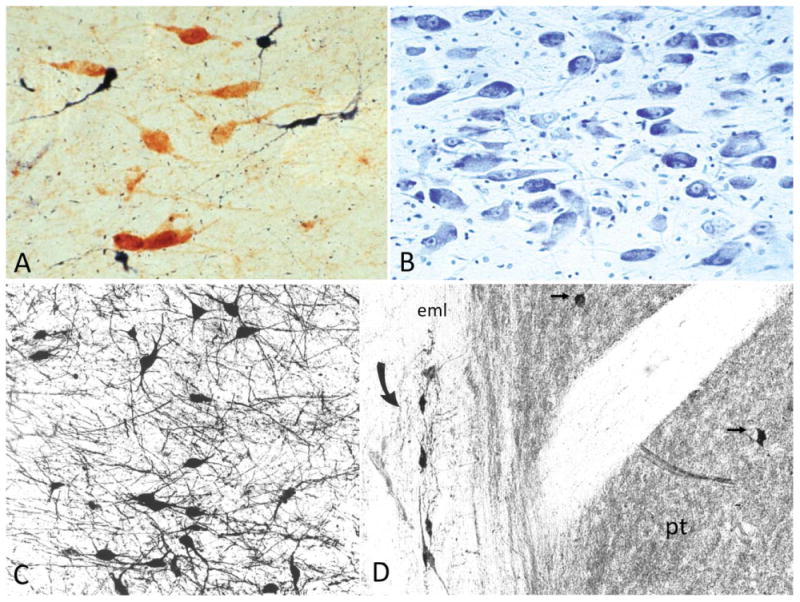
Cytological detail of the human nucleus basalis and Ch4. A: Cholinergic (ChAT-positive, brown) and noncholinergic (NADPH-positive, blue) neurons are intermingled in the nucleus basalis. The Ch4 designation applies only to the cholinergic contingent, whereas the term nucleus basalis refers to all neuronal populations in the nucleus. Control human brain. ×275. B: Cresyl violet stain of the nucleus basalis at the level of Figure 4A. Note the polymorphism, heterochromia, and prominent nucleoli of the magnocellular neurons. From a 60-year-old control brain. ×150. C: ChAT-positive cholinergic neurons in Ch4a. From an 82-year-old control brain. ×100. D: ChAT-positive interstitial cholinergic neurons (curved arrow) embedded within the white matter or the external medullary lamina of the globus pallidus. The abutting putamen (pt) is characterized by a ChAT-rich matrix and contains ChAT-positive neurons (straight arrows). The interstitial Ch4 neurons are larger and more elongated than the radially more symmetrical putaminal cholinergic neurons. Medial is to the left, dorsal to the top. From a 76-year-old control brain. ×120. eml, External medullary lamina of the globus pallidus.
There are no strict boundaries between the nucleus basalis and adjacent cell groups such as those of the olfactory tubercle, preoptic area, hypothalamic nuclei, nuclei of the diagonal band, amygdaloid nuclei, and globus pallidus. In addition to this “open” nuclear structure, reminiscent of the brainstem reticular system, the neurons of the human nucleus basalis display physiological and morphological heterogeneity. They are generally magnocellular and hyperchromic and have prominent nucleoli (Fig. 5B). Perikaryal shapes range from complex multipolar to fusiform and pyramidal. Dendritic trees arborize profusely, overlap with each other, extend into fiber tracts traversing the basal fore-brain, and do not display a common orientation (Fig. 5C). In addition to the compact sectors located within the nucleus basalis, Ch4 also contains interstitial neurons embedded within the anterior commissure, internal and external medullary laminae of the globus pallidus, ansa peduncularis, ansa lenticularis, and even the internal capsule (Figs. 4, 5D). The Ch4 neurons of the human brain also express AChE, the vesicular acetylcholine transporter, calbindin-d28k, high-affinity nerve growth factor receptor trkA, and low-affinity p75 nerve growth factor receptor (NGFr; Geula et al., 1993; Gilmor et al., 1999; Kordower et al., 1994; Mufson et al., 1989).
The noncholinergic neurons of the septum, diagonal band nuclei, and nucleus basalis have been studied most intensively in the rodent brain, where they have been shown to be γ-aminobutyric acid (GABA)-ergic, glutamatergic, peptidergic, and tyrosine hydroxylase (TH)-positive (Gouras et al., 1992; Gritti et al., 1993; Henderson, 1987; Henny and Jones, 2008; Mesulam et al., 1989; Walker et al., 1989; Wisniowski et al., 1992). Some of the noncholinergic neurons are inter-neurons; others project to the cerebral cortex (Freund and Meskenaite, 1992; Gritti et al., 1993). Noncholinergic NADPH-positive and peptidergic neurons have been identified in the human nucleus basalis as well (Geula et al., 1993; Mufson et al., 2003). The transmitter profiles and projection patterns of noncholinergic neurons in the human septum, diagonal band nuclei, and nucleus basalis remain mostly unknown.
Cortical Cholinergic Projections from the Nucleus Basalis
Tracer experiments based on the retrograde labeling of cholinergic perikarya in the monkey brain show that Ch1 and Ch2 provide the major cholinergic innervation for the hippocampal complex and the hypothalamus, Ch3 for the olfactory bulb, and Ch4 for the rest of the cerebral cortex and the amygdala (Mesulam et al., 1983a, 1986). Experiments with the rat show that individual nucleus basalis neurons have restricted projection fields of 1-1.5 mm in diameter (Price and Stern, 1983). In the monkey, retrograde labeling with multiple fluorescent tracers has failed to show collateralization of nucleus basalis projections to interconnected cortical areas (Morecraft et al., 1993). In keeping with this relative specificity of projection targets, individual cortical areas have been shown to receive their major cholinergic input from different sectors of the Ch4 complex. For example, Ch4am provides the major source of cholinergic input to medial cortical areas, including the cingulate gyrus; Ch4al to frontoparietal cortex, opercular regions, and the amygdaloid nuclei; Ch4i to laterodorsal frontoparietal, peristriate, and midtemporal regions; and Ch4p to the superior temporal and temporopolar areas (Mesulam et al., 1983a). The topography is nowhere as differentiated as in the projection fields of specific thalamic nuclei, and there is a great deal of overlap.
The detailed topography of Ch4 projections to the human cerebral cortex remains largely unknown. However, the white matter trajectories of cholinergic axons can be traced as they course from the nucleus basalis-Ch4 complex to the cerebral cortex. These fibers contain AChE, ChAT, and NGFr. Because the only NGFr-positive perikarya of the primate forebrain are located in Ch1-4, axonal NGFr immunoreactivity identifies a fiber as both cholinergic and also of basal forebrain origin. The examination of whole-hemisphere sections labeled with these cholinergic markers helped to identify a medial and lateral cholinergic NGFr-positive pathway coursing from the basal forebrain to the cortical mantle (Selden et al., 1998). The medial pathway joins the white matter of the gyrus rectus; curves around the rostrum of the corpus callosum; enters the cingulum bundle; and supplies the parolfactory, cingulate, and retrosplenial cortices. The lateral pathway has a capsular division traveling in the external capsule and uncinate fasciculus and a perisylvian division traveling within the claustrum and extreme capsule. Branches of the perisylvian division supply the frontoparietal operculum, insula, and superior temporal gyrus, whereas branches of the capsular division supply the remaining parts of the frontal, parietal, and temporal neocortex (Fig. 6). Diffusion tensor imaging appears to allow the general trajectory of some of these pathways to be visualized in vivo (Hong and Jang, 2010; Teipel et al., 2011).
Figure 6.
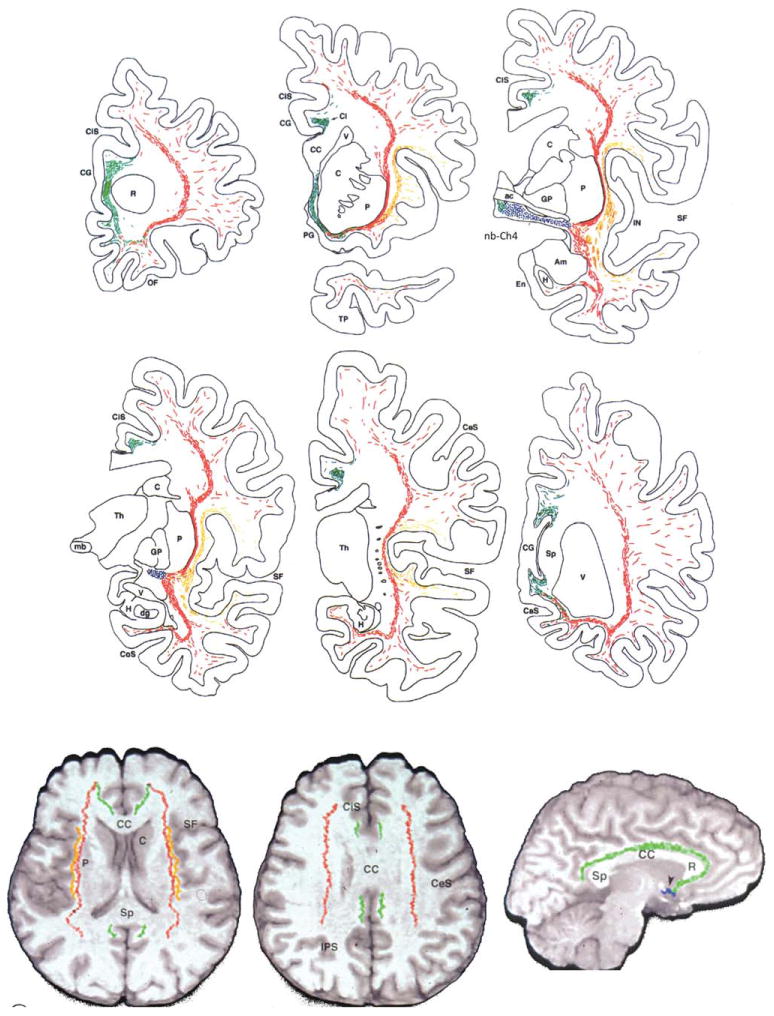
Trajectory of Ch4 projections. Top two rows show electronic plotter maps of cholinergic fibers in the white matter of the human brain as identified in whole-hemisphere sections processed for AChE histochemistry and ChAT and NGFr immunocytochemistry. Sections represent increasingly more caudal coronal levels. The Ch4 neurons are represented in blue, the medial cholinergic pathway in green, the capsular branch of the lateral pathway in red and the perisylvian branch of the lateral pathway in orange. The bottom row shows a reconstruction of the trajectories in a 3D volume of a control magnetic resonance image (MRI) of the whole brain. The two figures at left show axial views and the third at right a parasaggital view, ac, Anterior commissure; Am, amygdala; C, caudate; CaS, calcarine sulcus; CC, corpus callosum; CeS, central sulcus; Ci, cingulum; CiS, cingulate sulcus; CoS, collateral sulcus; dg, dentate gyrus; En, entorhinal cortex; GP, globus pallidus; H; hippocampus; IN, insula; mb, mammillary body; OF, orbitofrontal cortex; P, putamen; PG, parolfactory gyrus; R, rostrum of corpus callosum; SF, Sylvian fissure; Sp, splenium of corpus callosum; Th, thalamus; TP, temporal pole; V, ventricle. From Selden et al. (1998) with permission.
There are no intrinsic cholinergic neurons in the human cerebral cortex, amygdala, or hippocampus. In cortical areas where this has been investigated, ChAT-positive and NGFr-positive axons tend to show similar patterns of distribution and density, supporting the conclusion that the cholinergic innervation of the cerebral cortex originates almost exclusively from Ch1-4 (Mesulam et al., 1992b). In the rat, a population of Ch4 neurons is NGFr negative and preferentially innervates the nucleus of the lateral olfactory tract and the basolateral nucleus of the amygdala (Heckers and Mesulam, 1994). An analogous organization may exist in the human as suggested by the relatively low ratio of NGFr-positive to ChAT-positive axons in the basolateral nucleus of the amygdala (Mufson et al., 1989).
Cholinergic Neurons of the Striatum and Upper Brainstem
The human putamen and caudate contain multipolar ChAT-positive neurons with extensive dendritic ramifications. They correspond in size and morphology to the large, aspiny striatal cholinergic neurons that have also been identified in the monkey (DiFiglia, 1987; Selden et al., 1994; Yelnik et al., 1991). These neurons are embedded in a dense matrix of cholinergic processes and project only within the striatum (Fig. 5D). Additional cholinergic neuronal groups are located in the regions of the pedunculopontine and laterodorsal tegmental nuclei of the brainstem. Each of these nuclei contains a mixture of cholinergic and noncholinergic neurons. The group of cholinergic neurons centered around the pedunculopontine nucleus is designated Ch5, and the group of cholinergic neurons centered around the laterodorsal tegmental nucleus is designated Ch6 (Mesulam et al., 1989). Retrograde transport experiments in the rat show the vast majority of cholinergic labeling to be within Ch5-6 following tracer injections in the thalamus (Mesulam et al., 1983b). These two cell groups appear to provide the major cholinergic innervation for thalamic nuclei and therefore constitute a major brainstem component of the ascending reticular activating system. In the rat and monkey, cortical injections yield only minor retrograde labeling in Ch5-6 (Mesulam et al., 1983b). The brainstem contribution to cortical cholinergic innervation is therefore likely to be quite small.
Although the vast majority of the striatal cholinergic innervation originates intrinsically from the large aspiny ChAT-positive neurons, the striatum also receives a weak NGFr-positive cholinergic input from Ch1-4. This is more prominent in the putamen than in the caudate. In the human thalamus, which also lacks intrinsic cholinergic neurons, the majority of the cholinergic innervation is NGFr negative and can be assumed to originate in Ch5-6. However, there is an additional NGFr-positive contingent of cholinergic afferents in the thalamus, especially within the intralaminar, reticular, and mediodorsal nuclei (Heckers et al., 1992b). These nuclei are thus under dual cholinergic influence, raising the possibility that nucleus basalis neurons can influence thalamic function as well. In keeping with this possibility, nucleus basalis stimulation in the rat has been shown to facilitate thalamocortical synaptic transmission (Metherate and Ashe, 1993).
Inputs into the Nucleus Basalis
Electron microscopy of immunostained sections in the monkey showed that dendrites of Ch4 neurons make synaptic contact with cholinergic (ChAT-positive), GABAergic (GAD-positive), and dopaminergic (TH-positive) terminals (Fig. 7A-C; Smiley and Mesulam, 1999). The cholinergic contacts form predominantly large, asymmetric synapses and could represent local collaterals or projections from Ch5-6. The GABAergic input occurs through mostly symmetric synapses and could represent input from inhibitory neurons within the nucleus basalis or extrinsic afferents. The human nucleus basalis contains numerous varicose axons that are TH positive (dopaminergic), serotonergic, and dopamine β-hydroxylase (DBH) positive (noradrenergic; Fig. 7D-F; Smiley et al., 1999). These axons likely represent afferents from the dopaminergic neurons of the ventral tegmental area/substantia nigra, the serotonergic neurons of the midbrain raphe, and the noradrenergic neurons of the nucleus locus coeruleus. In the rat, each of these nuclei as well as the cholinergic Ch5-6 cell groups has been shown to project to Ch4 (Jones and Cuello, 1989). Galanin, glutamate, and estrogen receptors have also been reported for the human Ch4 (Mufson et al., 2003). The glutamatergic receptors likely represent the postsynaptic component of extrinsic afferents to Ch4.
Figure 7.
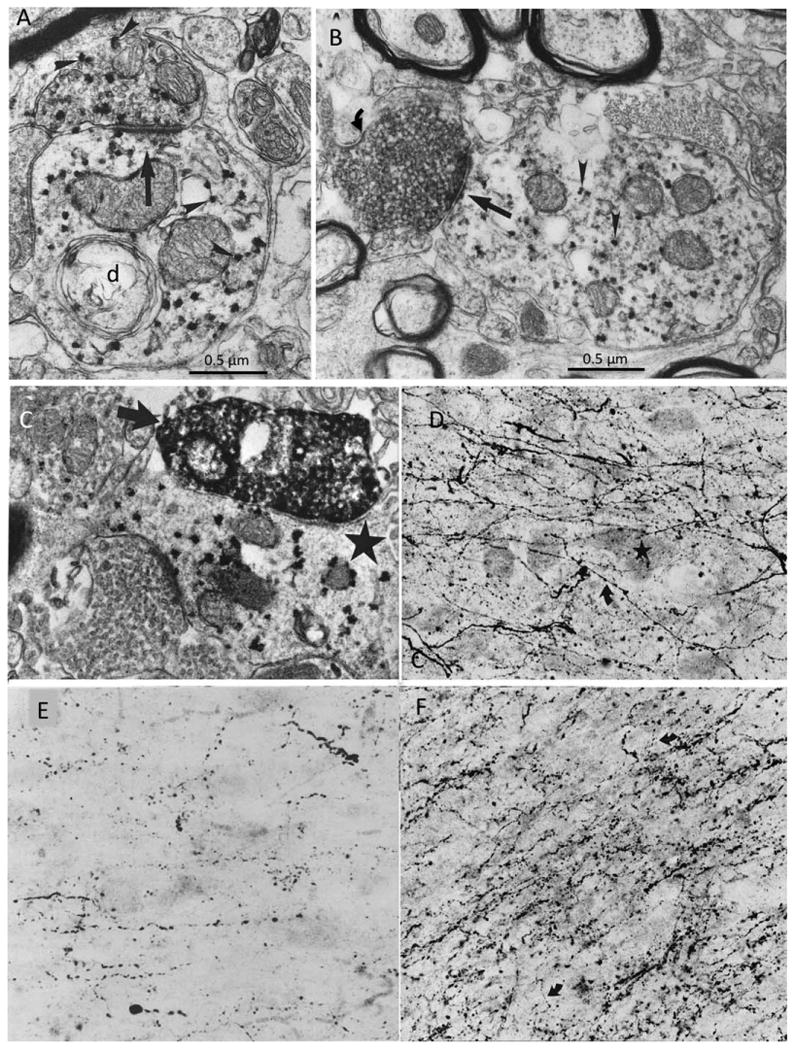
Transmitter-specific input into the monkey and human Ch4. A: ChAT immunoreactivity is visualized with the punctate VIP red reaction product (arrowheads) in the macaque monkey nucleus basalis. The ChAT axon on top is forming an asymmetric synapse (straight arrow) onto a postsynaptic ChAT dendrite (d) in the monkey Ch4. B: Double labeling of GAD (with diffuse DAB reaction product) and ChAT (with punctate VIP red reaction product) shows a GAD-positive GABAergic bouton (curved arrow) making a symmetric synapse (straight arrow) onto a nucleus basalis dendrite containing ChAT (arrowheads) in the macaque monkey brain. C: Double labeling of TH (with the diffuse DAB reaction product) and ChAT (with the punctate VIP red reaction product) showing a TH-immunoreactive bouton (arrow) forming a fine synapse onto a ChAT-immunoreactive nucleus basalis dendrite (star) in the macaque monkey. ×34,000. D: TH immunohistochemistry in the Ch4i sector of an autopsy specimen from a 27-year-old man showing multiple fine, varicose, TH-positive dopaminergic axons (curved arrow) coursing through unlabeled Ch4 perikarya (star). ×250. E: Serotonin immunohistochemistry showing serotonergic axonal varicosities in the Ch4a sector of the human brain. ×250. F: DBH immunohistochemistry in the Ch4i sector of an 82-year-old brain shows a dense plexus of thick and thin, varicose noradrenergic axons (curved arrows) coursing through the nucleus basalis perikarya. ×250. From Smiley and Mesulam (1999) and Smiley et al. (1999) with permission. Scale bars = 0.5 μm.
All cortical areas receive Ch4 projections, but not all project back to Ch4. In the monkey, tritiated amino acid injections in 37 cerebra showed anterograde transport to Ch4 from piriform cortex, orbitofrontal cortex, frontopolar cortex, anterior insula, temporal pole, entorhinal cortex, and perhaps anterior cingulate (Fig. 8). In contrast, tracer injections in parietal cortex, peristriate cortex, lateral temporal cortex, and dorsolateral prefrontal cortex did not results in anterograde transport to Ch4 (Mesulam and Mufson, 1984). It appears, therefore, that the cortical inputs to Ch4 are very selective and that they originate in areas with predominantly limbic-paralimbic affiliations. In keeping with this pattern of connectivity, Ch4 also receives inputs from the hypothalamus and amygdala (Mesulam and Mufson, 1984; Price and Amaral, 1981). Through this circuitry, the Ch4 cell group can influence cholinergic transmission in all parts of the cerebral cortex in a manner that is selectively sensitive to neural activity within components of the limbic system. In keeping with this putative role, single-unit recordings in the monkey show that the nucleus basalis neurons are selectively sensitive to motivational relevance (DeLong, 1971; Wilson and Rolls, 1990). The selectivity of cortical projections to the nucleus basalis has not been documented in the human brain. This question could potentially be addressed through the postmortem visualization of anterograde degeneration in cases with focal cortical lesions.
Figure 8.
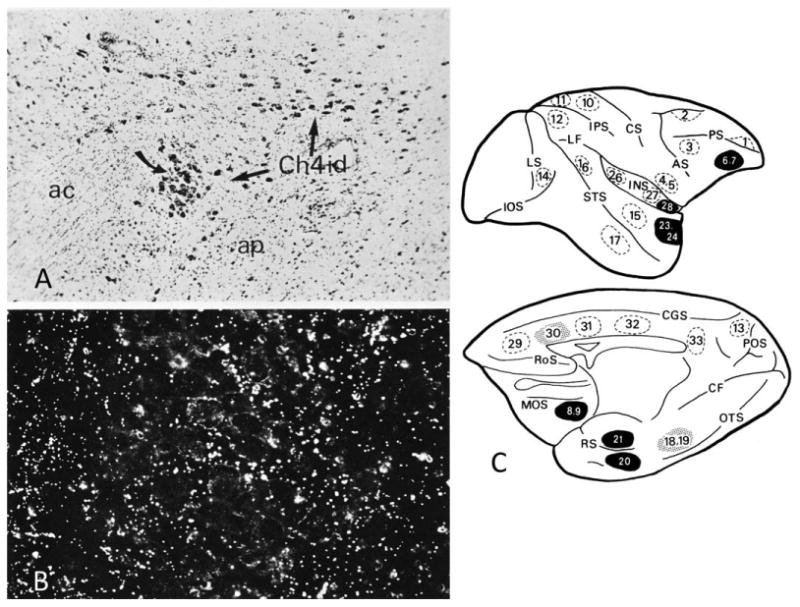
Projections into the Ch4 of macaque monkeys following tritiated amino acid injections into entorhinal cortex. A: Brightfield view at ×70 of Ch4id (arrows) and their relationship to the anterior commissure (ac) and ansa peduncularis (ap). B: Darkfield view at ×500 of anterogradely transported tracer (white dots) within the cell island designated by the curved arrow in A. C: Tritiated amino acid injection sites. Those that resulted in definite projections to the nucleus basalis are shown in black, and those that resulted in questionable projections are stippled. Dashed circles represent sites where injections did not cause anterograde transport to the nucleus basalis. Numbers refer to individual cases, not to architectonic designations. AS, arcuate sulcus; CF, calcarine fissure; CGS, cingulate sulcus; CS, central sulcus; IOS, inferior occipital sulcus; IPS, intraparietal sulcus; LF, lateral fissure; LS, lunate sulcus; MOS, medial orbitofrontal sulcus; OTS, occipitotemporal sulcus; POS, parietooccipital sulcus; PS, sulcus principalis; RoS, Rostral sulcus; RS, rhinal sulcus; STS, superior temporal sulcus. From Mesulam and Mufson (1984) with permission.
Regional Variations of Cortical Cholinergic Innervation
Cortical cholinergic fibers are unmyelinated, enter the cortical mantle through the underlying white matter, and undergo branching as they course toward the pial surface (Aston-Jones et al., 1985; Mesulam et al., 1992a). In the human brain, the axons contain multiple varicosities that represent transmitter release sites (Fig. 9). They also display an orderly gradient of density that mirrors the hierarchical organization of pathways that link sensory areas to association and limbic regions of the brain (Mesulam and Geula, 1992; Mesulam et al., 1992a). According to tract tracing experiments in the monkey brain, primary sensory areas first project to upstream (parasensory) unimodal areas, which then project to downstream unimodal areas and heteromodal cortex. In turn, heteromodal association areas and downstream sectors of unimodal association areas provide the major sources of sensory information into para-limbic and limbic areas of the brain (Mesulam, 1998; Pandya and Yeterian, 1985; Van Hoesen et al., 1972). Observations in the human brain show that the density of cholinergic innervation is lower within primary, unimodal, and heteromodal association areas then in paralimbic areas of the brain (Fig. 10A vs. B). In the unimodal areas, moreover, the downstream sectors tend to have a higher density of cholinergic innervation than the upstream (parasensory) sectors. For example, the posterior part of area 22, which is an upstream auditory association area, has a lower density of cholinergic fibers than the anterior part of area 22, which is a downstream auditory association area (Fig. 10C vs. D). Even within the same anatomical region, such as orbitofrontal cortex, homotypical (granular) sectors of association cortex have a lower cholinergic fiber density than immediately adjacent dysgranular sectors that are synaptically closer to core limbic structures (Fig. 11). Core limbic areas such as the hippocampus and especially the amygdala contain the highest densities of cholinergic innervation. This gradient of densities suggests that sensory information is likely to come under progressively greater cholinergic influence as it is conveyed along the multisynaptic pathways leading to the limbic system. This is consistent with the modulatory role of cortical cholinergic innervation, insofar as cortical areas that occupy more downstream levels of the sensory-limbic hierarchy are known to play a gradually more important role in the attentional, emotional, and motivational editing of sensory experience.
Figure 9.
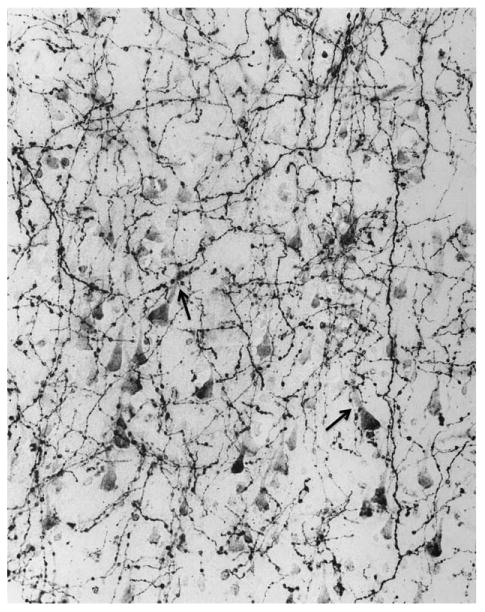
This photomicrograph of AChE histochemistry based on a modified Karnovsky-Roots method shows cholinergic axons in layer 3 of inferotemporal cortex in the brain of a 22-year-old control specimen. The multiple varicosities represent putative sites of ACh release and are closely associated with AChE-rich cholinoceptive pyramidal neurons. The arrows point to two examples where the varicosities are apposed to the apical dendrite of cholinoceptive neurons. ×415. From Mesulam and Geula (1988) with permission.
Figure 10.
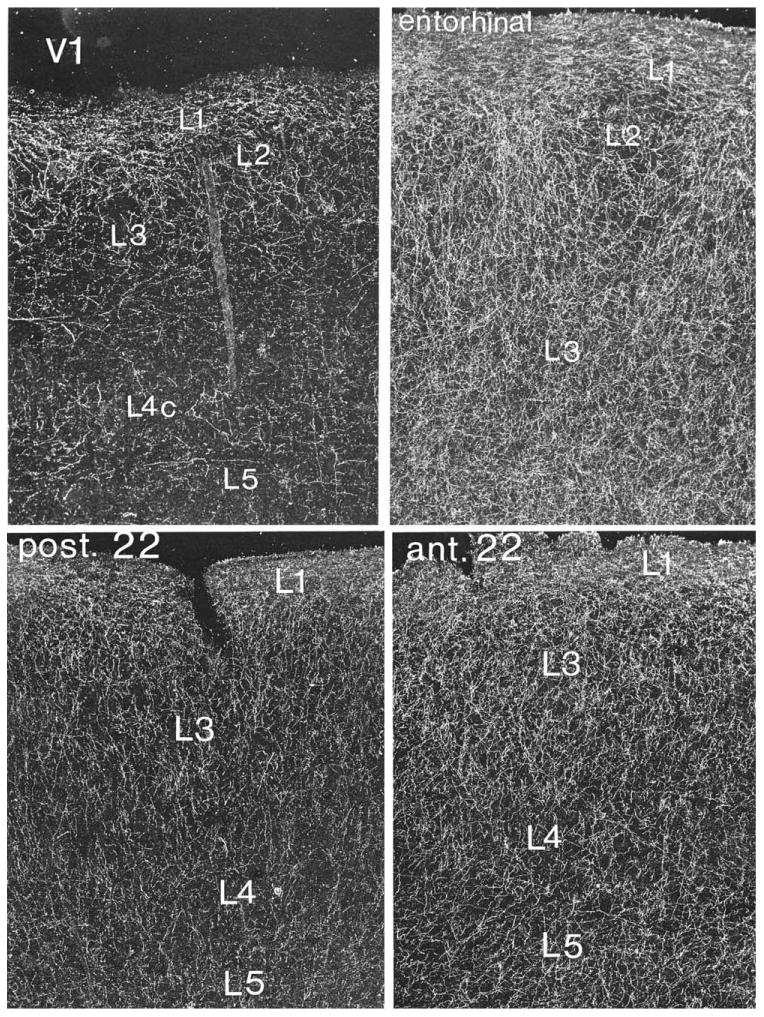
Darkfield photomicrograph of ChAT-positive axons in the human cerebral cortex illustrating regional variations in the density of afferents from Ch4. V1 designates primary visual cortex; post. 22 designates the posterior part of Brodman area 22 where initial synaptic relays in the hierarchy of auditory association pathways are located; ant. 22 designates a synaptically more downstream component of this hierarchy. ×45. From Mesulam et al. (1992a) with permission.
Figure 11.
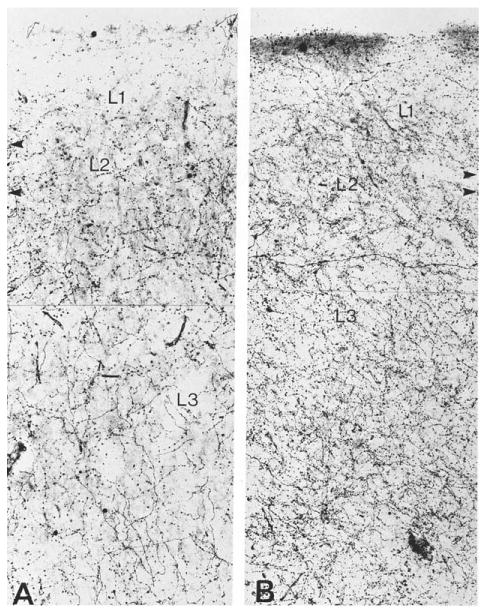
Brightfield photomicrograph of ChAT-positive axons in the granular (A) and dysgranular (B) sectors of the human orbitofrontal cortex. The density is higher in the dysgranular sector, which is synaptically closer to core limbic structures. Arrowheads mark laminar boundaries. ×266. From Mesulam et al. (1992a) with permission.
The ACh that is released by cholinergic axons exerts its influence on the cerebral cortex by interacting with multiple species of metabotropic muscarinic and ionotropic nicotinic receptors. Antibodies raised against heterogeneous muscarinic and nicotinic receptor preparations have revealed large numbers of immunopositive, presumably cholinoceptive, neurons in the human cerebral cortex (Schröder et al., 1989, 1990). The pharmacologically defined M1 (or molecularly identified ml) subtype of muscarinic receptor is the most numerous species of cholinergic receptor in the mammalian cerebral cortex. At postsynaptic M1 receptors, a major action of ACh is to trigger a relatively prolonged suppression of potassium conductance that presumably makes the cholinoceptive neuron more responsive to other excitatory inputs (McCormick and Prince, 1985). This mode of action is consistent with the modulatory role attributed to cholinergic innervation. Most cholinergic varicosities in the rodent are not associated with identifiable synapses, leading to the inference that cholinergic activity may rely predominantly on volume transmission (Umbriaco et al., 1994; Yamasaki et al., 2010). The situation seems to be different in the human brain. In the temporal cortex, an ultrastructural investigation of serial sections immunostained for ChAT showed that 67% of ChAT-positive varicosities established synaptic specializations (Fig. 12). These were usually quite small and symmetrical and were located predominantly on the dendritic shafts and spines of ChAT-negative pyramidal neurons (Smiley et al., 1997).
Figure 12.
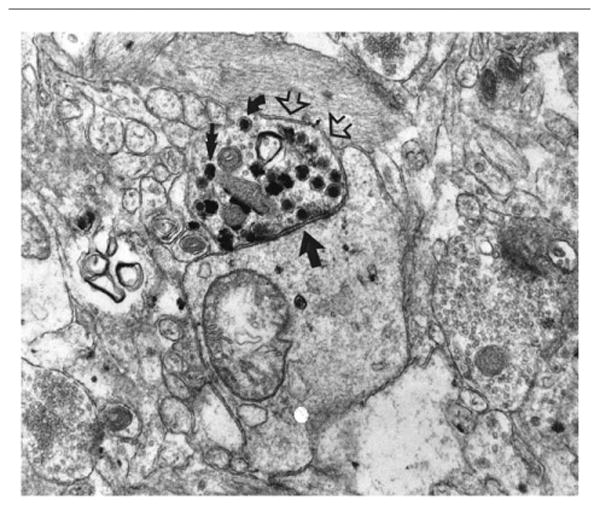
Surgically removed temporal lobectomy specimen from a 20-year-old subject. The electronmicrograph shows a ChAT-positive varicosity labeled with the VIP reaction product (open arrows) forming a fine symmetric synapse (single straight arrow) with an unlabeled (noncholinergic) dendrite. ×32,000. From Smiley et al. (1997) with permission.
The cerebral cortex contains several sets of cholinoceptive neurons. Some are interneurons, others projection neurons. Some are excitatory, others inhibitory (McCormick and Prince, 1985). In the human, a subset of cholinoceptive pyramidal neurons is characterized by an AChE-rich reaction that displays a unique developmental pattern (Kostovic et al., 1988; Mesulam and Geula, 1991). The AChE-rich histochemical pattern in these neurons appears to be absent in childhood, emerges during adolescence, and seems to increase in intensity in adulthood and old age (Fig. 13). The physiological significance of this developmental change is unknown but suggests the presence of age-related changes in the way in which the cerebral cortex reacts to ACh. This group of AChE-rich pyramidal neurons is much less conspicuous in monkey and rat brains.
Figure 13.
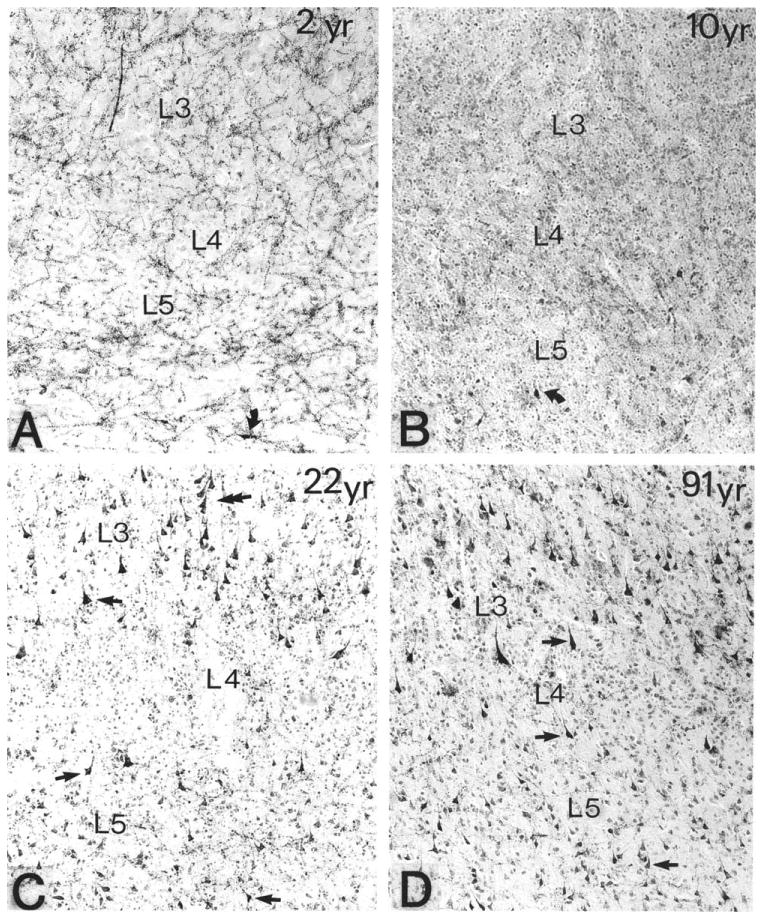
Age-related differences in the density of AChE-rich neurons in the banks of the superior temporal sulcus in four human brains that came to postmortem examination at 2, 10, 22, and 91 years of age (A-D, respectively). The histochemical reaction was obtained with a modified Koelle-Friedenwald method, which provides better visualization of cell bodies than of axons. Straight arrows point to AChE-rich neurons. ×100. From Mesulam and Geula (1991) with permission.
Vulnerability to AD
Aging is the single most important risk factor for the extracellular deposition of β-amyloid plaques and the intracellular formation of neurofibrillary tangles (NFTs), the two critical markers of Alzheimer's pathology. The relationship of the amyloidopathy to the tauopathy that underlies NFT formation remains conjectural, especially in late-onset and sporadic forms of the disease (Jack et al., 2013; Mesulam, 1999). Although the amyloidopathy undoubtedly contributes to the overall pathogenic cascade, regional variations of NFT densities are much more closely correlated with patterns of neuronal loss and resultant clinical impairments than regional variations in plaque density (Gefen et al., 2012; Gómez-lsla et al, 1997).
The neuropathological components of AD display a complex temporal evolution. An initial asymptomatic period during which amyloid plaques and few NFT emerge in the absence of detectable clinical consequences is followed by an intermediate stage of intensifying pathology accompanied by mild cognitive impairments (MCI). The final stage is characterized by major behavioral and cognitive impairments together with widespread plaque and tangle accumulations.
In the vast majority of cases, NFTs are first detected in basotemporal regions of the brain and then gradually spread into surrounding cortical areas as if they were propagating along corticocortical axonal projection pathways (Braak and Braak, 1996; de Calignon et al., 2012; Liu et al., 2012; Mesulam, 1999). Within the context of this evolution, the nucleus basalis-Ch4 complex stands out as one of the most vulnerable structures to neurofibrillary degeneration (Mesulam et al., 2004; Sassin et al., 2000). In postmortem specimens at early and presymptomatic stages of Alzheimer's pathology, the nucleus basalis contains NFT densities that are as high as, and sometimes even higher than, those of any other region in the brain (Fig. 14A,B). The nucleus basalis can thus be included within an uninterrupted band of nonisocortical basotemporal areas such as the entorhinal region, hippocampal formation, amygdala, and piriform cortex, where the tauopathy and neurofibrillary degeneration of Alzheimer's pathology originate and from which they seem to spread centrifugally toward other parts of the cerebral cortex.
Figure 14.
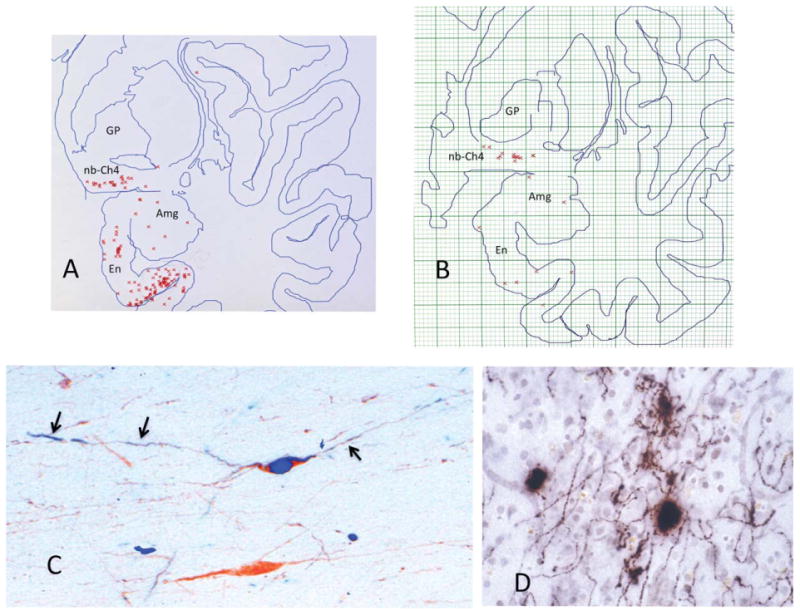
Early neurofibrillary degeneration of Ch4. A,B: Raw data obtained through the electronic plotter mapping of thioflavin-S stained whole-hemisphere sections in two autopsy cases aged 71 (A) and 72 (B). Neither had dementia or neurological disease. Each red cross marks a single NFT. These plots show that the nucleus basalis is at least as vulnerable to NFT formation as the entorhinal cortex and the amygdala. In A, there were no NFTs outside of the medial temporal lobe in that section. In B, the one additional NFT was encountered in the dorsal insula. C: Double immunostaining for ChAT (brown) and early neurofibrillary degeneration labeled with AT-8 (blue), an antibody that recognizes abnormally phosphorylated tau at early stages of neurofibrillary degeneration. The neuron on top is alive but undergoing neurofibrillary degeneration, whereas the one on the bottom is spared. The tauopathy has extended into the processes of the affected neuron (arrows). D: AChE histochemistry with a modified Karnovsky-Roots method in peristriate cortex of a 99-year-old woman who had no known dementia. Varicose cholinergic axons display dystrophic swollen profiles, which could represent early stages of cortical cholinergic degeneration and perhaps also aberrant attempts at regeneration. ×650. Amg, amygdala; En, entorhinal cortex; GP, globus pallidus; nb, nucleus basalis.
The tauopathy and neurofibrillary degeneration in Ch4 undergo several stages of severity (Sassin et al., 2000). Initially, the Ch4 cell body accumulates hyper-phosphorylated tau that can be identified immunohistochemically by antibodies such as AT-8. In time, the abnormal tau undergoes conformational changes and aggregation into beta-pleated intracellular NFTs that bind thioflavin-S. There is a period of unknown length during which abnormally phosphorylated tau, pretangles, and early tangles exist within a surviving but undoubtedly ailing neuron. The neuron eventually dies and leaves behind an extracellular ghost tangle.
As the perikaryal neurofibrillary pathology progresses during the initial stages of this evolution, the abnormal tau invades neuronal processes emanating from the perikaryon (Fig. 14C). This is presumably the stage at which the cortically directed cholinergic axons of the affected Ch4 perikarya start to undergo degeneration. Although this is not observed in every case, the initial stages of this degeneration may include dystrophic swellings along the intracortical segment of the cholinergic Ch4 axon (Fig. 14D). This early dystrophy of the cholinergic axon may represent degeneration as well as attempts at regeneration, a possibility in keeping with the reorganization of AChE fibers and paradoxical increases of ChAT activity that have been reported at some stages of AD (DeKosky et al., 2002; Geula et al., 2008; Hyman et al., 1987).
The timing of Ch4 tauopathy and its potential relevance to cognitive function have been explored in postmortem specimens from subjects who were rigorously assessed in life and who died at mild stages of Alzheimer's pathology (Fig. 15). Such investigations have shown low numbers of NFT in the nucleus basalis even in elderly individuals with no detectable cognitive impairment, whereas higher numbers are associated with mild cognitive impairments and early dementia. At the early stages of pathology illustrated in Figure 15, most nucleus basalis neurons are unaffected by tauopathy and many of the affected neurons remain alive as shown by the persistence of ChAT immunoreactivity (Fig. 14C). In fact, Ch4 neuronal counts in patients with MCI and mild AD may appear normal, although quantitative structural MRI at those stages can detect early pathology in the from of nucleus basalis and substantia innominata atrophy (Gilmor et al., 1999; Grothe et al., 2012). Although the data shown in Figure 15 cannot establish causality, they do illustrate the high degree of vulnerability of Ch4 to tauopathy within the age-MCI-AD continuum and also imply that the progression of neurofibrillary degeneration in this nucleus may have a functionally significant impact on cognitive function, even at early stages of Alzheimer's pathology (Mesulam et al., 2004; Vana et al., 2011).
Figure 15.
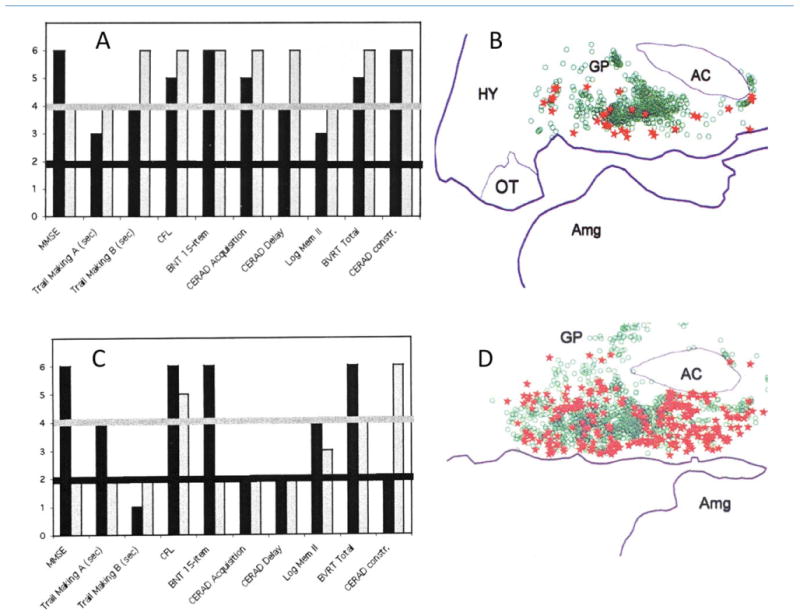
Cognitive correlates of tangles in the nucleus basalis. A and B are from a patient who died at the age of 95 years. A shows her test scores 3 years (black bars) and 4 months (gray bars) before death. On this graph, a level of 1 or 2 indicates performance that is impaired for age, levels 3 and 4 performance that is normal for age, and level 5 or 6 performance that is superior for age. In this patient, none of the scores was abnormal at either of the testing sessions. She was considered cognitively unimpaired. B, based on electronic plotting of stained tissue sections, shows normal neurons in her nucleus basalis (green circles) as well as those that contain NFT (red stars). C and D are from a patient who died at the age of 91 years. He was tested 2 years (black bars) and then 1 year (gray bars) before death. C shows that there has been a decline in performance and that he had multiple cognitive impairments (scores at level 2) at the last testing. Clinical notes indicate that the patient had progressed from a stage of mild cognitive impairment (MCI) at the first testing to early dementia of the AD type in the second. D shows his nucleus basalis at postmortem. Normal neurons are shown in green and neurons with NFT in red. Abbreviations for tests in frames A and C (from left to right): MMSE, Mini-Mental State Examination; Trail Making A and B, tests of executive function; CFL, test of verbal fluency; BNT, Boston naming test; CERAD Acquisition and Delay, tests of memory for words; Log Mem II, recall of a short story; BVRT, Benton visual retention test; CERAD constr., visuomotor test. AC, anterior commissure; Amg, amygdala; GP, globus pallidus; HY, hypothalamus; OT, optic tract. From Mesulam et al. (2004) with permission.
As AD progresses, nearly all of the neurons in this nucleus become invaded by NFTs (Fig. 16A). Some of these NFTs are intracellular; others take the form of ghost tangles, marking the site of dead neurons. This is the stage at which cortical cholinergic axons are severely depleted (Fig. 16B,C). As shown by the absence of plaques in Figure 16A, the nucleus basalis is not a site of particularly prominent amyloid deposition, and there is no correlation between plaque density and severity of cholinergic axonal loss in cortical areas (Geula et al., 1998). The cholinergic lesion in AD is therefore more closely related to the tauopathy than to the amyloid plaques.
Figure 16.
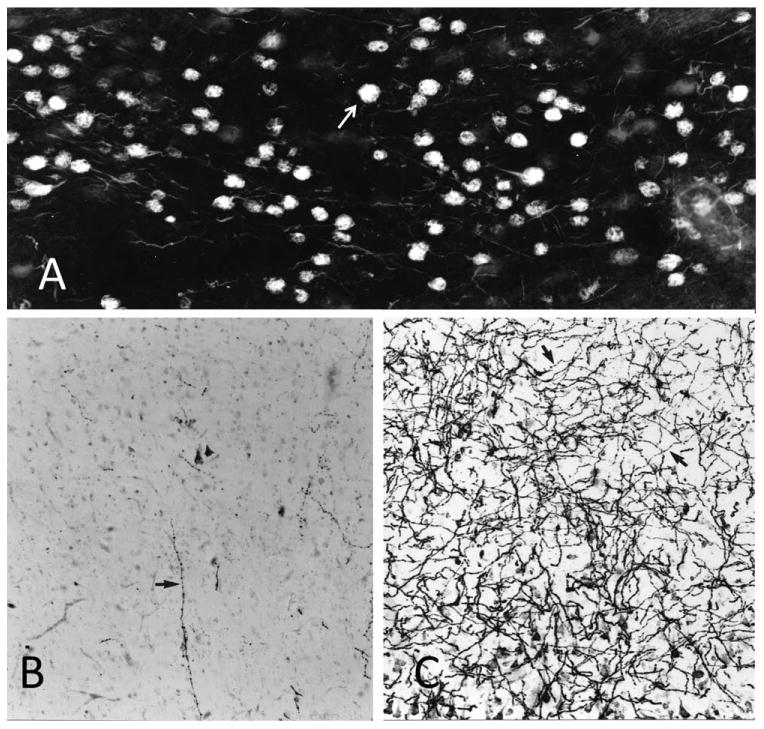
Neurofibrillary tangles and cholinergic innervation in AD. A: Nucleus basalis of a woman who died at the age of 84 years with clinical dementia and postmortem evidence of AD pathology. Histofluorescence in this thioflavin-S-stained section indicates that nearly all nucleus basalis neurons have been invaded by NFTs (arrow). Most are intracellular; others are ghost tangles. B: AChE histochemistry with a modified Karnovsky-Roots method shows nearly complete loss of cholinergic fibers in the middle temporal gyrus of the same patient. The arrow points to the one remaining axon in the field of view. C: Same region of the brain stained in the same fashion as in B but from a control subject who died at the age of 89 years with no evidence of dementia. The arrows point to two examples of cholinergic axons. ×100.
There are considerable regional variations in the magnitude of the cholinergic denervation in AD. It is usually most extensive in the temporal lobe, including the hippocampus, the entorhinal cortex, and the lateral and accessory basal nuclei of the amygdala, but much less conspicuous in primary sensorimotor cortices (Emre et al., 1993; Geula and Mesulam, 1996). The postsynaptic correlates of the cholinergic denervation in the cerebral cortex include a loss of AChE in cholinoceptive neurons and of nicotinic receptors but relative preservation of M1 receptors (Heckers et al., 1992a; Mash et al., 1985; Whitehouse and Kellar, 1987). Even at the height of cortical cholinergic denervation in the cerebral cortex, the cholinergic innervation of the striatum, derived predominantly from intrinsic ChAT-positive neurons, and of the thalamus, derived predominantly from Ch5-6 in the brainstem, remains relatively spared. The cholinergic lesion of Alzheimer's pathology therefore reflects the selective vulnerability of the basal forebrain and of its Ch4 contingent specifically rather than of cholinergic neurons in general.
Although the nucleus basalis is no more that 16 × 18 mm in size, it is the source of dense projections to all cortical areas. Its vulnerability to neurofibrillary degeneration creates a setting in which the earliest stages of Alzheimer's pathology can impact the physiological integrity of the entire cerebral cortex. However, the exact contribution of the cholinergic denervation to the cognitive impairment is almost impossible to determine, because it unfolds on a background of amyloid deposition and neurofibrillary pathology elsewhere in the brain. Circumstantial evidence for the relevance of the cholinergic denervation to the cognitive and behavioral changes in AD comes from the statistically significant, though practically modest, symptomatic improvements that have been achieved through the use of cholinomimetic drugs that inhibit AChE. Even this inference is highly conjectural, because there is hardly any neuronal or axonal AChE left to inhibit in the cerebral cortex of established AD (Fig. 16B,C). The alternative possibility must be entertained that the positive responses to AChE inhibitors could reflect cholinomimetic effects in thalamic nuclei that receive their AChE-rich cholinergic input from Ch5-6, two cell groups that remain relatively immune to Alzheimer's pathology.
Conclusions
From a strictly neuroanatomical vantage point, it is difficult to conceive of a structure more strategically situated than the nucleus basalis (Fig. 17). Although the locus coeruleus and the raphe nuclei also send widespread projections to the cerebral cortex, neither receives much cortical input (Mesulam, 1987). The circuitry of the nucleus basalis allows it to influence rapidly every part of the cerebral cortex in response to afferents not only from the brainstem but also from the cerebral cortex. There is a distinct limbic emphasis throughout this circuitry. For example, cortical components of the limbic system, such as allocortical and mesocortical cortices, tend to receive heavier cholinergic inputs than homotypical isocortices. Within the amygdala, cholinergic innervation from Ch4 is light within the lateral nucleus, which is interconnected predominantly with association neocortex but becomes much stronger within the immediately adjacent basolateral nucleus, which is more heavily interconnected with limbic and paralimbic cortices. The inputs to Ch4 are also quite selective. In addition to core limbic afferents from the hypothalamus and amygdala, Ch4 receives the vast majority of its cortical input from limbic and paralimbic cortices, not from homotypical isocortex. Cortical cholinergic innervation is thus in a position to modulate the neural impact of ambient events and mental experiences in accordance with their motivational and emotional significance as detected by the limbic system. Exactly how this happens and the nature of the modulatory influence are incompletely understood. This is not surprising considering the fact that the entire cortical mantle is involved and that the action of the released ACh encompasses multiple presynaptic and postsynaptic receptor species located on multiple types of cortical neurons, axons, and probably also blood vessels.
Figure 17.
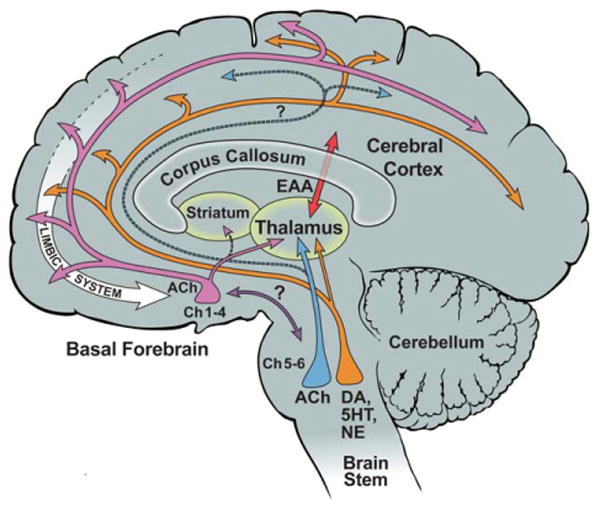
Cholinergic circuitry of the human nucleus basalis. ACh, acetylcholine; DA, dopamine; EAA, excitatory amino acids; NE, norepinephrine; 5HT, serotonin. Question marks indicate that the connection has not been confirmed in the human brain.
The aging-MCI-AD continuum is associated with a gradually intensifying degeneration of cortical cholinergic innervation. The process starts with a tauopathy and neurofibrillary degeneration of Ch4 perikarya that can be identified even during the clinically silent prodromal period of the disease. The contribution of amyloid toxicity to this neurodegenerative process remains to be clarified. In time, the underlying tauopathy extends centrifugally from the perikaryon and eventually causes dystrophy and destruction of cortical cholinergic axons, especially in the temporal lobes. The root cause of this vulnerability does not seem to be the cholinergic nature of Ch4 but its anatomical location within a continuous band of basolimbic structures, including the amygdala, hippocampus, and entorhinal cortex, which are collectively at highest risk of neurofibrillary degeneration in the course of events that lead to AD. Many questions concerning the functionality of Ch4 in the healthy brain and of its vulnerability to AD and other neurological diseases remain to be resolved. Nonetheless, its elaborate anatomical organization singles out the cholinergic circuitry of the nucleus basalis as one of the most pivotal modulatory systems of the human cerebral cortex.
Acknowledgments
Numerous colleagues have participated in the research described here. Elliott Mufson, Changiz Geula, John Smiley, and Deborah Mash made key contributions over the years.
Grant sponsor: Javits Neuroscience Award from the National Institute of Neurological Disorders and Stroke; Grant number: NS20285; Grant sponsor: Alzheimer's Disease Center grant from the National Institute on Aging; Grant number: AG05134.
Footnotes
Conflict of Interest Statement: The author has no conflicts of interest to disclose.
Literature Cited
- Arendt T, Bigl V, Tennstedt A, Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer's disease. Neuroscience. 1985;14:1–14. doi: 10.1016/0306-4522(85)90160-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shaver R, Dinan TG. Nucleus basalis neurons exhibit axonal branching with decreased impulse conduction velocity in rat cerebrocortex. Brain Res. 1985;325:271–285. doi: 10.1016/0006-8993(85)90323-3. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Heckers S, Mesulam MM, Wiley RG, Lappi DA, Sharma M. Differential effects upon spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J Neurosci. 1994;14:4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand. 1996;165(Suppl):3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Kyriazis DA, Baxter MG. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci. 2011;14:1510–1512. doi: 10.1038/nn.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Synaptic organization of cholinergic neurons in the monkey neostriatum. J Comp Neurol. 1987;225:245–258. doi: 10.1002/cne.902550208. [DOI] [PubMed] [Google Scholar]
- Divac I. Magnocellular nuclei of the basal forebrain project to neocortex, brain stem and olfactory bulb. Review of some functional correlates. Brain Res. 1975;93:385–398. doi: 10.1016/0006-8993(75)90178-x. [DOI] [PubMed] [Google Scholar]
- Emre M, Heckers S, Mash DC, Geula C, Mesulam MM. Cholinergic innervation of the amygdaloid complex in the human brain and its alterations in old age and Alzheimer's disease. J Comp Neurol. 1993;336:117–134. doi: 10.1002/cne.903360110. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. γ-Amynobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Nat Acad Sci U S A. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, Wieneke C, Bigio E, Geula C, Mesulam MM. Clinically concordant variations of Alzheimer patology in aphasic versus amnestic dementia. Brain. 2012;135:1554–1565. doi: 10.1093/brain/aws076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer's disease. Cereb Cortex. 1996;6:165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- Geula C, Schatz CR, Mesulam MM. Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience. 1993;54:461–476. doi: 10.1016/0306-4522(93)90266-i. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Saroff DM, Wu CK. Relationship between plaques, tangles, and loss of central cholinergic fibers in Alzheimer's disease. J Neuropathol Exp Neurol. 1998;57:63–75. doi: 10.1097/00005072-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:1–10. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey Al. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer's disease. J Comp Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- Gómez-lsla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Rance NE, Young WS, III, Koliatsos VE. Tyrosine-hydroxylase containing neurons in the primate basal forebrain magnocellular complex. Brain Res. 1992;584:287–293. doi: 10.1016/0006-8993(92)90907-q. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer's disease. Biol Psychiatry. 2012;71:805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Mesulam MM. Two types of cholinergic projections to the rat amygdala. Neuroscience. 1994;60:383–397. doi: 10.1016/0306-4522(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Heckers S, Geula C, Mesulam MM. Acetylcholinesterase-rich pyramidal neurons in Alzheimer's disease. Neurobiol Age. 1992a;13:455–460. doi: 10.1016/0197-4580(92)90072-6. [DOI] [PubMed] [Google Scholar]
- Heckers S, Geula C, Mesulam MM. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol. 1992b;325:68–82. doi: 10.1002/cne.903250107. [DOI] [PubMed] [Google Scholar]
- Henderson Z. A small proportion of cholinergic neurones in the nucleus basalis magnocellularis of ferret appear to stain positively for tyrosine hydroxylase. Brain Res. 1987;412:363–369. doi: 10.1016/0006-8993(87)91144-9. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Jang SH. Neural pathway from nucleus basalis of Meynert passing through the cingulum in the human brain. Brain Res. 2010;1346:190–194. doi: 10.1016/j.brainres.2010.05.088. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Kromer LJ, Van Hoesen GW. Reinnervation of the hippocampal perforant pathway zone in Alzheimer's disease. Ann Neurol. 1987;21:259–267. doi: 10.1002/ana.410210307. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer' disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz DM, Palkovits M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. J Comp Neurol. 1974;157:13–28. doi: 10.1002/cne.901570103. [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic-catecholamine, serotonin and acetylcholine-neurons. Neuroscience. 1989;31:37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Karczmar AG. Cholinergic influences on behavior. In: Waser PG, editor. Cholinergic mechanisms. New York: Raven Press; 1975. pp. 501–529. [Google Scholar]
- Karczmar AG. Exploring the vertebrate central cholinergic system. New York: Springer; 2007. [Google Scholar]
- Kievit J, Kuypers H. Basal forebrain and hypothalamic connections to frontal and parietal cortex in the rhesus monkey. Science. 1975;187:660–662. doi: 10.1126/science.1114317. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Sladek JR, Jr, Mufson EJ. TRKimmunoreactivity in the monkey central nervous system: forebrain. J Comp Neurol. 1994;349:20–35. doi: 10.1002/cne.903490103. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Skavic J, Strinovic D. Acetylcholinesterase in the human frontal associative cortex during the period of cognitive development: early laminar shifts and late innervation of pyramidal neurons. Neurosci Lett. 1988;90:107–112. doi: 10.1016/0304-3940(88)90795-1. [DOI] [PubMed] [Google Scholar]
- Krnjevic K. Cellular mechanisms of cholinergic arousal. Behav Brain Sci. 1981;4:484–485. [Google Scholar]
- Krnjevic K, Silver A. A histochemical study of cholinergic fibers in the cerebral cortex. J Anat. 1965;99:711–759. [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. Plos One. 2012;7:1–9. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi O. Über humorale Übertragbarkeit der Herznervenwirkung. Pflugers Arch Ges Physiol. 1921;189:239–242. [Google Scholar]
- Mash D, Flynn D, Potter LT. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer's disease and experimental cholinergic denervation. Science. 1985;228:1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic responses to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Asymmetry of neural feedback in the organization of behavioral states. Science. 1987;237:537–538. doi: 10.1126/science.3110953. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275:216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Acetylcholinesterase-rich neurons of the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol. 1991;306:193–220. doi: 10.1002/cne.903060202. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons in human cerebral cortex. Brain Res. 1992;577:112–120. doi: 10.1016/0006-8993(92)90543-i. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984;107:253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW. Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res. 1976;109:152–157. doi: 10.1016/0006-8993(76)90385-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey Al, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey Al. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-6) Neuroscience. 1983b;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey Al, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984;12:669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH. Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Res. 1986;367:301–308. doi: 10.1016/0006-8993(86)91607-0. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–633. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Hersh LB, Mash DC, Geula C. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 1992a;318:316–328. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mash D, Hersh L, Bothwell M, Geula C. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J Comp Neurol. 1992b;323:252–268. doi: 10.1002/cne.903230209. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Architecture of connectivity within a cingulofrontoparietal neurocognitive network for directed attention. Arch Neurol. 1993;50:279–284. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Bothwell M, Hersh LB, Kordower JH. Nerve growth factor receptor immunoreactive profiles in the normal, aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol. 1989;285:196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: Chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cereb cortex. New York: Plenum Press; 1985. pp. 3–61. [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Stern R. Individual cells in the nucleus basalis-diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res. 1983;269:352–356. doi: 10.1016/0006-8993(83)90145-2. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis. Nat Rev Neurosci. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassin I, Schultz C, Thai DR, Rüb U, Arai K, Braak E, Braak H. Evolution of Alzheimer's disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Schröder H, Zilles K, Maelicke A, Hajós F. Immunohistoand cytochemical localization of cortical cholinoceptors in rat and man. Brain Res. 1989;502:287–295. doi: 10.1016/0006-8993(89)90624-0. [DOI] [PubMed] [Google Scholar]
- Schröder H, Zilles K, Luiten PGM, Strosberg AD. Immunocytochemical visualization of muscarinic cholinoceptors in the human cerebral cortex. Brain Res. 1990;514:249–258. doi: 10.1016/0006-8993(90)91420-l. [DOI] [PubMed] [Google Scholar]
- Selden N, Geula C, Hersh L, Mesulam MM. Human striatum: chemoarchitecture of the caudate nucleus, putamen and the ventral striatum in health and Alzheimer's disease. Neuroscience. 1994;60:621–636. doi: 10.1016/0306-4522(94)90491-x. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Silver A. The biology of cholinesterases. New York: Elsevier; 1974. pp. 8–10. [Google Scholar]
- Smiley JF, Mesulam MM. Cholinergic neurons of the nucleus basalis of Meynert (Ch4) receive cholinergic, catecholaminergic, and GABAergic synapses: an electron microscopic investigation in the monkey. Neuroscience. 1999;88:241–255. doi: 10.1016/s0306-4522(98)00202-4. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM. Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol. 1997;144:361–368. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Subramanian M, Mesulam MM. Monoaminergic-cholinergic interactions in the primate basal forebrain. Neuroscience. 1999;93:817–829. doi: 10.1016/s0306-4522(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Grinberg L, Grothe M, Cantero JL, Reiser MF, Möller HJ, Heinsen H, Hampel H. The cholinergic system in mild cognitive impairment and Alzheimer's disease: an in vivo MRI and DTI study. Hum Brain Mapp. 2011;32:1349–1362. doi: 10.1002/hbm.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35:567–574. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Loftus W, Thomas CE, Green RL, Mott LA, Gazzaniga MS. Surface area of human cerebral cortex and its gross morphological subdivisions: in vivo measurements in monozygatic twins suggest differential hemisphere effects of genetic factors. J Cognit Neurosci. 1995;7:292–301. doi: 10.1162/jocn.1995.7.2.292. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol. 1994;348:351–373. doi: 10.1002/cne.903480304. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Cortical afferents to the entorhinal cortex of the rhesus monkey. Science. 1972;175:1471–1473. doi: 10.1126/science.175.4029.1471. [DOI] [PubMed] [Google Scholar]
- Vana L, Kanaan NM, Ugwu IC, Wuu J, Mufson EJ, Binder LI. Progression of tau pathology in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer's disease. Am J Pathol. 2011;179:2533–2550. doi: 10.1016/j.ajpath.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayashankar N, Brody H. A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol. 1979;38:490–497. doi: 10.1097/00005072-197909000-00004. [DOI] [PubMed] [Google Scholar]
- Walker LC, Koliatsos VE, Kitt CA, Richardson RT, Rökaeus Å, Price DL. Peptidergic neurons in the basal fore-brain magnocellular complex of the rhesus monkey. J Comp Neurol. 1989;280:272–282. doi: 10.1002/cne.902800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Kellar KJ. Nicotinic and muscarinic cholinergic receptors in Alzheimer's disease and related disorders. J Neural Transmiss. 1987;24:175–182. [PubMed] [Google Scholar]
- Wilson FAW, Rolls ET. Neuronal responses related to novelty and familiarity of visual stimuli in the substantia innominata, diagonal band of Broca and periventricular region of the primate basal forebrain. Exp Brain Res. 1990;80:104–120. doi: 10.1007/BF00228852. [DOI] [PubMed] [Google Scholar]
- Wisniowski L, Ridley RM, Baker HF, Fine A. Tyrosine hydroylase-immunoreactive neurons in the nucleus basalis of the common marmoset (Callithrix jaccus) J Comp Neurol. 1992;325:379–387. doi: 10.1002/cne.903250305. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Matsui M, Watanabi M. Preferential localization of muscarininc M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci. 2010;30:4408–4418. doi: 10.1523/JNEUROSCI.5719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelnik J, Francois C, Percheron G, Tande D. Morphological taxonomy of the neurons of the primate striatum. J Comp Neurol. 1991;313:273–294. doi: 10.1002/cne.903130207. [DOI] [PubMed] [Google Scholar]


