Abstract
Significance: Patients with diabetes mellitus suffer an excess of cardiovascular complications and recover worse from them as compared with their nondiabetic peers. It is well known that microangiopathy is the cause of renal damage, blindness, and heart attacks in patients with diabetes. This review highlights molecular deficits in stem cells and a supporting microenvironment, which can be traced back to oxidative stress and ultimately reduce stem cells therapeutic potential in diabetic patients. Recent Advances: New research has shown that increased oxidative stress contributes to inducing microangiopathy in bone marrow (BM), the tissue contained inside the bones and the main source of stem cells. These precious cells not only replace old blood cells but also exert an important reparative function after acute injuries and heart attacks. Critical Issues: The starvation of BM as a consequence of microangiopathy can lead to a less efficient healing in diabetic patients with ischemic complications. Furthermore, stem cells from a patient's BM are the most used in regenerative medicine trials to mend hearts damaged by heart attacks. Future Directions: A deeper understanding of redox signaling in BM stem cells will lead to new modalities for preserving local and systemic homeostasis and to more effective treatments of diabetic cardiovascular complications. Antioxid. Redox Signal. 21, 1620–1633.
Introduction
A growing body of research indicates new roles for reactive oxygen species (ROS) in health and disease. Among pathologies related to an excess of ROS, diabetes mellitus (DM) occupies a prominent position. In fact, the high associated risk for cardiovascular morbidity and mortality makes DM one of the major threats to human health in the 21 century. From 2005 to 2008, 25.8 million patients (8.3% of the population) were diagnosed with DM in the United States. An additional 79 million had impaired fasting glycemia indicative of prediabetes (12). If current trends are confirmed, the prevalence of DM among adults will reach the figure of 33% by 2050. Moreover, DM and its complications impose a public burden of economic costs (23). In 2007, the total cost of DM in the United States was estimated to be $174 billion, $116 billion in direct medical costs and $58 billion in indirect costs due to disability, work loss, and premature death (12).
Cardiovascular disease (CVD), including coronary artery disease, stroke, peripheral arterial disease, and cardiomyopathy, are acknowledged for being the cause of death in ≈65% of patients with DM. To make the problem worse, when patients with DM develop cardiovascular complications, they bear a poorer course compared with CVD patients without DM. One possible explanation is that healing mechanisms are dampened by the metabolic disorder. For instance, a number of studies highlight the dysfunction of resident vascular cells and circulating angiogenic cells (30, 68, 84, 92). This translates into impaired reparative angiogenesis, the process of new vessel formation by local endothelial cells (ECs) and mural cells, and vasculogenesis, which consists of recruitment and incorporation of angiogenic cells in the nascent neovasculature.
Investigation on the role of circulating angiogenic cells in CVD is complicated by the large heterogeneity of cells with direct and indirect pro-angiogenic capacities (117). Indeed, this pool includes CD34+ progenitor cells, Tie2 expressing monocytes, and mesenchymal stem cells (MSCs) from bone marrow (BM) and non-BM sources (26). There is, however, a consensus on the fact that circulating angiogenic cells are particularly reduced in diabetic patients who manifest vascular complications of the highest degree of severity (27, 28). These observations suggest a pathogenic link between the deficit in vasculogenesis-driven repair and poor prognosis of diabetic patients with CVD complications.
The reasons for the shortage of circulating angiogenic cells in patients with DM remain unclear. Different possibilities have been considered, including a general reduction in hematopoietic stem cells (HSCs) or a defect in HSCs becoming monocytes or other progenitors within the BM, a reduction in circulating monocytes, or a specific incapacity of monocytes to become circulating angiogenic cells. In this regard, recent studies suggest that molecular modifications caused by chronic hyperglycemia might endanger stem cells and their progeny, that is, lineage committed progenitors, in their primitive niches (91). Furthermore, the lack in progenitor cell mobilization and preferential differentiation toward a pro-inflammatory phenotype have been reported in patients with DM (25, 41, 70, 84, 113). Importantly, the possibility that disruption of the normal redox balance participates in the damage of BM stem cells and their supportive microenvironment is gaining much attention.
In this review, we illustrate current knowledge of the mechanisms by which DM impinges on stem cell functions, including survival, self-renewal, differentiation, proliferation, migration, and mobilization. We also focus on DM as a disease model in which excessive ROS formation can jeopardize stem and stromal cells of the marrow niche. Advancing our understanding on how disruption of the redox balance induces excessive stimulation of stem cell differentiation, activation of apoptosis and, eventually, exhaustion of BM regenerative capacity is fundamental for establishing preventive measures in patients at risk of cardiovascular complications. It is also hoped that research will provide a new scope for preservation of BM integrity during normal and pathologic aging. Furthermore, managing ROS levels could be a rational means for better utilization of stem and progenitor cells in supply-side cell therapies of regenerative medicine for patients with manifested CVD.
Organization of BM Microenvironment
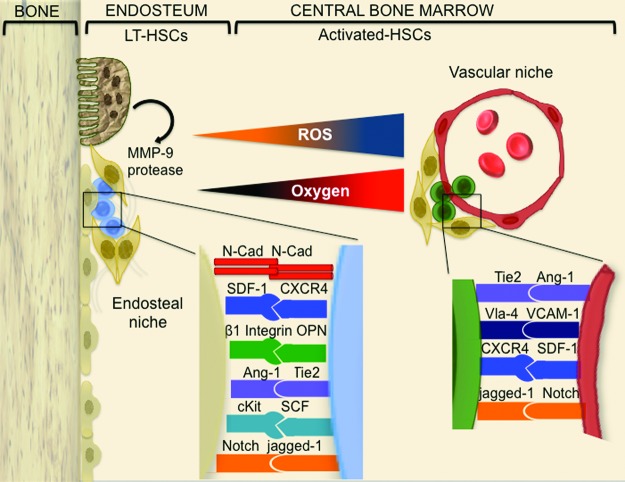
HSCs were the first adult stem cells employed for therapeutic purposes. The use of BM stem cells has recently expanded from treatment of hematologic disorders to initial clinical trials of cardiovascular regenerative medicine. Along with an increasing number of clinical applications, knowledge is growing on the presence of different functional compartments for stem cell residence. In the BM, stem and progenitor cells are distributed in distinct microenvironments called “niches” (93) constituted by a frame of stromal cells, macrophages, regulatory T cells, and the extracellular matrix (33, 72, 115). Two distinct niches have been identified: the osteoblastic niche (also named endosteal niche) lining on the endosteal bone surface and the vascular niche located near the sinusoids, which constitute the vast majority and most peculiar component of BM vasculature (Fig. 1). The existence of two niches creates a polarized gradient for stem cell self-renewal, maturation, and relocation into the systemic circulation. Several vascular sinusoids are recognized in close proximity to the endosteum, which indicates an intricate relationship between the two niches (86).
FIG. 1.
BM microenvironment organization. The BM is spatially organized into different niches. The osteoblastic niche (left) resides in the inner part of BM, lining on the endosteum. It includes the most primitive stem cells and a frame of stromal cells, principally osteoblasts. The vascular niche (right) is constituted by committed progenitor elements that are embedded in a frame of several kinds of stromal cells (ECs, macrophages, perycites, etc.). In both niches, stem and progenitor cells establish cell–cell contacts that are instrumental to reciprocal control of cell function. Ang-1, angiopoietin 1; BM, bone marrow; cKit, stem cell factor receptor; EC, endothelial cell; MMP-9, metalloproteinase 9; N-Cad, N-cadherin; SCF, also known as kit-ligand, stem cell factor; SDF-1, stromal cell-derived factor-1; SDF-1 receptor, CXCR4, C-X-C chemokine receptor type 4; Tie2, angiopoietin receptor 2; VCAM-1, integrin receptor vascular cell adhesion molecule-1; very late antigen-4, Vla-4, integrin alpha4beta1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Heterogeneity of BM Perfusion, Oxygenation, and ROS Content Creates Specialized Areas for Stem Cell Self-Renewal and Differentiation
It is now becoming increasingly evident that the HSC compartment consists of several subpopulations with distinct expansion and differentiation behaviors. This heterogeneity is attributed to the existence of stem cell-intrinsic programs under the influence of different milieus (74). One major milieu element is represented by the peculiar gradient of perfusion and oxygenation across the marrow (79, 81). The presence of high and low oxygenic areas creates the conditions for a differential production of ROS in different niches. In recent years, the interest on ROS as a key determinant of the marrow microenvironment heterogeneity has significantly increased, especially in the light of its recognition as a second messenger in growth factor-mediated angiogenic signaling and hypoxia-induced mobilization of angiogenic cells (88, 89) as well as a stimulus skewing the balance away from stem cell self-renewal toward differentiation (50, 103). In line with this, ROS levels are higher in terminally differentiated ECs than in endothelial progenitors (17). Furthermore, the low oxygenic, low ROS zone abundantly contains undifferentiated cells with high self-renewal potential (Fig. 1) (50, 75, 83, 96, 97). Conversely, the relatively high ROS concentration found in the vascular niche is instrumental to stem cell maturation (58).
Intracellular levels of ROS are important not only to instruct BM cell behavior but also for determining cell relocation (56, 57) and egression into the bloodstream (38, 51, 85, 99). Importantly, ROS play a key role in BM mononuclear cell (MNC) and angiogenic cell mobilization on induction of peripheral ischemia in experimental models (109). Thus, ROS acts as a second messenger that facilitates stress-induced mobilization of stem and progenitor cells as a part of host defense and repair mechanisms.
Sources of ROS and ROS-Mediated Mechanisms
Although the role of ROS in regulation of stem cell biology is well acknowledged, it is not yet completely clear “who-does-what” among different types of ROS. The short half life and easy diffusion of ROS along with limitations of many dyes currently used for measuring ROS represent an obstacle to mechanistic understanding (53). On the other hand, it is possible to reconstruct the physiological scenario by studying the different sources of ROS and downstream pathways.
ROS generators are mainly represented by the mitochondria and NADPH oxidase machinery. In mitochondria, ROS are a by-product of the respiratory chain enzymes complex I and complex III. The tuberous sclerosis complex (TSC)–mammalian target of rapamycin (mTOR) pathway maintains the quiescence of HSCs by repressing mitochondrial biogenesis and the local production of ROS. Conversely, mitochondrial generation of ROS drives HSCs from quiescence into rapid cycling (14). Further supporting the link between mitochondrial ROS and stem cell biology, Liu et al. showed that BM cells from polycomb repressor Bmi-1 knock-out mice have mitochondrial dysfunction, reduced ATP, increased ROS levels, and subsequent DNA damage (66) Of note, mitochondrial DNA is particularly susceptible to oxidative stress because of its vicinity to the electron transport chain and also due to the fact that it lacks protective histones. Accumulation of damage in the mitochondrial genome contributes to cellular aging. Accordingly, mutations of mitochondrial DNA accelerate the stem cell functional decline by influencing mobilization of energy and the balance between production and disposal of mitochondrial-derived ROS (4, 36).
NADPH oxidases are a family of enzymes that catalyze the transfer of electrons from NADPH to molecular oxygen via their “NOX” catalytic subunit, thereby generating superoxide and hydrogen peroxide and controlling numerous biological and pathological processes (8, 61). Among NOX isoforms, NOX1 is expressed in vascular smooth muscle cells and other vascular cells. NOX2, previously known as gp91phox, is present in endothelial and phagocytic cells (39, 105). NOX4 is constitutively expressed in ECs, vascular smooth muscle cells, cardiac cells, and phagocytic cells (3, 44). HSCs express NOXs 1, 2, and 4, which generate low levels of ROS to regulate cell growth and differentiation (82). NOX2-derived ROS is involved in ischemia-induced mobilization of and neovascularization afforded by progenitor cells and circulating angiogenic cells (88, 109). Thus, NOXs play a pivotal role in maintaining ROS levels within a limited range in order to regulate physiological functions of BM stem and progenitor cells and circulating angiogenic cells.
Molecular Pathways Involved in ROS Signaling
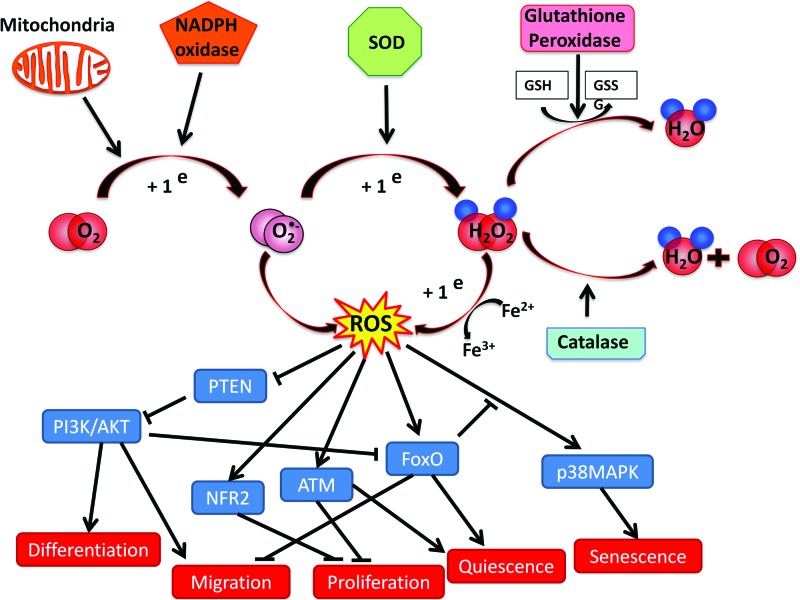
There are many downstream actors involved in regulation of the BM microenvironment and stem cell biology by ROS (Fig. 2). Of course, cells of the vascular niche and HSCs have different ROS signaling mechanisms and targets. In vascular cells, ROS are responsible for modulation of angiogenesis and vascular permeability as well as for cell-extrinsic regulation of stem cell maturation (vide infra); whereas, in HSCs, ROS signaling participates in cell-intrinsic regulation of hematopoiesis. Recent evidence from studies in genetically modified animals indicates that ROS is a key modulator of the PI3K–Akt signaling pathway in HSCs. In 2010, Juntilla et al. investigated physiologic hematopoiesis in Akt1−/−/Akt2−/− mice. Interestingly, serial transplantation experiments suggested that Akt is important for maintaining long-term HSCs throughout generation of ROS (52). Moreover, Kharas et al. showed that rapid induction of Akt signaling causes expansion of the HSCs compartment and increased HSC cycling, whereas a sustained Akt signaling in myr-AKT mice causes depletion of HSCs and increased apoptosis, eventually resulting in myeloproliferative disorders (55). Phosphatase and tensin homolog (PTEN) is a negative regulator of the PI3K–Akt pathway; thus, it does not come as a surprise that PTEN−/− mice show a phenotype similar to that of animals with activated Akt (122). The effects of PTEN are, in part, dependent on ROS production in HSCs. In addition, downstream inhibitory targets of Akt, such as the forkhead box transcription factors (FOXOs), are critical mediators of the cellular responses to oxidative stress in HSCs (95). In particular, mice lacking FOXO3a show altered HSC proliferation and differentiation patterns, with increased susceptibility to damage induced by stress and myeloid bias with aging, a defect associated to increased ROS level and p38 mitogen-activated protein kinase (MAPK) phosphorylation (73, 104). Furthermore, activation of p38 MAPK in response to increasing levels of ROS limits the lifespan of HSCs in vivo (49). Accordingly, FOXO3a deficiency is counterbalanced by antioxidant treatment with N-acetylcysteine (104).
FIG. 2.
Oxidative stress network in BM microenvironment. In BM, the sources of ROS are principally represented by the mitochondria and NADPH oxidase catalytic subunit NOX. Superoxide produced is then converted by superoxide dismutase into hydrogen peroxide and scavenged by catalase or glutathione peroxidase. ROS can enhance or decrease stem cell functions by activating or inhibiting different signaling pathways. For example, ROS induce differentiation and migration through the PTEN/PI3K/Akt pathway, sustain quiescence via FOXOs and ATM, and induce senescence through p38 MAPK pathway. ATM, ataxia telangiectasia mutated protein; FOXOs, forkhead box transcription factors; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositide 3-kinase; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The nuclear factor erythroid-2-related factor 2 (Nrf2), a master regulator of the antioxidant response, is required for maintenance of HSC homeostasis with a mechanism partially independent of ROS levels (45, 107). Along with FOXO3a and Nrf2, the ataxia telangiectasia mutated (ATM) gene exerts a protective role in HSCs against oxidative stress, as it maintains genomic stability by activating cell-cycle checkpoint, as demonstrated in ATM−/− mice (2, 48, 64). Similar to FOXO3a−/− mice, antioxidant treatment rescues HSC alteration in ATM−/− mice (48). Lastly, another factor regulating HSCs quiescence is the tumor suppressor gene p53. HSCs from mouse double minute 2 homolog (Mdm2, an ubiquitin ligase that targets p53 for degradation) knockout mice are characterized by elevated ROS, cell cycle arrest, senescence, and cell death in the hematopoietic compartment. This phenotype is partially rescued by antioxidants (1).
Although studies from genetically modified mice are certainly informative, it should be noted that the complete ablation of a specific pathway might not reflect the real pathophysiologic situation. Furthermore, the pool of HSCs is heterogeneous and, therefore, alteration of ROS levels in distinct populations could significantly change the scenario predicted by studies in which total BM cells carry a genetic mutation responsible for redox unbalance.
Implication of ROS in Physiological Aging of BM Stem Cells
Although HSCs can persist in vivo longer than a whole organism's life-span, the hematopoietic compartment is not spared by aging, which results in reduced stem cell regenerative potential, diminished adaptive immune competence, myeloid bias, and myeloproliferative disease predisposition. Among different factors responsible for stem cell senescence, genomic DNA damage accrual and epigenetic modification of chromatin components are thought to limit the regenerative response of HSCs from old mice (9, 43). Generalized expansion of the high ROS zone to the whole marrow may also contribute to the unbalance between self-renewal and differentiation in aging individuals.
Excessive ROS Production in Diabetes
Vascular ECs are particularly exposed to damage, because they are unable to downregulate the uptake of glucose when extracellular glucose concentrations are elevated. The high levels of intracellular glucose cause mitochondrial superoxide overproduction (106, 121). In turn, superoxide induces the activation of five major pathways involved in the pathogenesis of complications: polyol pathway flux, increased formation of advanced glycation end-products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C isoforms, and over-activity of the hexosamine pathway (21). Increased ROS production in the mitochondria causes DNA strand breaks and mutations, activating poly(ADP-ribose) polymerase (PARP), which, in turn, is responsible for further stimulation of toxic metabolic pathways (21).
In addition to the mitochondria, NAPDH oxidase has been credited as one of the main sources of ROS production in DM. Hyperglycemia triggers PKC-α-induced NAPDH oxidase activation via intracellular AGE formation (100). A sustained NAPDH oxidase activation may lead to exhaustion of intra-cellular NAPDH reservoir, which is fundamental for the activity of endothelial nitric oxide synthase (eNOS) and several antioxidant systems. Thus, NADPH oxidase could work as a double-edged sword, with transient activation providing a feedback against excessive ROS generation through the activation of antioxidant redox-sensitive Nrf2-Keap1 signaling pathway. In DM, this balanced mechanism seems to be disrupted: ROS overproduction leads to eNOS uncoupling, mitochondrial dysfunction, and impaired antioxidant defenses, resulting in depletion of intracellular NADPH (35).
Microangiopathy in BM of Diabetic Mice Jeopardizes Stem Cell Homeostasis
The vascular system pervades all organs of the body. Hence, the systemic nature of diabetic damage is strictly related to microangiopathy, which, along with diffuse atherosclerotic disease, represents the main feature of diabetic vasculopathy. While the consequences of microangiopathy for the retina, heart, brain, kidneys, and lower extremities are very well known, no specific research was performed until recently about the BM microvasculature and the possible impact of diabetic microangiopathy on SC homeostasis.
There are good reasons for considering the vascular niche as a primary site of increased oxidative stress in diabetic BM. This is a high oxygenic region compared with low perfused endosteum. In addition, DM could primarily increase oxidative stress in ECs because of lack of control in glucose influx in these cells. Accordingly, we found that ROS levels are already increased in BM ECs at 14 weeks after induction of type 1 DM (69), whereas oxidative stress was extended to HSCs after ∼20 weeks of DM in the same animal model (79). However, to the best of our knowledge, no study has addressed the issue of whether DM increases ROS levels in HSCs directly or through mediation of cells of the vascular niche.
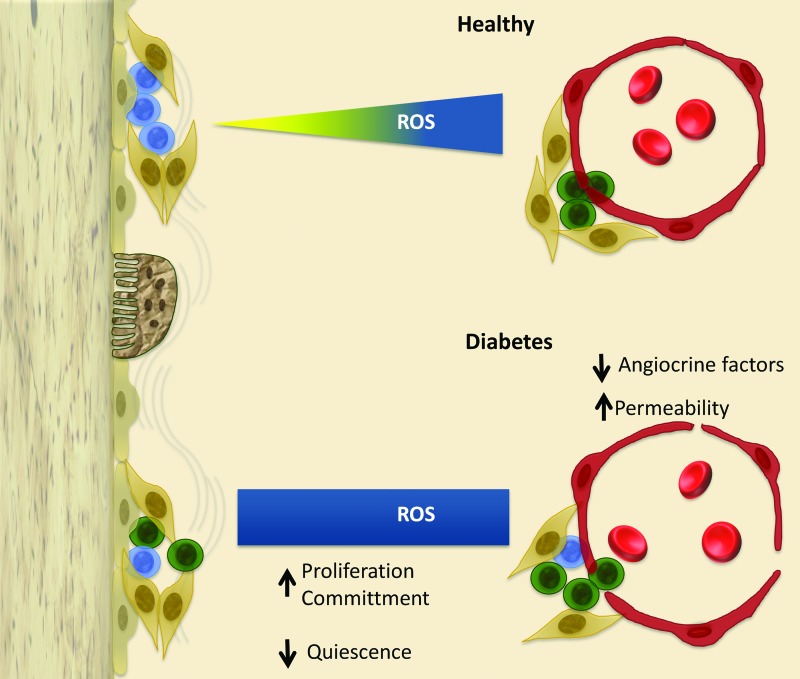
We were the first to demonstrate that diabetic microangiopathy in streptozotocin (STZ)-induced type 1 diabetic (T1D) mice might be the cause of BM dysfunction through the creation of an hostile environment where stem cells, namely Lin−/Sca-1+/c-Kit+ (LSK) cells, undergo oxidative stress-induced damage, accelerated senescence, and death by apoptosis (Fig. 3) (79). The LSK cell population showed a greater ROS content assessed by CM-H2DCFDA. Likewise, BM MNCs isolated from trabecular bone showed an increased oxidative stress at mitochondrial level. Oxidative stress causes DNA damage, thereby reducing the lifespan of BM stem cells. In line, the levels of phosphorylated histone H2AX, a marker of double DNA strand breaks, were 2.5-fold higher in BM cells from T1D mice compared with nondiabetic age-matched controls. Since H2AX is phosphorylated by ATM, we analyzed the expression in T1D BM cells and found that this gene is 2.6-fold upregulated compared with BM cells of nondiabetic mice. Furthermore, flow cytometry analysis of Annexin V-positive cells unraveled the increased apoptosis of LSK cells from BM of T1D mice.
FIG. 3.
Proposed model of microenvironmental alteration in diabetic BM. Representation of endosteal and vascular niches in healthy and diabetic conditions. Different colors identify HSCs (blue), HPCs (green), MSCs (beige), and ECs (brown). Erythrocytes inside the marrow vessels are represented in red. Excessive production of ROS in the marrow microenvironment tends to cause a disruption of stem and progenitor cell homeostasis. The ROS gradient is lost, thus triggering stem cell proliferation and differentiation in areas of the marrow that are devoted to maintaining stem cell quiescence. Furthermore, failure of sinusoidal barrier function causes unselective mobilization, skewed toward inflammatory cells instead of regenerative cells. In addition, microvascular rarefaction causes hypoperfusion and deprives stem cells of necessary trophic inputs, leading to stem cells apoptosis. Altogether, these changes jeopardize the regenerative capacity of BM with consequences for peripheral complications. HPCs, hematopoietic progenitor cells; HSCs, hematopoietic stem cells; MSCs, mesenchymal stem cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
What is the link between microangiopathy and BM cell damage and does BM remodeling contribute to peripheral complications? We found that vascular density is drastically decreased in the BM of diabetic mice at the capillary, sinusoid, and arteriole level. Microvascular rarefaction was associated to a profound reduction of the marrow perfusion as assessed by fluorescent microspheres. We next determined the relative position of LSK cells with regard to in vivo Hoe dye perfusion gradient and the distribution of MECA32+ ECs. Hoe was injected intravenously and then, the degree of uptake of the dye by BM cells from different locations was evaluated by flow cytometry. We found that the Hoelow perfusion region contained 53% of total LSK cells in BM of nondiabetic mice, but this fraction was reduced to 21% in T1D BM. Reversing the gating procedure, we analyzed the abundance of LSK cells in total cells and lympho-monocyte fraction of each Hoe perfusion area. Results showed that LSK cells were depleted in the low-perfused zone of T1D BM, but preserved in the high-perfused zone, which corresponds to the predominant localization of MECA32+ BM ECs (e.g., the vascular niche). Altogether, these data suggest that the hypo-perfusion caused by contraction of the vascular niche is a major determinant of stem cell reduction in BM of diabetic mice. However, in a similar study conducted by Orlandi et al. in C57BL/6 STZ-induced diabetic mice, no microvascular rarefaction was observed, probably due to the different mouse strain analyzed and shorter duration of DM (80).
Microvascular rarefaction, reduction of the hematopoietic fraction, and adiposity were also observed by us in BM of leptin receptor transgenic mice, which develop obesity and type 2 diabetes (T2D) as they reach adulthood. The adipose tissue deposition in BM of diabetic mice merits attention. Interestingly, oxidative stress is a major factor impairing MSC function, resulting in decreased osteogenesis in favor of adipogenesis (7). MSCs differentiate into osteoblasts or adipocytes according to multiple signaling pathways, including those influenced by heme oxygenase-1 and -2 (111). Adipose tissue not only replenishes the space left by hematopoietic tissue shrinkage but may also act as a negative regulator of the BM microenvironment through secretion of pro-inflammatory cytokines (77).
Oxidative Stress Causes Endothelial Barrier Dysfunction in Diabetic BM
Besides providing perfusion and oxygenation, the vascular niche exerts trophic support to cells of the niche and acts as a gatekeeper for passage of cells and endocrine factors from and to the peripheral circulation.
Therefore, we have investigated the status of BM endothelium by in vivo assessment of vascular permeability, in vitro fluorescent-labeled dextrans, and we found an increased vascular permeability in BM of diabetic mice. Furthermore, BM ECs isolated from T1D mice showed a variety of functional alterations, including diminished production of factors that exert trophic actions on hematopoietic cells, impairment of migration and network formation capacity, and altered barrier function. The latter manifested as increased permeability to macromolecules and spontaneous trans-endothelial migration of BM MNCs, but reduced directed trans-endothelial migration of the same cells toward chemoattractants. The analysis of differentially expressed transcripts in BM ECs from T1D and nondiabetic mice showed an effect of DM on signaling pathways controlling cell death, migration, and cytoskeletal rearrangement. We also found a remarkable increase in mitochondrial ROS levels in diabetic endothelium. Increased oxidative stress was associated to upregulation of PARP and downregulation of Nfr2. It is known that oxidative stress induces DNA strain breaks, which, in turn, activate PARP; whereas Nfr2 exerts antioxidant activity to protect vascular cells from glucose-induced damage. In contrast, the expression of NADPH oxidase isoform 2, another important source of ROS, was similar in BM ECs from diabetic and control mice. Altogether, these data suggest that oxidative stress in BM endothelium is attributable to increased ROS production in mitochondria and reduced antioxidant defense.
Redox-dependent activation of small guanosinetriphosphatases (GTPases), including RhoA and its associated protein kinases ROCK1/ROCK2, is implicated in DM-induced endothelial dysfunction (90, 110). PKC is reportedly implicated in RhoA activation by ROS (13, 22, 112). Using a RhoA–GTP-bound pulldown assay, we documented that DM increases Rho activity in BM ECs. Importantly, ROS scavengers inhibit RhoA activation in this cellular setting. Moreover, both RhoA knocking down with a dominant negative form and ROCK inhibition rescued endothelial dysfunction, restoring Akt-dependent production of angiocrine factors, vascular permeability, and trans-endothelial migration of BM MNCs.
Small GTPases are not the only determinants of altered vascular permeability in BM of diabetic mice. We found that diabetic BM ECs have a higher phosphorylation levels of VE-cadherin at tyrosine 731, which is the binding site for β-catenin, and proline rich kinase 2 (Pyk2) at tyrosine 402 (i.e., its auto-phosphorylation site). Phosphorylation of VE-cadherin and Pyk2 favors the disassembly of adherens junctions and BM MNC extravasation (69). Along with Pyk2, DM increases the activity of other kinases, including p38, JNK, MEK1, and ERK1/2, which regulate EC viability, proliferation, and barrier function (120).
In summary, BM-specific endothelial dysfunction may play a relevant role in diabetic complications. In fact, microvascular rarefaction damages BM stem cells through reduction of perfusion and suspension of paracrine trophic signaling (58). Increased production of ROS in ECs may transfer oxidative stress to other cellular components of the niche, thereby propagating cellular damage to HSCs (69, 79). On the other hand, interaction of HSCs with stromal cells facilitates the ROS flux between neighboring cells, thus reducing the negative effect of excessive ROS production through redistribution. For instance, HSCs can prevent senescence by transferring oxidative stress to stromal cells through gap junction's component connexins-43 (47, 98).
ROS is a key determinant in causing the failure of the BM endothelium to modulate the regular passage of macromolecules and cells (11, 32). The loss of barrier function leads to plasma extravasation and increased interstitial pressure, which are particularly harmful for tissues such as the marrow contained in nonexpandable bone (Fig. 3). Altered vascular barrier function has negative consequences for regulated stem cell liberation as confirmed by studies of diabetic mobilopathy (vide infra).
Studies of Human BM Indicate Remodeling of the Vascular Niche
Although the pauperizing effect of DM on circulating angiogenic cells is well recognized, investigation on BM of diabetic patients is limited to aspirate samples. By a flow cytometry approach, Fadini et al. showed a decrease in the abundance of CD34+ cells in a group of 10 diabetic patients and 10 nondiabetic controls (24). A retrospective evaluation of BM biopsies from patients with suspected hematologic disorders confirms the depletion and impaired mobilization capacity of BM in DM. Furthermore, diabetic patients undergoing BM transplantation showed slow or unsuccessful engraftment compared with nondiabetic patients (31). These data suggest that DM reduces the regenerative capacity of BM stem cells but do not clarify whether the delayed engraftment was also due to damage of the niche.
We recently performed a case-control study in large cohorts of T2D diabetic subjects and nondiabetic controls without suspect of hematologic disorders, who were recruited on the occasion of orthopedic surgery. In parallel, we investigated the BM of T2D patients undergoing amputation for critical limb ischemia (CLI) (91). Employing both flow cytometry of isolated BM cells and in situ immunofluorescence staining, we showed the depletion of hematopoietic and pro-angiogenic progenitors, namely CD34+, CD34/CD133+, CD34/KDR+, and CD34/CD14/KDR/CXCR4+ cells, but not of mature hematopoietic cells. Moreover, in situ detection of DNA fragmentation by terminal deoxy nucleotidyl transferased UTP nick end labeling assay indicates activation of apoptosis in BM CD34+ cells of diabetic patients compared with controls. The marrow was replaced by accumulating fat, especially in patients with CLI. Notably, we also demonstrated the presence of microangiopathy in BM of diabetic patients, thus confirming our earlier findings in T1D mice. We found that both microvascular rarefaction and depletion of CD45dimCD34+cells in BM could be predicted by duration of DM and fasting glucose in a multiple regression analysis. Therefore, we speculate that the failing microvasculature, which feeds the marrow niche, could reduce the production of progenitor cells, including pro-angiogenic cells. Nonetheless, prospective studies are required to verify the cause-effect relationship between these correlates.
DM Impacts Stem Cell Viability by Inhibiting Hematopoietic microRNA
High glucose could damage BM stem cells directly by upregulating a pro-apoptosis pathway. In fact, investigation of underpinning mechanisms revealed that DM impacts microRNAs (miRs), which are small RNA molecules controlling gene expression and hence biological functions. In particular, miR-155 is drastically reduced in CD34+ progenitor cells from diabetic patients. The miR-155 is implicated in maintenance of BM cell stemness, by holding HSCs at an early stem-progenitor stage through inhibition of differentiation-associated molecules, such as CCAAT/enhancer-binding protein-β, cAMP response element-binding protein, JUN, and FOS (37, 71).
In addition, we found that miR-155 downregulation is associated to induction and nuclear localization of FOXO3a in BM stem cells of diabetic patients and consensual upregulation of p27kip1. Likewise, in vitro exposure of healthy CD34+ cells to high glucose reproduced the transcriptional changes induced by DM, with this effect being reversed by forced expression of miR-155. How to reconcile these data with results from genetically modified mice showing that the abrogation of FOXO3a results in increased susceptibility to stress-induced damage of BM stem cells? One possible explanation is that in hematopoietic cells with incurred DNA damage, FOXO3a induces cell cycle arrest and apoptosis, via transcriptional regulation of the cyclin-dependent kinase inhibitor p27Kip1 and pro-apoptotic Bcl-2 family member Bim (59, 62, 94, 119). Thus, by inhibiting miR-155, DM might exert a negative impact on HSC survival. It should be noted that our study provides an instantaneous picture of the histological and molecular alterations incurred by the BM of patients with long-term DM, but does not allow us to draw a conclusion on the time course of these features.
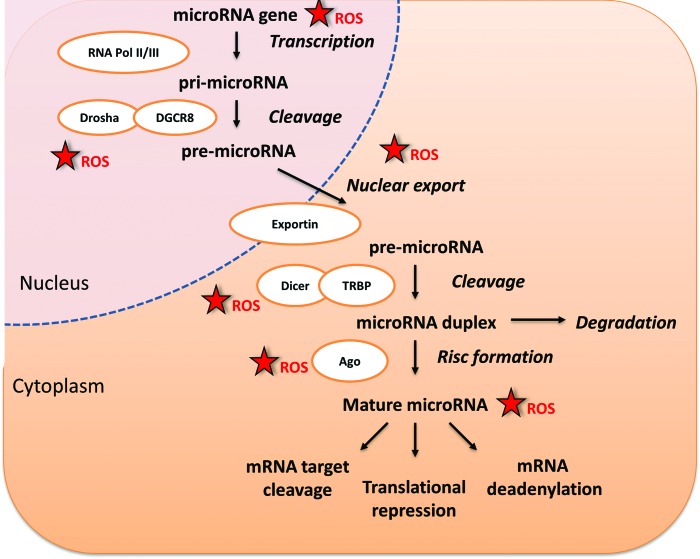
These data are intriguing, as increasing evidence points to miRs as master regulators of hematopoietic cell functions (37). In this regard, high levels of miR-155 have been recently found in circulating CD34+ cells after stimulation of mobilization by systemic chemokine administration in healthy nonhuman primates (20). Furthermore, miR-155 confers lymphoma cells and splenocytes with increased migratory activity in response to stromal cell-derived factor-1 (SDF-1) (15). Therefore, miR-155 downregulation might contribute to the defective mobilization of stem cells in patients with DM. Additional investigation is needed to elucidate whether miR-155 is under control of ROS and whether ROS can affect stem cells and cells of the niche through modulation of other redox-sensitive miRs. A recent study showed that short-term stimulation of cardiac stem cells with hydrogen-peroxide induces miR-155, which, in turn, attenuates necrotic cell death by targeting receptor interacting protein 1 (RIP1) (67). However, it is not clear how the acute ROS elevation activates miR-155 and whether this represents a reactive defense response that can be overwhelmed by persistent oxidative stress. Another investigation showed that ROS inhibits miR-199a and miR-125b expression through increasing the promoter methylation of the miR-199a and miR-125b genes by DNA methyltransferase 1 (42). The generation of miRs is dependent on the RNase III enzyme Dicer that cleaves the pre-miR transported from the nucleus to a ∼22 nt duplex miR. Interestingly, Dicer is inhibited by multiple stresses, including ROS, phorbol esters, and the Ras oncogene (114). In addition, ROS can induce direct oxidative inactivation of mRNAs and miRs. Thus, ROS could influence miR activity via epigenetic changes influencing their transcription, maturation, and processing (Fig. 4).
FIG. 4.
Influence of ROS on microRNA generation. Recent evidence indicates that miRs control several functions of stem cells. ROS can influence the regulation of gene expression through interference of miRs. This influence is exerted at different levels of miR generation and processing. miR, microRNA. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
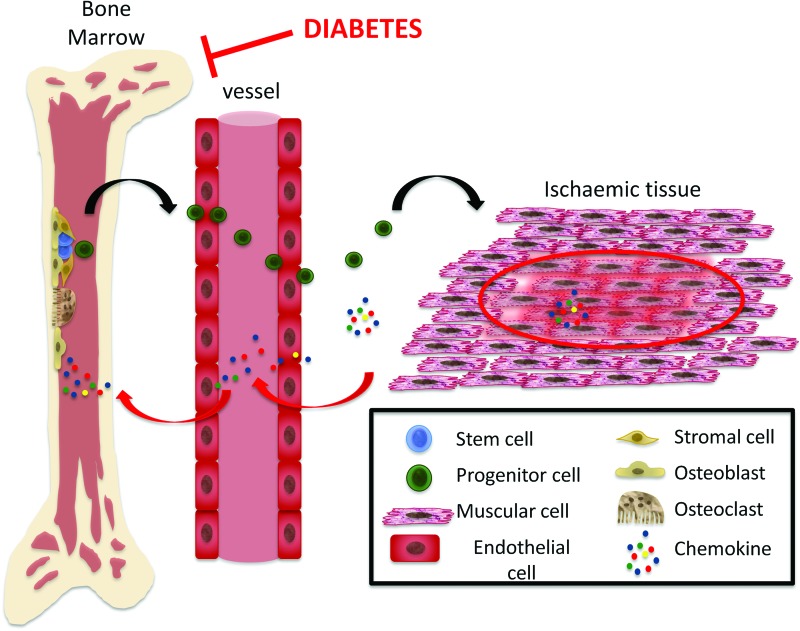
Diabetic Mobilopathy
HSCs detach from BM niches and mobilize into circulation. Recent studies demonstrate the defective mobilization of LSK cells in rodent models of DM (16, 29). Similarly, diabetic patients show impaired mobilization of stem cells after stimulation with granulocyte colony-stimulating factor. The new term “mobilipathy” was coined to illustrate this DM-induced stem cell deficit (Fig. 5) (19, 31). Stem cell mobilization is mediated by several cytokines, growth factors, and hormones and is dependent on the intrinsic motility of stem cells and permeability of the endothelial barrier, both of which are potentially influenced by ROS levels. Stem cells isolated from murine diabetic BM show eNOS uncoupled and inactivation (101). This nitric oxide generating enzyme is crucial for proper mobilization. Hence, its inactivation might account for diabetic mobilopathy. Interestingly, one of the major causes of eNOS uncoupling is represented by sustained oxidative stress generated by NAPDH oxidase (63). Conversely, several growth factors trigger progenitor cell mobilization via NOX2 (88). In particular, the SDF-1/CXCR4 duo is the most important axis in regulating HSC mobilization according to circadian fluctuations (87). Those fluctuations are in anti-phase with SDF-1 levels in the BM and are strongly affected by light exposure or “jet lag.” Autonomic noradrenergic fibers regulate stem cell release and expression of SDF-1 (87). In fact, the BM niche is innervated by an abundant plexus of noradrenergic and peptidergic fibers, which reach the different niche elements (76). Furthermore, nociceptive signaling from ischemic organs can activate the release of pro-angiogenic cells from the BM (5). Osteoclasts have been demonstrated to be necessary for HSC mobilization. It is known that ROS is a potent inducer for differentiation of monocytes into osteoclasts (118).
FIG. 5.
Diabetes-induced mobilopathy. Stem and progenitor cell mobilization from BM is a multistep process triggered by a gradient of chemokines generated from ischemic tissues. Chemokines also promote proteolytic activity in the marrow environment, thus favoring stem and progenitor cell relocation from the endosteal niche to the vascular niche and then the passage to the bloodstream. Oxidative stress generated in stem cells by activation of NOX2 acts as a mediator of those chemokines. However, sustained and excessive oxidative stress can lead to eNOS uncoupling, thereby compromising stem cell mobilization. eNOS, endothelial nitric oxide synthase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Altogether, these data indicate that ROS is necessary for proper mobilization. Disruption of the redox balance might result in disordered mobilization in response to peripheral stimuli.
Therapeutic Perspectives
The quest for treatment of BM damage induced by DM is challenging. A tight control of glycemia is certainly useful as documented by us in a T1D diabetic model using insulin implants, which resulted in prevention of BM microangiopathy and remodeling (69). Furthermore, since ROS is implicated in these processes, the use of ROS scavengers seems to be also obvious (46, 65, 123). However, a recent meta-analysis of 68 randomized trials showed no evidence of benefit on mortality, but, rather surprisingly, an increased risk of death by several common anti-oxidant dietary supplements (10). Alternatively, increasing the activity of anti-oxidant enzymes, such as the use of superoxide dismutase (SOD) mimetics, is being considered (78). Of course, the treatment should be carefully designed, in order to match the correct range of ROS levels necessary for physiological stem cell functions (40, 102, 108). Conversely, short-term ROS supplementation could be useful in acute situations to boost reparative angiogenesis in ischemia. Ex vivo preconditioning of stem cells with ROS could also reinvigorate their regenerative potential before transplantation into ischemic tissues (18, 60).
Systemic administration of agents that are able to contain chronic oxidative stress by interfering with key steps of the metabolic disorder could be considered. Benfotiamine, a vitamin B1 derivative, blunts the consequences of oxidative stress by diverting the excess of glucose toward the pentose pathway. Interestingly enough, benfotiamine proved to prevent stem cell depletion in BM (79) and hearts of diabetic mice (54), while concurrently exerting therapeutic effects of peripheral microvascular complications (34). Similarly, statins are attractive for their pleiotropic action, which include the preservation of pro-angiogenic cells in DM (6). Statins treatment improves stem cell mobilization and endothelial commitment (6). In addition, statin could have a direct effect on BM endothelium and niche-resident stem cells through its inhibitory effect on the Rho-ROCK pathway (69).
Finally, physical exercise training has been reported to improve clinical outcome, and, thus, it has been implemented into care guidelines to cardiovascular patients. The relevance of regular physical exercise for maintenance of BM fitness is seemingly due to an increased expression and activity of eNOS, as well as to a lower expression of NADPH oxidase in angiogenic cells (116).
Conclusions
The role of ROS in regulation of stem cell biology is firmly established. We foresee that a deeper understanding of redox signaling in those precious cells will translate soon into new modalities for preserving local and systemic homeostasis as well as into safer and more effective treatments of diabetic cardiovascular complications.
Abbreviations Used
- AGE
advanced glycation end-product
- Ang-1
angiopoietin 1
- ATM
ataxia telangiectasia mutated
- BM
bone marrow
- CLI
critical limb ischemia
- CVD
cardiovascular disease
- CXCR4
C-X-C chemokine receptor type 4
- DM
diabetes mellitus
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- FOXO3A
forkhead box, subclass O3a
- GTPases
guanosinetriphosphatases
- HSC
hematopoietic stem cell
- LSK
Lin−/Sca-1+/c-Kit+
- MAPK
mitogen-activated protein kinase
- Mdm2
mouse double minute 2 homolog
- miRs
microRNAs
- MMP-9
metalloproteinase 9
- MNC
mononuclear cells
- MSC
mesenchymal stem cell
- mTOR
mammalian target of rapamycin
- N-Cad
N-cadherin
- Nrf2
nuclear factor erythroid-2-related factor 2
- PARP
poly(ADP-ribose) polymerase
- PI3K
phosphatidylinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- Pyk2
proline rich kinase 2
- RIP1
receptor interacting protein 1
- ROS
reactive oxygen species
- SCF
stem cell factor
- SDF-1
stromal cell-derived factor-1
- SOD
superoxide dismutase
- STZ
streptozotocin
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- Tie2
angiopoietin receptor 2
- TSC
tuberous sclerosis complex
- VCAM-1
integrin receptor vascular cell adhesion molecule-1
- Vla-4
very late antigen-4/integrin alpha4beta1
- VE-cadherin
vascular endothelial-cadherin
Acknowledgments
This article is supported by a grant from the National Institute Health Research (NIHR) Biomedical Research Unit (BRU) and a grant from the British Heart Foundation “Bone marrow dysfunction alters vascular homeostasis in diabetes.”
References
- 1.Abbas HA, Maccio DR, Coskun S, Jackson JG, Hazen AL, Sills TM, You MJ, Hirschi KK, and Lozano G. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell 7: 606–617, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham RT. and Tibbetts RS. Cell biology. Guiding ATM to broken DNA. Science 308: 510–511, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, and Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, Tyynismaa H, Larsson NG, Wartiovaara K, Prolla T, Trifunovic A, and Suomalainen A. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab 15: 100–109, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Amadesi S, Reni C, Katare R, Meloni M, Oikawa A, Beltrami AP, Avolio E, Cesselli D, Fortunato O, Spinetti G, Ascione R, Cangiano E, Valgimigli M, Hunt SP, Emanueli C, and Madeddu P. Role for substance p-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation 125: 1774–1786, S1–S19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, Spyridopoulos I, Zeiher AM, and Dimmeler S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res 92: 1049–1055, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Barbagallo I, Vanella A, Peterson SJ, Kim DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo GA, Di Raimondo F, Abraham NG, and Asprinio D. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab 28: 276–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, and Rossi DJ. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 12: 413–425, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, and Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297: 842–857, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Boueiz A. and Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res 77: 26–34, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Boyle JP, Thompson TJ, Gregg EW, Barker LE, and Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8: 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, and Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol 165: 506–519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, and Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 205: 2397–2408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagan LN, Jiang X, Bhatt S, Cubedo E, Rajewsky K, and Lossos IS. miR-155 regulates HGAL expression and increases lymphoma cell motility. Blood 119: 513–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Falco E, Avitabile D, Totta P, Straino S, Spallotta F, Cencioni C, Torella AR, Rizzi R, Porcelli D, Zacheo A, Di Vito L, Pompilio G, Napolitano M, Melillo G, Capogrossi MC, and Pesce M. Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus. J Cell Mol Med 13: 3405–3414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, and Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104: 3591–3597, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Diebold I, Djordjevic T, Petry A, Hatzelmann A, Tenor H, Hess J, and Gorlach A. Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ Res 104: 1169–1177, 2009 [DOI] [PubMed] [Google Scholar]
- 19.DiPersio JF. Diabetic stem-cell “mobilopathy”. N Engl J Med 365: 2536–2538, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, and Stroncek DF. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood 114: 2530–2541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, and Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112: 1049–1057, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, and Caldwell RW. Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp Diabetes Res 2010: 247861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, and Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med 140: 945–950, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Fadini GP, Boscaro E, de Kreutzenberg S, Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A, and Avogaro A. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 33: 1097–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadini GP, de Kreutzenberg SV, Boscaro E, Albiero M, Cappellari R, Krankel N, Landmesser U, Toniolo A, Bolego C, Cignarella A, Seeger F, Dimmeler S, Zeiher A, Agostini C, and Avogaro A. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia 56: 1856–1866, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Fadini GP, Losordo D, and Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, and Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 45: 1449–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, and Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol 26: 2140–2146, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, and Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 49: 3075–3084, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, Mangoni M, Rizzoli V, Sykes SM, Lin CP, Frenette PS, Quaini F, and Scadden DT. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 3: 104ra101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med 51: 967–977, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, Sato T, Cote D, Sykes M, Strom TB, Scadden DT, and Lin CP. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474: 216–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadau S, Emanueli C, Van Linthout S, Graiani G, Todaro M, Meloni M, Campesi I, Invernici G, Spillmann F, Ward K, and Madeddu P. Benfotiamine accelerates the healing of ischaemic diabetic limbs in mice through protein kinase B/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis. Diabetologia 49: 405–420, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Gao L. and Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82: 9–20, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Garrison BS. and Rossi DJ. Reactive oxygen species resulting from mitochondrial mutation diminishes stem and progenitor cell function. Cell Metab 15: 2–3, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Georgantas RW, 3rd, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM, and Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A 104: 2750–2755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y, Lapid K, Shivtiel S, Morris AJ, Ratajczak MZ, and Lapidot T. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood 119: 2478–2488, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, and Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87: 26–32, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Menard C, Perez G, Maingrette F, and Rivard A. Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol 29: 1522–1528, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Hazra S, Jarajapu YP, Stepps V, Caballero S, Thinschmidt JS, Sautina L, Bengtsson N, Licalzi S, Dominguez J, Kern TS, Segal MS, Ash JD, Saban DR, Bartelmez SH, and Grant MB. Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia 56: 644–653, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He J, Xu Q, Jing Y, Agani F, Qian X, Carpenter R, Li Q, Wang XR, Peiper SS, Lu Z, Liu LZ, and Jiang BH. Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep 13: 1116–1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hidalgo I. and Gonzalez S. New epigenetic pathway for stemness maintenance mediated by the histone methyltransferase Ezh1. Cell Cycle 12: 383–384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, and Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Hochmuth CE, Biteau B, Bohmann D, and Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8: 188–199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iino K, Iwase M, Sonoki K, Yoshinari M, and Iida M. Combination treatment of vitamin C and desferrioxamine suppresses glomerular superoxide and prostaglandin E production in diabetic rats. Diabetes Obes Metab 7: 106–109, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa ET. and Cancelas JA. Lack of communication rusts and ages stem cells. Cell Cycle 11: 3149–3150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, and Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431: 997–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, and Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446–451, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Jang YY. and Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, Pitson SM, Rettig M, DiPersio JF, Bradstock KF, and Bendall LJ. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood 119: 707–716, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, and Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115: 4030–4038, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, and Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katare R, Oikawa A, Cesselli D, Beltrami AP, Avolio E, Muthukrishnan D, Munasinghe PE, Angelini G, Emanueli C, and Madeddu P. Boosting the pentose phosphate pathway restores cardiac progenitor cell availability in diabetes. Cardiovasc Res 97: 55–65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, and Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 115: 1406–1415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiel MJ, Acar M, Radice GL, and Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4: 170–179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiel MJ, Radice GL, and Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1: 204–217, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi H, Butler JM, O'Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, and Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 12: 1046–1056, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, and Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Kubo M, Li TS, Suzuki R, Ohshima M, Qin SL, and Hamano K. Short-term pretreatment with low-dose hydrogen peroxide enhances the efficacy of bone marrow cells for therapeutic angiogenesis. Am J Physiol Heart Circ Physiol 292: H2582–H2588, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Lassegue B. and Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Lei H. and Quelle FW. FOXO transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol Cancer Res 7: 1294–1303, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Li H. and Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol 13: 161–167, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Li TS. and Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 28: 1178–1185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin J, Bierhaus A, Bugert P, Dietrich N, Feng Y, Vom Hagen F, Nawroth P, Brownlee M, and Hammes HP. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia 49: 1089–1096, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira II, Xu XL, van Lohuizen M, Motoyama N, Deng CX, and Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459: 387–392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, van Mil A, Vrijsen K, Zhao J, Gao L, Metz CH, Goumans MJ, Doevendans PA, and Sluijter JP. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J Cell Mol Med 15: 1474–1482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, and van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Mangialardi G, Katare R, Oikawa A, Meloni M, Reni C, Emanueli C, and Madeddu P. Diabetes causes bone marrow endothelial barrier dysfunction by activation of the rhoa-rho-associated kinase signaling pathway. Arterioscler Thromb Vasc Biol 33: 555–564, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mangialardi G, Monopoli A, Ongini E, Spinetti G, Fortunato O, Emanueli C, and Madeddu P. Nitric oxide-donating statin improves multiple functions of circulating angiogenic cells. Br J Pharmacol 164: 570–583, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masaki S, Ohtsuka R, Abe Y, Muta K, and Umemura T. Expression patterns of microRNAs 155 and 451 during normal human erythropoiesis. Biochem Biophys Res Commun 364: 509–514, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, and Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, and Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1: 101–112, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Muller-Sieburg CE, Sieburg HB, Bernitz JM, and Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood 119: 3900–3907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naka K, Muraguchi T, Hoshii T, and Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 10: 1883–1894, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Nance DM. and Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21: 736–745, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, and Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460: 259–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohshima M, Li TS, Kubo M, Qin SL, and Hamano K. Antioxidant therapy attenuates diabetes-related impairment of bone marrow stem cells. Circ J 73: 162–166, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, and Madeddu P. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol 30: 498–508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, and Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol 105: 703–712, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Parmar K, Mauch P, Vergilio JA, Sackstein R, and Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A 104: 5431–5436, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piccoli C, Ria R, Scrima R, Cela O, D'Aprile A, Boffoli D, Falzetti F, Tabilio A, and Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem 280: 26467–26476, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Raaijmakers MH. and Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol 15: 301–306, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Saito H, Yamamoto Y, and Yamamoto H. Diabetes alters subsets of endothelial progenitor cells that reside in blood, bone marrow, and spleen. Am J Physiol Cell Physiol 302: C892–C901, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, and Griffin JD. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood 93: 2928–2935, 1999 [PubMed] [Google Scholar]
- 86.Scadden DT. The stem-cell niche as an entity of action. Nature 441: 1075–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Scadden DT. Circadian rhythms: stem cells traffic in time. Nature 452: 416–417, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, and Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res 105: 537–544, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Schroder K, Schutz S, Schloffel I, Batz S, Takac I, Weissmann N, Michaelis UR, Koyanagi M, and Brandes RP. Hepatocyte growth factor induces a proangiogenic phenotype and mobilizes endothelial progenitor cells by activating Nox2. Antioxid Redox Signal 15: 915–923, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Spindler V, Schlegel N, and Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 87: 243–253, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Spinetti G, Cordella D, Fortunato O, Sangalli E, Losa S, Gotti A, Carnelli F, Rosa F, Riboldi S, Sessa F, Avolio E, Beltrami AP, Emanueli C, and Madeddu P. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the microRNA-155/FOXO3a signaling pathway. Circ Res 112: 510–522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spinetti G, Fortunato O, Caporali A, Shantikumar S, Marchetti M, Meloni M, Descamps B, Floris I, Sangalli E, Vono R, Faglia E, Specchia C, Pintus G, Madeddu P, and Emanueli C. MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circ Res 112: 335–346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spradling A, Drummond-Barbosa D, and Kai T. Stem cells find their niche. Nature 414: 98–104, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, and Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol 168: 5024–5031, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal 14: 593–605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takubo K, Ohmura M, Azuma M, Nagamatsu G, Yamada W, Arai F, Hirao A, and Suda T. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell 2: 170–182, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Takubo K. and Suda T. Roles of the hypoxia response system in hematopoietic and leukemic stem cells. Int J Hematol 95: 478–483, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, and Cancelas JA. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc Natl Acad Sci U S A 109: 9071–9076, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, Shivtiel S, Kalinkovich A, Caione L, Gammaitoni L, Laurenti E, Buss EC, Shezen E, Itkin T, Kollet O, Petit I, Trumpp A, Christensen J, Aglietta M, Piacibello W, and Lapidot T. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 117: 419–428, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, and Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 57: 460–469, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, and Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 56: 666–674, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, and Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Tothova Z. and Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell 1: 140–152, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, and Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, and Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem 265: 11409–11412, 1990 [PubMed] [Google Scholar]
- 107.Tsai JJ, Dudakov JA, Takahashi K, Shieh JH, Velardi E, Holland AM, Singer NV, West ML, Smith OM, Young LF, Shono Y, Ghosh A, Hanash AM, Tran HT, Moore MA, and van den Brink MR. Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol 15: 309–316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turgeon J, Haddad P, Dussault S, Groleau J, Maingrette F, Perez G, and Rivard A. Protection against vascular aging in Nox2-deficient mice: impact on endothelial progenitor cells and reparative neovascularization. Atherosclerosis 223: 122–129, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, and Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Heijden M, Versteilen AM, Sipkema P, van Nieuw Amerongen GP, Musters RJ, and Groeneveld AB. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis 13: 404–412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vanella L, Sanford C, Jr., Kim DH, Abraham NG, and Ebraheim N. Oxidative stress and heme oxygenase-1 regulated human mesenchymal stem cells differentiation. Int J Hypertens 2012: 890671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Li Q, Du J, Chen B, Huang X, Guo X, and Huang Q. Advanced glycation end products induce moesin phosphorylation in murine retinal endothelium. Acta Diabetol 49: 47–55, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Westerweel PE, Teraa M, Rafii S, Jaspers JE, White IA, Hooper AT, Doevendans PA, and Verhaar MC. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One 8: e60357, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiesen JL. and Tomasi TB. Dicer is regulated by cellular stresses and interferons. Mol Immunol 46: 1222–1228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, and Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116: 4815–4828, 2010 [DOI] [PubMed] [Google Scholar]
- 116.Witkowski S. and Hagberg JM. Progenitor cells and age: can we fight aging with exercise? J Appl Physiol 102: 834–835, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, and Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia 26: 23–33, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Xu Y, Morse LR, da Silva RA, Odgren PR, Sasaki H, Stashenko P, and Battaglino RA. PAMM: a redox regulatory protein that modulates osteoclast differentiation. Antioxid Redox Signal 13: 27–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamamoto M, Kondo E, Takeuchi M, Harashima A, Otani T, Tsuji-Takayama K, Yamasaki F, Kumon H, Kibata M, and Nakamura S. miR-155, a modulator of FOXO3a protein expression, is underexpressed and cannot be upregulated by stimulation of HOZOT, a line of multifunctional Treg. PLoS One 6: e16841, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, Zhu W, Wu D, and Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol 28: 835–840, 2008 [DOI] [PubMed] [Google Scholar]
- 121.Yao D. and Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59: 249–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, and Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441: 475–482, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Zherebitskaya E, Akude E, Smith DR, and Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes 58: 1356–1364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]