Abstract
Despite the prevalence of the N-H aziridine motif in bioactive natural products and the clear advantages of this unprotected parent structure over N-protected derivatives as a synthetic building block, no practical methods have emerged for direct synthesis of this compound class from unfunctionalized olefins. Here, we present a mild, versatile method for the direct stereospecific conversion of structurally diverse mono-, di-, tri- and tetra-substituted olefins to N-H aziridines using O-(2,4-dinitrophenyl)hydroxylamine (DPH) via homogeneous rhodium catalysis with no external oxidants. This method is operationally simple (i.e., one-pot), scalable and fast at ambient temperature, furnishing N-H aziridines in good-to-excellent yields. Likewise, N-alkyl aziridines are prepared from N-alkylated DPH derivatives. Quantum-mechanical calculations suggest a plausible Rh-nitrene pathway.
Aziridines, the triangular, comparably highly-strained nitrogen analogues of epoxides, are important synthetic intermediates (i.e., building blocks) en route to structurally complex molecules due to their versatility in myriad regio- and stereoselective transformations (ring openings and expansions as well as rearrangements).(1–6) The aziridine structural motif, predominantly N-H and to a lesser extent N-alkyl, also appears in biologically active natural products (e.g., azinomycins and mitomycins).(7–9) As a result, the synthesis and chemistry of aziridines have been the subject of intense research during the past 25 years, resulting in multiple aziridination methods.(10–23) The majority of these methods rely either on the transfer of substituted nitrenes, which are generated using strong external oxidants, to the C=C bond of olefins or the transfer of substituted carbenes to the C=N bond of imines. Normally, the result is an aziridine bearing a strongly electron-withdrawing N-protecting group (e.g., Ts = para-toluenesulfonyl, Ns = para-nitrophenylsulfonyl); removal of these N-sulfonyl protecting groups is problematic as it often results in the undesired opening of the aziridine ring. In addition, the high reactivity of N-protected nitrenes might give rise to non-productive allylic C-H amination products as well as the loss of stereospecificity. Clearly, the direct synthesis of N-H (i.e., N-unprotected) and N-alkyl aziridines would alleviate the above problems. However, a practical, functional group-tolerant and environmentally benign direct preparation of N-H aziridines from structurally diverse olefins has so far eluded synthetic chemists.(24–31) Herein, we report an operationally simple, inherently safe, chemoselective and stereospecific conversion of a wide range of olefins to the corresponding N-H/N-Me aziridines via a rhodium-catalyzed pathway free of external oxidants.
Recently, we developed a metal-free protocol for primary amination of arylboronic acids using only O-(2,4-dinitrophenyl)hydroxylamine (DPH, 1a, Fig. 1) as the stoichiometric aminating agent.(32) The transformation proceeds under neutral or basic conditions and can be conducted on a multi-gram scale to provide structurally diverse primary arylamines. The versatility and robustness of 1a prompted us to explore other uses of this aminating agent, specifically for the direct functionalization of readily available and inexpensive olefins. Our investigations began by subjecting 1:1.5 mixtures of cis-methyl oleate (7)/1a as well as styrenes (3a & 3b)/1a to a vigorous screening with a variety of transition metal complexes (see Tables S1 & S2, Supplementary Materials). This initial screen identified Rh2(OAc)4 as a promising catalyst for vic-amino-oxyarylation of olefins. Further evaluation of dimeric rhodium dicarboxylate complexes (see Table S3, Supplementary Materials), revealed that just 1 mol% loading of Du Bois’ catalyst(33–36) (2, Fig. 1) in acetonitrile (MeCN) leads to amino-oxyarylated styrenes 4a and 4b at room temperature in 56% and 75% isolated yields, respectively. These promising results prompted us to conduct a thorough solvent screen.
Fig. 1.
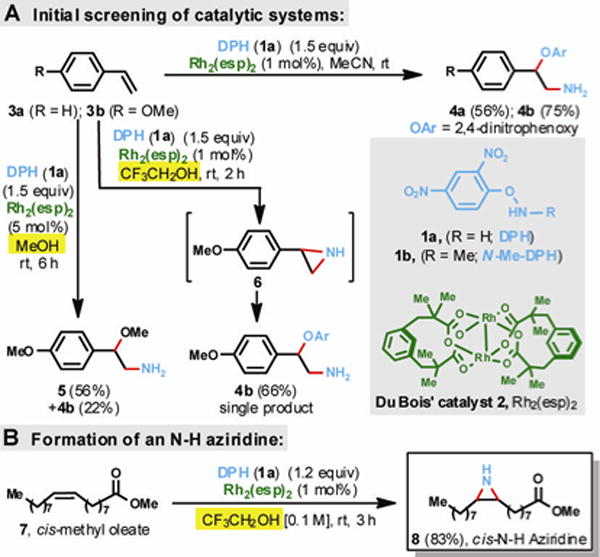
Exploration of DPH (1a) as a versatile aminating agent. (A) Rh2(esp)2 is an effective catalyst for olefin difunctionalization. (B) In 2,2,2-trifluoroethanol (TFE or CF3CH2OH), 7 undergoes direct aziridination to N-H aziridine 8 in excellent isolated yield.
In methanol, we observed the incorporation of the MeO group at the benzylic position (5) in addition to the amino-oxyarylated product 4b; these compounds were isolated in a combined yield of 78%. At this juncture, we reasoned a highly polar, hydroxylic and non-nucleophilic solvent such as 2,2,2-trifluoroethanol (CF3CH2OH, TFE) would completely avoid the incorporation of solvent into the products. Indeed, 3b was cleanly amino-oxyarylated in TFE and 4b was isolated in 66% yield. It was unclear if the transformation 3b→4b involved the opening of a highly reactive aziridine (6) or an alternative process. Surprisingly, when 7 was reacted in trifluoroethanol as solvent, cis-N-H aziridine 8 was isolated in excellent yield (83%) instead of the expected amino-oxyarylated product. The transformation proceeded with complete stereospecificity as no traces of the trans-N-H aziridine were detected by 1H- and 13C-NMR analysis (2% sensitivity).
Encouraged by this unexpected, yet most welcome result, a systematic study was initiated using representative aliphatic olefins with a wide range of substitution patterns and functionalities (Fig. 2). Terminal aliphatic olefin substrates (entries 1–3, Fig. 2) either did not react or reacted sluggishly (i.e., days) when 1 mol% of catalyst 2 was used; however, increasing the catalyst loading to 5 mol% led to rapid conversion at room temperature to the corresponding N-H aziridines (10a–c). We empirically found that in some of the reactions (i.e., entries 4, 5, 7, 9, 11, 14 & 20) addition of the catalyst in several 1 mol% portions minimized decomposition of both the catalyst and aminating agent and invariably led to higher isolated yield of product. Remarkably, the N-H aziridination took place efficiently in the presence of a labile terminal epoxide (10c) as well as an unprotected primary alcohol (10a); these functionalities typically interfere with currently used aziridination protocols. In case of the transformation 9c→10c, only the product was detected in the crude reaction mixture by NMR analysis. In the presence of 1 mol% of catalyst 2, both cis- and trans-1,2-disubstituted aliphatic olefins (entries 4–10, Fig. 2) underwent smooth and stereospecific N-H aziridination at room temperature as established by 13C-NMR analysis (≤2% sensitivity). The presence of an unprotected secondary alcohol in substrate 9i (entry 9) did not influence the stereochemical outcome of the N-H aziridination and 10i was isolated as a 1:1 mixture of diastereomers.
Fig. 2.
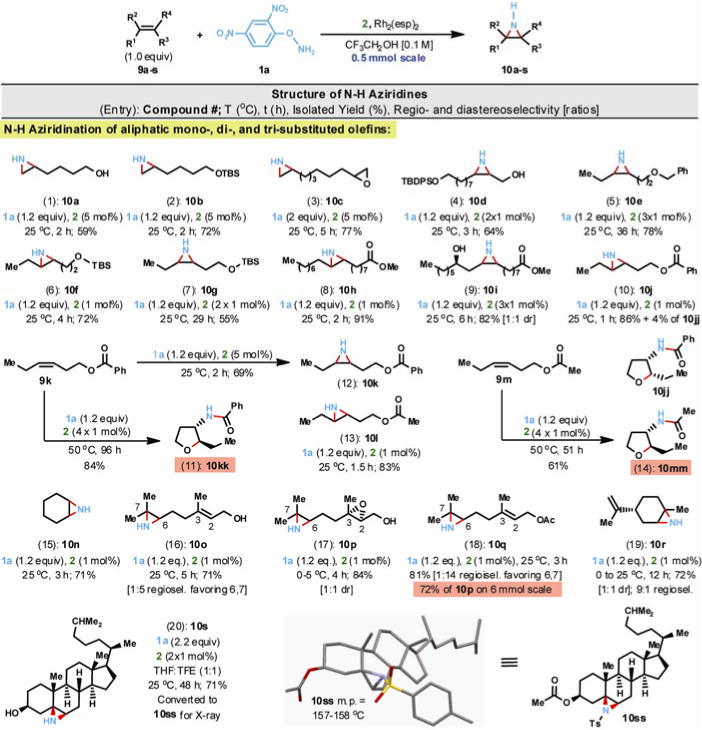
Direct and stereospecific N-H aziridination of olefins. Reactions were conducted at 0.1M using 2,2,2-trifluoroethanol as solvent and at 0.5 mmol scale unless otherwise indicated. To obtain crystalline material, 10s was O-acetylated and N-tosylated (Ts = para-toluenesulfonyl) to afford derivative 10ss.
Benzoyloxy and acetyloxy cis-olefins 9k and 9m (entries 11 & 14), when exposed to 1 mol% of catalyst 2 and 1.2 equiv of aminating agent 1a at 50 °C, were smoothly aziridinated followed by an in situ aziridine ring-opening (via transacylation) to yield the corresponding trans-2,3-disubstituted furans 10kk and 10mm in 84% and 61% yields, respectively. On the other hand, when olefin 9k was exposed to 5 mol% loading of catalyst 2 and 1.2 equivalents of 1a at 25 °C, the expected N-H aziridine 10k (entry 12) was formed in just 2 hours and isolated in 69% yield. As anticipated, when the rate of N-H aziridination is slow and elevated temperatures are used, secondary processes (i.e., intramolecular annulation) that consume the initially formed N-H aziridines can dominate. Apparently, a five-fold increase in catalyst loading increased the rate of N-H aziridination sufficiently that it could take place rapidly at ambient temperature.
Cyclohexene 9n (entry 15) was aziridinated at room temperature to afford cyclic N-H aziridine 10n; no traces of allylic C-H amination (i.e., 1-amino-2-cyclohexene) could be detected by 1H-NMR analysis (2% sensitivity), in sharp contrast with other metal nitrene-based aziridination methods.(37) Geraniol (9o, entry 16) and geranyl acetate (9q, entry 18), which incorporate two trisubstituted C=C double bonds, were N-H aziridinated regioselectively, favoring the double bond at the Δ6,7-position over the Δ2,3-position in both cases.
The shift of the regioisomeric ratio from 1:5 in 10o to 1:14 in 10q suggests a subtle directing effect of the free allylic alcohol and/or an inductive deactivation by the acetate; perhaps the extent of H-bonding in the solvent also plays a role. Entry 17 stands as a testament to the extraordinarily mild reaction conditions as trisubstituted olefin 9p, which possesses a highly sensitive epoxy alcohol, was aziridinated rapidly and efficiently to epoxy N-H aziridine 10p in excellent yield. The transformation 9q→10q (entry 18) could be readily scaled up (6 mmol) with minimal erosion of the isolated yield to provide gram quantities of 10q. N-H aziridination of limonene 9r (entry 19) favored the trisubstituted ring double bond with 9:1 regioselectivity; however, the chiral center had no evident influence on the diastereoselectivity (1:1 dr). In contrast with the lack of stereoselectivity in 9i, cholesterol 9s (entry 20) exclusively yielded the β-N-H aziridine 10s in 71% yield; this unexpected stereochemical outcome, confirmed by single crystal X-ray analysis of 10ss (a crystalline derivative of 10s), suggests a directing effect by the adjacent C(3)-β-alcohol not observed in conformationally more mobile acyclic molecules such as 9i. The success with cholesterol and other natural products (7, 9h, 9i, 9o and 9r, Fig. 1 & 2) highlights the prospective utility of this method in the straightforward elaboration of molecules of biomedical interest (e.g., for 15N-labeling studies).
Next, we turned our attention to the direct N-H aziridination of di-, tri- and tetra-substituted styrenes and stilbene (entries 21–28, Fig. 3A). In general, styrenes were more reactive than aliphatic olefins, and often lower temperatures (−10 to 25°C) were adequate. Conspicuously, cis- β-methyl styrene 11d furnished the corresponding cis-2-Ph-3-Me N-H aziridine (12d, entry 24) without isomerization. Similarly, trans-β-methyl styrene 11c readily furnished trans-2-Ph-3-Me N-H aziridine (12c, entry 23) even on a 1 to 8 mmol scale. The N-H aziridine derived from 2-Me indene (12h, entry 28) was not isolated due to its high reactivity, but instead reduced in situ to amine 12hh. Evaluation of the effect of catalyst loading on the reaction 11f→12f (entry 26) revealed the lowest practical loading of catalyst 2, without decreasing the isolated yield or drastically increasing the reaction time, was 0.5 mol%. This low catalyst loading renders the process economical and environmentally friendly. A further five-fold reduction in catalyst loading (from 0.5 mol% to 0.1 mol%) resulted in a 25-fold increase in reaction time and a 30% drop in the isolated yield of 12f. To our delight, tetrasubstituted olefin 11g (entry 27) was easily N-H aziridinated at room temperature; 12g was isolated in 70% yield. The attempted direct N-H aziridination of 1-Ph-1-cyclopropylethene (11b) yielded only amino-oxyarylated product 12b; the complete lack of cyclopropane ring-opening products corroborate an aziridination pathway that does not involve long-lived radical or carbocation intermediates (see more detailed discussion of the mechanism in the computation section and also in Fig. 4).
Fig. 3.
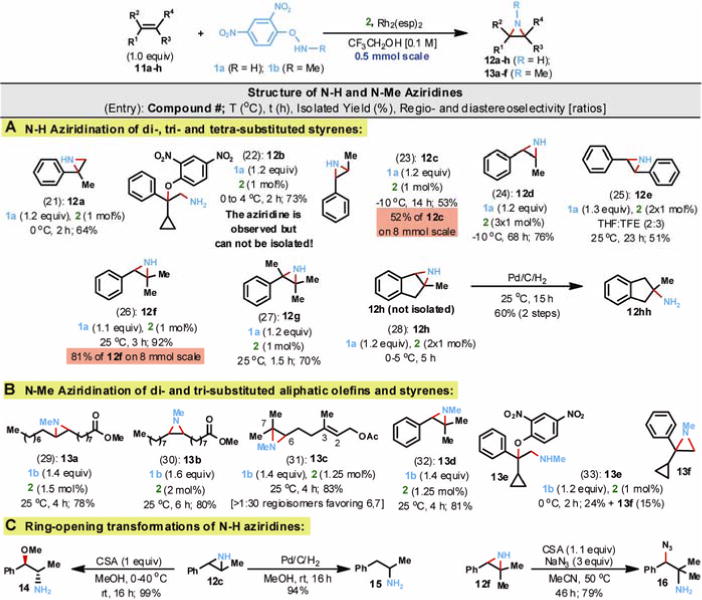
Direct and stereospecific N-H and N-Me aziridination of olefins. Reactions were conducted at 0.1M using 2,2,2-trifluoroethanol as solvent and at 0.5 mmol scale unless otherwise indicated. CSA = camphorsulfonic acid.
Fig. 4.
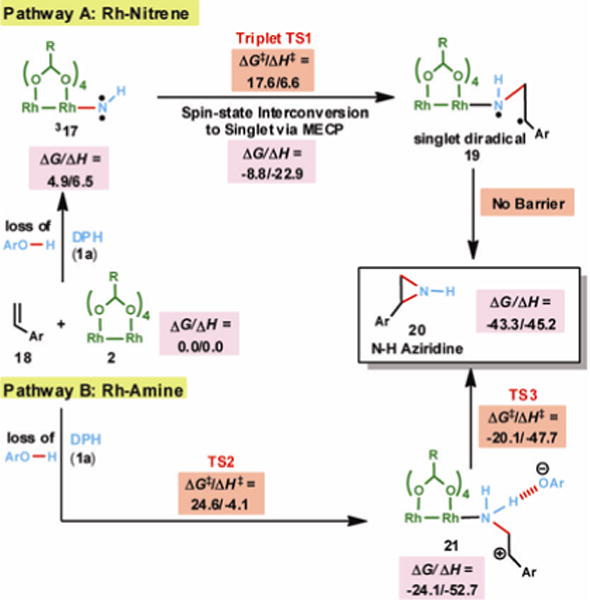
Selected DFT-examined pathways for N-H aziridination of styrene in 2,2,2-trifluoroethanol solvent. R = esp ligands. Energies in kcal/mol. MECP = minimum energy crossing point.
The practicality and broad scope of the preceding direct and stereospecific N-H aziridination of olefins (Fig. 2 & Fig. 3A) prompted an investigation of direct N-Me aziridination. Towards this end, several di- and trisubstituted aliphatic olefin and styrene substrates (entries 29–33, Fig. 3B) were examined in the presence of 1b as the stoichiometric aminating agent and 1 to 2 mol% of catalyst 2. The N-Me aziridination of olefins also proceeded stereospecifically (entries 29 & 30) and, in the case of geraniol acetate 9q, the regioselectivity increased from 1:14 (in 10q) to >1:30 (in 13c), favoring the Δ6,7-olefin in both cases.
Two of the N-H aziridine products (12c and 12f) were subjected to ring-opening transformations (Fig. 3C). Upon catalytic hydrogenation, aziridine 12c afforded a 94% yield of amphetamine 15, the active pharmaceutical ingredient (API) in Adderall™, an approved medication for attention deficit hyperactivity disorder (ADHD) as well as narcolepsy that is marketed as a mixture of enantiomers. Under acidic conditions, at slightly elevated temperature (40 °C) in MeOH, 12c was converted to O-Me-norephedrine 14 with complete regioselectivity and in nearly quantitative yield. Likewise, the ring-opening of trisubstituted N-H aziridine 12f with sodium azide furnished azidoamine 16 in 79% yield. These transformations by example illustrate how readily a nitrogen atom can be introduced into molecules.
We also examined prospective reaction mechanisms using quantum mechanical density-functional theory calculations (Fig. 4). Our (U)M06 calculations were carried out in Gaussian 09 (38) using a polarizable conductor continuum solvent model for trifluoroethanol. Details of calculated transition states and intermediates are given in the Supplementary Materials.
We first examined plausible rhodium nitrene pathways. Generation of a rhodium nitrene intermediate is possible if the amino group of 1a coordinates to Rh2(esp)2 followed by loss of dinitrophenol (Pathway A, Fig. 4). Calculations suggest that the triplet-spin state of the nitrene (317) is more than 8 kcal/mol lower in energy than the open-shell singlet and reaction pathways identified on the triplet-spin energy surface were found to be lower in energy than reaction pathways on the singlet-spin energy surface.(39, 40) Because the Rh2(esp)2 catalyst and aziridine product have singlet-spin ground states, the reaction pathway must involve spin interconversion. The mechanism outlined in Fig. 4 provides a route for stereospecific aziridination if 317 reacts with alkenes by forming the first C–N bond via triplet transition state TS1 followed by spin interconversion along the pathway to diradical intermediate 19 or fast spin interconversion at the diradical intermediate.(41) After spin interconversion, the second C–N bond is formed by the coupling of singlet-paired electrons without a barrier and leads directly to aziridine 20.
As alternatives to nitrene pathways we also explored polar mechanisms involving Rh-amine and Rh-alkene coordination modes (see Supplementary Materials). One of several possible polar mechanisms is outlined as Pathway B in Fig. 4. This pathway is akin to the mechanism proposed for amination of aryl boronic acids with 1a.(32) While this mechanism may account for amino-oxyarylated products (e.g., 4a & 4b) observed under some experimental conditions, the calculated barrier for this mechanism, as well as alternative polar mechanisms, are higher in energy than the nitrene mechanism presented in Pathway A.
Supplementary Material
Acknowledgments
J.R.F. thanks NIH (GM31278, DK38226) and the Robert A. Welch Foundation (Grant GL625910) for funding. L.K. gratefully acknowledges the generous financial support of the UT Southwestern Endowed Scholars in Biomedical Research Program (W.W. Caruth, Jr, Endowed Scholarship in Biomedical Research), the Robert A. Welch Foundation (Grant I-1764), the ACS-PRF (Grant 51707-DNI1) and the American Cancer Society & Simmons Cancer Center Institutional Research Grant (New Investigator Award in Cancer Research, ACS-IRG 02-196). D.H.E. thanks BYU and the Fulton Supercomputing Lab. M.Y. acknowledges a grant from UT Arlington to support the upgrade of the X-ray facility. We also thank Prof. Kevin Schug, Dr. Maciej Kukula and the Shimadzu Center for Advanced Analytical Chemistry (SCAAC, UT Arlington) for their assistance in the full characterization of new compounds. The generous donation of 100g of aminating agent 1a and 5g of aminating agent 1b by Corvinus Chemicals, LLC (Dallas, TX) as well as 20 kg of 2,2,2-trifluoroethanol (TFE) by Joyant Pharmaceuticals, Inc (Dallas, TX) is also gratefully acknowledged. We thank P.S. Baran, J. Du Bois, E.M. Carreira, A.J. Catino, M.P. Doyle, M. Krische and R. Sarpong for helpful commentary. Metrical parameters for the structure of compound 10ss are available free of charge from the Cambridge Crystallographic Data Centre under reference number CCDC-959664.
Footnotes
Materials and Methods
References and Notes
- 1.McCoull W, Davis FA. Recent synthetic applications of chiral aziridines. Synthesis. 2000:1347. [Google Scholar]
- 2.Sweeney JB. Aziridines: epoxides’ ugly cousins? Chem Soc Rev. 2002;31:247. doi: 10.1039/b006015l. [DOI] [PubMed] [Google Scholar]
- 3.Botuha C, Chemla F, Ferreira F, Perez-Luna A. Aziridines in natural product synthesis in. In: Majumdar KC, Chattopadhyay SK, editors. Heterocycles in Natural Product Synthesis. Wiley-VCH; 2011. pp. 3–39. [Google Scholar]
- 4.Ogawa C, Kobayashi S. Ring opening of epoxides and aziridines in. Science of Synthesis, Water in Organic Synthesis. 2012:579–599. [Google Scholar]
- 5.Chawla R, Singh AK, Yadav LDS. Organocatalysis in synthesis and reactions of epoxides and aziridines. RSC Adv. 2013;3:11385. [Google Scholar]
- 6.Njardarson JT. Catalytic ring expansion adventures. Synlett. 2013;24:787. [Google Scholar]
- 7.Lowden PAS. Aziridine natural products – discovery, biological activity and biosynthesis in. In: Yudin AK, editor. Aziridines and Epoxides in Organic Synthesis. Wiley-VCH; 2006. pp. 399–442. [Google Scholar]
- 8.Ismail FMD, Levitsky DO, Dembitsky VM. Aziridine alkaloids as potential therapeutic agents. Eur J Med Chem. 2009;44:3373. doi: 10.1016/j.ejmech.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Thibodeaux CJ, Chang W-c, Liu H-w. Enzymatic Chemistry of Cyclopropane, Epoxide, and Aziridine Biosynthesis. Chem Rev (Washington, DC, U. S.) 2012;112:1681. doi: 10.1021/cr200073d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn HMI, Sweeney J. The asymmetric synthesis of aziridines. Tetrahedron Asymmetry. 1997;8:1693. [Google Scholar]
- 11.Jacobsen EN. Compr Asymmetric Catal. I–III. Vol. 2. Springer; 1999. Aziridination in; pp. 607–618. [Google Scholar]
- 12.Mueller P, Fruit C. Enantioselective catalytic aziridinations and asymmetric nitrene insertions into CH bonds. Chem Rev (Washington, DC, U. S.) 2003;103:2905. doi: 10.1021/cr020043t. [DOI] [PubMed] [Google Scholar]
- 13.Moessner C, Bolm C. Transition Met Org Synth. 2. Vol. 2. Wiley; 2004. Catalyzed asymmetric aziridinations in; pp. 389–402. [Google Scholar]
- 14.Aggarwal VK, Badine DM, Moorthie VA. Asymmetric synthesis of epoxides and aziridines from aldehydes and imines. In: Yudin AK, editor. Aziridines and Epoxides in Organic Synthesis. Wiley-VCH; 2006. pp. 1–35. [Google Scholar]
- 15.Cardillo G, Gentilucci L, Tolomelli A. Asymmetric synthesis of three- and four-membered ring heterocycles. In: Royer J, editor. Asymmetric Synthesis of Nitrogen Heterocycles. Wiley-VCH; 2009. pp. 3–50. [Google Scholar]
- 16.Sweeney J. Aziridine Synthesis via Nucleophilic Attack of Carbene Equivalents on Imines: the Aza-Darzens Reaction. Eur J Org Chem. 2009;4911 [Google Scholar]
- 17.Pellissier H. Recent developments in asymmetric aziridination. Tetrahedron. 2010;66:1509. [Google Scholar]
- 18.Ye S, Tang Y. Cyclopropanation, epoxidation and aziridination reactions. In: Ma S, editor. Handbook of Cyclization Reactions. Vol. 2. Wiley-VCH; 2010. pp. 687–732. [Google Scholar]
- 19.Karila D, Dodd RH. Recent progress in iminoiodane-mediated aziridination of olefins. Curr Org Chem. 2011;15:1507. [Google Scholar]
- 20.Muchalski H, Johnston JN. Science of Synthesis, Stereoselective Synthesis. Vol. 1. Thieme; 2011. Aziridination; pp. 155–184. [Google Scholar]
- 21.Jenkins DM. Atom-economical C2 + N1 aziridination: progress towards catalytic intermolecular reactions using alkenes and aryl azides. Synlett. 2012;23:1267. [Google Scholar]
- 22.Jiang H, Zhang XP. Oxidation: C-N bond formation by oxidation (aziridines) In: Carreira EM, Yamamoto H, editors. Comprehensive Chirality. Vol. 5. Elsevier; 2012. pp. 168–182. [Google Scholar]
- 23.Rios R, Cordova A. C-N Bond formation: aziridine formation. In: Carreira EM, Yamamoto H, editors. Comprehensive Chirality. Vol. 6. Elsevier; 2012. pp. 399–413. [Google Scholar]
- 24.Bottaro JC. Stereospecific conversion of olefins into aziridines by p-tolylsulfonylhydroxylamine. J Chem Soc Chem Commun. 1980:560. [Google Scholar]
- 25.Andreae S, Schmitz E. Electrophilic aminations with oxaziridines. Synthesis. 1991:327. [Google Scholar]
- 26.Vedejs E, Sano H. Synthesis of N-methoxy and N-H aziridines from alkenes. Tetrahedron Lett. 1992;33:3261. [Google Scholar]
- 27.Ho CM, Lau TC, Kwong HL, Wong WT. Activation of manganese nitrido complexes by Bronsted and Lewis acids. Crystal structure and asymmetric alkene aziridination of a chiral salen manganese nitrido complex. J Chem Soc Dalton Trans. 1999:2411. [Google Scholar]
- 28.Koohang A, Stanchina CL, Coates RM. Regio- and stereoselective synthesis of N-H aziridines by N-N bond reduction of N-quinazolinyl aziridines. Tetrahedron. 1999;55:9669. [Google Scholar]
- 29.Lebel H, Lectard S, Parmentier M. Copper-catalyzed alkene aziridination with N-tosyloxycarbamates. Org Lett. 2007;9:4797. doi: 10.1021/ol702152e. [DOI] [PubMed] [Google Scholar]
- 30.Lu Z, Zhang Y, Wulff WD. Direct Access to N-H-Aziridines from Asymmetric Catalytic Aziridination with Borate Catalysts Derived from Vaulted Binaphthol and Vaulted Biphenanthrol Ligands. J Am Chem Soc. 2007;129:7185. doi: 10.1021/ja069371r. [DOI] [PubMed] [Google Scholar]
- 31.Varszegi C, Ernst M, van Laar F, Sels BF, Schwab E, De Vos DE. A micellar iodide-catalyzed synthesis of unprotected aziridines from styrenes and ammonia. Angew Chem Int Ed. 2008;47:1477. doi: 10.1002/anie.200704772. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C, Li G, Ess DH, Falck JR, Kürti L. Elusive metal-free primary amination of arylboronic acids: synthetic studies and mechanism by density functional theory. J Am Chem Soc. 2012;134:18253. doi: 10.1021/ja309637r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espino CG, Fiori KW, Kim M, Du Bois J. Expanding the Scope of C-H Amination through Catalyst Design. J Am Chem Soc. 2004;126:15378. doi: 10.1021/ja0446294. [DOI] [PubMed] [Google Scholar]
- 34.Davies HML, Manning JR. Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature (London, U K) 2008;451:417. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalatan DN, Du Bois J. Metal-catalyzed oxidations of C-H to C-N bonds. Top Curr Chem. 2010;292:347. doi: 10.1007/128_2009_19. [DOI] [PubMed] [Google Scholar]
- 36.Lebel H. Rhodium-catalyzed C-H aminations. In: Yudin AK, editor. Catalyzed Carbon-Heteroatom Bond Formation. Wiley-VCH; 2011. pp. 137–155. [Google Scholar]
- 37.Lebel H, Huard K, Lectard S. N-tosyloxycarbamates as a source of metal nitrenes: rhodium-catalyzed C-H insertion and aziridination reactions. J Am Chem Soc. 2005;127:14198. doi: 10.1021/ja0552850. [DOI] [PubMed] [Google Scholar]
- 38.Frisch MJ, et al. Gaussian 09, Revision B.01. Gaussian, Inc; 2009. [Google Scholar]
- 39.Lin X, Zhao C, Che CM, Ke Z, Phillips DL. A DFT study on the mechanism of Rh2(II,II)-catalyzed intramolecular amidation of carbamates. Chem – Asian J. 2007;2:1101. doi: 10.1002/asia.200700068. [DOI] [PubMed] [Google Scholar]
- 40.Lorpitthaya R, Xie ZZ, Sophy KB, Kuo JL, Liu XW. Mechanistic insights into the substrate-controlled stereochemistry of glycals in one-pot rhodium-catalyzed aziridination and aziridine ring opening. Chem – Eur J. 2010;16:588. doi: 10.1002/chem.200901727. [DOI] [PubMed] [Google Scholar]
- 41.Maestre L, Sameera WMC, Diaz-Requejo MM, Maseras F, Perez PJ. A general mechanism for the copper- and silver-catalyzed olefin aziridination reactions: concomitant involvement of the singlet and triplet pathways. J Am Chem Soc. 2013;135:1338. doi: 10.1021/ja307229e. [DOI] [PubMed] [Google Scholar]
- 42.Lie Ken Jie MSF, Syed-Rahmatullah MSK. Synthesis and spectroscopic properties of long-chain aza, aziridine and azetidine fatty esters. J Am Oil Chem Soc. 1992;69:359. [Google Scholar]
- 43.Furmeier S, Metzger JO. Fat-derived aziridines and their N-substituted derivatives: Biologically active compounds based on renewable raw materials. Eur J Org Chem. 2003:649. [Google Scholar]
- 44.Li P, Li J, Arikan F, Ahlbrecht W, Dieckmann M, Menche D. Stereoselective Total Synthesis of Etnangien and Etnangien Methyl Ester. J Org Chem. 2010;75:2429. doi: 10.1021/jo100201f. [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Nelson CG, Taber DF. Potassium hydride in paraffin: a useful base for Williamson ether synthesis. Tetrahedron Lett. 2010;51:3545. [Google Scholar]
- 46.Huang X, Craita C, Awad L, Vogel P. Silyl methallylsulfinates: efficient and powerful agents for the chemoselective silylation of alcohols, polyols, phenols and carboxylic acids. Chem Commun (Cambridge, U K) 2005:1297. doi: 10.1039/b417894g. [DOI] [PubMed] [Google Scholar]
- 47.De Luca RJ, Edwards JL, Steffens LD, Michel BW, Qiao X, Zhu C, Cook SP, Sigman MS. Wacker-Type Oxidation of Internal Alkenes using Pd(Quinox) and TBHP. J Org Chem. 2013;78:1682. doi: 10.1021/jo302638v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prepared using method described in ref. 47 using acetic anhydride instead of Benzoyl chloride. Analytical data are consistent with literature:; Bartoli G, Bosco M, Dalpozzo R, Marcantoni E, Massaccesi M, Sambri L. Zn(ClO4)2·6H2O as a powerful catalyst for a practical acylation of alcohols with acid anhydrides. Eur J Org Chem. 2003:4611. [Google Scholar]
- 49.Caiazzo A, Dalili S, Yudin AK. Design and Development of Cyclohexane-Based P,N-Ligands for Transition Metal Catalysis. Org Lett. 2002;4:2597. doi: 10.1021/ol026249y. [DOI] [PubMed] [Google Scholar]
- 50.Koohang A, Stanchina CL, Coates RM. Regio- and stereoselective synthesis of N-H aziridines by N-N bond reduction of N-quinazolinyl aziridines. Tetrahedron. 1999;55:9669. [Google Scholar]
- 51.Noe MC, Hawkins JM, Snow SL, Wolf-Gouveia L. A short enantioselective synthesis of N-Boc-(2R,3R)-3-methyl-3-hydroxypipecolic acid from geraniol. J Org Chem. 2008;73:3295. doi: 10.1021/jo800080t. [DOI] [PubMed] [Google Scholar]
- 52.Voronkov MV, Gontcharov AV, Kanamarlapudi RC, Richardson PF, Wang ZM. Scaleable Syntheses of Isomeric Limonene Aziridines from the Commercially Available Mixture of cis- and trans-Limonene Oxides. Org Process Res Dev. 2005;9:221. [Google Scholar]
- 53.Snatzke G, Veithen A. 19-Nor-5β-methyl steroids. IV. Nitrous acid deamination of 5α-amino steroids. Justus Liebigs Ann Chem. 1967;703:159. doi: 10.1002/jlac.19677030120. [DOI] [PubMed] [Google Scholar]
- 54.Barton DHR, Hay-Motherwell RS, Motherwell WB. Functionalization of saturated hydrocarbons. Part 1. Some reactions of a ferrous chloride-chloramine-T complex with hydrocarbons. J Chem Soc Perkin Trans. 1983;1445 [Google Scholar]
- 55.von Kieseritzky F, Lindstroem J. Aziridines in one step from hydantoins via Red-Al mediated ring-contraction. Tetrahedron Lett. 2011;52:4558. [Google Scholar]
- 56.Han P, Wang R, Wang DZ. Electronic polarizability-based stereochemical model for Sharpless AD reactions. Tetrahedron. 2011;67:8873. [Google Scholar]
- 57.Nishimura M, Minakata S, Takahashi T, Oderaotoshi Y, Komatsu M. Asymmetric N1 Unit Transfer to Olefins with a Chiral Nitridomanganese Complex: Novel Stereoselective Pathways to Aziridines or Oxazolines. J Org Chem. 2002;67:2101. doi: 10.1021/jo016146d. [DOI] [PubMed] [Google Scholar]
- 58.Arroyo Y, Meana A, Rodriguez JF, Sanz-Tejedor MA, Alonso I, Garcia Ruano JL. Stereoselective Control of Planar α-Dimethylsulfonium Benzyl Carbanions. Synthesis of Optically Pure trans-Aziridines. J Org Chem. 2009;74:4217. doi: 10.1021/jo900381b. [DOI] [PubMed] [Google Scholar]
- 59.Zhu M, Hu L, Chen N, Du DM, Xu J. Synthesis of NH-aziridines from vicinal amino alcohols via the Wenker reaction. Scope and limitation. Lett Org Chem. 2008;5:212. [Google Scholar]
- 60.Cuenoud B, Bruce I, Fairhurst RA, Beattie D. Indan-2-ylamine derivatives as β2-adrenoceptor agonists and their preparation and use in the treatment of obstructive and inflammatory airway diseases (Novartis AG, Switz.) 7622483. US. 2009
- 61.Sheradsky T, Salemnick G, Nir Z. Introduction of the aminooxy group onto nitroaromatic and heterocyclic rings. Synthesis and properties of 0-(nitroaryl)hydroxylamines. Tetrahedron. 1972;28:3833. [Google Scholar]
- 62.Bechara WS, Pelletier G, Charette AB. Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nat Chem. 2012;4:228. doi: 10.1038/nchem.1268. [DOI] [PubMed] [Google Scholar]
- 63.Mencel JJ, Fishbein P. Phenylethylamine derivatives and their preparation (Johnson Matthey Public Limited Company, UK) 20100125146. US. 2010
- 64.Rao AVR, Reddy ER, Sharma GVM, Yadagiri P, Yadav JS. A stereoselective synthesis of coriolic acid and dimorphecolic acid. Tetrahedron. 1986;42:4523. [Google Scholar]
- 65.Sheldrick GM. SHELXTL, version 6.14. Bruker Analytical X-ray Systems, Inc; Madison, WI: 2000. [Google Scholar]
- 66.Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 2008;120:215. [Google Scholar]
- 67.Zhao Y, Truhlar DG. Density Functionals with Broad Applicability in Chemistry. Acc Chem Res. 2008;41:157. doi: 10.1021/ar700111a. [DOI] [PubMed] [Google Scholar]
- 68.Harvey JN, Aschi M, Schwarz H, Koch W. The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor Chem Acc. 1998;99:95. [Google Scholar]
- 69.Harvey JN, Aschi M. Spin-forbidden dehydrogenation of methoxy cation: a statistical view. Phys Chem Chem Phys. 1999;1:5555. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


