Abstract
For more than 2 billion years, microbes have reigned on our planet, evolving or outlasting many obstacles they have encountered. In the 20th century, this trend took a dramatic turn with the introduction of antibiotics and vaccines. Nevertheless, since then, microbes have progressively eroded the effectiveness of previously successful antibiotics by developing resistance, and many infections have eluded conventional vaccine design approaches. Moreover, the emergence of resistant and more virulent strains of bacteria has outpaced the development of new antibiotics over the last few decades. These trends have had major economic and health impacts at all levels of the socioeconomic spectrum – we need breakthrough innovations that could effectively manage microbial infections and deliver solutions that stand the test of time. The application of nanotechnologies to medicine, or nanomedicine, which has already demonstrated its tremendous impact on the pharmaceutical and biotechnology industries, is rapidly becoming a major driving force behind ongoing changes in the antimicrobial field. Here we provide an overview on the current progress of nanomedicine in the management of microbial infection, including diagnosis, antimicrobial therapy, drug delivery, medical devices, and vaccines, as well as perspectives on the opportunities and challenges in antimicrobial nanomedicine.
Keywords: Nanomedicine, Microbial infection, Diagnosis, Therapy, Drug delivery, Medical device, Vaccine
1. Introduction
Antibiotics and vaccines are arguably the most important medical innovations in human history. The introduction of a broad range of antibiotics and immunizations drastically reduced the morbidity and mortality from infectious diseases over the last century [1,2]. Between 1900 and 1996, mortality from infectious diseases in the United States fell remarkably from 797 to 59 deaths per 100,000 with the lowest mortality rate of 36 deaths per 100,000 in 1980. However, multiple concerning trends have emerged in the last several decades that threaten to deeply undermine progress. By far the most worrisome has been the relentless emergence of antimicrobial resistance, which has been widely documented to worsen clinical outcomes and increase the economic burden of infectious diseases [3]. While many factors appear to be involved in its emergence, the excessive and improper use and abuse of antibiotics has been shown to play a major role. The continued evolution of drug resistance, which has already invalidated many routinely used antibiotics, has reached a fevered pitch and is a serious public health threat, with some even warning of the possibility of the 21st century becoming the “post-antibiotic” era [4]. Moreover, some bacterial strains are capable of developing or acquiring resistance to multiple antimicrobial agents, a phenomenon known as multidrug resistance (MDR) [5]. MDR pathogens are notoriously difficult to treat using conventional methods and can be clinically untreatable. One serious example of MDR is the increasing proportion of methicillin-resistant Staphylococcus aureus (S. aureus), or MRSA, acquiring resistance to vancomycin (vancomycin-resistant S. aureus, or VRSA), making the treatment a daunting medical challenge since vancomycin is often regarded as the last resort for S. aureus infections [6]. In the ongoing race between the emergence of drug resistance and the development of novel antimicrobial agents, microbes appear to be pulling ahead [7]. To make the situation worse, the pharmaceutical industry has decreased its efforts to develop new antibiotics due to low returns on investment and shifts in R&D priorities; the pipeline of new antibiotics, in particular for MDR Gram-negative superbugs, is running dry [8,9].
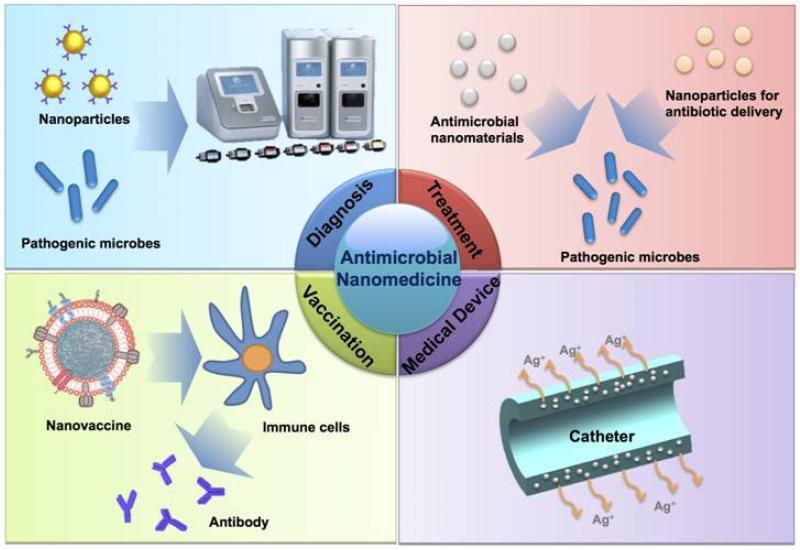
The application of nanotechnologies to medicine, also named nanomedicine, has profoundly altered the landscape of the pharmaceutical and biotechnology industries [10-13], with around 100 nanomedicine products already approved for clinical use ranging from drug delivery and imaging to implantable biomaterials and medical devices [14]. Nanotechnologies have also shown impressive potential in tackling almost every aspect of microbial infection (Figure 1). More than 10 nanoparticle-based products have been marketed for bacterial diagnosis, antibiotic delivery, and medical devices (Table 1). With their unique physicochemical characteristics, nanomaterials have played a critical role in the fast, sensitive, and selective detection of microbial infections. Many inorganic and organic nanomaterials have also been demonstrated to possess potent inherent antimicrobial properties that are rarely expressed in their bulk form. More importantly, some of these nanomaterials can combat antibiotic resistance by compromising existing resistance mechanisms. Furthermore, nanoparticles for antimicrobial drug delivery also offer distinct advantages in overcoming resistance and causing fewer side effects than conventional antibiotics. In addition, the incorporation of antimicrobial nanomaterials in medical devices can prevent microbial adhesion and infection. Last, but not least, using nanomaterials as vaccine adjuvants and/or delivery vehicles can evoke more efficient immune responses against microbial infection. Herein we provide an overview of all these aspects of antimicrobial nanomedicine and discuss the opportunities and challenges of this exciting area.
Figure 1.
Nanomedicine applications in the management of microbial infection.
Table 1.
Examples of clinical stage nanotechnology-based products for antimicrobial management
| Name | Company/Sponsor | Composition | Application | Stage of Development |
|
|---|---|---|---|---|---|
| Diagnosis | Verigene | Nanosphere | Oligonucleotide-conjugated Au nanoparticle |
Bacterial infection and drug resistance diagnosis |
Marketed |
| T2 Candida | T2 Biosystems | Oligonucleotide-conjugated SPION |
Blood detection for sepsis (candidemia) |
Clinical trial | |
| Drug Delivery |
Abelcet | Enzon Pharmaceutical |
Amphotericin B-lipid complex |
Fungal infection | Marketed |
| AmBisome | Gilead Sciences | Liposomal Amphotericin B | Fungal infection | Marketed | |
| Amphotec | Kadmon Pharmaceuticals |
Liposomal Amphotericin B | Fungal infection | Marketed | |
| Fungisome | Lifecare Innovations |
Liposomal Amphotericin B | Fungal infection | Marketed | |
| Arikace | Insmed | Liposomal amikacin | Chronic pseudomonas aeruginosa infection Pulmonary nontuberculous mycobacterial lung disease |
Clinical trial (phase 3) Clinical trial (phase 2) |
|
| Medical Device |
SilvaSorb | AcryMed | Ag nanoparticle-embedded hydrogel |
Wound dressing | Marketed |
| Acticoat | Smith & Nephew | Nanosilver-coated high- density polyethylene mesh |
Wound dressing | Marketed | |
| KoCarbonAg | Bio-medical Carbon Technology |
Ag nanoparticle-coated carbon fiber cloth and PE membrane |
Wound dressing | Clinical trial | |
| ON-Q SilverSoaker |
I-Flow | Ag nanoparticle-coated polyvinylchloride |
Catheter for the delivery of local anesthetics |
Marketed | |
| VentriGuard | Neuromedex | Ag nanoparticle-embedded nonmetallic porous materials |
Ventricular catheter for cerebrospinal fluid drainage |
Marketed | |
| AGENTO I.C. |
C.R. Bard | Ag nanoparticle-distributed hydrophilic polymer |
Endotracheal tube | Marketed | |
| LogiCathAg Tive |
Smiths | Ag nanoparticle-embedded polyurethane |
Central venous catheter | Marketed | |
| Silverline | Spiegelberg | Ag nanoparticle- and insoluble silver salt- incorporated polyurethane or silicone |
Catheter for internal CSF- drainage |
Marketed | |
| IABN | Hadassah Medical Organization |
Quaternary ammonium poly(ethylene imine) nanoparticle-embedded resin |
Root canal sealer, and dental restorative materials |
Clinical trial (phase 2) |
|
| Vaccine | CAF01 | Statens Serum Institut |
Cationic liposome-based adjuvant |
Tuberculosis | Clinical trial (phase 1 completed) |
2. Nanotechnologies for Microbial Diagnosis
Infectious diseases caused by contagious microbes are subject to transmission from either an infected individual or vector to a healthy individual. Rapid, sensitive, and specific detection of pathogens is therefore critical for identifying the source of infection, improving patient care with proper treatment, and controlling the spread of disease[15,16]. The complexity and broad variety of microbes, as well as the incubation period before clinical symptoms appear (ranging from a couple of minutes to years after the initial infection), make the diagnosis of some of these conditions very challenging. Modern molecular techniques for microbial infection diagnosis, including enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR), possess high sensitivity and reproducibility. Nevertheless, these techniques require laborious sample preparation processes and have long readout times, possibly delaying the time-critical diagnosis of and response to urgent situations in infection, such as bacterial sepsis. Moreover, the sophisticated instrumentation required and short shelf life of some reagents limit the application of these detection techniques in developing countries and rural areas of developed countries, where microbial infectious diseases are more likely a major health problem.
Nanotechnology presents a great opportunity for the development of fast, sensitive, specific, and cost-effective techniques for the diagnosis of microbial infection [17]. Sensitive and specific detection requires selective capturing and distinguishing of target molecules/microbes from other substances in a complex sample matrix. Nanotechnology can facilitate both of these processes, and the unique physicochemical properties of nanomaterials potentially enable the recording a single binding event. Targets can be labeled or captured by nanoparticles coupled with affinity probes (e.g., antibodies and nucleic acids), which can selectively recognize microbe biomarkers. In addition, the development of surface patterning techniques and nanoscale arrays of pathogen-targeting ligands may further revolutionize pathogen detection. Several different types of nanomaterials have been used for microbial diagnosis, including magnetic, gold (Au), and fluorescent nanoparticles.
2.1. Magnetic Nanoparticles
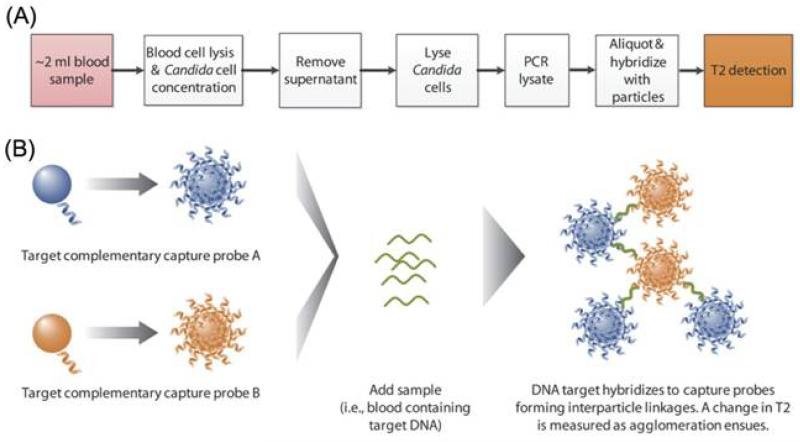
Magnetic nanoparticles, in particular superparamagnetic iron oxide nanoparticles (SPIONs), have been well studied as magnetic resonance imaging contrast agents for medical applications [18]. A great deal of effort has also been focused on the application of probe-decorated magnetic nanoparticles for microbial diagnosis. In one recent study, Lowery and co-workers developed a T2 magnetic resonance (T2MR)-based SPION diagnostic platform that can rapidly and reproducibly detect five Candida species in whole blood within 3 h [19]. Hybridization of oligonucleotide-decorated SPIONs with amplified Candida DNA yields large changes in the sample’s T2MR signal (Figure 2). Notably, the product based on this system, T2Candida, is currently in clinical trials, with data expected in 2014. Meanwhile, Weissleder and colleagues reported the development of magneto-DNA nanoparticles that can target bacterial ribosomal RNA for profiling of pathogens in clinical samples [20]. Coupled with a miniaturized nuclear magnetic resonance (NMR) system for signal readout, the assay was capable of simultaneously detecting and phenotyping a panel of 13 bacterial species in clinical specimens within 2 h.
Figure 2.
(A) Assay workflow for detection of Candida with T2MR. (B) Schematic depicting the T2MR detection particle reagent. Oligonucleotide probes are covalently conjugated to SPIONs. For each target, two populations of nanoparticles were generated, each bearing a distinct target-complementary probe. Upon hybridization to the target strand amplified in excess by asymmetric PCR, these nanoparticles cluster, leading to a change of the sample’s T2MR signal. The extent of clustering increases with the target DNA concentration. Reprinted with permission from [19]. Copyright 2013, American Association for the Advancement of Science.
Magnetic nanoparticles can also be externally manipulated by controlled magnetic fields, enabling the enrichment, washing, and resuspension of targets from a complex biological matrix. Application of this unique profile of magnetic nanoparticles in conjunction with novel detection techniques offers limitless potential in sensitive and multiplex detection of bacteria. For instance, matrix-assisted laser desorption/mass spectrometry (MALDI-MS) has recently been adopted as a fast and reliable method for bacterial identification based on the mass spectrometry profiles of commonly encountered bacterial species [21]. Integrated workflow combining the magnetic nanoparticle-based sample preparation/concentration with MALDI-MS detection enables rapid analysis of bacteria in clinical samples, such as whole blood [21,22]. Besides, ligand-modified magnetic nanoparticles have also been combined with magnetic microfluidic devices for clearing bacteria and endotoxins from the bloodstream [23]. Magnetic nanoparticles modified with a synthetic ligand bis-Zn-DPA can remove Escherichia coli (E. coli) from bovine whole blood with almost 100% clearance at flows as high as 60 mL/h.
In addition to the diagnosis of bacterial infection, magnetic nanoparticles were also utilized to determine bacterial metabolic activity and antimicrobial susceptibility by monitoring the consumption rate of nutrients (e.g., starch). Perez and co-workers developed two SPION-based assays to assess the bacterial susceptibility to antibiotics in blood with magnetic relaxation [24]. At low metabolic activity or bacterial growth rates, the unconsumed starch, which has a high binding affinity to Concanavalin A (Con A), can induce the aggregation of Con A-conjugated SPIONs or dextran-coated SPIONs supplemented with free Con A, leading to a change of T2MR. The susceptibility of different microbes to ampicillin can be determined within 2.5 h with a dextran-coated SPION competition assay in the presence of free Con A, or within 5 min with a Con A-conjugated SPION non-competition assay. This strategy is as sensitive and reliable as the turbidity method, which requires 24 h cell incubation for the measurement of antimicrobial susceptibility.
2.2. Au Nanoparticles
Au nanoparticles have distinctive optical and electrochemical properties that bulk Au does not possess and can be easily surface-functionalized with probes, both advantages that have triggered widespread interest in their application as sensing materials [25]. Since the pioneering work by Mirkin and colleagues [26], oligonucleotide-functionalized Au nanoparticles have been widely used as probes for fast identification of pathogens whose genome sequence is known to contain unique nucleic acid signatures. Hybridization of oligonucleotide-Au nanoparticles with target nucleic acids allows the formation of a polymeric network with a concomitant distinct shift in the plasmon resonance peak [26]. Utilizing the distance-dependent optical properties of Au nanoparticles, Storhoff and co-workers also developed a ‘spot-and-read’ colorimetric detection method for identifying the mecA gene found in MRSA strains [27]. Hybridization of these nanoparticles created a visually detectable color change when the solutions were spotted onto an illuminated glass waveguide, with zeptomole sensitivities in detecting nucleic acids.
With a Raman tag as a narrow-band spectroscopic fingerprint and silver coating as a surface-enhanced Raman scattering promoter, Au nanoparticle probes labeled with oligonucleotides and Raman-active dyes have been utilized for multiplexed detection of oligonucleotide targets with high sensitivity and selectivity [28]. Six dissimilar DNA targets were simultaneously distinguished with six Raman-labeled Au nanoparticle probes with a detection limit of 20 femtomolar. Using this detection modality, Mirkin and colleagues further developed a bio-barcode assay for extraordinarily sensitive analysis of nucleic acid and protein targets [29]. As shown in Figure 3, the targets of interest form a sandwich structure with Au nanoparticles and magnetic microparticles for magnetic separation and dithiothreitol (DTT)-mediated release of barcode strands, which are subsequently identified and quantified on a microarray. Based on this diagnostic technology, the Verigene test was successfully developed by Nanosphere, Inc. and approved by FDA for in vitro bloodstream identification of both Gram-positive and Gram-negative bacteria. This test is ~ two to three orders of magnitude more sensitive than ELISA-based methods and can provide results within 2 - 2.5 h of blood culture positivity, as compared to ~ 2 - 4 days required by conventional microbiological methods [30].
Figure 3.
Bio-barcode assay for DNA and protein detection. Schematic representation of (A) protein detection using the bio-barcode assay; (B) nucleic acid detection using the bio-barcode assay; and (C) the scanometric detection method. Au-NP, gold nanoparticle; MMP, magnetic microparticle. Reprinted with permission from[29]. Copyright 2006, Nature Publishing Group.
Au nanomaterials labeled with other affinity probes than oligonucleotides have also been reported for bacterial diagnosis. Au nanoclusters embedded within lysozymes that can bind with peptidoglycans on bacterial cell walls, were developed to concentrate pathogenic bacteria for MALDI-MS-based identification[31]. Human serum albumin- or its binding peptide motif-stabilized Au nanoclusters also demonstrated specific affinity with S. aureus and MRSA for their selective detection[32]. Moreover, similar to magnetic nanoparticles, Au nanoparticles can also be used to determine antimicrobial susceptibility by measuring the shifts in the surface plasmon band, upon the Con A-induced clustering of dextran-coated Au nanoparticles in the presence of starch in bacterial suspension [33].
2.3. Fluorescent Nanoparticles
Nanomaterials with fluorescent properties or nanoparticles labeled/encapsulated with fluorescent dyes have also been applied for microbial detection. Using antibody-conjugated silica nanoparticles that encapsulate thousands of fluorescent dye molecules for signal amplification, Tan and co-workers developed an assay tool for in situ detection of single bacterium cells in less than 20 min [34]. They also developed multicolored FRET (fluorescence resonance energy transfer) silica nanoparticles by co-encapsulating three tandem dyes that emit unique colors upon excitation with a single wavelength [35]. Simultaneous detection of multiple bacterial targets was achieved with different monoclonal antibody-conjugated FRET silica nanoparticles. Quantum dots (QDs), a type of fluorescent semiconductor nanoparticle, exhibit many characteristics that make them superior to conventional fluorophores, such as photobleaching resistance and size-tunable broad absorption spectra with narrow emission [36]. These optical qualities along with versatile surface chemistry make QDs a good modality for the analysis of complex samples and have been applied for the detection of bacteria such as Listeria monocytogenes [37].
The chemical and physical versatility of these inorganic nanoparticles, together with the unique interactions of affinity probes with molecular targets or pathogens, make them very promising for robust and high-throughput microbial diagnosis of biological and environmental samples. Miniaturized devices that require lower sample volumes and produce faster readouts with higher sensitivity and accuracy will be designed. However, it should be mentioned that most of these nanoparticle-based diagnostic strategies depend upon the recognition of known bacterial genome sequences/biomarkers by targeting probes, and thus may not identify mutated and/or new bacteria strains. As drug-resistant strains continue to emerge, another important direction is the development of diagnostic nanotechnology capable of not only sensing the presence of pathogens, but also determining the susceptibility of the pathogens to antimicrobial drugs at the same time.
3. Nanotechnologies in Antimicrobial Treatment
The rapid emergence of antimicrobial drug resistance has become a widespread challenge and a significant threat to public health. Bacteria acquire resistance by spontaneous mutation of existing genes or through horizontal gene transfer by transformation, conjugation, or transduction [38]. Resistance to antimicrobial drugs involves numerous mechanisms, including decreased uptake and increased efflux of drug from the microbial cell, increased production of a competitive inhibitor of antibiotics, and alteration of the substrate to which antibiotics bind [39]. Another very important challenge in antimicrobial therapy is the treatment of chronic infections, which are often caused by the formation of biofilms and/or by intracellular microbes (e.g., Mycobacterium leprae, Chlamydia, Listeria, and others) [40]. Biofilm is a matrix consisting of extracellular polymeric substance (EPS) that accumulates and surrounds bacterial cells [41], acting as a barrier of diffusion by trapping and degrading antibiotic molecules. Bacteria in a biofilm can exhibit up to 1000 times more resistance to multiple antibiotics than planktonic bacteria [42]. Intracellular microbes are well protected by the host cell, and thus have limited exposure to many antibiotics. Frequent administration of high-dose antibiotics is still often prescribed for chronic infections, but complete eradication is difficult.
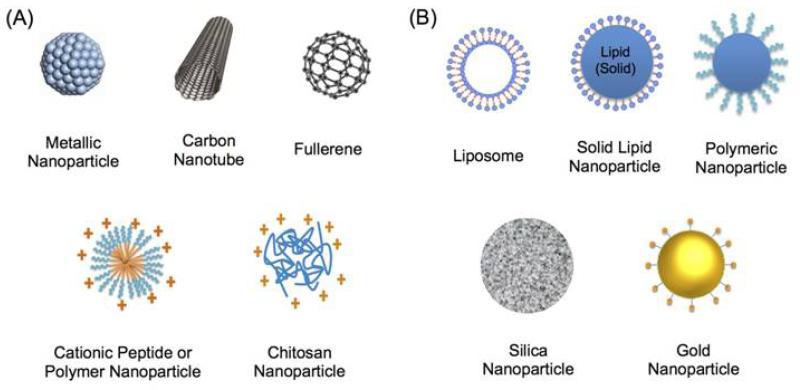
One prominent advantage of nanomedicine in antimicrobial treatment is the potential to address existing microbial resistance while avoiding its further development. This could be achieved by different strategies, such as concurrently targeting multiple pathways by antimicrobial nanomaterials, and increasing local antibiotic concentration by nanoparticle-based delivery. Moreover, some antimicrobial nanotherapeutics show promise in treating chronic infections through inhibiting the formation of biofilms and targeting intracellular microbes. Two major applications of nanomedicine in antimicrobial treatment are (i) the development of inorganic and organic nanomaterials with inherent antimicrobial properties (Figure 4A) and (ii) nanoparticle-based antimicrobial drug delivery (Figure 4B).
Figure 4.
Schematic of (A) nanomaterials with inherent antimicrobial properties, and (B) nanoparticle-based antimicrobial drug delivery systems.
3.1. Antimicrobial Nanomaterials
3.1.1. Inorganic nanoparticles
Metal and metal oxide
The inherent antibacterial properties of some metals and metal oxides have been known for centuries, causing them to be utilized extensively as bactericidal substances in infection control [43,44]. Metal and metal oxide nanoparticles exhibit striking physicochemical and biological properties distinct from their bulk forms, such as photocatalysis, photothermal effects, and reactive oxygen species (ROS)-stimulating activity [45]. Furthermore, the large surface-area-to-volume ratio of these nanomaterials also provides ample space for straightforward surface functionalization in developing more effective antibacterial agents.
Silver (Ag) nanoparticles are the most intensely studied metal nanomaterial. They are capable of killing both Gram-positive and Gram-negative bacteria and are effective against many drug-resistant microbes, such as Pseudomonas aeruginosa (P. aeruginosa), ampicillin-resistant E. coli O157:H7, and erythromycin-resistant Streptococcus pyogenes [46]. While many attempts have been made to clarify the mode of action of Ag upon microbes, our understanding remains incomplete. Multiple mechanisms may be involved, including direct interactions between Ag compounds and (i) bacterial cell membrane, (ii) DNA, and/or (iii) enzymes and proteins, and indirect interactions through the formation of ROS [45]. The antimicrobial activity of Ag depends heavily upon effective delivery of Ag+ ions, which are formed when Ag is exposed to atmospheric O2 and dissolved in aqueous solution. Therefore, size appears to have a very important effect on antimicrobial activity by influencing Ag+ release rate; higher activity is often associated with smaller Ag nanoparticles, which possess a larger surface-area-to-volume ratio [47,48]. This is also why Ag nanoparticles have usually shown higher antimicrobial activity than metallic bulk Ag. Many other factors can also influence the antimicrobial capabilities of Ag nanoparticles, such as surface roughness, hydrophobicity, oxidation state, and surface functionalization [45,49]. For example, surface modification of glucosamine on Ag nanoparticles greatly enhanced their antimicrobial effect against both Gram-negative and Gram-positive bacteria, through glucosamine-mediated penetration of the nanoparticles into bacterial cells [50].
Besides Ag, other metal nanomaterials have also been studied for antimicrobial treatment, including tellurium (Te) and bismuth (Bi). Interestingly, Te nanoparticles were reported to exhibit higher antibacterial activity and lower toxicity than Ag nanoparticles [51]. Moreover, metal oxide nanomaterials have also shown antimicrobial properties, such as zinc oxide (ZnO), copper oxide (CuO), titanium dioxide (TiO2), aluminum oxide (Al2O3),and ceriumoxide (CeO2) [43,47,52]. ZnO nanoparticles were recently shown to exhibit inhibitory effects against foodborne pathogens such as E. coli O157:H7 [53]. The antibacterial mechanisms of metal oxide nanoparticles generally include: (i) photocatalytic production of ROS (which damages cellular components), (ii) compromised integrity of bacterial membranes, (iii) interruption of energy transduction and transport processes, and (iv) inhibition of respiratory enzyme activity and DNA synthesis [43,54,55].
An important advantage of using metal and metal oxide nanoparticles as antimicrobial agents is that it is difficult for microbes to develop resistance to them. The primary reason is that metals/metal oxides have multiple modes of action, which significantly reduces the chance for microbes to gain resistance unless multiple mutations occur simultaneously. A 50-day microcosm exposure experiment indicated that Ag nanoparticles did not elicit increased resistance among naturally occurring bacteria in estuarine sediments [56]. Equally important is the anti-biofilm activity of some metal and metal oxide nanomaterials, such as Ag, Bi, ZnO, and TiO2 nanoparticles [57-59]. For example, Bi nanoparticles inhibited Streptococcus mutans growth by 69% and achieved 100% inhibition of biofilm formation [60].
It is noteworthy, however, that metal and metal oxide nanoparticles are primarily applied to medical devices (including wound dressings) for preventing bacterial adhesion and infection, which will be discussed below. Their limited application as antimicrobial therapeutic agents may be partially due to safety concerns [61,62]. For instance, ZnO and TiO2 have been demonstrated to cause DNA damage, and the high toxicity of CuO nanoparticles actually caused oxidative lesions [62]. It has also been reported that repeated injection resulted in significant accumulation of Ag nanoparticles in liver, lung, and spleen, which might cause damage to these organs [63]. All these findings suggest that extra attention should be paid to potential toxicity after chronic exposure. In addition, other possible risks from some metal and metal oxide nanomaterials also need to be carefully considered. For example, Al2O3 nanoparticles were shown to promote horizontal conjugative transfer of MDR genes, which could in turn increase antibiotic resistance [64].
Carbon
Carbon-based nanomaterials such as single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs), and fullerene have also been utilized in antimicrobial applications, although they are still in early stages of development [65]. These nanomaterials may exert antibacterial activity through cell membrane damage upon direct contact or through their photothermal/photodynamic properties upon irradiation [66,67]. The strong antimicrobial activity of SWCNTs against both Gram-positive and Gram-negative bacteria was due to oxidative stress influencing both membrane integrity and metabolic activity of bacteria [68]. Fullerene was reported to exhibit strong antibacterial activity towards several microbes. However, both that activity and its possible mechanisms are controversial, as some studies suggest that the toxicity may be attributed to oxidative by-products created during fullerene preparation [69,70]. Hydrophilic fullerene derivatives can be used as photosensitizers in antimicrobial photodynamic therapy (PDT) due to their highly efficient ROS production. Antimicrobial PDT exhibits broad-spectrum activity against microbial pathogens upon illumination regardless of their drug-resistance status, and repeated treatment induces no intrinsic resistance [71].
3.1.2. Peptide- and polymer-based nanoparticles
Cationic peptides
Cationic antimicrobial peptides (CAPs) are short amphipathic peptides presenting in virtually every life form as nature’s antibiotics, and are potent against a broad spectrum of microbes and MDR bacteria [72]. CAPs are also considered an integral part of the ancestral system of defense against microbial infection in higher multicellular organisms [73]. The antimicrobial properties are generally based on the cationic and hydrophobic nature of CAPs, which can physically damage negatively charged microbial membranes. Although hundreds of CAP sequences have been identified, their antimicrobial application is limited by the inherent drawbacks of cationic peptides, including cytotoxicity (e.g., hemolysis), enzymatic instability, and immune surveillance [74]. Delivery strategies such as loading CAPs on silica or paramagnetic nanoparticles have thus been proposed to protect the peptides from proteolytic degradation and immune recognition [74,75].
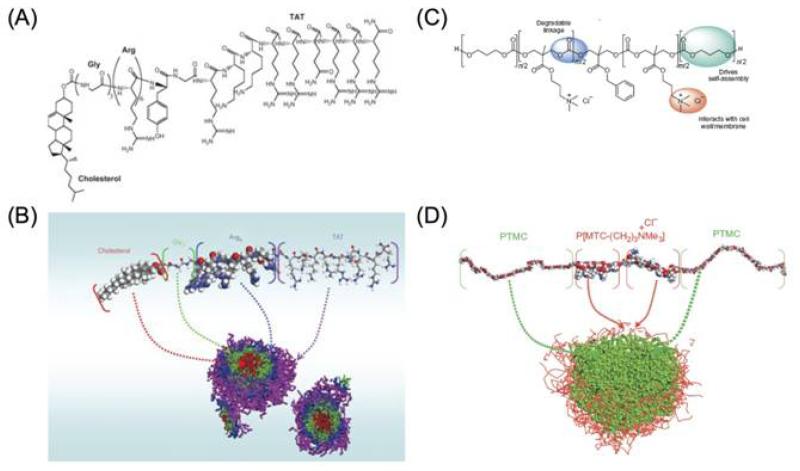
Interestingly, the cationic and amphipathic properties of some CAPs allow self-assembly into different types of nanostructures, which can reduce toxicity and enhance therapeutic index against bacteria in vivo, as compared to their unassembled peptide counterparts [65,76]. In addition, variations in morphology among nanostructures were associated with differences in bioactivity, suggesting that the nanostructure itself might also play a major role in the antimicrobial activity [77]. Yang and colleagues developed an amphiphilic peptide composed of cell-penetrating peptide TAT, six arginine residues, and cholesterol, which can self-assemble to form core-shell nanoparticles (Figures 5A and 5B) [76]. Not only can these nanoparticles have stronger antimicrobial properties than the unassembled peptides, but they have also been shown to cross the blood-brain barrier and inhibit bacterial growth in infected brains in a S. aureus-infected rabbit model. One very recent study also demonstrated that the combination of hydrophobically modified CAPs with rifampicin offered a synergistic effect against both multi-drug resistant and non-resistant tuberculosis, and delayed the emergence of rifampicin resistance[78]. It is thus expected that the development of CAP nanostructures for simultaneous encapsulation and delivery of antibiotics may further enhance the therapeutic efficacy of such combination strategies.
Figure 5.
Chemical structure of (A) the designed peptide with cholesterol, glycine, arginine, and TAT; and (C) cationic amphiphilic polycarbonate. (B) and (D) are the formation of micelles of (A) and (C), respectively, simulated through molecular modeling using Materials Studio software. Reprinted with permission from [76] and [80]. Copyright 2009, Nature Publishing Group [76]. Copyright 2011, Nature Publishing Group [80].
Synthetic cationic polymers
Designing synthetic polymer mimics of CAPs is of great interest due to their relatively low cost and better enzymatic stability [79]. Polymers with quaternary ammonium and phosphonium groups structurally mimic CAPs and share similar antimicrobial mechanisms. Figures 5C and 5D show that rationally designed amphiphilic, CAP-mimicking, biodegradable triblock polymers can form micellar nanoparticles by self-assembly [80].These nanoparticles can inhibit the growth of Gram-positive bacteria, MRSA, and fungi by selectively disrupting microbial membranes, while avoiding significant hemolysis over a wide range of concentrations. In another study, CAP-mimicking poly[2-(tert-butylamino)ethyl methacrylate] nanofibers incorporated with Ag nanoparticles were also reported to have excellent antibacterial performance against Gram-negative E. coli and Gram-positive S. aureus [81].
Chitosan
Apart from synthetic polymers, a natural cationic polysaccharide polymer, chitosan, has also shown antimicrobial activity. The antimicrobial properties of chitosan and its derivatives are mainly due to their polycationic character. Several mechanisms have been proposed, such as increased permeability of microbial wall via electrostatic interaction, and inhibition of enzymes by chelating necessary trace metals [82]. Impressively, nanoscale chitosan has been shown to be a more effective antimicrobial agent than chitosan solution, as a result of the higher surface-area-to-volume ratio and increased affinity to microbes [83]. Minimum inhibitory concentration (MIC) of chitosan nanoparticles against E. coli and S. aureus was less than 0.25 μg/mL, while MIC for conventional chitosan molecules was reported to be 20 μg/mL. Chitosan nanoparticles are also more effective against Gram-positive bacteria than Gram-negative bacteria, and are excellent against fungi [82,84]. In addition, one recent work by Friedman and colleagues showed that nanoparticles composed of chitosan and alginate exhibited direct bactericidal effect against Propionibacterium acnes (P. acnes), a bacterium linked to the pathogenesis of acne, as well as anti-inflammatory properties by inhibiting P. acnes-induced cytokine production [85]. When encapsulated with benzoyl peroxide, a commonly used anti-acne drug, these nanoparticles further demonstrated high potential for topical treatment of some dermatologic conditions. Moreover, the hydrophilic and polycationic nature of chitosan makes it a suitable carrier for antibiotic delivery or a suitable coating biomaterial for stabilizing other nanomaterials like metallic nanoparticles [86,87].
3.2. Drug Delivery
Another very significant application of nanoparticle technologies in antimicrobial treatment is the delivery of antimicrobial agents. Nanoparticle-based drug delivery could overcome some important challenges in the treatment of infections, such as systemic toxic effects of antibiotics, decreased uptake and increased efflux of drugs, biofilm formation, and intracellular bacterial infection. Targeted nanoparticle delivery to the infection site could also be achieved by surface modification with targeting ligands or by microenvironment responsiveness, both of which may further improve therapeutic efficacy and reduce the side effects of antimicrobial drugs. Other advantages of nanoparticle delivery of antimicrobial drugs include (but are not limited to) improved solubility of hydrophobic drugs, prolonged systemic circulation time and drug half-life, and sustained drug release, all of which may eventually reduce systemic side effects and lower administration frequency [43]. To this end, various nanoparticle platforms have been developed, including liposomes, solid lipid nanoparticles, polymeric nanoparticles, silica nanoparticles, and Au nanoparticles [43,88]. Notably, a few liposomal/lipid complex platforms for antibiotic delivery have been approved for use in human patients, including Abelcet, AmBisome, Amphotec, and Fungisome (Table 1).
3.2.1. Non-targeted nanoparticles for antibiotic delivery
Reduced uptake and increased efflux of drugs are two important mechanisms of antibiotic resistance that prevent the concentration of antimicrobial drugs from reaching toxic levels within microbial cells [38]. The outer membrane of Gram-negative bacteria like P. aeruginosa and E. coli may also provide an extra barrier against uptake, lowering susceptibility to some hydrophobic antibiotics (e.g., beta-lactams and macrolides) [89]. Increased efflux by the overexpression of different transmembrane pumps could confer MDR in microbes and is often related to resistance against chloramphenicol, fluoroquinolones, and macrolides [38,89]. A variety of nanoparticle delivery vehicles have been reported to compromise these resistance mechanisms, as summarized in two recent review works [39,90]. For example, fusogenic liposomes that consist of certain lipids could fuse quickly with the plasma membrane of microbial cell and release a high concentration of drug into the microbial cytoplasm, which could saturate the transmembrane pumps [91,92].
Nanoparticle-based antibiotic delivery also shows promise to combat biofilms and intracellular microbes – two common reasons for chronic infections that are difficult to treat with regular antimicrobial therapies. Nanocarriers, such as liposomes and lipid-/polymer-based nanoparticles, have been demonstrated to significantly enhance the efficacy of antibiotics against biofilm-forming bacteria, attributed to the protection of antibiotics from enzymes and increased permeation [93,94]. Certain lipids including phosphatidylinositol and stearylamine also exhibited specific affinity with biofilms, and thus could increase the biofilm adhesion of liposomes [95,96]. For intracellular infection treatment, the small size of nanoparticles facilitates their entering into host cells through endocytic/phagocytic pathways, and subsequent releasing of the antibiotic payload to the localities of infection. A beneficial fact is that many different types of intracellular bacteria reside in the mononuclear phagocyte system composed primarily of monocytes and macrophages, which is also responsible for the clearance of nanoparticles administered in the body [97]. For example, it has been shown that polyethylenimine-coated mesoporous silica nanoparticles loaded with rifampin can be efficiently internalized by human macrophages, and exhibited greater efficacy against Mycobacterium tuberculosis-infected macrophages than free rifampin [98]. Several most recent review papers have well summarized the application of nanoparticles for anti-biofilm and intracellular infection treatment [39,97,99].
As the effect of some antibiotics depends on their interaction with surface components of the bacterium, stronger antibacterial effects could be obtained through the polyvalent effect achieved by conjugating multiple antibiotic copies on nanomaterial surface. For instance, Au nanoparticles provide a stable surface for the attachment of different antibiotic drugs and could greatly enhance their antibacterial activity through stronger interactions with cell walls [100].Vancomycin-capped Au nanoparticles exhibited a 64-fold higher antibacterial activity than vancomycin itself against vancomycin-resistant Enterococcus and E. coli [101]. In addition to improvement in antibacterial activity of antibiotics, some studies have also shown that introduction of molecules that are inactive or less active as antibiotics on inert nanoparticles can trigger significant antibacterial effects. One study demonstrated that after conjugation on an Au nanoparticle surface, amino-substituted pyrimidine that is completely inactive by itself, exhibited strong antibacterial activity against MDR clinical isolates and induced bacterial resistance more slowly than conventional antibiotics [102].
Nanoparticles have also facilitated the delivery of a specific short-lived gaseous antimicrobial agent, nitric oxide (NO). NO exhibits antimicrobial effects mainly through the direct interference with DNA replication and cell respiration, or the formation of reactive nitrogen intermediates [103]. These mechanisms of action make the development of bacterial resistance to exogenous NO treatments very unlikely [39,104]. Thus far, different nanoparticle platforms have been created for effective NO delivery, and discussed in recently published review papers [90,103]. For example, silica nanoparticles prepared with NO donors (e.g., diazeniumdiolate) showed not only excellent antibacterial effect, but also great efficacy (≥99.9%) against biofilm formation of P. aeruginosa and E. coli[105,106]. Moreover, when biomaterials such as PAMAM dendrimer and chitosan that possess inherent antibacterial properties were used for the encapsulation of NO donors, this type of NO nanoparticles exhibited even better bactericidal and anti-biofilm efficacy [107,108]. Besides the encapsulation of NO-donating chemicals, Friedman and colleagues also developed a sol-gel-based nanoparticle system that can carry gaseous NO through the thermal reduction of nitrite, and release it in a controlled and sustained manner [103,109]. These NO nanoparticles showed antimicrobial activity against a broad spectrum of bacteria, including some drug-resistant bacteria. More interestingly, this system retains NO in a stable form when dry, and allows the release of gaseous NO upon exposure to moisture. Promising applications have been explored for the topical treatment of wounds and affected areas[110,111], and confirmed by the accelerated wound closure and less bacterial burden in a MRSA-infected murine wound model [112].
Combination antibiotic therapy appears to hold a great deal of potential not only in tackling existing mechanisms of drug resistance but in preventing its development in the first place [113]. Combining multiple drugs can result in higher potency and higher antimicrobial efficacy by additive or synergistic effects. Development of resistance to multiple agents, each of which has different mechanisms of action, requires multiple simultaneous gene mutations in the same bacterial cell, the chances of which are considered slim. Nanoparticles could facilitate the co-delivery of multipleantibiotics as well as the combination of antibiotics with antimicrobial nanomaterials, while avoiding synergistic/additive off-target toxicities from these combinations. PLGA nanoparticles loaded with rifampin and azithromycin were more effective against chlamydial infections than those loaded with either of the individual drugs [114]. Mesoporous silica simultaneously loaded with peracetic acid and Ag nanoparticles allowed the sustained release of both agents and showed a considerable synergistic bactericidal effect on antibiotic-resistant and biofilm-forming S. Aureus [115].
3.2.2. Targeted nanoparticle delivery
Compared with non-targeted nanoparticles, targeted delivery of antimicrobial drugs could achieve even higher doses of drug at the site of infection with fewer adverse effects. It may also increase the success rate of therapy for chronic and persistent infections, such as slow-growing or even dormant bacterial infections, which are important challenges in antimicrobial therapy and require frequent administration of high-dose antibiotics [116,117]. Modification of nanoparticles with ligands that bind to specific receptors on the bacterial wall is the traditional mode of targeting. For example, PLGA nanoparticles conjugated with folate were used to deliver azithromycin and rifampicin drug combinations for infections with Chlamydia, which has upregulated folate receptor expression[118]. Mannose-conjugated liposomes into which ciprofloxacin was incorporated also exhibited high selectivity for alveolar macrophages and were used in the treatment of respiratory intracellular infections [119].
In addition to ligand-targeted nanoparticle delivery, other targeting strategies have utilized the unique microenvironment at the site of infection, such as low pH, enzyme overexpression, and bacterial toxins. Localized acidity occurs due to the combined actions of bacterial metabolism and host immune response and often reduces the activity of antibiotics [120]. Based on this mechanism, we have recently reported pH-responsive, surface-charge-switching nanoparticles that can shield non-specific interactions at pH 7.4 but bind avidly to bacteria at pH 6.0 (Figures 6A and 6B) [120]. Vancomycin encapsulated within the nanoparticles also retains more efficacy at low pH compared with free drugs (Figure 6C). In a different case, by adsorbing carboxyl-modified gold nanoparticles to the outer phospholipid layer, the fusion activity of liposomes with bacteria can be switched off at neutral pH, and resumed at acidic environments due to the detach of Au nanoparticles [121]. By integrating this Au nanoparticle-modified liposome system with hydrogel, it has also shown potential for sustained topical drug delivery [122].
Figure 6.
(A) Schematic representation of the designed nanoparticle-mediated drug targeting to bacterial cell walls. The nanoparticles avoid uptake or binding to nontarget cells or blood components at physiologic pH 7.4 due to a slight negative charge and surface PEGylation. The weakly acidic conditions at sites of certain infections activate the surface charge-switching mechanism, resulting in nanoparticle binding to negatively charged bacteria. (B) Nanoparticle zeta potential vs. pH demonstrates notable switching from anionic to cationic with decreases in pH in PLGA-PLH-PEG but not PLGA-PEG nanoparticles. (C) Minimum inhibitory concentrations (MIC) of the different vancomycin formulations in S. aureus. Reprinted with permission from [120]. Copyright 2012, American Chemical Society.
Unique biomolecules secreted by bacteria, such as enzymes and bacterial toxins, can also be utilized for the environment-targeting delivery. With the pore-forming property of bacterial toxins and their tremendous viability at the infection site, Zhang and colleagues developed innovative liposomes that can selectively deliver antibiotics to the sites of bacterial infections upon contact with the existing bacterial toxins, which can trigger the release of encapsulated therapeutic agents by inserting themselves into the liposome membranes [123]. In another example, Wang and colleagues reported a lipase-sensitive polymeric nanogel for the selective delivery of vancomycin only in the presence of lipase-secreting bacteria [124]. The polymeric nanogel contains a polyphosphoester core and a poly(ε-caprolactone) fence, which can be degraded by bacteria-secreted lipase, thus triggering drug release. Furthermore, by being conjugated with macrophage-targeting ligands (i.e., mannose), the polymeric nanogel first binds to macrophages, accumulates at bacterial infection sites through macrophage-guided transport, and then triggers antibiotic release upon contact with lipase-secreting bacteria [125].
Despite the enormous potential of targeted nanoparticles, their translation into clinical development has faced considerable challenges, such as (i) the difficulty of identifying highly selective targeting ligands, (ii) the development or adaptation of simple, robust, and reproducible processes that can facilitate scale-up and manufacturing, and (iii) the rapid optimization of the biophysicochemical properties of nanoparticles for maximal efficacy [126,127]. Thus, many efforts have been focused on developing nanoparticles through self-assembly and high-throughput processes to facilitate their screening and optimization as well as subsequent scale-up and manufacturing [11,128]. To precisely engineer targeted nanoparticles in a simple and scalable manner, we have recently developed an innovative strategy by first pre-functionalizing polymer components with targeting ligands and then self-assembling with other nanoparticle components [128]. With the capability of eliminating the need for post-particle modification and enabling the formulation of distinct targeted nanoparticles with narrow variations for optimization, this technique has been successfully translated into the development of a targeted polymeric nanoparticle product (BIND-014) currently in Phase 2 trials for cancer treatment, and a synthetic vaccine candidate (SEL-068) currently in Phase 1 trials for smoking cessation and relapse prevention [11,126,129].
4. Antimicrobial Nanotechnologies in Medical devices
Medical devices play an important role in modern healthcare practice, but their application may increase the risks of nosocomial infection. According to a report by the Centers for Disease Control and Prevention, device-related infection accounts for approximately 45% of all nosocomial infections, with associated costs estimated at $16 to $20 billion per year in the United States alone [130]. The leading device-associated infections are associated with catheters, implants, and sutures. Up to 54% of all catheters have been shown to be infected with bacteria and can cause many serious complications such as urinary tract infection, bloodstream infection, and even death [131]. Implant-associated infection occurs at low to moderate rates (7.4% of cardiovascular and 4.3% of orthopedic implants infected in the United States [132]) but is very problematic, since treatment is implant removal and replacement if standard antibiotic treatment is not successful [44]. The initiation of device-related infection usually involves bacterial adhesion to the device or the patient-derived glycoprotein coating (conditioning film), subsequent microbial proliferation, and the development of biofilms. The pathogens most commonly found in infected devices include S. epidermidis, S. aureus, and P. aeruginosa [44]. These bacteria can become extremely resistant to antibiotic treatment due to the formation of biofilms, and systemic administration of antibiotics usually does not show satisfactory results [133].
Medical devices with inherent antimicrobial properties have been studied for decades, with the goal of good integration with host cells while preventing any bacterial adhesion or biofilm formation. Most relevant studies have utilized the intrinsic antimicrobial effect of metals (in particular Ag). Interestingly, some studies suggested that the application of bulk metallic or metal-coated devices failed to prevent bacterial infection, especially in vivo [45]. Possible reasons may be the poor release of metallic ions and/or the formation of patient-derived conditioning film [134]. To address this concern, many nanomaterial-coated or -embedded medical devices have been developed (Figure 7), and several have been approved for clinical use, including catheters, endotracheal tubes, and wound dressings (Table 1) [45,135].
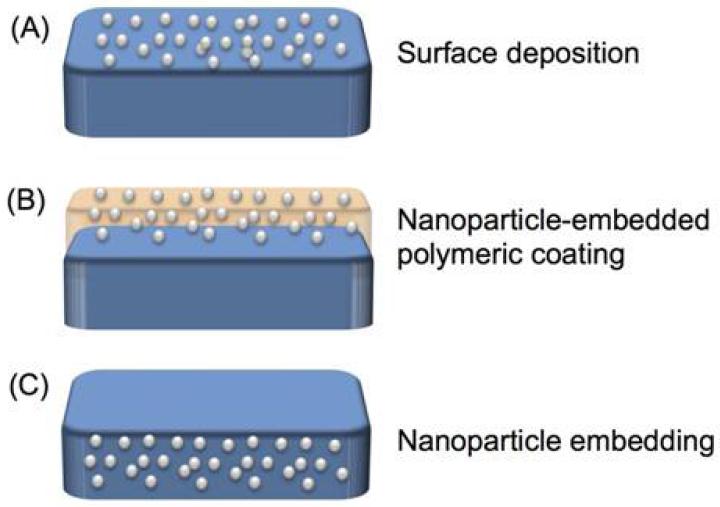
Figure 7.
Strategies of the application of antimicrobial nanomaterials in medical devices, including (A) surface coating by direct deposition, (B) surface coating by blending antibacterial nanomaterials into a polymer coating, and (C) embedding of antimicrobial nanomaterials into the bulk matrix of medical devices.
4.1. Surface Coating
Surface coating by direct deposition (Figure 7A) of antimicrobial metal nanomaterials has proven an effective strategy for the prevention of bacterial attachment. Bacterial adhesion and infection were reduced, and biofilm formation was prevented by different metal nanoparticle-coated devices, such as wound dressings, heart valves, central venous catheters, and urinary catheters [44]. Acticoat, the first nanocrystalline Ag-coated wound dressing, showed better wound healing capacity and shorter healing times compared with silver sulfadiazine, and achieved scarless healing in animal models [136]. In clinical studies of burn wounds, sepsis and secondary bacteremia were less frequent in patients treated with Anticoat dressing than in those treated with gauze soaked in 0.5% silver nitrate solution [137].
Another strategy for surface coating is the blending of antibacterial nanomaterials into a polymer coating (Figure 7B). A variety of polymers exhibit excellent film- and coating-forming properties, and antimicrobial nanoparticles can be easily trapped within the polymer film to form nanocomposites by either physical attachment or chemical bonding. In fact, synergistic antibacterial effects between some polymers and metal nanomaterials have been identified. For example, chitosan was shown to greatly enhance the antimicrobial properties of incorporated TiO2, ZnO, and Ag nanoparticles by forming membranes or nanogels[138,139]. Moreover, coatings may be made multifunctional by incorporating different polymers. Combining the inherent antimicrobial activity of chitosan and Ag nanomaterials with the anticoagulation effect of heparin, coating poly(ethylene terephthalate) (PET) with Ag nanoparticle-containing layer-by-layer polyelectrolyte films (chitosan and heparin) yielded excellent anti-microbial and anti-coagulant activities [140]. This is an additional benefit of using PET in cardiovascular implants: it can reduce thrombogenicity and the chance of infection, both of which pose significant risk of morbidity and mortality [141]. Polymeric hydrogels containing metal nanomaterials have also been used to coat medical devices such as dental and orthopedic implants [142]. The hydrophilic properties of the hydrogel reduce the toxicity of the imbedded nanoparticles and make the implant better integrated with the host cells. One example is alginate hydrogel containing Ag nanoparticles, which displayed effective bactericidal activity against both Gram-positive and Gram-negative bacteria but no cytotoxicity in three different eukaryotic cell lines [143].
4.2. Embedding
Compared to surface coating, embedding of antimicrobial nanoparticles into the bulk matrix of medical devices (Figure 7C) could offer several distinct advantages. First, impregnation of nanomaterials could enhance the physical and chemical properties of the bulk matrix [144]. Second, the whole device would be protected, not just the surface. Third, embedding nanoparticles rules out the possible inactivation of their antimicrobial effect by the formation of plasma protein conditioning film from the host, which is one proposed reason for the unsatisfactory clinical results obtained with some devices coated with antimicrobial nanomaterial [134]. Meanwhile, incorporation of antimicrobial nanoparticles in the device matrix has also been reported to offer sustained release of the antimicrobial agents, a desirable quality in an implant usually expected to stay in the body for years. On the other hand, this finding may also suggest that this strategy is applicable only to permeable matrices. For example, an Ag-impregnated non-permeable poly(urethane) catheter was no different from an Ag-free poly(urethane) catheter in the prevention of abscess, while Ag-impregnated permeable silicon catheters created considerably fewer abscesses [145].
Incorporating nanoparticles into medical devices composed of polymer matrix could be achieved by different techniques, including in situ synthesis of nanoparticles in the polymer matrix, polymerization of the matrix around the nanoparticles, and direct incorporation with the aid of a blending solvent. Metal nanomaterials frequently demonstrated not only increased antimicrobial activity and reduced toxicity after immobilization in polymer matrix, but aggregation was avoided as well [146]. Poly(methyl methacrylate) bone cement with Ag nanoparticles showed low cytotoxicity and impressive antimicrobial activity and could be used to attach joint prostheses in replacement surgery [147]. Xu and colleagues also developed a dentin primer containing Ag nanoparticles and 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) as antibacterial agents, combating residual bacteria in tooth cavities [148]. Primers with either Ag nanoparticles or MDPB can greatly reduce the biofilm viability, but both agents together had a much stronger effect than either agent alone.
The incorporation of metal nanomaterials into medical devices made of solid inorganic materials can combine the properties of both the nanomaterials and the inorganic matrices, even producing novel characteristics beyond those of the individual components. One example is the application of Ag nanomaterial-Ti matrix composites. Ti-based materials are commonly used for implants due to good biocompatibility, mechanical strength, and corrosion resistance. The antimicrobial activity of Ag nanoparticles against C. albicans and Aspergillus was enhanced in the Ag-Ti composite, while the mechanical properties of Ti matrix were usually not significantly affected [149,150]. Meanwhile, adding Ag nanoparticles to the TiO2 matrix also enhanced the photoactivity of TiO2 by producing electron-hole pairs, which show promising antimicrobial activity under visible light irradiation [151]. Hydroxyapatite (HA) is a naturally occurring mineral form of calcium apatite that shows good biocompatibility and is an excellent candidate for bone repair and substitution. However, HA’s strong adsorption properties attract bacteria. Incorporating Cu and Zn nanoparticles into HA effectively inhibits the adsorption of E. coli, S. aureus, and the pathogenic yeast Candidaalbicans in both solid and liquid media [152].
5. Antimicrobial Nanovaccine
Enlisting the host’s immune system to recognize and target microbes has been demonstrated to be very effective in protecting human against microbial infection [153]. When the pathogens breach the host’s physical barriers, they could be recognized by the innate immune system through microbial characteristics called pathogen-associated molecular patterns. The subsequent activation of antigen-presenting cells (APCs) induces antigen-specific adaptive immune responses against bacterial infections, which may mount a protective response as long as decades after the initial contact. Even if the protective response fails to prevent infection, it may extend the window of opportunity for antibiotic treatment by delaying the onset of bacteremia and septic shock [154]. However, various existing vaccines for microbes display considerable variation in immunogenicity and safety [155]. Concerns with the application of live attenuated bacterial vaccines include the possible reversion of pathogenicity and the pre-existing immunity to the vector, as well as the safety risks to immune-compromised individuals [156].
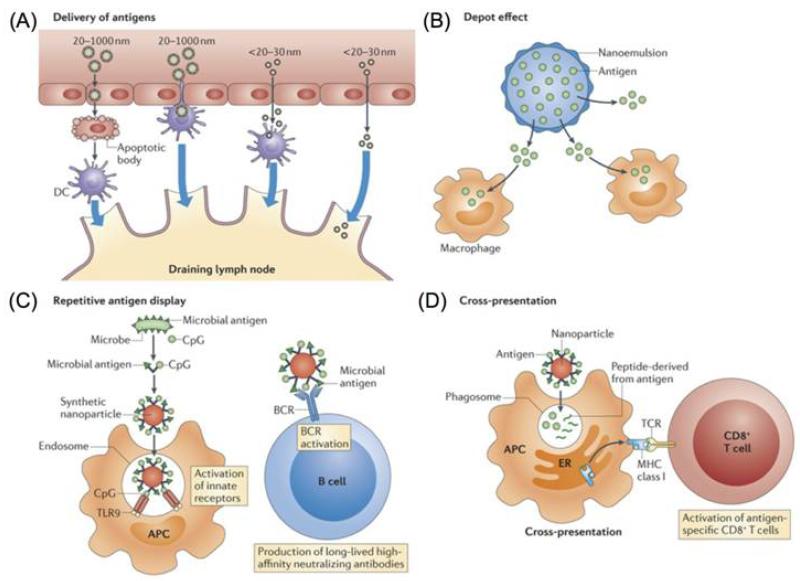
Advances in biotechnology enable the production of next-generation bacterial vaccines, including isolated proteins, polysaccharides, and naked DNA [157]. However, such novel vaccines are often less immunogenic than traditional vaccines, such as those using live attenuated microbes. To address this challenge, the application of nanotechnologies to enhance the immune responses of these vaccines has attracted great interest. Nanoparticle-based antigen delivery induces a broad spectrum of immune responses, including cellular immune responses of Th1, Th2, and Th17, and humoral immunes responses of IgG and IgA antibodies, both systemically and locally [158]. Nanoparticles exert immunostimulatory effects that could be attributed to several different mechanisms (Figure 8), including (i) better tissue penetration, access to the lymphatics and preferential uptake by APCs; (ii) depot effect by stabilizing the antigens and controlling their sustained release; (iii) repetitive antigen and adjuvant display on the particle surface, which can facilitate B cell receptor co-aggregation, triggering, and activation; and (iv) cross-presentation enabled by the nanoparticle-mediated escape of antigens into the cytosol after being taken up by the phagosome[158,159]. Some nanoparticle systems also show adjuvant properties by themselves.
Figure 8.
Mechanisms by which nanoparticles alter the induction of immune responses. Reprinted with permission from [158]. Copyright 2013, Nature Publishing Group.
Nanoparticles have also been shown to be effective delivery systems for mucosal vaccination. Mucosal surfaces are a part of the first line of defense and contain almost 80% of all immunocytes in the body [160]. It is estimated that 70% of infectious agents invade the body through mucosal surfaces [161]. A protective, long-lasting mucosal immune response is thus very important to protect the host from potential bacterial infection. Subcutaneously or intramuscularly administered vaccines elicit only a weak mucosal immune response, while mucosal vaccination leads to both mucosal and systemic immunity [162]. Therefore, mucosal administration through intranasal, inhalational, or gastrointestinal routes is becoming a favored route of vaccination. Nevertheless, the effectiveness of mucosal immunization is still limited, since the antigen has to pass through several barriers before reaching the APCs. Different nanoparticle delivery vehicles have been proposed to enhance mucosal vaccination through their immunostimulatory activities [162]. Moreover, these nanoparticles could be engineered to facilitate delivery to the organized mucosa-associated lymphoid tissue (MALT), the main sites of mucosal immune activation. For example, antigen-loaded nanoparticles modified with UEA-1 lectin that targets M cells in MALT elicited a two- to four-fold increase in antibody titers compared with unmodified ones [163].
5.1. Nanoadjuvant
Nanoemulsions, or oil-in-water emulsions formed by isotropic mixtures of oil and surfactant with droplet diameter in the nanometer scale, are effective non-inflammatory mucosal adjuvants [164]. The adjuvanticity of nanoemulsions has been suggested to contribute to increased cellular uptake of antigens, recruitment of monocytes and granulocytes, and enhanced release of cytokines and chemokines [158]. Intranasally administered recombinant anthrax protective antigen mixed in nanoemulsion induced both serum IgG and bronchial IgA and IgG antibodies after one or two mucosal administrations in mice and guinea pigs [165]. In comparison, commercially available human anthrax vaccine requires six subcutaneous injections over 18 months and yearly boosters. In another work, using nanoemulsion as a novel mucosal adjuvant for the intranasal delivery of Burkholderia multivorans outer membrane proteins antigen, robust serum IgG and mucosal secretory IgA immune responses with cross-neutralizing activity against Burkholderia were elicited in vaccinated mice [166].
Cationic liposomes have also been used as vaccine adjuvants. CAF01, a cationic liposome-based adjuvant being evaluated in clinical studies, has been proven to enhance immune responses of a series of different vaccine candidates [167]. In a study to develop more potent and safer tuberculosis vaccines, CAF01 incorporated with a synthetic mycobacterial glycolipid induces strong and protective Th1 and Th17 responses [168]. The potent adjuvanticity of CAF01 was associated with prolonged dendritic cell (DC) uptake and activation. Cationic liposomes complexed with non-coding plasmid DNA were also reported to be effective as parenteral and mucosal vaccine adjuvants. The use of liposome-DNA complex as mucosal adjuvant with heat-killed Burkholderia pseudomallei (B. pseudomallei) effectively generated potent IgG and IgA antibody responses and increased the survival rate of BALB/c mice to 100% after lethal pulmonary challenge with B. pseudomallei[169].
5.2. Vaccine Delivery
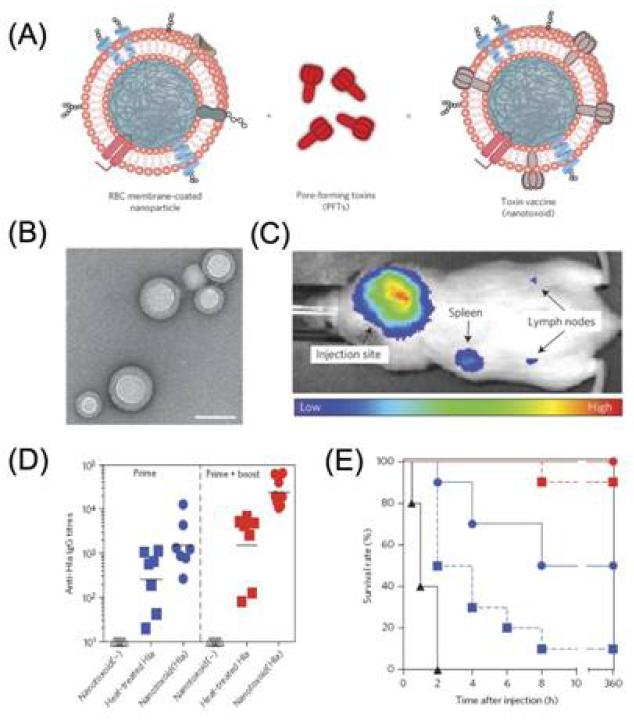
Polymeric nanoparticles can serve as delivery vehicles for a wide range of agents including small molecules, peptides, proteins, and nucleic acids. Synthetic polymers alone do not usually possess an immunostimulatory effect, but can enhance immunization via facilitating the delivery of antigens and/or adjuvants [47]. For example, PLGA nanoparticles incorporating a recombinant major outer membrane protein of Chlamydia trachomatis(C. trachomatis) induced elevated numbers of CD4+ and CD8+ T cell subsets and a 64-fold higher Th1 than Th2 antibody titer [170]. Toxoid vaccines, which are based on inactivated bacterial toxins, have been widely used to promote antitoxin immunity for the prophylaxis and treatment of microbial infections. However, it is still a significant challenge to eliminate toxin virulence while retaining faithful antigenicity presentation. Using erythrocyte membrane-coated polymeric nanoparticles, Zhang and colleagues successfully developed a nanoparticle-based toxin-detainment strategy that can safely deliver non-disrupted pore-forming toxins for immune processing (Figure 9) [171]. Mice vaccinated with the nanoparticle-detained toxin showed superior protective immunity against toxin-mediated adverse effects; effective virulence neutralization of toxins and 100% survival rate were achieved. Moreover, natural polymers such as chitosan and pullulan have also been utilized for delivery of antigen against bacteria such as C. trachomatis and Streptococcus pneumoniae[172,173]. Interestingly, chitosan was also reported to have adjuvant properties through the promotion of cytokine production. Increased serum (IgG) and mucosal (IgA) antibody responses were obtained by modifying antigen-loaded poly(ε-caprolactone) nanoparticles with chitosan [174].
Figure 9.
(A) Schematic preparation of nanoparticle-detained toxins, consisting of substrate-supported RBC membranes into which PFTs can spontaneously incorporate.(B) TEM image of the particle vectors with uranyl-acetate staining (scale bar, 80 nm). (C) Live, whole-body fluorescent imaging of nanotoxoid(Hla) at 1h after subcutaneous administration. (D) Anti-Hla IgG titres at day 21 (n=7). Black lines indicate geometric means. Anti-Hla titres from mice vaccinated with non-toxin loaded particle vectors (nanotoxoid(-)) were monitored as controls (open triangles). (E) Unvaccinated mice (black triangles, solid line) and mice vaccinated with heat-treated Hla (prime; blue squares, dashed line), nanotoxoid(Hla) (prime; blue circles, solid line), heat-treated Hla (prime tboost; red squares, dashed line) or nanotoxoid(Hla) (prime + boost; red circle, solid line) received intravenous or subcutaneous administration of Hla. Survival rates of mice over a 15-day period following intravenous injections of 120 mg kg−1 Hla on day 21 via the tail vein (n=10). Reprinted with permission from [171]. Copyright 2013, Nature Publishing Group.
Self-assembling peptide nanoparticles (SAPNs) are icosahedral symmetric assemblies of different protein oligomerization that are analogous to viral capsids, and are often called “virus-like particles (VLPs)”. SAPNs provide a repetitive scaffold structure to achieve the conformational high-density display of inserted protein epitopes or domains in a highly exposed configuration. With different antigens inserted, SAPNs are a powerful platform to generate antibody responses against antigens with poor immunogenicity [175]. Without using adjuvant, SAPNs integrated with an immunodominant B cell epitope from the malaria parasite Plasmodium berghei circumsporozoite protein induced the production of high-avidity and long-lasting T cell-dependent antibodies in mice [176]. Immunized mice were protected against both primary and long-term secondary challenges with live sporozoites.
Immune-stimulating complexes (ISCOMs) are micelle-based antigen delivery systems composed of cholesterol, phospholipid, and the inbuilt adjuvant saponin [177], and have a cage-like structure with a diameter of ~ 40 nm. Suggested mechanisms underlying the adjuvant properties of ISCOMs include the induction of antigen presentation by both MHC class I and class II pathways, and the activation of IL-12-dependent aspects of the innate immune system. In addition, ISCOMs exhibited significant potential as mucosal vaccines, especially for intranasal administration [178]. ISCOMs have been shown to evoke protective immune responses with numerous antigens from bacteria and parasites including Helicobacter pylori, Anaplasma marginale, Mycoplasma mycoides, Mycobacterium tuberculosis, Corynebacterium diphtheriae, Streptococcus pyogenes, Moraxella bovis, and C. trachomatis [177].
6. Opportunities and Challenges
The potential impact of nanotechnology on microbial infectious diseases has already been demonstrated by the clinical approval of many nanotechnology-based products for the detection of bacterial infection, the delivery of antibiotics, and the development of medical devices with antimicrobial coatings (Table 1). Nanoparticles with unique physiochemical properties have enabled the diagnosis of microbial disease with high sensitivity and selectivity, as well as rapid readout. Liposome-mediated antibiotic delivery has also been validated through reducing the side effects of the previously approved drug Amphotericin B. In addition, the coating of medical devices with antimicrobial nanomaterials, in particular Ag, has drastically reduced device-associated bacterial infection and biofilm formation, and enhanced wound healing when used in dressings. However, despite these fascinating achievements, the full potential of nanotechnology in managing microbial infection, particularly in the areas of antimicrobial therapy and vaccines, is far from being reached. Below are several areas, from our perspective, that have not been well explored, but may generate break throughs in the exciting field of antimicrobial nanomedicine for years to come.
Gene silencing technologies such as the antisense strategy and RNA interference (RNAi) have shown significant promise in medical applications, through which the expression of target genes can be specifically inhibited by nucleic acid-based molecules including antisense oligonucleotide, small interfering RNA, and microRNA [179,180]. Though still in its early stages, the antisense strategy has attracted considerable attention in the antimicrobial field, with the potential to facilitate the study of microbial functional genomics and the development of new antibacterials against emerging drug-resistant strains [181,182]. It has been demonstrated very recently that selective killing of particular bacteria in mixed culture can be achieved by using species-specific antimicrobial antisense oligonucleotides [183]. Different from eukaryotic cells that can employ RNAi pathway to target mRNA, bacteria do not have homologous RNAi machinery, but can utilize the clustered regularly interspaced short palindromic repeat (CRISPR) system to modulate gene expression [184]. It has been demonstrated that, by reprogramming the small guide RNAs of the various CRISPR-Cas complexes, the CRISPR system could be redirected to silence the expression of endogenous DNA or RNA of interest [185]. Nonetheless, one major hurdle for clinical applications of nucleic acid-mediated antimicrobial therapy is the safe and effective delivery to bacterial cells [181]. Using nanoparticle technologies, it may be possible to increase penetration and/or target delivery to infection sites more effectively. Indeed, we have recently designed and screened a combinatorial library of lipid-like materials for the delivery of nucleic acids including antisense oligonucleotides[186], and developed hybrid lipid-polymer nanoparticle platforms for sustained, long-term gene silencing and synergistic delivery of nucleic acids and small molecular drugs [187-189]. We speculate that the identification of delivery materials and nanoparticles that have favorable pharmacokinetics and can readily cross bacterial cell walls will pave the way for the clinical application of gene silencing therapy against microbes. In addition, such nanoparticle strategies could also become an important tool for investigating mechanisms of bacterial pathogenesis and identifying valid targets for drug development.
Vascular permeability at infection sites is another important issue that has not been well exploited but could be significant in the development of systemic nanoparticle drug delivery. In cancer nanotechnology, macromolecules and nanoparticles can effectively extravasate from the leaky tumor microvasculature and accumulate in the tumor tissue. This enhanced permeability and retention (EPR) effect [190] is the keystone in the successful development of cancer nanotherapeutics. Interestingly, several features of infection-induced inflammation resemble the tumor environment in terms of pathological processes that result in the EPR effect. It has also been demonstrated that a clinically significant EPR effect is present during infection by major pathogenic bacterial species [191]. Therefore, taking full advantage of the EPR effect in infection sites may lead to new nanotherapeutic approaches for the management of infectious diseases.
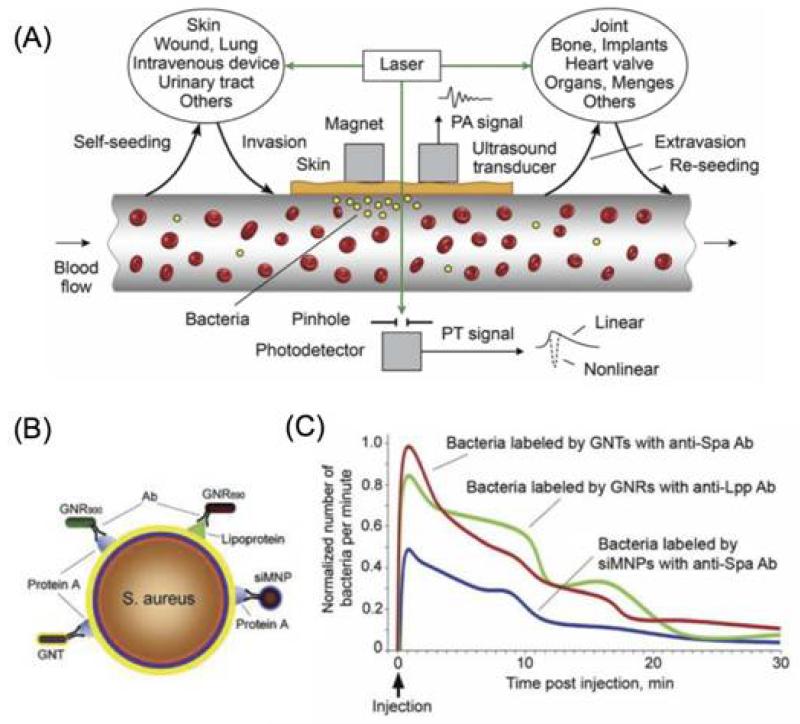
The novel field of theranostics has well-recognized potential for personalized cancer therapy. By combining diagnostic and therapeutic agents, theranostic nanoparticles hold the promise of enabling pre-screening of patients and real-time monitoring and evaluation of nanotherapeutics [192]. Simultaneous real-time detection and therapy may also be beneficial for anti-microbial management. One pioneering work in this field is the development of a theranostic platform based on two-color Au and multilayered magnetic nanoparticles modified with an antibody cocktail for S. aureus targeting [193]. With giant amplifications of photoacoustic (PA) and photothermal (PT) contrasts of these nanoparticles, this platform was demonstrated to have ultrasensitive PA detection, in vivo magnetic enrichment, and PT eradication of circulating bacteria cells or CBCs (Figure 10). Real-time monitoring of therapeutic efficacy could also be achieved by counting the CBCs. While more efforts are required to validate this strategy, theranostic nanotechnologies might change the paradigm of antimicrobial treatment.
Figure 10.
(A) Principle of in vivo integrated PA-PT nanotheranostics of bacteria in blood and distant infected sites. (B) Multiplex targeting S. aureus surface biomarker protein A (Spa) and lipoprotein (Lpp) by siMNPs, GNRs and GNTs functionalized with either anti-Spa or anti-Lpp antibody (Ab). (C) In vivoPA monitoring of CBCs labeled with Ab-functionalized NP sin vitro prior to injection. siMNPs, silica-coated magnetic nanoparticles; GNRs, gold nanorods; GNTs, golden carbon nanotubes. Reprinted with permission from [193]. Copyright 2012, Public Library of Science.
Nanotechnologies have been exploited for safer and more effective mucosal vaccination against microbes. Interestingly, the mechanisms underlying the potent immunity elicited by mucosal nanovaccines are not well understood, and may depend upon the nanoparticle platform, administration route, adjuvant/antigen presentation, and other variables. Very recently, we designed a mucosal vaccine consisting of inactivated C. trachomatis attached to nanoparticles that contain TLR7 agonist [194]. Results show that genital and intranasal, but not subcutaneous, immunization with this vaccine construct resulted in protection when mice were genitally challenged with infectious C. trachomatis up to six months after immunization. Mechanistic studies suggest that the induction of a long-lived protective mucosal memory T cell response is critical. Considering that there is no vaccine available for C. trachomatis, this research may be essential for translating mucosal vaccines against this and possibly other sexually transmitted infections in humans. More importantly, we believe that a deeper understanding of the underlying principles will provide valuable insights for the successful design and clinical development of mucosal nanovaccines.
While nanotechnology is becoming the driving force behind a variety of changes in the antimicrobial field, the clinical development of antimicrobial nanotechnologies still faces considerable challenges. The rapid emergence of clinically invalidated new biomaterials and nanomaterials will require more careful examination of their biocompatibility and long-term safety. The mass production of complex nanotechnologies (e.g., targeted multifunctional nanoparticles), with minimal batch-to-batch variation, remains a significant barrier to their development and commercialization. High-throughput technologies are also in great demand for the rapid in vitro and in vivo screening of nanoparticle biophysicochemical properties (e.g., composition, size, shape, surface charge, targeting ligand and its density, etc.), and optimization of the interplay of nanomaterials with payloads (e.g., loading efficiency, release kinetics, and stability), matrices (in the case of medical devices), and tissues (e.g., pharmacokinetics, biodistribution, and immune surveillance). Furthermore, the successful translation of antimicrobial nanotechnologies can be facilitated by developing more clinically relevant animal models, identifying the mechanisms of microbial pathogenesis and new biomarkers, understanding the microenvironment of bacterial infection sites, and reducing the regulatory barriers. With continual advancements in antimicrobial nanomedicine, we can expect that many more nanotechnology-based products will be brought into the clinic to manage every aspect of microbial infection.
Highlights.
Microbial infections remain a major cause of morbidity and mortality in the world.
Nanomedicine is a major driving force behind ongoing changes in the antimicrobial field.
We overview the current progress of nanomedicine in the management of microbial infection, including diagnosis, antimicrobial therapy, drug delivery, medical devices, and vaccines.
We provide perspectives on the opportunities and challenges in antimicrobial nanomedicine.
Acknowledgments
This work was supported by NIH grants R00CA160350 (J.S.) and U54-CA151884 (R.L.); David Koch – Prostate Cancer Foundation Program in Nanotherapeutics (R.L.); Movember-PCF Challenge Award (J.S.); and PCF Young Investigator Award (J.S.). X.Z. acknowledges financial support from the State Scholarship Fund of China. We also thank Dr. Omid C. Farokhzad and Danny Liu for critical reviewing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
R.L. has a financial interest in BIND Therapeutics, Selecta Biosciences, and T2 Biosystems, three biotechnology companies developing nanoparticle technologies for medical applications.
References
- [1].Cohen ML. Nature. 2000;406:762–767. doi: 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- [2].Armstrong GL, Conn LA, Pinner RW. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization . The Evolving Threat of Antimicrobial Resistance: Options for Action. WHO Press; Geneva: 2012. [Google Scholar]
- [4].Kåhrström CT. Nat. Rev. Microbiol. 2013;11:146. doi: 10.1038/nrmicro2978. [DOI] [PubMed] [Google Scholar]
- [5].Alekshun MN, Levy SB. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [6].Limbago BM, Kallen AJ, Zhu W, Eggers P, McDougal LK, Albrecht VS. J. Clin. Microbiol. 2014;52:998–1002. doi: 10.1128/JCM.02187-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schäberle TF, Hack IM. Trends Microbiol. 2014;22:165–167. doi: 10.1016/j.tim.2013.12.007. [DOI] [PubMed] [Google Scholar]
- [8].Taubes G. Science. 2008;321:356–361. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- [9].Boucher HW, Talbot GH, Benjamin DK, Jr., Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. Clin. Infect. Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- [11].Shi J, Xiao Z, Kamaly N, Farokhzad OC. Acc. Chem. Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- [12].Wagner V, Dullaart A, Bock AK, Zweck A. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- [13].Shi J, Votruba AR, Farokhzad OC, Langer R. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. Nanomedicine: NBM. 2013;9:1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilson BA, Salyers AA, Whitt DD, Winkler ME. Bacterial pathogenesis: A molecular approach. American Society for Microbiology; Washington: 2011. [Google Scholar]
- [16].Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- [17].Kaittanis C, Santra S, Perez JM. Adv. Drug Deliv. Rev. 2010;62:408–423. doi: 10.1016/j.addr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xie J, Liu G, Eden HS, Ai H, Chen X. Acc. Chem. Res. 2011;44:883–892. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, Blanco M, Demas V, Skewis LR, Anagnostou T, Coleman JJ, Wellman P, Mylonakis E, Lowery TJ. Sci. Transl. Med. 2013;5:182ra154. doi: 10.1126/scitranslmed.3005377. [DOI] [PubMed] [Google Scholar]
- [20].Chung HJ, Castro CM, Im H, Lee H, Weissleder R. Nat. Nanotechnol. 2013;8:369–375. doi: 10.1038/nnano.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bizzini A, Durussel C, Bille J, Greub G, Prod’hom G. J. Clin. Microbiol. 2010;48:1549–1554. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lin Y-S, Tsai P-J, Weng M-F, Chen Y-C. Anal. Chem. 2005;77:1753–1760. doi: 10.1021/ac048990k. [DOI] [PubMed] [Google Scholar]
- [23].Lee JJ, Jeong KJ, Hashimoto M, Kwon AH, Rwei A, Shankarappa SA, Tsui JH, Kohane DS. Nano Lett. 2014;14:1–5. doi: 10.1021/nl3047305. [DOI] [PubMed] [Google Scholar]
- [24].Kaittanis C, Nath S, Perez JM. PLoS One. 2008;3:e3253. doi: 10.1371/journal.pone.0003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Uehara N. Adv. Mater. 2010;26:1219–1228. doi: 10.2116/analsci.26.1219. [DOI] [PubMed] [Google Scholar]
- [26].Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- [27].Storhoff JJ, Lucas AD, Garimella V, Bao YP, Muller UR. Nat. Biotechnol. 2004;22:883–887. doi: 10.1038/nbt977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cao YC, Jin R, Mirkin CA. Science. 2002;297:1536–1540. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- [29].Hill HD, Mirkin CA. Nat. Protoc. 2006;1:324–336. doi: 10.1038/nprot.2006.51. [DOI] [PubMed] [Google Scholar]
- [30].Scott LJ. Mol. Diagn. Ther. 2013;17:117–122. doi: 10.1007/s40291-013-0021-z. [DOI] [PubMed] [Google Scholar]
- [31].Chan PH, Wong SY, Lin SH, Chen YC. Rapid Commun. Mass Spectrom. 2013;27:2143–2148. doi: 10.1002/rcm.6674. [DOI] [PubMed] [Google Scholar]
- [32].Chan PH, Chen YC. Anal Chem. 2012;84:8952–8956. doi: 10.1021/ac302417k. [DOI] [PubMed] [Google Scholar]
- [33].Nath S, Kaittanis C, Tinkham A, Perez JM. Anal. Chem. 2008;80:1033–1038. doi: 10.1021/ac701969u. [DOI] [PubMed] [Google Scholar]
- [34].Zhao X, Hilliard LR, Mechery SJ, Wang Y, Bagwe RP, Jin S, Tan W. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15027–15032. doi: 10.1073/pnas.0404806101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang L, Zhao W, O’Donoghu MB, Tan W. Bioconjugate Chem. 2007;18:297–301. doi: 10.1021/bc060255n. [DOI] [PubMed] [Google Scholar]
- [36].Zrazhevskiy P, Sena M, Gao X. Chem. Soc. Rev. 2010;39:4326–4354. doi: 10.1039/b915139g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tully E, Hearty S, Leonard P, O’Kennedy R. Int. J. Biol. Macromol. 2006;39:127–134. doi: 10.1016/j.ijbiomac.2006.02.023. [DOI] [PubMed] [Google Scholar]
- [38].Jayaraman R. Curr. Sci. 2009;96:1475–1484. [Google Scholar]
- [39].Pelgrift RY, Friedman AJ. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- [40].Ray K, Marteyn B, Sansonetti PJ, Tang CM. Nat. Rev. Microbiol. 2009;7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- [41].Mah TF, O’Toole GA. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- [42].Huang L, Dai T, Xuan Y, Tegos GP, Hamblin MR. Antimicrob. Agents Chemother. 2011;55:3432–3438. doi: 10.1128/AAC.01803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huh AJ, Kwon YJ. J. Controlled Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- [44].Tran N, Tran PA. ChemPhysChem. 2012;13:2481–2494. doi: 10.1002/cphc.201200091. [DOI] [PubMed] [Google Scholar]
- [45].Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM. Chem. Rev. 2013;113:4708–4754. doi: 10.1021/cr300288v. [DOI] [PubMed] [Google Scholar]
- [46].Lara HH, Ayala-Núnez NV, Turrent L.d.C.I., Padilla CR. World J. Microbiol. Biotechnol. 2010;26:615–621. [Google Scholar]
- [47].Blecher K, Nasir A, Friedman A. Virulence. 2011;2:395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- [48].Knetsch ML, Koole LH. Polymers. 2011;3:340–366. [Google Scholar]
- [49].Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, Alvarez PJ. Water Res. 2008;42:4591–4602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- [50].Veerapandian M, Lim SK, Nam HM, Kuppannan G, Yun KS. Anal. Bioanal. Chem. 2010;398:867–876. doi: 10.1007/s00216-010-3964-5. [DOI] [PubMed] [Google Scholar]
- [51].Zare B, Faramarzi MA, Sepehrizadeh Z, Shakibaie M, Rezaie S, Shahverdi AR. Mater. Res. Bull. 2012;47:3719–3725. [Google Scholar]
- [52].Wang Q, Perez JM, Webster TJ. Int. J. Nanomedicine. 2013;8:3395–3399. doi: 10.2147/IJN.S50292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. J. Appl. Microbiol. 2009;107:1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x. [DOI] [PubMed] [Google Scholar]
- [54].Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Nanomedicine: NBM. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- [55].Huang Z, Zheng X, Yan D, Yin G, Liao X, Kang Y, Yao Y, Huang D, Hao B. Langmuir. 2008;24:4140–4144. doi: 10.1021/la7035949. [DOI] [PubMed] [Google Scholar]
- [56].Mühling M, Bradford A, Readman JW, Somerfield PJ, Handy RD. Mar. Environ. Res. 2009;68:278–283. doi: 10.1016/j.marenvres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- [57].Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet J-B, Antimicrob J. Chemother. 2008;61:869–876. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- [58].Lellouche J, Kahana E, Elias S, Gedanken A, Banin E. Biomaterials. 2009;30:5969–5978. doi: 10.1016/j.biomaterials.2009.07.037. [DOI] [PubMed] [Google Scholar]
- [59].Kwak S-Y, Kim SH, Kim SS. Environ. Sci. Technol. 2001;35:2388–2394. doi: 10.1021/es0017099. [DOI] [PubMed] [Google Scholar]
- [60].Hernandez-Delgadillo R, Velasco-Arias D, Diaz D, Arevalo-Niño K, Garza-Enriquez M, De la Garza-Ramos MA, Cabral-Romero C. Int. J. Nanomedicine. 2012;7:2109–2113. doi: 10.2147/IJN.S29854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2:544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- [62].Karlsson HL, Cronholm P, Gustafsson J, Möller L. Chem. Res. Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- [63].Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, Van Eijkeren JC, Geertsma RE, De Jong WH. Biomaterials. 2010;31:8350–8361. doi: 10.1016/j.biomaterials.2010.07.045. [DOI] [PubMed] [Google Scholar]
- [64].Qiu Z, Yu Y, Chen Z, Jin M, Yang D, Zhao Z, Wang J, Shen Z, Wang X, Qian D. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4944–4949. doi: 10.1073/pnas.1107254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Veerapandian M, Yun K. Appl. Microbiol. Biotechnol. 2011;90:1655–1667. doi: 10.1007/s00253-011-3291-6. [DOI] [PubMed] [Google Scholar]
- [66].Kang S, Herzberg M, Rodrigues DF, Elimelech M. Langmuir. 2008;24:6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- [67].Kotagiri N, Lee JS, Kim J-W, Biomed J. Nanotechnol. 2013;9:1008–1016. doi: 10.1166/jbn.2013.1531. [DOI] [PubMed] [Google Scholar]
- [68].Kang S, Pinault M, Pfefferle LD, Elimelech M. Langmuir. 2007;23:8670–8673. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- [69].Lyon DY, Adams LK, Falkner JC, Alvarez PJ. Environ. Sci. Technol. 2006;40:4360–4366. doi: 10.1021/es0603655. [DOI] [PubMed] [Google Scholar]
- [70].Xia XR, Monteiro-Riviere NA, Riviere JE. Toxicol. Lett. 2010;197:128–134. doi: 10.1016/j.toxlet.2010.05.010. [DOI] [PubMed] [Google Scholar]
- [71].Rajesh S, Koshi E, Philip K, Mohan A. J. Indian Soc. Periodontol. 2011;15:323–327. doi: 10.4103/0972-124X.92563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hancock RE, Sahl H-G. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- [73].Peschel A, Sahl H-G. Nat. Rev. Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- [74].Eby DM, Farrington KE, Johnson GR. Biomacromolecules. 2008;9:2487–2494. doi: 10.1021/bm800512e. [DOI] [PubMed] [Google Scholar]
- [75].Blin T, Purohit V, Leprince J.r.m., Jouenne T, Glinel K. Biomacromolecules. 2011;12:1259–1264. doi: 10.1021/bm101547d. [DOI] [PubMed] [Google Scholar]
- [76].Liu L, Xu K, Wang H, Tan PJ, Fan W, Venkatraman SS, Li L, Yang Y-Y. Nat. Nanotechnol. 2009;4:457–463. doi: 10.1038/nnano.2009.153. [DOI] [PubMed] [Google Scholar]
- [77].Makovitzki A, Baram J, Shai Y. Biochemistry. 2008;47:10630–10636. doi: 10.1021/bi8011675. [DOI] [PubMed] [Google Scholar]
- [78].Khara JS, Wang Y, Ke XY, Liu S, Newton SM, Langford PR, Yang YY, Ee PL. Biomaterials. 2014;35:2032–2038. doi: 10.1016/j.biomaterials.2013.11.035. [DOI] [PubMed] [Google Scholar]
- [79].Engler AC, Wiradharma N, Ong ZY, Coady DJ, Hedrick JL, Yang Y-Y. Nano Today. 2012;7:201–222. [Google Scholar]
- [80].Nederberg F, Zhang Y, Tan JP, Xu K, Wang H, Yang C, Gao S, Guo XD, Fukushima K, Li L, Hedrick JL, Yang Y-Y. Nat. Chem. 2011;3:409–414. doi: 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- [81].Song J, Kang H, Lee C, Hwang SH, Jang J. ACS Appl. Mater. Interfaces. 2011;4:460–465. doi: 10.1021/am201563t. [DOI] [PubMed] [Google Scholar]
- [82].Kong M, Chen XG, Xing K, Park HJ. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- [83].Qi L, Xu Z, Jiang X, Hu C, Zou X. Carbohydr. Res. 2004;339:2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [84].Rabea EI, Badawy ME-T, Stevens CV, Smagghe G, Steurbaut W. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- [85].Friedman AJ, Phan J, Schairer DO, Champer J, Qin M, Pirouz A, Blecher-Paz K, Oren A, Liu PT, Modlin RL, Kim J. J. Invest. Dermatol. 2013;133:1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jain D, Banerjee R. J. Biomed. Mater. Res. B Appl. Biomater. 2008;86:105–112. doi: 10.1002/jbm.b.30994. [DOI] [PubMed] [Google Scholar]
- [87].Potara M, Jakab E, Damert A, Popescu O, Canpean V, Astilean S. Nanotechnology. 2011;22:135101. doi: 10.1088/0957-4484/22/13/135101. [DOI] [PubMed] [Google Scholar]
- [88].Zhang L, Pornpattananangku D, Hu CM, Huang CM. Curr. Med. Chem. 2010;17:585–594. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- [89].Poole K. J. Appl. Microbiol. 2002;92(Suppl):55S–64S. [PubMed] [Google Scholar]
- [90].Singh R, Smitha MS, Singh SP. J. Nanosci. Nanotechnol. 2014;14:4745–4756. doi: 10.1166/jnn.2014.9527. [DOI] [PubMed] [Google Scholar]
- [91].Huang CM, Chen CH, Pornpattananangkul D, Zhang L, Chan M, Hsieh MF, Zhang L. Biomaterials. 2011;32:214–221. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nicolosi D, Scalia M, Nicolosi VM, Pignatello R. Int. J. Antimicrob. Agents. 2010;35:553–558. doi: 10.1016/j.ijantimicag.2010.01.015. [DOI] [PubMed] [Google Scholar]
- [93].Alipour M, Suntres ZE, Lafrenie RM, Omri A. J. Antimicrob. Chemother. 2010;65:684–693. doi: 10.1093/jac/dkq036. [DOI] [PubMed] [Google Scholar]
- [94].Cheow WS, Chang MW, Hadinoto K. Colloids Surf. A Physicochem. Eng. Asp. 2011;389:158–165. [Google Scholar]
- [95].Jones MN, Kaszuba M, Reboiras MD, Lyle IG, Hill KJ, Song YH, Wilmot SW, Creeth JE. Biochimica et biophysica acta. 1994;1196:57–64. doi: 10.1016/0005-2736(94)90295-x. [DOI] [PubMed] [Google Scholar]
- [96].Sanderson NM, Guo B, Jacob AE, Handley PS, Cunniffe JG, Jones MN. Biochimica et biophysica acta. 1996;1283:207–214. doi: 10.1016/0005-2736(96)00099-5. [DOI] [PubMed] [Google Scholar]
- [97].Abed N, Couvreur P. Int. J. Antimicrob. Agents. 2014 doi: 10.1016/j.ijantimicag.2014.02.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [98].Clemens DL, Lee BY, Xue M, Thomas CR, Meng H, Ferris D, Nel AE, Zink JI, Horwitz MA. Antimicrob. Agents Chemother. 2012;56:2535–2545. doi: 10.1128/AAC.06049-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. J. Controlled Release. 2014 doi: 10.1016/j.jconrel.2014.03.055. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [100].Pissuwan D, Cortie CH, Valenzuela SM, Cortie MB. Trends Biotechnol. 2010;28:207–213. doi: 10.1016/j.tibtech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [101].Gu H, Ho P, Tong E, Wang L, Xu B. Nano Lett. 2003;3:1261–1263. [Google Scholar]
- [102].Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X. J. Am. Chem. Soc. 2010;132:12349–12356. doi: 10.1021/ja1028843. [DOI] [PubMed] [Google Scholar]
- [103].Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ. Virulence. 2012;3:271–279. doi: 10.4161/viru.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Privett BJ, Broadnax AD, Bauman SJ, Riccio DA, Schoenfisch MH. Nitric Oxide. 2012;26:169–173. doi: 10.1016/j.niox.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Biomaterials. 2009;30:2782–2789. doi: 10.1016/j.biomaterials.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH. ACS Nano. 2008;2:235–246. doi: 10.1021/nn700191f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Worley BV, Slomberg DL, Schoenfisch MH. Bioconjugate Chem. 2014 doi: 10.1021/bc5000719. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [108].Lu Y, Slomberg DL, Schoenfisch MH. Biomaterials. 2014;35:1716–1724. doi: 10.1016/j.biomaterials.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. PLoS One. 2009;4:e7804. doi: 10.1371/journal.pone.0007804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Free Radic. Biol. Med. 2010;49:530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, Friedman JM. Nitric Oxide. 2008;19:12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [112].Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, Friedman JM. J. Invest. Dermatol. 2009;129:2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- [113].Chow JW, Yu VL. Int. J. Antimicrob. Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- [114].Toti US, Guru BR, Hali M, McPharlin CM, Wykes SM, Panyam J, Whittum-Hudson JA. Biomaterials. 2011;32:6606–6613. doi: 10.1016/j.biomaterials.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Carmona D, Lalueza P, Balas F, Arruebo M, Santamaría J. Microporous Mesoporous Mater. 2012;161:84–90. [Google Scholar]
- [116].Hurdle JG, O’Neill AJ, Chopra I, Lee RE. Nat. Rev. Microbiol. 2010;9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ranjan A, Pothayee N, Seleem MN, Boyle SM, Kasimanickam R, Riffle JS, Sriranganathan N. FEMS Microbiol. Lett. 2012;332:1–9. doi: 10.1111/j.1574-6968.2012.02566.x. [DOI] [PubMed] [Google Scholar]
- [118].Benchaala I, Mishra MK, Wykes SM, Hali M, Kannan RM, Whittum-Hudson JA. Int. J. Pharm. 2014;466:258–265. doi: 10.1016/j.ijpharm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- [119].Chono S, Tanino T, Seki T, Morimoto K. J. Controlled Release. 2008;127:50–58. doi: 10.1016/j.jconrel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- [120].Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. ACS Nano. 2012;6:4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Pornpattananangkul D, Olson S, Aryal S, Sartor M, Huang CM, Vecchio K, Zhang L. ACS Nano. 2010;4:1935–1942. doi: 10.1021/nn9018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Gao W, Vecchio D, Li J, Zhu J, Zhang Q, Fu V, Li J, Thamphiwatana S, Lu D, Zhang L. ACS Nano. 2014;8:2900–2907. doi: 10.1021/nn500110a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang C-M, Zhang L. J. Am. Chem. Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Xiong M-H, Bao Y, Yang X-Z, Wang Y-C, Sun B, Wang J. J. Am. Chem. Soc. 2012;134:4355–4362. doi: 10.1021/ja211279u. [DOI] [PubMed] [Google Scholar]
- [125].Xiong MH, Li YJ, Bao Y, Yang XZ, Hu B, Wang J. Adv. Mater. 2012;24:6175–6180. doi: 10.1002/adma.201202847. [DOI] [PubMed] [Google Scholar]
- [126].Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Sci. Transl. Med. 2012;4:128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- [130].Scott RD. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. GA: Centers for Disease Control and Prevention; Atlanta: 2009. [Google Scholar]
- [131].Trerotola SO. Radiology. 2000;215:651–658. doi: 10.1148/radiology.215.3.r00jn23651. [DOI] [PubMed] [Google Scholar]
- [132].Darouiche RO. N. Engl. J. Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- [133].Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M. Kidney Int. 2002;61:1136–1142. doi: 10.1046/j.1523-1755.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- [134].Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Bayston R, Brown PD, Winship PD, Reid HJ. J. Antimicrob. Chemother. 2004;54:1019–1024. doi: 10.1093/jac/dkh478. [DOI] [PubMed] [Google Scholar]
- [135].Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. Chem. Rev. 2011;111:3407–3432. doi: 10.1021/cr1003166. [DOI] [PubMed] [Google Scholar]
- [136].Khundkar R, Malic C, Burge T. Burns. 2010;36:751–758. doi: 10.1016/j.burns.2009.04.008. [DOI] [PubMed] [Google Scholar]
- [137].Wong KK, Liu X. MedChemComm. 2010;1:125–131. [Google Scholar]
- [138].Anisha B, Biswas R, Chennazhi K, Jayakumar R. Int. J. Biol. Macromol. 2013;62:310–320. doi: 10.1016/j.ijbiomac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- [139].Sudheesh Kumar P, Lakshmanan V-K, Anilkumar T, Ramya C, Reshmi P, Unnikrishnan A, Nair SV, Jayakumar R. ACS Appl. Mater. Interfaces. 2012;4:2618–2629. doi: 10.1021/am300292v. [DOI] [PubMed] [Google Scholar]
- [140].Fu J, Ji J, Fan D, Shen J. J. Biomed. Mater. Res. A. 2006;79:665–674. doi: 10.1002/jbm.a.30819. [DOI] [PubMed] [Google Scholar]
- [141].Wang J, Huang N, Yang P, Leng Y, Sun H, Liu Z, Chu P. Biomaterials. 2004;25:3163–3170. doi: 10.1016/j.biomaterials.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [142].Travan A, Marsich E, Donati I, Benincasa M, Giazzon M, Felisari L, Paoletti S. Acta Biomater. 2011;7:337–346. doi: 10.1016/j.actbio.2010.07.024. [DOI] [PubMed] [Google Scholar]
- [143].Travan A, Pelillo C, Donati I, Marsich E, Benincasa M, Scarpa T, Semeraro S, Turco G, Gennaro R, Paoletti S. Biomacromolecules. 2009;10:1429–1435. doi: 10.1021/bm900039x. [DOI] [PubMed] [Google Scholar]
- [144].Manias E. Nat. Mater. 2007;6:9–11. doi: 10.1038/nmat1812. [DOI] [PubMed] [Google Scholar]
- [145].Böswald M, Mende K, Bernschneider W, Bonakdar S, Ruder H, Kissler H, Sieber E, Guggenbichler J-P. Infection. 1999;27:S38–S42. doi: 10.1007/BF02561616. [DOI] [PubMed] [Google Scholar]
- [146].Nirmala R, Nam KT, Park DK, Woo-il B, Navamathavan R, Kim HY. Surf. Coat. Technol. 2010;205:174–181. [Google Scholar]
- [147].Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- [148].Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, Bai Y, Xu HH. J. Dent. 2013;41:464–474. doi: 10.1016/j.jdent.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nino-Martinez N, Martinez-Castanon G, Aragon-Pina A, Martinez-Gutierrez F, Martinez-Mendoza J, Ruiz F. Nanotechnology. 2008;19:065711. doi: 10.1088/0957-4484/19/6/065711. [DOI] [PubMed] [Google Scholar]
- [150].Santillán M, Quaranta N, Boccaccini A. Surf. Coat. Technol. 2010;205:2562–2571. [Google Scholar]
- [151].Seery MK, George R, Floris P, Pillai SC. J. Photochem. Photobiol. A. 2007;189:258–263. [Google Scholar]
- [152].Stanić V, Dimitrijević S, Antić-Stanković J, Mitrić M, Jokić B, Plećaš IB, Raičević S. Appl. Surf. Sci. 2010;256:6083–6089. [Google Scholar]
- [153].Plotkin SA. Nat. Med. 2005;11:S5–11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Choh L-C, Ong G-H, Vellasamy KM, Kalaiselvam K, Kang W-T, Al-Maleki AR, Mariappan V, Vadivelu J. Front. Cell. Infect. Microbiol. 2013;3:1–18. doi: 10.3389/fcimb.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Curtiss R. J. Clin. Invest. 2002;110:1061–1066. doi: 10.1172/JCI16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Carleton HA. Yale J. Biol. Med. 2010;83:217–222. [PMC free article] [PubMed] [Google Scholar]
- [157].Peek LJ, Middaugh CR, Berkland C. Adv. Drug Deliv. Rev. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Smith DM, Simon JK, Baker JR., Jr Nat. Rev. Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- [160].Holmgren J, Czerkinsky C. Nat. Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [161].Neutra MR, Pringault E, Kraehenbuhl JP. Annu. Rev. Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- [162].Kammona O, Kiparissides C. J. Controlled Release. 2012;161:781–794. doi: 10.1016/j.jconrel.2012.05.040. [DOI] [PubMed] [Google Scholar]
- [163].Manocha M, Pal PC, Chitralekha K, Thomas BE, Tripathi V, Gupta SD, Paranjape R, Kulkarni S, Rao DN. Vaccine. 2005;23:5599–5617. doi: 10.1016/j.vaccine.2005.06.031. [DOI] [PubMed] [Google Scholar]
- [164].Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR. Microbiol. Res. 2001;156:1–7. doi: 10.1078/0944-5013-00069. [DOI] [PubMed] [Google Scholar]
- [165].Bielinska AU, Janczak KW, Landers JJ, Makidon P, Sower LE, Peterson JW, Baker JR. Infect. Immun. 2007;75:4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Makidon PE, Knowlton J, Groom JV, Blanco LP, LiPuma JJ, Bielinska AU, Baker JR., Jr. Med. Microbiol. Immunol. 2010;199:81–92. doi: 10.1007/s00430-009-0137-2. [DOI] [PubMed] [Google Scholar]
- [167].Martel CJ-M, Agger EM, Poulsen JJ, Jensen TH, Andresen L, Christensen D, Nielsen LP, Blixenkrone-Møller M, Andersen P, Aasted B. PLoS One. 2011;6:e22891. doi: 10.1371/journal.pone.0022891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kamath AT, Rochat A-F, Christensen D, Agger EM, Andersen P, Lambert P-H, Siegrist C-A. PLoS One. 2009;4:e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Henderson A, Propst K, Kedl R, Dow S. Vaccine. 2011;29:5304–5312. doi: 10.1016/j.vaccine.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Fairley SJ, Singh SR, Yilma AN, Waffo AB, Subbarayan P, Dixit S, Taha MA, Cambridge CD, Dennis VA. Int. J. Nanomedicine. 2013;8:2085–2099. doi: 10.2147/IJN.S44155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hu CM, Fang RH, Luk BT, Zhang L. Nat. Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kong IG, Sato A, Yuki Y, Nochi T, Takahashi H, Sawada S, Mejima M, Kurokawa S, Okada K, Sato S, Briles DE, Kunisawa J, Inoue Y, Yamamoto M, Akiyoshi K, Kiyono H. Infect. Immun. 2013;81:1625–1634. doi: 10.1128/IAI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cambridge CD, Singh SR, Waffo AB, Fairley SJ, Dennis VA. Int. J. Nanomedicine. 2013;8:1759–1771. doi: 10.2147/IJN.S42723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Florindo H, Pandit S, Lacerda L, Gonçalves L, Alpar H, Almeida A. Biomaterials. 2009;30:879–891. doi: 10.1016/j.biomaterials.2008.10.035. [DOI] [PubMed] [Google Scholar]
- [175].Schroeder U, Graff A, Buchmeier S, Rigler P, Silvan U, Tropel D, Jockusch BM, Aebi U, Burkhard P, Schoenenberger C-A. J. Mol. Biol. 2009;386:1368–1381. doi: 10.1016/j.jmb.2008.11.023. [DOI] [PubMed] [Google Scholar]
- [176].Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, Tropel D, Aebi U, Burkhard P, Lanar DE. J. Immunol. 2009;183:7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Sun H-X, Xie Y, Ye Y-P. Vaccine. 2009;27:4388–4401. doi: 10.1016/j.vaccine.2009.05.032. [DOI] [PubMed] [Google Scholar]
- [178].Hu K-F, Lövgren-Bengtsson K, Morein B. Adv. Drug Deliv. Rev. 2001;51:149–159. doi: 10.1016/s0169-409x(01)00165-x. [DOI] [PubMed] [Google Scholar]
- [179].Gleave ME, Monia BP. Nat. Rev. Cancer. 2005;5:468–479. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- [180].Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. Nat. Rev. Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Meng J, Wang H, Hou Z, Chen T, Fu J, Ma X, He G, Xue X, Jia M, Luo X. Antimicrob. Agents Chemother. 2009;53:2871–2878. doi: 10.1128/AAC.01542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE. Nat. Biotechnol. 2001;19:360–364. doi: 10.1038/86753. [DOI] [PubMed] [Google Scholar]
- [183].Mondhe M, Chessher A, Goh S, Good L, Stach JE. PLoS One. 2014;9:e89082. doi: 10.1371/journal.pone.0089082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rusk N. Nat. Methods. 2012;9:220–221. doi: 10.1038/nmeth.1916. [DOI] [PubMed] [Google Scholar]
- [185].Terns RM, Terns MP. Trends Genet. 2014;30:111–118. doi: 10.1016/j.tig.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Shi J, Xiao Z, Votruba AR, Vilos C, Farokhzad OC. Angew. Chem., Int. Ed. 2011;123:7165–7169. doi: 10.1002/anie.201101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Shi J, Xu Y, Xu X, Zhu X, Pridgen E, Wu J, Alexander V, Zetter BR, Farokhzad OC. Nanomedicine: NBM. 2014;10:897–900. doi: 10.1016/j.nano.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Xu X, Xie K, Zhang X-Q, Pridgen EM, Park GY, Cui DS, Shi J, Wu J, Kantoff PW, Lippard SJ, Langer R, Walker GC, Farokhzad OC. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18638–18643. doi: 10.1073/pnas.1303958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Fang J, Nakamura H, Maeda H. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [191].Azzopardi EA, Ferguson EL, Thomas DW. J. Antimicrob. Chemother. 2013;68:257–274. doi: 10.1093/jac/dks379. [DOI] [PubMed] [Google Scholar]
- [192].Zhu X, Anquillare ELB, Farokhzad OC, Shi J. In: Cancer Theranostics. Chen X, Wong S, editors. Academic Press; Oxford: 2014. pp. 419–436. [Google Scholar]
- [193].Galanzha EI, Shashkov E, Sarimollaoglu M, Beenken KE, Basnakian AG, Shirtliff ME, Kim J-W, Smeltzer MS, Zharov VP. PLoS One. 2012;7:e45557. doi: 10.1371/journal.pone.0045557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Stary G, Radovic-Moreno A, Gandek D, Olive A, Basto P, Farokhzad O, Langer R, Stambach M, von Andrian U. J. Invest. Dermatol. 2013;133:S16. [Google Scholar]