Abstract
Background
Oganotypic hippocampal slices (OHS) are commonly used to screen for neuroprotective effects of pharmacologic agents relevant to pediatric brain injury. The importance of donor rat pup age and N-methyl-d-aspartate (NMDA) receptor subunit composition have not been addressed. In this study we evaluated the age-dependent effect of oxygen-glucose deprivation (OGD) in the developing rat brain and determined whether OGD modulates the NMDA receptor subunit composition.
Methods
OHS were prepared from rat pups on postnatal days (PND) 4, 7, 14 and 21 and cultured 7 days in vitro. The slices were exposed to OGD for durations of 5– 60 min. After 24 and 72hours, OHS survival and NMDA subunit composition were assessed.
Results
Cell death was evident in OHS prepared from PND 14 and 21 rat pups (p<0.001) with OGD durations of 5 and 10 min, respectively. In OHS prepared from PND7 rat pups, neurodegeneration was not evident until 20 min OGD (p<0.001). Exposure to OGD in OHS prepared from PND4 and PND7 rat pups was associated with a transition in the NMDA receptor subunit composition from NR2B predominant to NR2A predominant subunit composition.
Conclusions
This in vitro neonatal rat pup investigation using OHS supports both an age and an NMDA receptor subunit composition-dependent relationship between OGD and neuronal cell death.
INTRODUCTION
N-methyl-d-aspartate (NMDA) receptors are heterotetramers of NMDA receptor 1 (NR1) and one or more of the NR2 subunits: NR2A-D (1, 2). The functional and pharmacological properties of the NMDA receptor (NR) are determined by its subunit composition, specifically the type of NR2 subunit incorporated (3, 4). During development, NMDA receptors play a critical role in postsynaptic stabilization in the cortex and hippocampus (5). Age-dependent changes in the subunit composition in the developing rat brain have been determined using mRNA and NMDA receptor subunit-specific antagonists. NR2B is expressed at birth and through the first 2 weeks. NR2A is expressed in the second week and gradually increases, thereafter. Therefore, the NR2A/NR2B ratio increases with maturation (6, 7, 8).
The developmental change in the NMDA receptor subunit composition is associated with a change in receptor properties and thus functionality (9, 10). For example, different subunits are involved in activity-dependent synaptic plasticity e.g., excitatory long-term enhancement and inhibitory long-term depression (11, 12, 13). In the developing hippocampus, the former is NR2B-dependent and the latter NR2A. Excitatory or inhibitory balances that are developmentally regulated can become unregulated, and thus promote susceptibility to brain injury (14–17).
NMDA-induced neurotoxicity reflects a calcium imbalance related to the developmental changes in NMDA receptor subunit composition, specifically NR2B (18, 19). The NR2B predominance early in development is an adaptive response that protects the fetus during hypoxic conditions normally seen in utero (20–21). However, the response of the NMDA receptor subunit composition to acute hypoxia has not been demonstrated fully in a developing brain model (14, 19, 22–26).
Using organotypic hippocampal slices (OHS) prepared from postnatal day (PND) 4, 7, 14 and 21 rat pups, we evaluated the effect of ontogenetic development of the NMDA receptor subunit composition in the response of neural tissue to oxygen-glucose deprivation (OGD). OHS offer a model of intact neural circuits and avoid some of the variables inherent in an in vivo investigation in small rodents (27, 28).
METHODS
OHS cultures
All studies were approved by the Duke University and the University of Colorado Health Sciences Center Institutional Animal Care and Use Committees. OHS cultures were prepared according to the methods described by Stoppini et al. (27) with some modification (28). PND 4, 7, 14 and 21 Sprague Dawley rat pups (Zivic Laboratories, Pittsburgh, PA) were anesthetized using an intraperitoneal injection of ketamine (10mg/kg) and diazepam (0.2 mg/kg). The pups were decapitated and the hippocampi were removed and placed in 4 °C Gey’s Balanced Solution (Sigma-Aldrich, St. Louis MO) with 100 μM adenosine. Using a MX-TS brain slicer (Siskiyou Design Instruments, Grants Pass, OR), the hippocampi were cut transversely (300 μm thickness) and transferred to 30-mm diameter membrane inserts (Millicell-CM, Millipore, Bedford, MA). Approximately 3–5 slices were placed on the insert within each well of a 6-well culture tray with media where they remained for 7 days. The culture media consisted of 50% Minimal Essential Media (MEM) (Invitrogen, Carlsbad, CA ), 25% Earle’s Balanced Salt Solution (Invitrogen, Carlsbad, CA) and 25% Hyclone Heat Inactivated Horse Serum (Perbio, Cell Culture Division, South Logan, UT) with 6.5 mg/ml glucose and 5 mM KCl. The media was exchanged after the second day in culture and twice a week thereafter. OHS were cultivated in a humidified atmosphere at 37°C and 5% CO2. No antibiotics or antimitotics were used.
Oxygen and glucose deprivation
During OGD, the media in each cell culture well was exchanged for glucose-free MEM (Invitrogen, Carlsbad, CA) (15). Hypoxic gas (5% O2 and 95% N2, 6 liters/m) was delivered to a 3L air-tight chamber, (Billup’s Rothenberg, San Diego, CA) housed within a water jacketed incubator, for 30 min. The hypoxic gas was heated and humidified to 37 °C (Fisher-Paykel, Laguna Hills, CA). After 30 min the inserts containing OHS were placed in the hypoxic tissue culture wells above the hypoxic/glucose-free media, so as to allow exposure to media on one surface and the gas mixture on the opposing surface. The flow rate of hypoxic gas was decreased and maintained at 700 ml/min for a period of 5–60 min, determined by random assignment. Oxygen and CO2 concentrations were continuously monitored via a sampling port connected to a medical gas analyzer (Datex Instruments Corporation, Tewksbury, MA). The pH of the media before and after exposure was 7.4 ± 0.4. After exposure to OGD, the slices with inserts were removed and returned to their glucose-containing normoxic media and maintained in the incubator for 3 days and then imaged for cell death at 24 and 72 hours.
Evaluation of cell death
Sytox (Molecular Probes, Eugene, OR) is a high affinity nucleic acid stain specific for neurons with compromised plasma membranes and does not penetrate live neurons (28, 29). At 24 h after OGD, the media was exchanged with media containing 5 μM SytoxR. The OHS with Sytox R were imaged using a Leica inverted microscope (2.5X) (Wetzlar, Germany) and fluorescent digital images were taken using a CoolSnap digital camera (Image Processing Solutions, North Reading, MA). The variables for imaging were standardized for each OHS. Both light and fluorescent microscopic images were made simultaneously for each slice to confirm region identification.
Images were obtained 24 and 72 h post–OGD and stored for later analysis. Using MCID software (Imaging Research, Inc. St. Catherines, Ontario), CA1, CA2, CA3 and dentate gyrus were manually outlined on the images obtained by light microscopy of each OHS. These outlines were then superimposed on the fluorescent images of the corresponding OHS. Excitation wavelength was 490 nm and emission was 590 nm. The fluorescence (relative units) was then measured for each hippocampal region. OHS with intense fluorescence in hippocampal CA2 in baseline (control) or OGD groups were excluded from analysis. These represented nonviable slices, which constituted approximately 5–10% of the OHS population. Analysis of the images was performed by an investigator blinded to group assignment and after completion of each experiment for consistency in measurement.
NMDA receptor subunit composition
To determine baseline values, OHS were maintained in culture for 7 days after which time the slices were freshly frozen without further treatment in liquid nitrogen and stored at −80 °C until further processing.
To assess effects of OGD, OHS were maintained in culture for 7 days and exposed to OGD as described above for 10 or 30 min. The slices were then returned to their original media and 24 hour later the slices were frozen in liquid nitrogen and stored at −80 °C until further processing.
Protein Preparations and Western Blot Analysis
Protein from hippocampal slices was homogenized with PowerGen Tissue homogenizer (Fisher Scientific, Pittsburgh, PA) in buffer containing 50 mM Tris-HCl, pH 7.4, 1% SDS, 2 mM EDTA and one Complete Protease Inhibitor Cocktail Tablet (Roche Diagnostics, Chicago, Ill), briefly boiled, and centrifuged at 13,000 x g for 30 m. Supernatants were aliquoted and stored at −80oC. Protein concentration was determined using the bicinchoninic (Smith) protein assay (Peirce; Rockford, Ill).
Western blot analyses were performed on protein extracts. Protein was loaded at a concentration of 50 μg sample/lane using Invitrogen (Carlsbad, CA) NuPage 4–12% Bis-Tris pre-poured gel. The protein was then transferred to polyvinylidene difluouride membranes (Immobilon -P Transfer Membrane, Millipore, Bedford, MA) briefly soaked in methanol. Protein transfer was performed using the semi-dry immunoblot method (Owl Model HEP-1 Panther Semi-Dry Electroblotter - #HEP-3). Membranes were blocked with 5% Milk PBS-tween, followed by overnight incubation with primary antibodies (anti-NMDA receptor, subunit 2A rabbit IgG Fraction; 1:200, anti-NMDA receptor, subunit 2B rabbit IgG Fraction; 1:200, Molecular Probes, Eugene, OR). After PBS-tween washes, membranes were incubated with horseradish peroxidase conjugated secondary antibodies and bands were visualized using SuperSignalR West Dura Extended Duration Substrate (Pierce Biotechnology, Inc., Rockford, Ill). Imaging was performed using a UVP BioChem Imaging System and densitometry was performed using LabWorks 4.0 software. The protein loading internal control was s-Actin Clone A-15, (Sigma, St. Louis, MO). Data are expressed as a ratio of the NMDA subunits to s-Actin.
Statistics
Experiments for neurodegeneration and NMDA receptor subunit composition were not performed concurrently and therefore were analyzed separately. Within each experiment, fluorescence intensity was compared initially with 2-way ANOVA (PND X duration of hypoxia exposure) and multivariate analysis (PND X duration of hypoxia exposure X DIV). Each hippocampal region (CA1, CA3, and dentate gyrus) was analyzed independently. Approximately 15 OHS were used for each experimental condition. The critical exposure duration for each PND was defined as the shortest OGD duration at which statistically different optical densities were observed as compared to age-matched controls. OHS with 100% neuronal cell death in CA2 were excluded from the analysis. When indicated by a significant F ratio, post hoc testing was performed using Scheffe’s test. Significance was assumed when P ≤ 0.05.
RESULTS
Neuronal Cell Death
Comparison to control group by region and duration
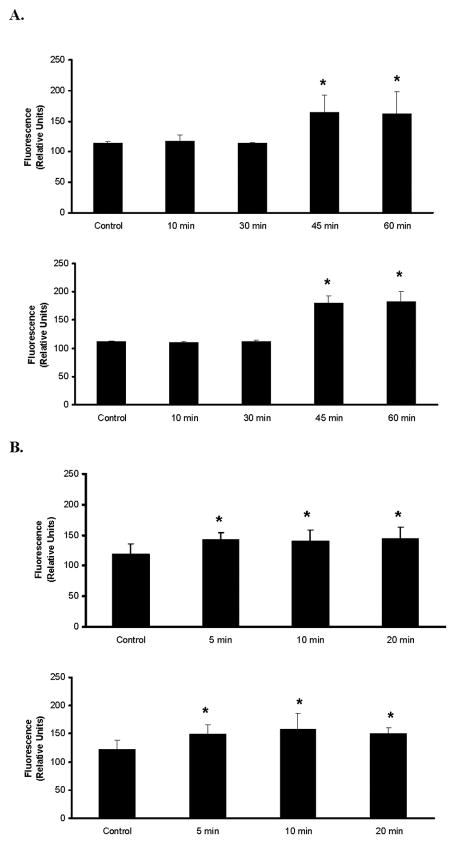
In CA1, critical exposure duration for neurodegeneration was 5 min OGD in PND21 (p=0.005), 10 min OGD in PND14 OHS (p=0.004) 20 min OGD in PND7 OHS (p=0.04), and 45 min OGD in PND4 OHS (p<0.0001) (Figure 1). In CA3, critical exposure duration was 5 min in PND21 (p=0.008), 10 min in PND14 (0.019), 20 min in PND7 (p=0.04) and 45 min PND4 OHS (p=<0.001) (Figure 1). In the dentate gyrus, there were no observed differences except in the 45 min PND4 OHS (p<0.001). The 24h and 48h observations were not statistically different regardless of region evaluated.
Figure 1. Comparison of region and PND.
A: Neuronal cell death in hippocampal CA1 (upper graph) and CA3 (lower graph) at 24 h post-oxygen-glucose deprivation (OGD) in postnatal day (PND4) organotypic hippocampal slices (OHS) exposed to 10, 30, 45 or 60 min OGD compared to PND-matched controls. Neuronal death increased with OGD duration. Forty-five min reflects the critical exposure duration to produce neuronal cell death that is statistically different than that observed in control OHS. B: Neuronal cell death in CA1 (upper graph) and CA3 (lower graph) at 24 h post-OGD in PND21 OHS exposed to 5, 10, or 20 min OGD compared to PND-matched controls. Neuronal death increased with OGD duration. In both A and B statistical significance was absent between cell death in CA1 as opposed to CA3. Data are displayed as fluorescence (relative units) ± SD. * denotes statistical significance p<0.05 from control values.
Comparison to PND4 by age and duration
When compared to PND4 OHS significant differences in the ischemic duration and the resultant cell death were noted in all groups, 10 min PND21 (p=0.0145), 20 min PND14 (p=0.03), and 30 min PND7 OHS (p=0.04) (Figure 2). This effect on neuronal cell death was independent of the region and the time of observation
Figure 2. Comparison of Postnatal Day (PND) and Oxygen-Glucose Deprivation (OGD) Duration.

A: Neuronall death in hippocampal CA1 in PND 4, 7, 14 and 21 organotypic hippocampal slices (OHS), exposed to 10 min oxygen-glucose deprivation (OGD) at 24 h post-OGD. Neuronal death increased as a function of increased PND. B: CA1 neuronal death at 24 h post-OGD in PND 4, 7, 14 and 21 OHS exposed to 20 min OGD. Neuronal death increased with PND. C: CA1 neuronal death at 24 h post-OGD in PND 4, 7 and 14 OHS exposed to 30 min OGD. Data are displayed as fluorescence (relative units) ± SD. * denotes statistical significance p<0.05 from PND4 values.
Western blot analysis of NMDA receptor subunit composition
Baseline NMDA Receptor Subunit Composition
In OHS, in the absence of OGD, baseline NMDA receptor subunit composition changed as a function of postnatal age. In OHS the ratio of NR2B to NR2A receptor subunit composition was NR2B-predominant in PND4 and PND7 OHS but transitioned to a NR2A-predominant subunit composition by PND14 (Figure 3).
Figure 3. Western blot analysis of NMDA receptor subunit composition NR2A as compared to NR2B in organotypic hippocampal slices (OHS) prepared from rat pups at postnatal day (PND) 4, 7, 14, and 21 DIV7 in the absence of oxygen-glucose deprivation.
An age-related change in N-methyl-D-aspartate (NMDA) subunit composition was observed. NR2B (stippled) NR2A (solid) data expressed in densitometry units, mean ± SD as a ratio to β-actin. PND 4 OHS showed a predominance of NR2B. PND7 OHS showed NR2B predominance with more NR2A than PND4. PND14 and PND21 OHS showed a change to NR2A predominance. Data demonstrate protein pooled from approximately 50 OHS for each PND. For each NMDA receptor subunit composition, 2 –3 Western blot analyses were performed. Analysis was normalized to tissue β-actin.
Change in NMDA Receptor Subunit Composition in Response to OGD
In order to compare all postnatal age groups, both 10 min and 30 min OGD were evaluated. These OGD durations were selected based on the critical exposure durations of each PND group, spanning 5 min for PND21 and to 45 min for PND4. Exposure to OGD for 10 or 30 min decreased the NR2B subunit composition in PND 4 and PND7 OHS and reversed the NR2B to NR2A predominance in PND4 and PND7 OHS that was observed in control (no OGD) OHS (Figure 4).
Figure 4.
Western blot analysis of N-methyl-D-aspartate (NMDA) receptor subunit composition NR2B and NR2A in organotypic hippocampal slices (OHS) prepared from rat pups at postnatal day (PND) 4, 7, 14, and 21 exposed to oxygen-glucose deprivation (OGD) for 10 or 30 min. Data shows a change in NMDA subunit composition with respect to age and OGD. NR2B (stippled) and NR2A (solid) data are expressed in densitometry units, mean ± SD, as a ratio to β-actin. There is a receptor subunit change in response to 10 min and 30 min of OGD. In PND4 and 7 OHS, the NR2B to NR2A predominance is reversed from that observed in controls not exposed to OGD (Figure 3).
DISCUSSION
This study demonstrates that OGD-induced neuronal death is dependent on the exposure duration of OGD and the age of rat pup used for OHS preparation. As shown in our work and other neonatal rat models (18, 30–33), in the absence of OGD, PND7 is the stage in rat development when a transition from NR2B to NR2A receptor subunit composition occurs. We also show that the NMDA receptor subunit composition changes in PND 4 and PND7 OHS to a NR2A predominance after OGD instead of the NR2B predominance seen in control PND 4 and 7 OHS (no OGD). When evaluating cell death 24 and 48 h after OGD in both the CA1 and CA3 regions of the hippocampus, we found that the PND 14 and 21 slices were similar for the OGD duration required to cause significant injury (10 and 5min, respectively). In OHS derived from younger rat brains (PND 4 and 7), OHS cell death was evident only after longer durations (45 and 30 min, respectively).
It is important to note that the observations made by our investigation are specific to the hippocampus. In human preterm infants developing white matter is vulnerable to hypoxic-ischemic injury. The vulnerability of gray matter, cortex and basal ganglia increases towards term in the early postnatal period (34). Thus, responses may be different in other brain regions (9). Synaptogenesis peaks in at PND7 in the rat brain (30, 35). Ontogenetically, this corresponds to 32–36 weeks gestation in humans and may extend throughout the brain growth spurt (6 month to 2-years-of-age) (36, 37). Using mRNA, expression of the NMDA receptor subunit composition in the hippocampus has been determined to be a function of postnatal age in humans (36). The pattern of NMDA receptor subunit composition development in the human hippocampus parallels that seen in the rodent (36). We did not evaluate region-specific differences in NMDA subunit composition, but this has been described in rodents (38).
The responsiveness to NMDA-induced neuronal death as a result of duration in culture in OHS has been described (39). The responsiveness to NMDA-induced cell death in OHS of the same postnatal age cultured for 1 and 2 weeks differed from those cultured for 3 weeks. This difference was not a function of NR2A and 2B subunit composition but the responsiveness of the receptor to NMDA. This investigation further demonstrated that OHS from PND6 pups, regardless of culture duration, did not differ in the NR2A or NR2B subunit composition. Thus, the ratio of the NR2A and NR2B subunit composition was preserved regardless of culturing conditions. However, the NR1 receptor increased after 3 weeks in culture. As determined by western blot analysis, we have shown the preservation of the NR2A to NR2B ratio in our OHS prepared from rat pups of differing postnatal age.
Changes in NMDA receptor subunit expression in response to hypoxia-ischemia in a neonatal piglet model have been reported (25). NR2B levels were increased at 24 hours post hypoxia-ischemia. Thus, differences in our investigation could relate to difference in species, region evaluated (forebrain versus hippocampus) (40), mechanism of insult or postnatal age Indeed, investigation in younger aged piglets found no change in the receptor subunit composition in response to one hour of hypoxia (24). Like the rat model, the distribution of NR2A and 2B receptor subunits in neonatal piglets is greatest in the forebrain and hippocampus (24, 33, 40, 41). Regardless, our investigation in a rat model demonstrates a postnatal age-related and OGD duration-dependent change in NMDA receptor subunit composition.
Although NMDA receptor density increases with increasing postnatal age and NMDA receptor density decreases with acute hypoxia (24,32), we normalized our results to the total β-actin in the sample, thus eliminating receptor density (protein) as a variable with postnatal age. It is important to note that, because of this normalization of our data to β-actin, the amount of NR2A is less in control PND14 and PND21 OHS as compared to control PND4 and 7 OHS. However, the ratio of NR2B to NR2A is the focus of our investigation, as detailed by the responses of the NMDA receptor subunit composition to acute hypoxia. By normalizing to β-actin we can further account for the total protein differences as an effect of OHS slice size which increases with increasing postnatal age. Our protein analysis did not define regional differences in CA1 as opposed to CA3 in terms of relative subunit composition (25). Tissue from those regions were pooled to provide sufficient sample for analysis.,
Indeed, the NMDA receptor subtype expressed varies with development. NR1 is necessary for receptor function (7, 33, 41). NR1, though initially paired with NR2B or NR2D early in life, becomes paired with NR2A or NR2C later. This transition parallels the decrease in hypoxia tolerance with increasing postnatal age. Transition in NMDA receptor subunit composition occurs at PND7 in rat pups with completion at PND14 (6, 7, 41). We have demonstrated the preservation of this transition in NR2B to NR2A receptor subunit composition in our OHS. Thus, the tolerance to hypoxia as a function of postnatal age is not unique to our investigation. However, our investigation is the first to confirm this preservation of NR2A to NR2B ratio in rat OHS of differing postnatal age using protein as opposed to mRNA analysis (18, 31, 32, 36).
In summary, using an OHS model, we demonstrated an age-related change in the NMDA receptor subunit composition at baseline that parallels that seen in vivo in rodents and in humans. Age-dependent NMDA receptor subunit composition is altered by acute exposure to OGD. As a result, when using OHS to investigate NMDA receptor responses to hypoxia, the stage of ontogenetic development in tissue used for analysis can be expected to have an influence on results obtained. An understanding of developmental differences and the affect of acute hypoxia on the NMDA subunit composition in the model being used may help us better understand the neurotoxic/neuroprotective effect of a pharmacologic agent in the presence of hypoxic-ischemic injury.
Implications.
N-methyl-d-aspartate receptor subunit composition in rat organotypic hippocampal slices changes in response to developmental age and oxygen-glucose deprivation.
Acknowledgments
This work was supported by American Heart Association National Scientist Development Grant, Foundation for Anesthesia Education and Research Mentored Research Award, NIH Grants T32 GM08600-09 and RO1 GM067139-03.
The technical assistance of Ms. Virginia E. Beckey is greatly appreciated.
References
- 1.Forrest D, Yusaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Conner JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 2.Hardingham GE, Bading H. The yin and the yang of NMDA receptor signaling. TRENDS in Neurosciences. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 3.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 4.Cull-Candy S, Leszkiewicz DN. Role of distinct NMDA receptor subtypes in central synapses. Sci STKE. 2004;255:re 16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 5.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 6.Moyner H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 7.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 8.Groc L, Heine M, Cousins SL. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci USA. 2006;103:18769–74. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babb TL, Mikuni N, Najm I, Wylie C, Olive M, Dollar C, MacLennan H. Pre-and postnatal expressions of NMDA receptors 1 and 2B subunit proteins in the normal rat cortex. Epilepsy Res. 2005;64:23–30. doi: 10.1016/j.eplepsyres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Wong TP, Pozza MF, Lingnehoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNA’s. NeuroReport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Gurd JW, Bissoon N, Beesley PW, Nakazawa T, Yamamoto T, Vannucci SJ. Differential effects of hypoxia-ischemia on subunit expression and tyrosine phosphorylation of the NMDA receptor in 7- and 21-day old rats. J Neurochem. 2002;82:848–856. doi: 10.1046/j.1471-4159.2002.01026.x. [DOI] [PubMed] [Google Scholar]
- 15.Ben Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;J8:91–102. Review. [PubMed] [Google Scholar]
- 16.Ikonomidou C, Mosinger JL, Salles KS, Labruyere J, Olney JW. Sensitivity of the developing rat brain to hypobaric/ischemic damage parallels sensitivity to N-methyl-D-aspartate neurotoxicity. J Neurosci. 1989;9:2809–2818. doi: 10.1523/JNEUROSCI.09-08-02809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26:2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Haniq JP, Slikker W., Jr Ontogeny of the N-methyl-d-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxic Sci. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Misha OP, Fritz KI, Delivoria-Papadopoulos M. NMDA receptor and neonatal hypoxic brain injury. Men Retard Dev Dis Res Rev. 2001;7:249–253. doi: 10.1002/mrdd.1034. [DOI] [PubMed] [Google Scholar]
- 20.Bickler PE, Fahlman CS, Taylor DM. Oxygen sensitivity of NMDA receptors: relationship to NR2 subunit composition and hypoxia tolerance of neonatal neurons. Neuroscience. 2003;118:25–35. doi: 10.1016/s0306-4522(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 21.Bickler PE, Hansen BM. Hypoxia-tolerant neonatal CA1 neurons: relationship to survival to evoked glutamate release and glutamate receptor mediated calcium changes in hippocampal slices. Dev Brain Res. 1998;106:57–69. doi: 10.1016/s0165-3806(97)00189-2. [DOI] [PubMed] [Google Scholar]
- 22.Grojean S, Pourie G, Vert P, Daval J. Differential neuronal fates in the CA1 hippocampus after hypoxia in newborn and 7 day-old rats: effects of pretreatment with MK-801. Hippocampus. 2003;13:970–977. doi: 10.1002/hipo.10171. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Lopez D, Martinez-Orgado J, Casanova I, Bonet B, Leza JC, Lorenzo P, Moro MA, Lizasoain I. Immature rat brain slices exposed to oxygen-glucose deprivation as an in vitro model of neonatal hypoxic-ischemic encephalopathy. J Neurosci Methods. 2005;30:145(1–2):205–12. doi: 10.1016/j.jneumeth.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Kim WT, Kuo MF, Mishra OP, Delivoria-Papadopoulos M. Distribution and expression of the subunits of N-methyl-D-aspartate (NMDA) receptors; NR1, NR2A and NR2B in hypxic newborn piglet brains. Brain Res. 1998;799:49–54. doi: 10.1016/s0006-8993(98)00464-8. [DOI] [PubMed] [Google Scholar]
- 25.Guerguerian AM, Bambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia-ischemia. Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 26.Gee CE, Benquet P, Raineteau O, Rietschin L, Kirbach SW, Gerber V. NMDA receptors and the differential ischemic vulnerability of hippocampal neurons. Eur J Neurosci. 2006;23:2595–2603. doi: 10.1111/j.1460-9568.2006.04786.x. [DOI] [PubMed] [Google Scholar]
- 27.Stoppini L, Buch PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 28.Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg. 2005;101:651–7. doi: 10.1213/01.ane.0000167382.79889.7c. [DOI] [PubMed] [Google Scholar]
- 29.Moldrich RX, Beart PM, Pascoe CJ, Cheung NS. Low-affinity kainite receptor agonists induce insult-dependent apoptosis and necrosis in cultured cortical neurons. J Neurosci Res. 2000;59:788–96. doi: 10.1002/(SICI)1097-4547(20000315)59:6<788::AID-JNR11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Hestrin S. Developmental regulation of NMDA receptor mediated at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhong J, Carrozza DP, Williams K, Britchett DB, Molshoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- 32.Goebel DJ, Poosch MS. NMDA receptor subunit composition in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1, NR2A, NR2B, NR2C, NR2D and NR3A. Mol Brain Res. 1999;69:164–170. doi: 10.1016/s0169-328x(99)00100-x. [DOI] [PubMed] [Google Scholar]
- 33.Portera-Cailliau C, Price DL, Martin LJ. N-methyl-d-aspartate proteins NR2A and NR2B are differentially distributed in the developing rat central nervous system as revealed by subunit-specific antibodies. J Neurochem. 1996;66:692–700. doi: 10.1046/j.1471-4159.1996.66020692.x. [DOI] [PubMed] [Google Scholar]
- 34.Barkovich AJ, Gressens P, Evrard P, Lyon G, Vrard P. Formation and maturation and developmental disorders of brain white matter and neocortex. AJNR Am J Neuroradiol. 1992;13:423–61. [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez RM, Jensen FE. Maturational aspects of epilepsy mechanisms and consequences for the immature brain. Epilepsia. 2001;42:577–85. doi: 10.1046/j.1528-1157.2001.12000.x. [DOI] [PubMed] [Google Scholar]
- 36.Law AJ, Weickert CS, Webster MJ, Herman, Kleinman JE, Harrison PJ. Changes in NMDA receptor subunit mRNA’s and cyclophilin mRNA during development of the human hippocampus. Ann NY Acad Sci. 2003;1003:426–430. doi: 10.1196/annals.1300.043. [DOI] [PubMed] [Google Scholar]
- 37.Romijn HJ, Hoffman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Human Dev. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 38.Coultrap SJ, Nixon KM, Alvestad RM, Valenzuela CF, Browning MD. Differential expression of NMDA receptor subunits and splice variants among the CA1, CA3 and dentate gyrus of the adult rat. Molec Brain Res. 2005;135:104–111. doi: 10.1016/j.molbrainres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi T, Okada M, Kuno M, Kawasaki K. Dual mode of N-methyl-D-aspartate-induced neuronal death in hippocampal slice cultures in relation to N-methyl-d-aspartate receptor properties. Neuroscience. 1997;76:411–423. doi: 10.1016/s0306-4522(96)00403-4. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto A, Takeda A, Kogure V, Onodera H. NMDA receptor (NMDAR1) expression in the rat hippocampus after forebrain ischemia. Neurosci Lett. 1994;170:39–42. doi: 10.1016/0304-3940(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 41.Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]