Abstract
Propionic acidemia (PA) is an autosomal recessive inborn error of metabolism caused by deficiency of propionyl-CoA carboxylase (PCC). This enzyme is composed of six PCCA and six PCCB subunits and mediates a critical step in catabolism of odd chain fatty acids and certain amino acids. Current treatment options for PA are limited to stringent dietary restriction of protein consumption and some patients undergo elective liver transplantation. We previously generated a hypomorphic model of PA, designated Pcca−/−(A138T), with 2% of wild-type enzyme activity that mimics many aspects of the human disease. In this study, we used the differing tissue tropisms of adeno-associated virus (AAV) to probe the ability of liver or muscle-directed gene therapy to treat systemic aspects of this disease that affects many cell types. Systemic therapy with muscle-biased AAV1, liver-biased AAV8, and broadly tropic AAVrh10 mediated significant biochemical corrections in circulating propionylcarnitine (C3) and methyl citrate by all vectors. The innate tissue bias of AAV1 and AAV8 gene expression was made more specific by the use of muscle-specific muscle creatine kinase (specifically MCK6) and hepatocyte-specific transthyretin (TTR) promoters, respectively. Under these targeted conditions, both vectors mediated significant long-term correction of circulating metabolites, demonstrating that correction of muscle and likely other tissue types in addition to liver is necessary to fully correct pathology caused by PA. Liver-specific AAV8-TTR-PCCA mediated better correction than AAV1-MCK-PCCA. These data suggest that targeted gene therapy may be a viable alternative to liver transplantation for PA. They also demonstrate the effects of tissue-specific and broad gene therapy on a cell autonomous systemic genetic disease.
Introduction
Propionic acidemia (PA) is an autosomal recessively inherited organic acidemia that occurs as a result of mutations in either the PCCA or PCCB gene. The protein products of these genes are the α and β subunits of the mitochondrial propionyl-CoA carboxylase (PCC) enzyme (Huang et al., 2010). The propionyl-CoA substrate of PCC is generated by several mechanisms: (1) catabolism of the amino acids isoleucine, valine, methionine, and threonine; (2) beta oxidation of odd chain fatty acids (Lehnert et al., 1994); and (3) production by gut bacteria (Bain et al., 1988; Leonard, 1997). In patients with reduced PCC activity levels, propionyl-CoA accumulates in the body, eventually causing downstream elevations in several compounds, including propionylcarnitine (C3), methyl citrate (MeCit), and glycine.
The incidence of PA is approximately 1 in 100,000 live births in the United States, but can be as high as 1 in 1,000 births within select populations (Ravn et al., 2000). Patients with PA are usually identified within the first few days of life when newborn screening reveals high levels of C3 and MeCit (Turgeon et al., 2008, 2010). Clinical signs of PA generally manifest within the first few weeks of life with hypotonia, metabolic acidosis, lethargy, vomiting, poor feeding, and failure to thrive (Pena et al., 2012). Additional organ-specific pathologies present later in life and include cardiomyopathy, cardiac arrhythmias, neurological abnormalities, and acute pancreatitis (Pena and Burton, 2012).
There is no cure for PA. Treatment options are limited to reducing the production of propionyl-CoA from the previously mentioned sources of amino acids, odd-chain fatty acids, and gut bacteria. This is accomplished mainly through dietary restriction, particularly of the protein substrates. Even when strict dietary control is maintained, patients may still undergo metabolic decompensation in response to stresses like infections (Chapman et al., 2012a). Elective liver transplantation is being utilized to reduce the rate of serious complications, but transplantation itself is an invasive procedure with associated risks and does not actually cure the disease, as circulating C3, MeCit, and glycine remain elevated (Yorifuji et al., 2000; Chapman et al., 2012b). Additionally, patients receiving a donor liver will require long-term immunosuppression and risk a relatively high rate of posttransplant death and graft rejection (Barshes et al., 2006). The pathological involvement of multiple organ systems and lack of complete correction with liver transplantation indicates that systemic therapy treating additional tissues will provide a benefit and result in more complete treatment response than liver transplantation alone.
Gene therapy offers a possible alternative to current approaches. Until recently, the study of PA in animals and validation of gene therapy approaches was limited by the lack of a good animal model. Knockout mice lacking functional PCCA were developed, but these animals die within 36 hr of birth, making intravenous gene therapy very difficult to administer and monitor (Miyazaki et al., 2001; Hofherr et al., 2009).
We generated a hypomorphic model of PA by introducing a human PCCA transgene harboring an A138T mutation (Guenzel et al., 2013). This A138T PCCA protein with reduced activity was identified in PA patients with a mild form of the disease (Ugarte et al., 1999; Perez-Cerda et al., 2000; Desviat et al., 2004). Pcca−/−(A138T) mice survive to adulthood, but have significant elevations of circulating C3, MeCit, glycine, and ammonia along with cardiac defects consistent with PA (Guenzel et al., 2013). With this model, we were able to show that systemic gene therapy with either adenoviral (Ad5) and adeno-associated virus (AAV) serotype 8 (AAV8) vectors expressing human PCCA mediated significant reductions in C3 and MeCit levels. Single tail vein injection of 5×1011 vector genomes (vg) of AAV8-PCCA resulted in significant correction throughout the 13-week duration of the study (Guenzel et al., 2013).
Mutations in Pcca or Pccb have the potential to affect every cell in the body. Therefore, transplantation or gene therapy that targets correction to only one organ will not correct the cell autonomous disease in all affected tissues. Our goals in this study were to determine the degree to which correction of liver or muscle tissue is able to mediate the circulating metabolites associated with PA and determine whether genetic correction of large metabolic organs may serve as sites to eliminate some of the systemic byproducts of the deficiency to temper the worst aspects of the disease.
In this work, we used the known differences in AAV serotype tropism to probe how genetic correction in the liver and muscle influence PA therapy. We tested a muscle-biased AAV1 vector, a liver-biased AAV8 vector, and a broadly tropic AAVrh10 vector for their ability to reduce systemic metabolite profiles in the A138T mice. We next made these vectors more specific by adding tissue-specific transcriptional regulation to further restrict protein expression to liver and muscle tissue.
Materials and Methods
AAV vector production
AAV serotype 1, 8, and rh10 vectors carrying the Cre transgene were obtained from the University of Pennsylvania Vector Core (Philadelphia, PA). All PCCA expression vectors were made according to the previously published protocol using triple transfection of HEK293 cells and subsequent concentration of media by tangential flow filtration (Guenzel et al., 2013). All vectors were quantitated using TaqMan primer/probe sets recognizing sequence in the codon-optimized PCCA gene (Life Technologies, Grand Island, NY).
Cloning
The transthyretin (TTR) promoter in combination with an intron from the small minute virus of mice (Wu et al., 2008) was a kind gift from Dr. Paul E. Monahan (University of North Carolina at Chapel Hill). The muscle creatine kinase (MCK6) promoter was a kind gift from Dr. Jeffrey Chamberlain (University of Washington). pAAV-TTR-PCCA_co and pAAV-MCK-PCCA_co were generated by addition of an MluI site upstream of the TTR and MCK promoter regions and an EcoRI site downstream of the intron via PCR using Platinum Taq DNA Polymerase (Life Technologies). The PCR product was then cut with MluI and EcoRI, and the fragment was ligated into pAAV-PCCA_co in place of the CMV promoter and β-globin intron.
Intravenous vector administration
Animal experiments were conducted with Institutional Animal Care and Use Committee approval in accordance with National Institutes of Health guidelines. All animals were housed and bred in Department of Comparative Medicine animal facilities (Mayo Clinic, Rochester, MN). Intravenous administration of AAV vectors was performed by single tail vein injection to 5-week-old Pcca−/−(A138T) mice of 1×1012 vg of all AAV-cre vectors and 5×1011 vg of all AAV-PCCA vectors. AAV was administered in a volume of 100 μl for all injections. Groups of mice injected with AAV-PCCA vectors were composed of approximately 50% male and 50% female animals.
Confocal microscopy
Upon euthanasia, organs were quickly removed and fixed in 4% paraformaldehyde–PBS for 4 hr at room temperature. Tissue segments were then immersed overnight in 30% sucrose–PBS solution at 4°C and transferred to 10% sucrose–PBS for an additional overnight incubation at 4°C. Tissue sections were then embedded in optimal cutting temperature medium (Sakura Finetek USA, Torrance, CA) and flash-frozen in liquid nitrogen. Eight-micrometer sections were prepared using a Leica CM-850 cryostat, mounted on slides, and stained using VECTASHIELD with DAPI (Vector Laboratories, Burlingame, CA). Confocal imaging was then performed using a Zeiss Axiovert LSM510 laser confocal microscope (Carl Zeiss Jena, Jena, Germany) in the Optical Morphology Core facility at Mayo Clinic.
Metabolic assays
Quantitation of propionyl carnitine and MeCit was performed by spotting blood on to filter paper. Blood was collected by submandibular puncture of mice with a Goldenrod Lancet (MEDIpoint Inc., Mineola, NY) and spotted on a Whatman 903 Protein Saver card (GE Healthcare, Westborough, MA). Punches of blood spots were then taken, and acylcarnitine and MeCit levels were analyzed by tandem mass spectrometry as published previously (Turgeon et al., 2008, 2010).
PCCA protein assays
Tissues for PCCA protein analysis were removed from euthanized mice 25 days after vector administration and homogenized in T-Per Reagent and quantitated using Pierce BCA Protein Assay Reagent (Thermo Fischer Scientific, Rockford, IL). About 50 μg of total protein in equal volumes was loaded onto 7.5% Mini-PROTEAN TGX gels (Bio-Rad, Hercules, CA). Blots were then probed with anti-PCCA antibody (ProteinTech, Chicago, IL).
Data analysis
Statistical tests and graphing were performed with GraphPad Prism software.
Results
Tissue specificity of AAV serotypes
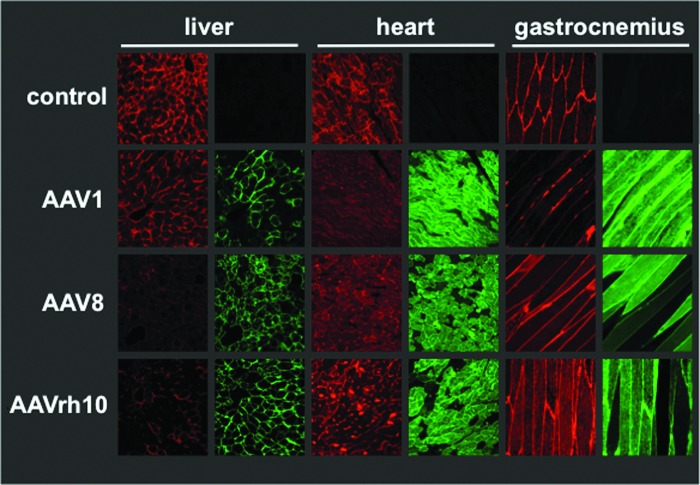
To evaluate the transduction of multiple tissue types, we used a sensitive Cre recombinase-based reporter system (Hillestad et al., 2012). In this model, all cells of the mouse express the gene for the membrane-targeted red fluorescent “tomato” protein (mT) flanked by LoxP sites followed by a gene-encoding membrane-targeted green fluorescence protein (mG). When Cre is expressed in the cell, the red fluorescence gene is excised and the green fluorescence gene is activated. If AAV successfully transduces a cell, this system gives two signals: (1) loss of red fluorescence combined with (2) activation of membrane-targeted GFP.
For this study, the Cre transgene was packaged with AAV1, 8, and rh10 capsid serotypes, and 1×1012 vg of each vector were administered intravenously to mice via tail vein injection. Twenty-one days after injection, the liver, heart, and gastrocnemius muscle were harvested to examine the tropism of each AAV serotype (Fig. 1). Examination at this dose revealed that all of the serotypes transduced all of the tissues, but with inherent biases. AAV serotype 8 produced transduction at all sites, but the highest levels of modification were observed in the liver. In contrast, the strongest signal in AAV1-Cre-treated mice occurred in skeletal and cardiac muscle, with reduced, but not eliminated transduction in the liver. AAV-rh10 was more broadly tropic than either AAV1 or 8. Some cells are observed that appear to have both red and green fluorescence. It is believed that these instances represent transduction of the main cell type (i.e., myofibers or hepatocytes) but lack of transduction of endothelium lining the blood vessels. In the heart and skeletal muscle images, it may also be because of the multinucleated nature of fused myofibers in which some nuclei may have been genetically altered by cre recombinase and other nuclei in the same myofiber have not been altered.
FIG. 1.
Reporter gene monitoring in liver, heart, and gastrocnemius. Pcca−/−(A138T) mice were intravenously injected with 1×1012 vg of AAV-cre vectors of indicated serotypes. A red-to-green color change in a cell of these mice indicates transduction of that cell by a cre recombinase expression vector. Twenty-five days after injection, tissues were fixed and imaged by confocal microscopy. Images shown are 400× magnification. AAV, adeno-associated virus; vg, vector genomes. Color images available online at www.liebertpub.com/hum
Endogenous PCCA expression in wild-type animals
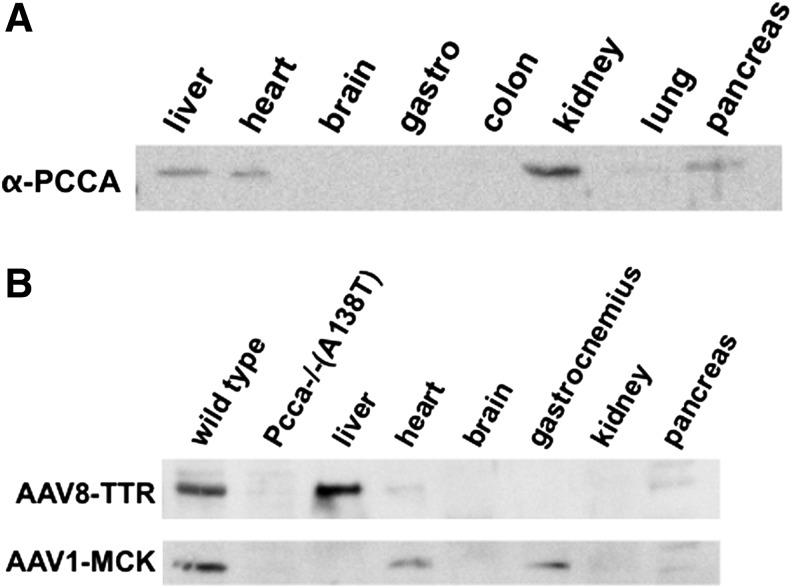
To clarify whether or not regulation of PCCA levels in nonhepatic tissues may provide benefit for the treatment of PA, PCCA levels were analyzed by Western blot in multiple tissue types of a Pcca+/+ mouse (Fig. 2a). Definitive PCCA expression was observed in the liver, heart, kidney, and pancreas. These tissues all represent possible treatment targets to restore normal PCC activity.
FIG. 2.
Tissue PCCA expression levels. (A) Indicated tissues were removed from an untreated Pcca+/+ mouse. Protein content was analyzed by BCA assay, and 50 μg of protein from each tissue was run on SDS-PAGE gels, blotted onto PVDF membrane, and then probed with anti-PCCA antibody. (B) Indicated tissues were removed from Pcca−/−(A138T) mice 25 days after IV injection of 5×1011 vg of AAV1-MCK-PCCA or AAV8-TTR-PCCA. Protein content was analyzed by BCA assay, and 50 μg of protein from each tissue was run on SDS-PAGE gels, blotted onto PVDF membrane, and then probed with anti-PCCA antibody. Wild-type and Pcca−/−(A138T) control lanes are 50 μg of protein from untreated liver tissue. BCA, bicinchoninic acid; IV, intravenous; PVDF, polyvinylidene fluoride; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Systemic gene therapy with AAV1, 8, and rh10
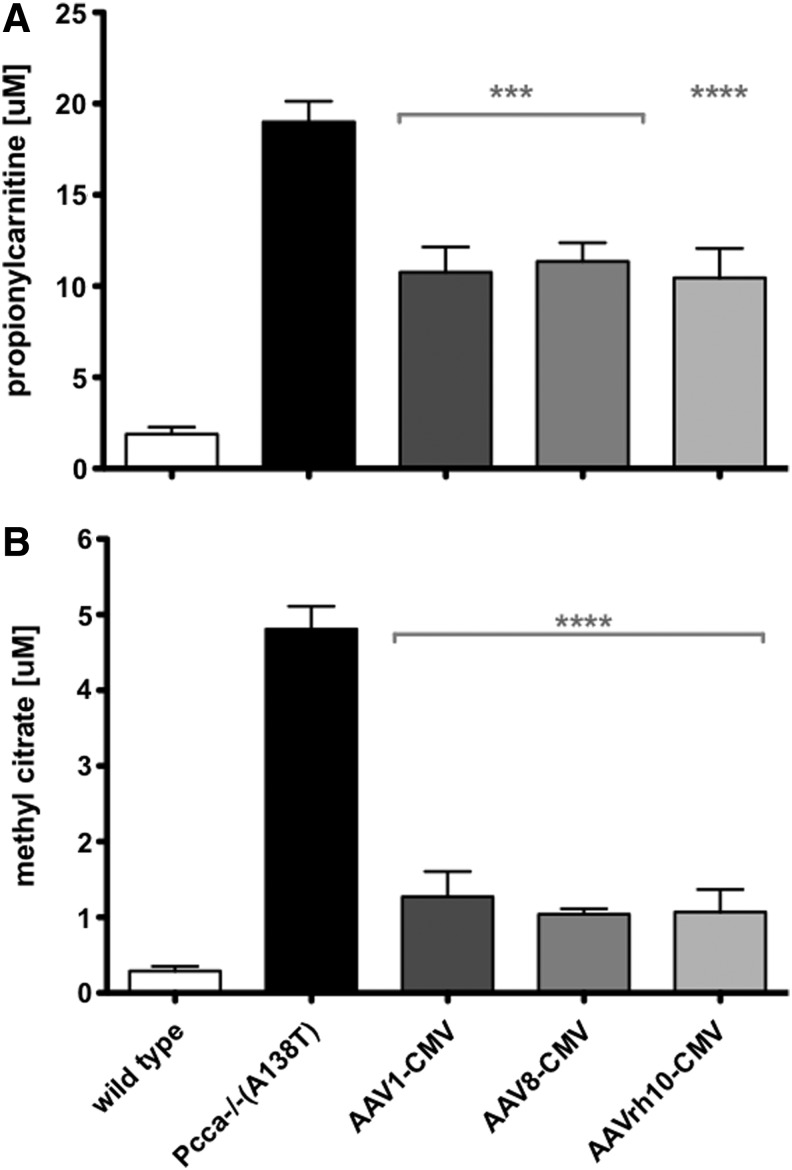
We previously demonstrated that AAV8 vectors expressing codon-optimized human PCCA under control of the CMV promoter could attenuate disease-associated metabolite profiles in A138T mice (Guenzel et al., 2013). To test alternate AAV serotypes, the same vector genome was packaged with AAV1, 8, and rh10 capsid serotypes. An amount of 5×1011 vg of each were injected intravenously via tail vein into 5-week-old Pcca−/−(A138T) mice. One week after administration, blood was collected on filter paper and assayed by tandem mass spectrometry for the PA biomarkers C3 and MeCit (Fig. 3a and b). All three of the AAV vectors mediated significant reductions in both C3 and MeCit compared with untreated mice (p<0.001 or 0.0001). While the degree of statistical significance in relation to untreated mice varied, there was no statistically significant difference between the AAV1, 8, and rh10 vectors expressing PCCA.
FIG. 3.
Biomarker concentrations in Pcca−/−(A138T) mice in response to treatment. C3 (A) and MeCit (B) were assayed by tandem mass spectrometry 1 week after IV administration of 5×1011 vg of AAV1 (n=7), AAV8 (n=10), and AAVrh10 (n=10) expressing PCCA cDNA. Age-matched wild-type mice were also analyzed (n=10). Asterisks indicate statistical significance relative to untreated Pcca−/−(A138T) mice (n=11). ***p<0.001, ****p<0.0001. Error bars depict SEM. MeCit, methyl citrate.
Transcriptional targeting of PCCA expression
To further restrict the expression of the PCCA protein, tissue-specific transcriptional regulatory elements were utilized in place of the promiscuous CMV enhancer/promoter. To obtain liver-specific expression, we utilized the TTR promoter in combination with an intron from the small minute virus of mice (Wu et al., 2008). This TTR vector was used in the context of the liver-biased AAV8 vector to encourage the highest liver PCCA expression levels possible. To obtain muscle-specific expression, the CK6 regulatory element of the MCK promoter was used to drive muscle-specific expression when delivered by the muscle-biased AAV1 vector. This MCK6 promoter was previously characterized and found to have 12% of the activity of a CMV promoter in muscle, but mediates a very muscle-restricted pattern of expression (Hauser et al., 2000).
Pcca−/−(A138T) mice were administered 5×1011 vg of each vector by tail vein, and 25 days after injection, liver, heart, brain, gastrocnemius, kidney, and pancreas tissues were assayed by Western blot (Fig. 2b). In contrast to the widespread expression mediated by CMV-PCCA vectors, AAV8-TTR-PCCA mediated expression restricted to the liver and AAV1-MCK-PCCA produced expression that was restricted to the skeletal and cardiac muscles. Muscle-specific expression driven by the MCK6 promoter was predictably much weaker than what we have observed from CMV promoters (Guenzel et al., Long-term sex-biased correction of propionic acidemia by adeno-associated virus vectors. Gene Therapy submitted), but heart PCCA levels in AAV1-MCK-treated mice were approximately equal to heart PCCA levels in wild-type mice (Fig 2a and b, heart lanes).
Therapeutic effects of tissue-restricted PCCA expression
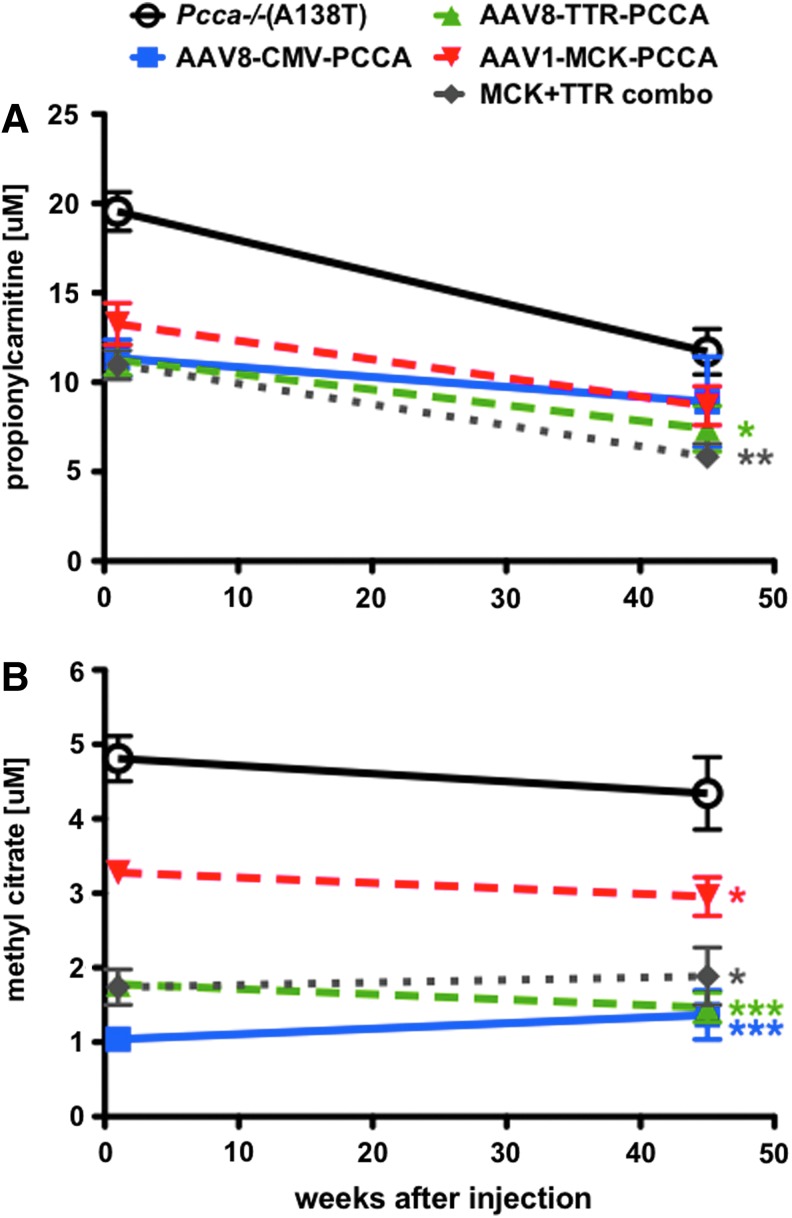
An amount of 5×1011 vg of each AAV1-MCK-PCCA and AAV8-TTR-PCCA vector were administered via single tail vein injection to 5-week-old Pcca−/−(A138T) mice, and disease-associated metabolites were measured in the blood at 1 week and 45 weeks after injection (Fig. 4). Both the liver-specific and muscle-specific AAV vectors mediated significant decreases in the levels of circulating MeCit compared with untreated mice. MeCit correction in the AAV1-MCK group was statistically less than that mediated by AAV8-TTR or the CMV vectors (p<0.001 for AAV8-CMV and AAV8-TTR compared with p<0.05). Propionylcarnitine levels were significantly lower in all treated animals than untreated Pcca−/−(A138T) mice at the 1-week time point and remained fairly constant throughout the 45-week study. However, the C3 concentration in the blood of untreated Pcca−/−(A138T) mice decreased naturally over time, making analysis at the late time point difficult. Additionally, mice were administered a combinatorial therapy including 5×1011 vg of both AAV1-MCK-PCCA and AAV8-TTR-PCCA in a single injection. The levels of both metabolites in response to the combination treatment were similar to that observed in the AAV8-TTR- and AAV8-CMV-treated mice.
FIG. 4.
Circulating biomarker response to tissue-specific therapy. C3 (A) and MeCit (B) were assayed by tandem mass spectrometry 1 and 45 weeks after injection of 5×1011 vg of AAV1-MCK-PCCA, AAV8-TTR-PCCA, AAV8-CMV-PCCA, or AAV1-MCK-PCCA/AAV8-TTR-PCCA combination treatment (n=10 each). Asterisks indicate statistical significance relative to untreated Pcca−/−(A138T) mice at the 45-week time point. *p<0.05 and ***p<0.001. Error bars depict SEM. Color images available online at www.liebertpub.com/hum
Discussion
PA is a genetic disease that affects PCC activity in many cell and tissue types in the body. Current approaches to PA treatment involve stringent dietary restriction and, more recently, elective liver transplantation. Organ transplantation is an invasive procedure that corrects only the phenotype in the liver and does not address defects elsewhere in the body. For PA, liver transplantation is able to reduce the frequency of neurologic side effects, but is unable to reduce the levels of PA metabolites. This lack of metabolite correction may explain why other phenotypes are not always corrected by liver transplantation. These observations in liver transplant recipients suggest that correction of PA in only the liver is inadequate to fully treat the disease. This work explores the role of treatment of nonhepatic tissues for PA as well as tissue-specific and nonspecific gene delivery by select AAV serotypes.
Our previous work and the work of others showed that treatment of PA mice with liver-biased AAV8 and Ad5 vectors is able to significantly reduce systemic PA metabolites (Chandler et al., 2011; Guenzel et al., 2013). However, liver-biased vectors are not absolutely liver specific, and so they may also deliver the PCCA protein to additional nonhepatic cells (Fig. 1). In this study, we explored the ability to modulate PA phenotypes by directing genetic correction to different organ and tissue systems—primarily the muscle.
To explore this question, we compared the ability of muscle-biased AAV1 with liver-biased AAV8 and the more broadly tropic AAVrh10 vector to reduce systemic PA metabolite levels. We found that regardless of their natural tissue bias, all three vectors mediated significant decreases in circulating levels of both C3 and MeCit at a dose of 5×1011 vg per mouse. Restricting PCCA transgene expression to the liver and muscle by use of TTR and MCK promoters showed that both were still able to reduce systemic MeCit and C3 levels. AAV8-TTR-PCCA reduced PA metabolites to concentrations nearly as low as in mice treated with the promiscuous AAV-CMV-PCCA vectors. Muscle-specific AAV1-MCK-PCCA also resulted in significant decreases in metabolites 1 week after injection, although not to the reduced levels that were observed after administration of AAV8-TTR-PCCA or the broadly active AAV-CMV vectors. At least some of these results are similar to work restricting genetic correction to muscle for evaluating therapeutics for the treatment phenylketonuria (Ding et al., 2008). Importantly, reductions in MeCit observed in mice treated with both tissue-specific vectors were maintained for the duration of the 45-week study. MeCit and C3 have been implicated in energetic inhibition leading to organ-specific defects as well as hyperammonemia and lactic acidemia in PA patients (Cheema-Dhadli et al., 1975; de Keyzer et al., 2009). We believe that MeCit more directly reflects propionyl-CoA levels, and since these two analytes have together been implicated in bioenergetic inhibition in PA disease, MeCit is likely more significant than propionylcarnitine for evaluating efficacy of PA treatment options.
The decrease in C3 and MeCit in response to treatment with AAV8-TTR-PCCA was expected and reinforces the notion that liver gene therapy provides a therapeutic benefit. The results with AAV1-MCK-PCCA showed that treatment of muscle alone is able to reduce circulating metabolite levels as well. We expected that the combination of AAV8-TTR-PCCA and AAV1-MCK-PCCA might mimic the results of the CMV vectors and would mediate better metabolite control than either vector alone. This was surprisingly not the case, as the combination, at least at this dose, was not statistically better than AAV8-TTR-PCCA alone. We hypothesize that treatment of the liver is still the best choice for a single-organ treatment and that liver correction may help to clear out some of the additional circulating C3 and MeCit produced in other tissues, but there are still locally produced C3 and MeCit that likely remain in the tissue and can cause pathology. However, it was interesting that the MCK/TTR combination treatment did provide the greatest reduction in propionylcarnitine over time.
An ideal treatment for PA would correct the genetic defect in every cell that normally expresses the protein. Since such a treatment does not exist, one must consider all of the causes of pathology relating to circulation of toxic metabolites and local accumulation of metabolites in the tissues that need to be reflected in the vector design.
If we are forced to treat one tissue, the liver is still the best choice for a single-organ treatment where it may serve to metabolize at least some C3 and MeCit produced by other tissues. The use of muscle-targeted therapy alone is not a preferred treatment option, but has been used in this case to demonstrate the need for expression in nonhepatic tissues. In that context, this study provides sound evidence that genetic correction of nonhepatic tissue mitigates systemic PA metabolites.
Local treatment of the cell autonomous aspects of PA may well provide benefits even if it does not affect systemic metabolite levels. It is likely that some aspects of PA result from local accumulation of disease metabolites. Preventing the buildup of propionyl-CoA and MeCit in the heart, skeletal muscle, or brain may reduce the amount of these metabolites in mitochondria and temper energetic inhibition that has been observed in these tissues. While systemic therapy likely mitigates neurologic damage caused by hyperammonemia, direct neurologic gene therapy may be the only effective method to protect the brain from locally produced toxic metabolites that may cause developmental delay, stroke, and other neurologic phenotypes.
A valid treatment option for PA must consider all of the causes of pathology relating to circulation of toxic metabolites and local accumulation of metabolites in the tissues that need to be reflected in the vector design. We believe that treating a wide array of tissues represents the best option for PA disease correction and therefore favors an approach with PCCA expression being driven by a constitutively active promoter delivered in a broadly tropic vector serotype to transduce as many tissues as possible. Given this, broadly tropic vector serotypes like AAV9 and rh10 may have value when used in combination for systemic as well as direct neurologic gene therapy. An ideal therapy could be administered by intravenous injection as shown in this study; however, intracranial treatment may also be necessary to effectively transduce brain tissue.
Acknowledgments
We thank the Clinical Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), Optical Microscopy Core, the Gene Expression Core, and the Advanced Genomics Technology Core at Mayo Clinic for assistance with the work. This work was supported by funding to M.A.B. from the Propionic Acidemia Foundation and the Organic Acidemia Association. This work was also supported by the Mayo Clinic Department of Laboratory Medicine and Pathology, the Liver Regeneration Program in the Center for Regenerative Medicine, and the Department of Molecular Medicine. M.L.H. received support from the Kidney Disease Research Training Program T32-DK007013. This Publication was made possible by CTSA Grant number UL1 TR000135 from NIH/NCATS.
Author Disclosure Statement
No competing financial interests exist.
References
- Bain M.D., Jones M., Borriello S.P., et al. (1988). Contribution of gut bacterial metabolism to human metabolic disease. Lancet 1, 1078–1079 [DOI] [PubMed] [Google Scholar]
- Barshes N.R., Vanatta J.M., Patel A.J., et al. (2006). Evaluation and management of patients with propionic acidemia undergoing liver transplantation: a comprehensive review. Pediatr. Transplant. 10, 773–781 [DOI] [PubMed] [Google Scholar]
- Chandler R.J., Chandrasekaran S., Carrillo-Carrasco N., et al. (2011). Adeno-associated virus serotype 8 gene transfer rescues a neonatal lethal murine model of propionic acidemia. Hum. Gene Ther. 22, 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.A., Gropman A., Macleod E., et al. (2012a). Acute management of propionic acidemia. Mol. Genet. Metab. 105, 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.A., Summar M.L., and Enns G.M. (2012b). Propionic acidemia: to liver transplant or not to liver transplant? Pediatr. Transplant. 16, 209–210 [DOI] [PubMed] [Google Scholar]
- Cheema-Dhadli S., Leznoff C.C., and Halperin M.L. (1975). Effect of 2-methylcitrate on citrate metabolism: implications for the management of patients with propionic acidemia and methylmalonic aciduria. Pediatr. Res. 9, 905–908 [DOI] [PubMed] [Google Scholar]
- De Keyzer Y., Valayannopoulos V., Benoist J.F., et al. (2009). Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr. Res. 66, 91–95 [DOI] [PubMed] [Google Scholar]
- Desviat L.R., Perez B., Perez-Cerda C., et al. (2004). Propionic acidemia: mutation update and functional and structural effects of the variant alleles. Mol. Genet. Metab. 83, 28–37 [DOI] [PubMed] [Google Scholar]
- Ding Z., Harding C.O., Rebuffat A., et al. (2008). Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol. Ther. 16, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzel A.J., Hofherr S.E., Hillestad M., et al. (2013). Generation of a hypomorphic model of propionic acidemia amenable to gene therapy testing. Mol. Ther. 21, 1316–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M.A., Robinson A., Hartigan-O'connor D., et al. (2000). Analysis of muscle creatine kinase regulatory elements in recombinant adenoviral vectors. Mol. Ther. 2, 16–25 [DOI] [PubMed] [Google Scholar]
- Hillestad M.L., Guenzel A.J., Nath K.A., and Barry M.A. (2012). A vector-host system to fingerprint virus tropism. Hum. Gene Ther. 23, 1116–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofherr S.E., Senac J.S., Chen C.Y., et al. (2009). Short-term rescue of neonatal lethality in a mouse model of propionic acidemia by gene therapy. Hum. Gene Ther. 20, 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.S., Sadre-Bazzaz K., Shen Y., et al. (2010). Crystal structure of the alpha(6)beta(6) holoenzyme of propionyl-coenzyme A carboxylase. Nature 466, 1001–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert W., Sperl W., Suormala T., and Baumgartner E.R. (1994). Propionic acidaemia: clinical, biochemical and therapeutic aspects. Experience in 30 patients. Eur. J. Pediatr. 153, S68–S80 [DOI] [PubMed] [Google Scholar]
- Leonard J.V. (1997). Stable isotope studies in propionic and methylmalonic acidaemia. Eur. J. Pediatr. 156Suppl 1, S67–S69 [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Ohura T., Kobayashi M., et al. (2001). Fatal propionic acidemia in mice lacking propionyl-CoA carboxylase and its rescue by postnatal, liver-specific supplementation via a transgene. J. Biol. Chem. 276, 35995–35999 [DOI] [PubMed] [Google Scholar]
- Pena L., and Burton B.K. (2012). Survey of health status and complications among propionic acidemia patients. Am. J. Med. Genet. A 158A, 1641–1646 [DOI] [PubMed] [Google Scholar]
- Pena L., Franks J., Chapman K.A., et al. (2012). Natural history of propionic acidemia. Mol. Genet. Metab. 105, 5–9 [DOI] [PubMed] [Google Scholar]
- Perez-Cerda C., Merinero B., Rodriguez-Pombo P., et al. (2000). Potential relationship between genotype and clinical outcome in propionic acidaemia patients. Eur. J. Hum. Genet. 8, 187–194 [DOI] [PubMed] [Google Scholar]
- Ravn K., Chloupkova M., Christensen E., et al. (2000). High incidence of propionic acidemia in greenland is due to a prevalent mutation, 1540insCCC, in the gene for the beta-subunit of propionyl CoA carboxylase. Am. J. Hum. Genet. 67, 203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon C., Magera M.J., Allard P., et al. (2008). Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin. Chem. 54, 657–664 [DOI] [PubMed] [Google Scholar]
- Turgeon C.T., Magera M.J., Cuthbert C.D., et al. (2010). Determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin. Chem. 56, 1686–1695 [DOI] [PubMed] [Google Scholar]
- Ugarte M., Perez-Cerda C., Rodriguez-Pombo P., et al. (1999). Overview of mutations in the PCCA and PCCB genes causing propionic acidemia. Hum. Mutat. 14, 275–282 [DOI] [PubMed] [Google Scholar]
- Wu Z., Sun J., Zhang T., et al. (2008). Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol. Ther. 16, 280–289 [DOI] [PubMed] [Google Scholar]
- Yorifuji T., Muroi J., Uematsu A., et al. (2000). Living-related liver transplantation for neonatal-onset propionic acidemia. J. Pediatr. 137, 572–574 [DOI] [PubMed] [Google Scholar]