Abstract
Stem cells are defined as cells that have the capacity to self-renew and exhibit multipotency or pluripotency, whereas progenitor cells are committed to selected lineages but retain their self-renewal capacity. The stem or progenitor cell niche refers to the microenvironment of the regenerative cells in the bone marrow (BM) or other tissues such as the heart. It can regulate self-renewal, differentiation, migration, and proliferation of regenerative stem/progenitor cells. The precise regulatory mechanisms by which the niche and the stem/progenitor cells interact are an active area of research. Reactive oxygen species (ROS) are one such niche regulatory mechanism. Quiescent stem cells in a hypoxic niche exhibit low ROS levels due to well-organized antioxidant defense systems, which protect stem cells from extrinsic oxidative stress, whereas high levels of ROS promote the differentiation or migration of stem/progenitor cells. In pathophysiological conditions such as diabetes, BM niche dysfunction induced by oxidative stress contributes to the reduction of the angiogenic and vasculogenic potential of BM-derived regenerative cells, thereby leading to less efficient healing and revascularization. Cells have evolved mechanisms to fine-tune ROS levels by tightly regulated metabolic pathways such as glycolysis rather than oxidative phosphorylation to reduce oxidative stress. This Forum will summarize the recent progress regarding the redox and metabolic regulation of hematopoietic and cardiac stem/progenitor cells, as well as their niche interactions involved in tissue regeneration and repair under physiological and pathological conditions. Understanding such mechanisms will contribute to the development of novel therapeutic strategies to enhance regeneration and repair of diseased tissues. Antioxid. Redox Signal. 21, 1587–1590.
Introduction
Stem cells are defined as cells that exhibit multipotency or pluripotency and have the capacity for self-renewal as well as clonal expandability. Hematopoietic stem cells (HSCs) and mesenchymal stem cells (also known as stromal cells) found in the bone marrow (BM) are among the most widely studied types of adult stem cells. The stem/progenitor cells in BM are distributed in distinct microenvironments (niches) consisting of stromal cells, endothelial cells, macrophages, regulatory T cells, and the extracellular matrix. Undifferentiated HSCs reside within the BM niche and are maintained in a quiescent state via interaction with stromal cells and macrophages, but can undergo self-renewing cell divisions and maintain blood production for their lifetime if they are stimulated by specific cues (8). There is increasing evidence that similar regulatory niche environments exist in many other adult tissues, including the heart, and these niches can also regulate the proliferation of differentiation of stem and progenitor cells, as well as migration and regeneration, similar to what has been previously shown for the BM niche (1).
Neovascularization is involved in wound healing as well as various pathophysiological states such as ischemic heart and peripheral artery diseases. This process depends on local tissue angiogenesis and may therefore involve resident regenerative stem or progenitor cells, stem/progenitor cells released from the BM, or circulating cells derived from these stem/progenitor cells, which home to foci of ischemia and promote blood vessel growth. BM-derived cells can also orchestrate tissue repair by paracrine mechanisms to augment blood vessel growth or repair tissues. Although BM stem/progenitor cells are being evaluated for regenerative therapies, the reduced angiogenic and regenerative function of stem/progenitor cells obtained from patients with chronic diseases such as diabetes can mitigate their regenerative function and represents an obstacle for implementing such experimental treatments. Identifying the regulatory factors, which regulate their regenerative function, would be of great value for therapeutic applications of stem/progenitor cells.
A vast array of studies have identified growth factors and chemokines that regulate stem/progenitor cell function and mobilization, but in recent years, a set of unlikely new players are emerging as an important regulator of regenerative cells. Reactive oxygen species (ROS) such as superoxide anion (O2•−) and hydrogen peroxide are not just by products of metabolic activity and other enzymatic reactions, but play a key role in determining the stem/progenitor cell function (7). ROS have a double-edged role in cellular processes. Excess ROS levels are detrimental and can induce various pathologies such as atherosclerosis, heart failure, diabetes, and cancer. In contrast, physiological levels of ROS function as signaling molecules, which mediate biological responses such as cell proliferation, migration, survival, differentiation, and gene expression.
This Forum will highlight how stem cell self-renewal, proliferation, and differentiation are regulated by physiological and pathological ROS levels and will also emphasize the emerging role of interactions with the respective stem cell niches. For example, a low level of ROS can maintain the quiescence of HSCs, whereas a higher level of ROS contributes to proliferation, senescence, or apoptosis, leading to a premature exhaustion of self-renewal in these cells (2, 7). Furthermore, physiological ROS levels are crucial for repair processes that involve differentiation and mobilization of stem/progenitor cells from the BM, as well as maintaining host immunity during steady-state and stress conditions. ROS derived from NADPH oxidase regulate oxygen consumption in the BM niche after ischemic injury, which promotes progenitor cell expansion and mobilization, leading to tissue regeneration and repair (5, 6). In pathophysiological states, excess ROS produced from inflammatory and oxidant stress microenvironments in the BM induce cell damage and apoptosis of stem/progenitor cells, as well as the BM niche itself, resulting in a less efficient wound healing in diabetes patients. Emerging evidence reveals that not only ROS but also cellular metabolism itself can regulate stemness versus undergoing lineage-specific differentiation, both via ROS as well as possibly by non-ROS mechanisms (3, 4, 9).
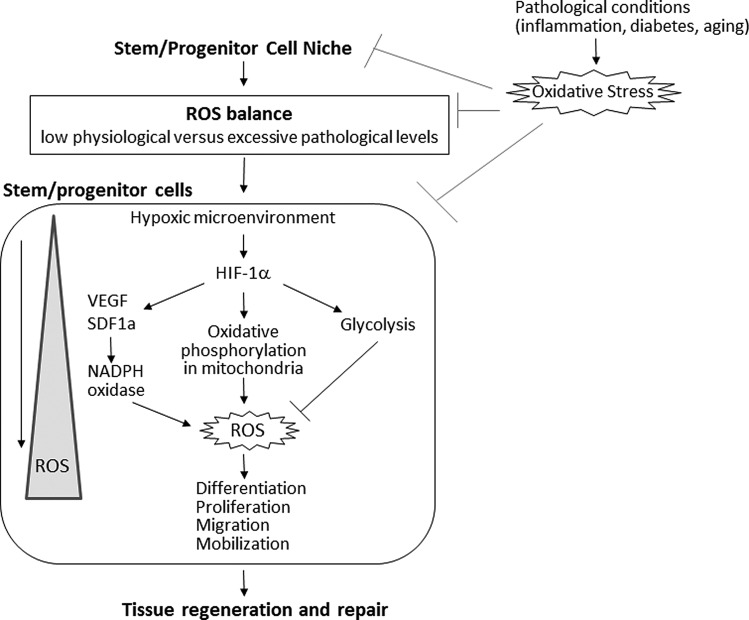
This Forum will summarize the recent progress regarding the redox and metabolic regulation of stem/progenitor cells and their niche in physiological and pathological conditions (Fig. 1). In the first review of this Forum, Tsvee Lapidot and coworkers discuss the role of ROS in the fate determination of HSCs and their interaction with the BM niche. Quiescent, long-term repopulating stem cells are characterized by low levels of ROS. The elevation of ROS levels in the stem cells enhances motility and short-term repopulation ability of the cells. Once ROS levels return back to baseline levels, stem cells can reduce their cycling rate and restore the long-term repopulation potential. On the other hand, if ROS are excessively elevated, they lead to irreversible damage such as senescence and apoptosis. In the BM microenvironment, ROS levels are regulated by mitochondria, NADPH oxidase, or the hypoxic HIF1α signaling pathway, as well as additional extrinsic factors. High ROS levels induced by stress or inflammation in HSCs and mesenchymal stromal cells promote stem cell differentiation and mobilization. It is essential to identify the threshold level above which ROS become detrimental or to understand how the effects of ROS are altered in normal and diseased conditions.
FIG. 1.
Regulation of stem/progenitor cells and their niche by reactive oxygen species (ROS) and metabolism involved in tissue regeneration and repair. The microenvironment (niche) in the bone marrow (BM) and other tissues, including the heart, regulates self-renewal, differentiation, migration, and proliferation of stem/progenitor cells. Quiescent stem cells in the hypoxic niche are characterized by low levels of ROS, while high levels of ROS promote the differentiation or migration of stem/progenitor cells. ROS levels are determined by the balance of ROS generating and antioxidant defense systems. Cells have evolved mechanisms to fine-tune ROS levels by tightly regulating metabolic pathways. In pathophysiological conditions such as diabetes mellitus, niche dysfunction induced by oxidative stress contributes to the reduction of the angiogenic and vasculogenic potential of stem/progenitor cells, thereby leading to a less efficient healing and revascularization.
In the second review, Paolo Madeddu and coworkers summarize the current knowledge of how diabetes induces BM microenvironmental defects such as microangiopathy through oxidative stress and disruption of the normal redox balance. This BM niche dysfunction induced by oxidative stress may suppress the angiogenic and vasculogenic potential of the regenerative cells, thereby leading to a less efficient healing and revascularization in diabetic patients. Understanding how disruption of the redox balance induces apoptosis and exhaustion of the BM regenerative capacity in diabetes opens up new avenues for therapeutic interventions. Thus, preservation of BM integrity and normal stem/progenitor cell function by maintaining ROS at optimal levels is a potentially important therapeutic strategy to restore wound healing and tissue regeneration in pathological conditions such as diabetes.
An important clinical application of ROS in regeneration is discussed by Katina M. Fosen and Stephen R. Thom in the third review: hyperbaric oxygen (HBO2) therapy. This novel therapeutic approach is already being implemented in clinical studies to improve wound healing, especially in patients with impaired wound healing due to chronic disease states such as diabetes. In a remarkable overview of a bench-to-bedside translation of antioxidant signaling, they describe how wound healing requires “waves” of regenerative signals—ROS, nitric oxide, and lactate—all of which are activated by HBO2. These signaling waves can simultaneously activate multiple regenerative pathways. Activation and mobilization of stem and progenitor cells appear to be key mediators of HBO2-induced wound healing. The lack of target specificity is sometimes seen as a major problem with therapies that modulate ROS signaling. However, the success of HBO2 makes a compelling case for also considering the benefits of concomitantly activating multiple distinct pathways, which can then synergize and accelerate regeneration.
The review by Andre Terzic and coworkers provides an important complementary perspective by evaluating recent findings in the emerging field of stem cell metabolism. Mitochondrial metabolism is a major source of ROS, but these are not just mere by-products of cellular metabolism. Instead, they play an active role in regulating cell fate because ROS can activate differentiation and force the cells to lose their stemness. It appears that undifferentiated pluripotent stem cells primarily rely on metabolic pathways, which minimize ROS generation, such as glycolysis and the pentose phosphate pathway. The specific mechanisms by which stem cells fine-tune their metabolism and adapt it during differentiation as well as the molecular targets of ROS that regulate stem cell fate have yet to be fully understood. Metabolic modulation of stem cells may be a critical and often overlooked approach for the successful differentiation of stem cells and engineering of tissues or organs.
In the final review, Hesham A. Sadek and coworkers introduce the redox and metabolic regulation of cardiac progenitor cells involved in cardiac regeneration. They propose that cycling cells in the heart may be cardiac progenitors, which utilize glycolysis rather than mitochondrial oxidative phosphorylation in a HIF1α-dependent manner. HIF2α upregulates several antioxidant enzymes to reduce cellular ROS levels. The homeodomain transcription factor Meis1 and its cofactor is essential for the activation of HIF1α and HIF2α. Thus, Meis1-HIFs-mediated metabolism and redox regulation are critical for the maintenance of stem cell quiescence. Modulating redox states or hypoxic signaling pathways in the heart could activate regenerative cells and, thus, accelerate cardiac repair or regeneration in cardiomyopathy or ischemic heart disease.
The original article by Paolo Madeddu and coworkers demonstrates that adventitia-derived progenitor cells from the vein remnants of coronary artery bypass graft surgery patients express the antioxidant enzyme extracellular superoxide dismutase, which is involved in resistance to oxidative stress-induced damage. This could potentially explain the therapeutic action of vascular wall-resident progenitor cells and be used to augment resilience of blood vessels to injury.
In summary, these five reviews and the original article demonstrate that ROS and metabolic pathway play important roles in regulating the function of various stem/progenitor cells (HSCs, cardiac- and adventitia-derived progenitor cells) and their niches, which are involved in tissue regeneration and repair (Fig. 1). ROS can both activate or suppress regeneration depending on their levels and sources, and it is therefore important to fine-tune the ROS levels in distinct compartments and at specific phases of the regenerative process to achieve maximal efficacy.
Abbreviations Used
- BM
bone marrow
- HBO2
hyperbaric oxygen
- HSC
hematopoietic stem cell
- ROS
reactive oxygen species
Acknowledgments
This article was supported by grants from the National Institutes of Health (NIH) R01 HL116976 and R21HL112293 to M.U.-F. and R01 GM094220 to J.R.
References
- 1.Morrison SJ. and Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pervaiz S, Taneja R, and Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal 11: 2777–2789, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med 88: 981–986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suda T, Takubo K, and Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9: 298–310, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Urao N, Inomata H, Rzvi M, Kim HW, Wary KK, Mckinney RD, Fukai T, and Ushio-Fukai M. Role of Nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urao N, McKinney RD, Fukai T, and Ushio-Fukai M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: role in reparative mobilization of progenitor cells. Stem Cells 30: 923–934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urao N. and Ushio-Fukai M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic Biol Med 54: 26–39, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson A. and Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6: 93–106, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Marboom G, Toth PT, and Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One 8: e77077, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]