Abstract
Thromboxane A2 (TXA2) is a biologically active metabolite of arachidonic acid formed by the action of the terminal synthase, thromboxane A2 synthase (TXA2S), on prostaglandin endoperoxide (PGH2). TXA2 is responsible for multiple biological processes through its cell surface receptor, the T-prostanoid (TP) receptor. Thromboxane A2 synthase and TP are the two necessary components for the functioning of this potent bioactive lipid. Thromboxane A2 is widely implicated in a range of cardiovascular diseases, owing to its acute and chronic effects in promoting platelet aggregation, vasoconstriction, and proliferation. In recent years, additional functional roles for both TXA2S and TP in cancer progression have been indicated. Increased cyclooxygenase (COX)-2 expression has been described in a variety of human cancers, which has focused attention on TXA2 as a downstream metabolite of the COX-2-derived PGH2. Several studies suggest potential involvement of TXA2S and TP in tumor progression, especially tumor cell proliferation, migration, and invasion that are key steps in cancer progression. In addition, the regulation of neovascularization by TP has been identified as a potent source of control during oncogenesis. There have been several recent reviews of TXA2S and TP but thus far none have discussed its role in cancer progression and metastasis in depth. This review will focus on some of the more recent findings and advances with a significant emphasis on understanding the functional role of TXA2S and TP in cancer progression and metastasis.
Keywords: Thromboxane synthase, Thromboxane receptor, Cyclooxygenase, Cancer progression, Metastasis, Angiogenesis, Cell migration, Apoptosis
1 Introduction
Thromboxane A2 (TXA2) was one of the first prostaglandins to be identified from washed platelets (in 1975) and has been widely implicated in a range of cardiovascular diseases, owing to its acute and chronic effects in promoting platelet aggregation, vasoconstriction, and proliferation [1–3]. TXA2 is a biologically active metabolite of arachidonic acid (AA) formed by the action of TXA2 synthase (TXA2S) on prostaglandin endoperoxide (PGH2) [1, 4, 5]. TXA2 is highly unstable in aqueous solution, where it spontaneously hydrolyzes to the biologically inactive hemiacetal thromboxane B2 (TXB2) with a half-life of 30 s [4]. Due to its short half-life, TXA2 primarily functions as an autocrine or paracrine mediator in the tissues surrounding its site of production. TXA2 is responsible for multiple biological processes through the cell surface TXA2 receptor, or T-prostanoid (TP) receptor [6, 7]. TXA2 biosynthesis and TP expression are elevated in numerous cardiovascular and inflammatory diseases [3, 8– 10]. It is felt that TXA2, through its receptors, plays a very important role in the pathogenesis of acute coronary artery syndrome, vessel remodeling, thrombosis, renal, pulmonary, and atherosclerotic cardiovascular diseases primarily through its action as a potent vasoconstrictor and an inducer of platelet aggregation and activation [8, 9, 11–15]. As such TXA2S inhibition and TP antagonism have become central to the therapy of many diseases including infarction, hypertension, stroke, and renal dysfunction [16–18]. Recently a role for TXA2 signaling in cancer has become apparent. This review will focus on some of the more recent findings and advances with a major emphasis on understanding the functional role of TXA2S and TP in cancer progression and metastasis.
1.1 Thromboxane A2 synthase
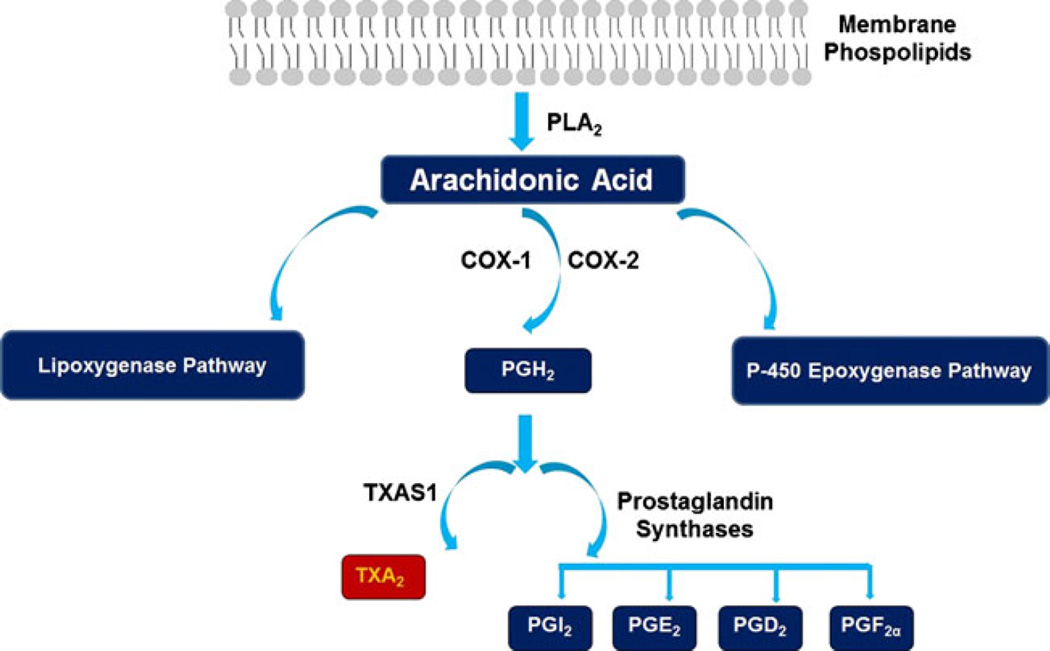
The cyclooxygenase enzymes, cyclooxygenase (COX)-1 and COX-2, are responsible for the conversion of AA to PGH2, the first step in the generation of TXA2. TXA2S is an endoplasmic reticulum membrane protein that belongs to the P450 epoxygenase family that catalyzes the conversion of the COX product PGH2 to TXA2 [19] (Fig. 1). TXA2S was first found as a microsomal enzyme in platelets (60 kDa) and is highly expressed in lung, platelets, kidney, stomach, duodenum, colon, and spleen [20–22]. TXA2S expression is reported to be closely associated with cardiovascular, renal, and inflammatory diseases [21, 23].
Fig. 1.
Generation of the prostanoids through metabolism of arachidonic acid. Arachidonic acid can be metabolized into different bioactive lipids by one of three distinct signaling pathways; the cyclooxygenase (COX), the lipoxygenase, and the P-450 epoxygenase pathways. The COX-isoforms-COX-1 and COX-2 are responsible for the conversion of AA to PGH2, the first step in the generation of TXA2. Thromboxane synthase (TXA2S) then catalyzes the conversion of the COX product, PGH2 to TXA2
1.2 Thromboxane A2 receptor
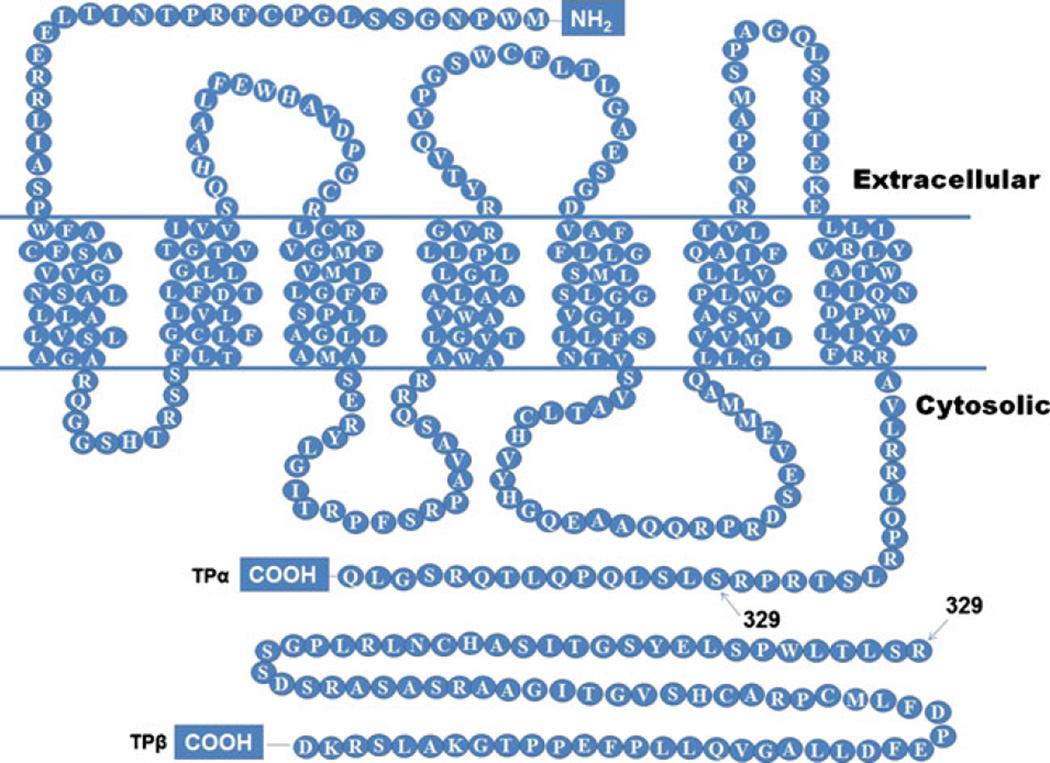
TXA2 is responsible for multiple biological processes through its cell surface receptor TP. Ligation of TP by TXA2 activates multiple downstream pathways, including phospholipase C, and raises intracellular Ca2+ levels leading to vasoconstriction and platelet aggregation [6, 21]. The TXA2 receptor (TP) is a typical G-protein-coupled receptor expressed as two different isoforms in humans— TP-alpha (TPα) and TP-beta (TPβ) [24, 25]. These TP isoforms arise via alternate splicing of a single gene and share the first 328 amino acids. TPα and TPβ differ in the length of the C-terminal cytoplasmic tail with TPα shorter than the TPβ isoform (15 versus 79 residues) [24–26] (Fig. 2). Ligand binding sites for the TP receptor are in its extracellular region and are identical in both splice variants. TP isoforms share both common and distinct signaling pathways depending on the G protein subunits bound to their C-terminal tail. Therefore, differences in function between TPα and TPβ may manifest due to the complement of G-proteins associated with the cytoplasmic tail. TP receptors are found in a large number of tissues and cell types. TP mRNAs are expressed widely in platelets and monocytes and in the lung, liver, kidney, cardiovascular system (myocytes, vascular smooth muscle cells, and endothelium), uterus, brain, spleen, thymus, and placenta [6, 26–29].
Fig. 2.
Structures of TPα and TPβ receptor proteins. The TXA2 receptor (TP) is a typical G-protein-coupled receptor that is expressed as two different isoforms in humans—TPα and TPβ. TP isoforms share the first 328 amino acids but differ in the length of the C-terminal cytoplasmic tail with TPα shorter than the TPβ isoform (15 versus 79 residues) [24, 25]
2 Role of thromboxane A2 and its receptors in cancer progression
In recent years, several studies have indicated functional roles for both TXA2S and TP in cancer progression. Increased COX-2 expression has been described in a variety of human cancers and downstream metabolites of COX-2, such as TXA2, have therefore also become of interest for their potential role in cancer progression [30– 33]. Multiple studies have documented roles for TXA2 signaling in the essential processes of neoplastic transformation, including enhanced tumor cell motility that are key steps in cancer progression. These effects are observed in multiple cancers indicating the extensive nature of these effects and the widespread clinical applicability of targeting these pathways as adjunct therapy in cancer [30–32, 34].
2.1 Role of thromboxane A2 signaling in prostate cancer
Prostate cancer (PCa) is one of the most common cancers among men in the USA and is the second leading cause of death in this population [35]. Strong correlations exist between diets high in fat (especially from red meat) and PCa [36–38]. As red meat is rich in AA, the presence of enzymes associated with AA metabolism might result in increased synthesis of downstream lipid mediators. Several metabolites of AA, such as 5-HETE and 12-HETE, have already been studied widely for their roles in PCa [39–42]. Numerous studies demonstrate increased COX-2 mRNA and protein expression in PCa correlate with poor prognosis [43, 44]. TXA2 is derived from the COX product PGH2 and, as a consequence, both TXA2S and TP have long been hypothesized to have a possible role in prostate cancer progression.
Prostate tumor progression involves several key steps including cell survival, cell migration, cell invasion, and metastasis. Human PCa cells express functionally active TXA2S and biosynthesize TXA2 [45]. As the expression of COX-2 and COX-1 in prostate cancer has been reported previously, Nie and colleagues investigated their role in synthesis of TXA2 [45]. Treatment of PC-3 cells with both COX-1 and COX-2 inhibitors reduced TXA2 synthesis by 95%, similar to the level achieved by direct TXA2S antagonism. These data suggest that human PCa cells express TXA2S and that both COX-1 and COX-2 are essential for providing the substrate for TXA2S mediated TXA2 biosynthesis in PCa cells [45]. Increased TXA2S expression and activity in PCa cells augmented cell migration but had minimal effect on cell cycle progression or survival indicating that TXA2S activity might contribute to PCa progression through modulating cell motility [45].
In a study conducted on tissue samples from 46 patients, with well-documented histological and molecular data, increased TXA2S and COX-2 expression were observed in tumors. In the same study, TP expression was higher in malignant tissues (high-grade prostatic intraepithelial neoplasia and cancer glands) [46]. Similarly, TXA2S mRNA and protein expression were higher in prostate carcinomas compared to matched normal tissues. In contrast, epithelial cells in non-tumoral glands displayed almost non-existent TXA2S and TP expression [46]. The degree of TXA2S expression correlated with the severity of prostate carcinoma lesions, with advanced stages and poorly differentiated forms having the highest expression levels [45, 46]. Moreover, a significant association between the expression of COX-2/TXA2S/TP and higher Gleason score/pathologic stage of the tumors was observed [46]. Within the cancer tissue, expression of TXA2S and TP were localized to areas of perineural invasion, a known mechanism by which PCa cells penetrate the prostatic capsule and spread to other tissues. Strong expression of TXA2S along perineural tracts might be an indicator for its potential involvement in tumor cell invasion and metastasis [45–47]. The enzyme was found to be involved in motility, but not proliferation or survival, of PCa cells [45].
Tumor cell migration is an important step in the metastatic cascade. At this time, tumor cells leave the primary organ, enter the circulation, and colonize distant tissues [48]. Rho GTPases are critical for the dynamic changes in cell shape and adhesion that drive cell migration. Nie and colleagues demonstrated that the TXA2-TP signaling axis regulated cell migration and cytoskeleton reorganization through promoting Rho-A activation [49]. Recent studies have indicated that the Gα12 family of heterotrimeric G proteins (Gα12 and Gα13) is up-regulated in PCa and that activation of Gα12 signaling promotes PCa cell invasion, through a Rho-dependent pathway. In addition, Gα12 signaling via Rho is required for TXA2 stimulated invasion of PCa cells [50]. As TP is known to couple to the Gα12 family of heterotrimeric G proteins, the up-regulation of both TP and Gα12 in PCa suggests a possible mechanism by which TP may drive the cell migration and invasion observed in high-grade PCa. This hypothesis needs to be investigated further.
A recent report provided evidence of a novel constitutive interaction between TPα/TPβ and protein kinase C-related kinases (PRK1) [51]. PRK1 is a Rho-A effector that has been widely implicated in androgen-associated PCas and ovarian serous carcinomas [51]. It was established that PRK1 directly interacts with endogenously expressed TPα and TPβ in both PC-3 and LNCaP cells, and disruption of PRK1 by siRNA substantially impairs cell migration in response to TXA2 agonist U46619 in these cells [51]. These findings all point towards a possible functional role of TP in cell migration in prostate cancer.
2.2 Role of thromboxane A2 signaling in breast cancer
Breast cancer is one of the most common cancers among American women and is the second leading cause of death among them in USA [35]. In a cancer-profiling array, TXA2S mRNA levels were increased in seven of nine breast tumors when compared to their matched normal tissues [45]. A larger study (120 patients) in normal breast and tumor tissues with well-documented histological and molecular data found transcripts of TP and TXA2S were differentially expressed by quantitative polymerase chain reaction (qPCR) [52]. TXA2S levels were similar in tumor and normal breast tissues; however, TXA2S expression was significantly lower in high-grade tumors compared to low-grade tumors. By comparison, higher total TP mRNA expression was observed in the tumor (grade 3 and above) compared with normal mammary tissues [52] and may indicate higher mortality and worse prognosis. TP expression correlated with estrogen receptor status in this study (p= 0.0128) but was independent of nodal involvement or primary tumor type (ductal versus lobular) [52]. As in prostate cancer, recent studies suggest the increased expression of Gα12 and TP in breast tissue may activate Rho-A to promote cell motility to further exacerbate the progression of breast cancer [53]. These data strongly suggest a role for TXA2 signaling in the development and progression of breast cancer. Moreover, the association of high TP expression in aggressive tumors with poor prognosis indicates TP may have significant prognostic value in clinical breast cancer.
Abraham et al. analyzed seven prostaglandin pathway genes for single nucleotide polymorphisms that may predispose to breast cancer and found that only PTGIS and TXA2S polymorphisms showed modest associations [54]. Collectively, these reports suggest that TP may play a role in breast cancer; however, the significance of this role remains uncertain due to the conflicting nature of the reports. The pathogenic role is made less clear by the realization that it is not necessary for both TXA2S and TP to be expressed in the same tissue to have a possible role in cancer progression. TXA2 can act as paracrine mediator due to the abundant expression of TXA2S in platelets and other cell types in the tumor microenvironment. Thus, further pre-clinical investigation into the role of TXA2 signaling is warranted to define the true role of this bioactive lipid in breast cancer.
2.3 Role of thromboxane A2 signaling in lung cancer
Lung cancer is the second leading cause of cancer in the USA every year and more people die from lung cancer than breast, colon, and prostate cancers combined [35]. The role of COX-2 and prostaglandins in lung cancer is now attracting considerable attention from cancer biologists and the public. A study conducted on 48 samples of non-small cell lung cancer (NSCLC) and matched normal lung tissues observed specific cellular expression patterns of the COX-isoenzymes and terminal synthases of prostanoid synthesis. Increased COX-2 and simultaneous down-regulation of COX-1 expression in NSCLC were identified by immunohistochemistry [55]. Moreover, high levels of TXB2, the stable metabolite of TXA2, have been detected in human lung cancer tissues indicating increased TXA2S activity in lung cancer [56]. An extension of the aforementioned work, in a larger sample size, compared the prostaglandin biosynthesis pathways in small cell lung carcinoma and NSCLC and further correlated their observations with angiogenesis and metastasis [57]. Expression of TXA2S, COX-1, COX-2, and microsomal prostaglandin-E synthase were significantly higher in the metastatic cases of NSCLC as compared to non-metastatic cases [57].
TPα expression has been documented in five out of six NSCLC cell lines [58]. Moreover, A549 cells with ectopic TPα expression exhibited greater tumor growth and increased vascularization than the control A549 cells when implanted into nude mice [58]. Over-expression of COX-2 in lung tumors has been widely reported and factors that may cause over-expression of COX-2 in lung tumors are not completely understood. Activation of TPα with the TXA2 mimetic IBOP induces the expression of COX-2 through activation of four signaling pathways (extracellular signal-regulated kinase (ERK), p38 MAPK, JAK, and β-catenin). In addition, transcription factors such as NFκB, cAMP response element-binding (CREB), C/EBP, and Stat3 are downstream signaling molecules that interact with the COX-2 promoter and play important roles in TPα-mediated expression of COX-2 [59].
Strong evidence suggests that Nurr1 is critical for the proliferation of lung carcinoma cells (H157) through regulation of cyclin D1 expression [60]. Studies have indicated that TP signaling induces Nurr1 expression through a mechanism that bypasses the epidermal growth factor receptor (EGFR) pathway. Moreover, TP agonist-induced proliferation in lung cancer cells promoted cyclin D1 expression through induction of Nurr1 [60]. Nie and colleagues showed that pulmonary metastasis of intravenously injected Lewis lung carcinoma (LLC) is attenuated by TXA2S inhibitors. Moreover, oral administration of TXA2S inhibitors CI or furegrelate reduced the number and size of metastatic colonies in the lung after injection of LLC cells [61]. These data confirm that TXA2S activity is required for development of lung tumor metastasis and that TXA2S inhibitors are potential anti-metastatic agents. These findings shed new light on the contribution of TPα signaling to lung tumorigenesis.
Evading apoptosis (or programmed cell death) is a hallmark of most types of cancer [62] and is a key step in cancer progression. TXA2S expression inhibits apoptosis in lung cancer cells and TXA2S inhibition induces apoptosis in NSCLC cells. Moreover, two specific TXA2 antagonists enhance the effects of cisplatin (a chemotherapeutic agent) [63]. The mechanism by which TXA2 signaling enhances cell survival and proliferation in lung cancer cells involves reduction of nuclear p27 levels which mitigates apoptosis [64]. While the mechanism inducing apoptosis is not fully defined treating NSCLC cells with the TXA2S inhibitor 1-BI induces ROS generation and decreases NF-κB activity and nuclear translocation by decreasing IκBα phosphorylation [65].
Given the well-established link between smoking and lung cancer and the role for TXA2 signaling in lung cancer, it will be obvious to examine if TXA2 signaling mediates the smoking–lung cancer connection. Indeed, it was shown that significantly higher TXB2 production in lung cancer tissues from smokers compared to cancer tissues from non-smokers [66]. To examine the causal role for TXA2 signaling in lung cancer, Huang and colleagues recently correlated TXA2S, TXB2, and carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers and non-smokers. As expected, significantly higher TXA2S expression was observed in tumor tissues compared to non-tumor tissues from the same patient. A more interesting link to NNK could be inferred from the observation that significantly fewer tumors from non-smokers showed TXA2S expression as compared to tumors from smokers. Further, 100% of tissues from smokers (tumors and non-tumoral tissue) were positive for TXA2S expression unlike non-smokers where 50% of non-tumoral and 25% of tumoral tissues were negative for TXA2S expression. NNK increased TXA2S, TXA2 synthesis, and TP activation in vivo and in lung cancer cells in vitro. The increased TXA2 may subsequently activate CREB through the PI3K/Akt and ERK pathways, thereby contributing to the promotion of survival and growth of lung cancer cells by NNK [67]. Moreover, specific TXA2S inhibitors and TP antagonists abolished growth and induced apoptosis in lung cancer cells [67] indicating the importance of TXA2- to NNK-induced tumor growth in lung cancer.
These data were sufficiently provocative that Cathcart and colleagues examined the expression of TXA2S in NSCLC in 204 patients, to identify if TXA2S was a prognostic and/or survival factor [68]. They also examined the role of TXA2S in mediating the migration and growth of NSCLC cells in vitro. Though they observed no prognostic role for TXA2S in NSCLC, they confirmed that both the plasma and protein level of TXA2S and TXB2 were significantly higher in tumor tissues than the matched “normal” tissues [68]. Equally their model system confirmed that enhanced TXA2S expression promotes proliferation, motility, invasiveness, and survival of cancer cells. Inhibition of TXA2S with selective inhibitors induced apoptosis confirming that it is indeed a potential therapeutic target in NSCLC [68].
2.4 Role of thromboxane A2 signaling in colon cancer
Colorectal cancer is one of the leading causes of cancer-related deaths in the United States [35]; however, early diagnosis often leads to a complete cure. As in the case of prostate cancer, studies have shown strong correlation between high-fat diets, associated with consumption of red meat, and colon cancer [69]. Consumption of foods rich in AA likely increases synthesis of prostaglandins, such as TXA2, in the presence of enzymes associated with AA metabolism. TXA2S and TXA2 biosynthesis are increased in colon cancer and cause detrimental effects by promoting TP signaling [70]. The recognition that COX signaling was a strong pro-cancer signal for colon tumors is not recent and the correlated expression of COX-2 with colon carcinoma [71] has reinforced the causal relationship between the two. Epidemiologic data showed that up to 40% reduction of mortality in colorectal cancer patients with regular nonsteroidal anti-inflammatory drugs (NSAIDs) use as compared to non-NSAID consumers [71]. Gene transfer of TXA2S increased colon cancer cell growth in vivo and enhanced angiogenesis [72] (see below). Strong evidence of a role for TXA2S in colorectal carcinoma was provided when a marked over-expression was observed in different grades of colorectal tumors compared to the paired-normal tissues. The same study found increased expression of TXA2S in colon cancer cell lines and that abrogating TXA2 signaling with TXA2S inhibitors, TXA2S anti-sense as well as TP antagonists reduced proliferation of the colon cancer cell lines [73, 74]. qPCR was used to quantify TP transcript expression from 62 tumors and adjacent normal colon tissues. Results indicated that TP was expressed at significantly higher levels in tumors compared to normal tissues but displayed lower levels of TP expression in cultured colorectal cancer cell lines (HT-29 and HCA-7) [73]. The aforementioned studies have started to carve a role for TXA2 signaling in colon cancer; however, the data presented are all from studies with small sample size and need to be validated in a larger cohort of patients with colon cancer.
2.5 Role of thromboxane A2 signaling in brain cancer
Gliomas are brain malignancies of glial cell origin, such as oligodendroglioma, that are notorious for recurrence and the rate at which they progress to high-grade malignancies [75]. The capacity of glial tumor cells to migrate and diffusely infiltrate normal brain makes the disease notoriously difficult to cure by surgical eradication. Thus, identification of genes associated with invasion may offer novel strategies for anti-invasive therapies. This prompted McDonough and colleagues to assess glioma cell lines with a migration advantage on a glioma-derived extracellular matrix [76]. The outcome was a genetically stable strain that showed a migration advantage, a slightly arrested growth rate, anchorage-independent growth and was comparable to the parental cells in their tumorigenicity in athymic nude mice. This migration-advantaged strain showed increased TXA2S expression [76]. When treated with TXA2S antagonists (such as dazmegrel and furegrelate) the migration of the strain with enhanced mobility was normalized to the rate of the parental cells, suggesting direct regulation of cellular motility by TXA2S [76]. Giese and colleagues showed TXA2S expression to be present in a large panel of glioma cell lines but not in normal human astrocytes [77]. Moreover, TXA2S expression was observed in the parenchyma of glial tumors and in reactive astrocytes but not in quiescent astrocytes and oligodendroglia of normal brain. A wide range of TXB2 formation in glioma cell lines was observed, the relative expression of which correlated with migration rates of these cells. Not surprisingly, TXA2S-specific inhibitors effectively blocked the migration advantage and decreased intercellular adhesion in glioma cells [77]. This was the first indication that TXA2 signaling may play a crucial role in glial tumors and represent a novel strategy for anti-invasive therapies.
Giese and colleagues also revisited phospholipid biosynthesis in glioma by assessing the metabolic activity of the upstream enzymes COX-1 and COX-2. In comparison to inhibiting TXA2S, the inhibition of COX isoforms produced no significant shift in the phenotype of glioma, despite the robust expression of COX in all grades of glioma [77]. This may highlight a functionally antagonistic role for other downstream metabolites of COX in cancer. In addition, TXA2S inhibition with furegrelate induced caspase activation, DNA fragmentation, and eventual apoptosis only in glioma-derived cells but not normal astrocytes or fibroblast [77]. These same effects of TXA2S inhibition were also observed on endothelial cells indicating that anti-TXA2S therapy might sensitize the invasive glioma cells, and angiogenic endothelium to apoptosis [77].
Diagnosis of invasive glioma is often too late for anti-invasive therapy to be effective. Thus, the identification of TXA2S inhibitors as regulators of glioma cell motility is of little consequence unless the invasive cells are also rendered susceptible to cyto-reductive treatments. In this regard TXA2 antagonism shows great promise as an adjunct to standard therapy. Pre-treatment of glioma cells with furegrelate, a specific TXA2S inhibitor, increased radiation sensitivity of cultured glioma cells [78]. The present standard of care in glioblastoma (the most malignant grade 4 glioma) is surgical resection followed by combined chemo-radiation therapy [79]. Schmidt and colleagues presented compelling data indicating that a single administration of furegrelate to in vivo orthotopic glioblastoma in mice significantly reduced tumor size, tumor cell proliferation, and angiogenesis and increased apoptotic cell death [80]. Combining TXA2S inhibition with an alkylating agent (BCNU) also showed a significant survival effect on an ectopic mouse model of glioma [80]. The ultimate proof of principle for the inclusion of TXA2S inhibition in glioma therapy will be to demonstrate significant improvements to clinically valid treatment regimens (such as radiation therapy followed by temozolomide treatment) in orthotopic mouse models. If successful, TXA2S inhibition is likely to be quickly implemented as an adjunct therapy in clinical trials, which raises hopes of finding an effective treatment for glioblastoma patients especially for those with unmethylated MGMT promoter [81].
2.6 Role of thromboxane A2 signaling in bladder cancer
Over the last decade, there has been an increasing awareness of the role of prostanoids in the development and progression of invasive bladder cancer. Susceptibility to invasive bladder cancer correlates with the −765G>C mutation in the COX-2 promoter [82]. This polymorphism results in an under active promoter and lower COX-2 expression. This change likely causes liberated AA to shunt into a COX-1-based pathway and potentially results in greater synthesis of prostaglandins like TXA2. TXA2 release is not observed from unstimulated or challenged “normal” urothelial cells [83] and TXA2S release and TXA2S expression are very low in normal bladder tissue [84, 85]. Conversely, TXA2S expression is an average of 11.6-fold higher (range 5–18-fold) in invasive bladder cancer and transformed bladder epithelial cell lines exhibit greater expression than non-transformed immortalized cells [85]. Focal TXA2S expression is first observed in carcinoma in situ and becomes widespread in invasive and high-grade carcinoma [84]. In both bladder cancer tissue and cell lines, TXA2S expression is transcriptionally regulated. TXA2S expression correlates significantly with tumor grade, stage, and overall survival and is an independent marker of invasiveness in the tumor [85].
TP expression is also increased in invasive bladder cancer compared to “normal” adjacent and remote bladder tissue. TP expression is increased at the protein, but not them RNA, level prompting speculation that post-translational mechanisms stabilize the protein [85]. However, the increased TP expression in bladder cancer was documented using antibodies/ probes that bind the common domain. More recently, the significance of individual TP isoforms in bladder cancer has been investigated. Increased expression of the humanspecific isoform TPβ, but not TPα, is enhanced in epithelial and stromal compartments in invasive bladder cancer and correlates with cancer grade. Like expression of TXA2S, TP expression, and in particular TPβ expression, correlates well with increased growth rate, invasiveness, and shorter survival time [86]. TPβ expression was only observed in bladder cancer cell lines and inhibition of TPβ produces apoptosis associated with suppression of ERK and focal adhesion kinase (FAK) signaling. Conversely, ectopic expression of TPβ in “normal” bladder epithelium stimulates growth, migration, and invasive potential [86]. Moreover, it enables “normal” bladder cancer to form xenografts in nude mice that result in highly de-differentiated tumors [86]. TP signaling through the Gα12 family was implicated in the enhanced motility of bladder cancer but no further information was provided on the pathways responsible for proliferation. This is one of the few cancers for which roles of the two TP isoforms have been identified and provides evidence for distinct roles for TPα and TPβ in the pathogenesis of cancer.
While the changes in TXA2/TP expression are pronounced in bladder cancer, the real question is: Does targeting this pathway arrest/perturb tumor growth? Cells overexpressing TXA2S or TP grow at an accelerated rate and are highly invasive [85, 86]. Pharmacological antagonism of TXA2S or TP in vitro slows proliferation of bladder cancer cell lines two- to three fold and ablates migration and invasion [85, 86]. Further, depriving bladder cancer cells from TP signaling (either pharmacological inhibition or small interfering RNA (siRNA) knockdown approaches) promotes caspase processing and the onset of apoptosis [84] and sensitizes the cells to the effects of other chemotherapeutic agents including paclitaxel and cisplatin [84]. These data suggest that enhanced TXA2 levels due to TXA2S overexpression were activating TP-dependent pathways of cell proliferation.
2.7 Modulation of endothelial cell migration/angiogenesis by thromboxane A2
Neovascularization, or the formation of new blood vessels, is central to the pathogenesis of most solid tumors and their sequelae, including metastasis. It occurs through a number of mechanisms including angiogenesis, vasculogenesis, and intussusception (reviewed in [87]). The level of new blood vessel growth is dependent upon the balance between proand anti-angiogenic factors present in the tissue. TXA2 has both pro- and anti-angiogenic effects in multiple experimental systems. We have found that TP stimulation reduces spontaneous endothelial cell (EC) migration by 58% and in vitro capillary formation by 85% [88] as well as abrogating the pro-angiogenic effects of vascular endothelial growth factor (VEGF)-A [89] and fibroblast growth factor-2 (FGF-2) [90] in vitro and in vivo. Moreover, TP stimulation results in the destruction of established EC networks through increased apoptosis [91]. The regulatory pathways for each of the effects are highly stimulus specific and share few features in common. Antagonism of nitric oxide and FAK [89], stabilization of p53/antagonism of integrin αvβ3 [90], and inhibition of intercellular communication [88] are just some of the mechanisms employed by TXA2 to prevent angiogenesis. A number of reports from other groups support the concept that TXA2 is an anti-angiogenic stimulus. TXA2 suppresses angiogenesis and promotes vascular degeneration in the retina through induction of calpain-dependent neuro-retinovascular EC death [92]. TP stimulation mediates EC apoptosis associated with diabetes [93, 94] and acts in an additive/synergistic manner with platelet releasate [95] and neurokinin B [96] to promote EC injury and inhibit angiogenesis. In addition, the TP ligands isoprostane 8-iso-PGF2α, 8-iso-PGE2, and 8-iso-PGA2 all inhibit spontaneous and VEGF-induced migration and differentiation of human coronary ECs in vitro and sprouting angiogenesis from cardiac explants ex vivo without influencing apoptosis [97]. These effects are sensitive to inhibition of Rho kinase suggesting deranged actin metabolism was involved, later proven by alterations to stress fiber formation. Collectively, these data support the hypothesis that TXA2 may discourage re-vascularization of infarcted/hypoxic tissue and may promote the regression of vessels through the induction of apoptosis in exposed vascular beds. Thus, blocking TP seems an appropriate course of action in diseases such as myocardial infarction, where re-vascularization is to be encouraged.
Conversely, a number of groups have shown TXA2 is pro-angiogenic. Robust TXA2S expression is observed in the tumor-associated endothelial cells of lung cancer but not normal lung tissue [57]. These findings support the view that TXA2 may promote angiogenesis, thereby accelerating cancer progression. Some of the most convincing data have correlated the overexpression of TXA2 synthase in tumor cells with enhanced angiogenesis, shortened survival time, and increased tumor growth rate [72]. However, an important limitation of these findings was that the authors did not distinguish whether the effects of TXA2 on angiogenesis were due to direct actions on endothelial cells or an autocrine effect on the tumor cells. Indeed, Tai and colleagues [58] reported that TXA2 stimulation of human lung cancer cells enhances production of the pro-angiogenic protein VEGF, a key stimulus for tumor angiogenesis. Stimulation of TPα increased VEGF expression at both the mRNA and protein levels via a mechanism involving activation of ERK, PKA, EGFR, and Src kinases. Xenografts of A549-TPα cells induced greater tumor growth and increased vascularization in nude mice than control A549 cells [58]. However, evidence of increased neovascularization was only macroscopic and could have easily resulted from hemorrhage into the growing tumors. These data indicate that the autocrine effects of TP on the tumor itself are not just a regulator of tumor growth but also a significant regulator of neovascularization.
TXA2 also has direct pro-angiogenic effects on the endothelium. Pro-angiogenic factors (FGF-2 and VEGF) promote TXA2 release from EC up to five fold [61]. TXA2 stimulation promotes sprouting in corneal neovascularization and rat aortic ring assays, induces vascularization of the rodent ovary, and stimulates the differentiation and migration of endothelial cells in vitro [46, 61, 72, 98, 99]. Furthermore, antagonizing TP/TXA2 synthesis attenuates endothelial chemotaxis to FGF-2 and VEGF, differentiation of endothelium on matrigel in vitro, and angiogenesis in the corneal neovascularization and rat aortic ring assays [61, 100] [98]. These activities are not overcome by incubation with VEGF [100] indicating the strength of the inhibition of angiogenesis by these agents and/or essential contribution of TXA2 to the pro-angiogenic effects of other factors. These antagonists do not affect EC viability or adhesion indicating their effects on quiescent ECs would be minimal [100]. If these data hold true, then the inhibition of TXA2 synthesis or TP signaling during diseases, such as cancer, would prevent vessel formation, slowing tumor growth, and prolonging survival.
TPα and TPβ have almost identical coupling to heterotrimeric G-proteins resulting in misplaced complacency regarding their distinct roles in disease. We believe the dichotomy in the reported effects of TP stimulation on angiogenesis result from the use of models that lack TPβ expression. The relative importance of both TP isoforms to EC biology, especially TPβ, is largely unexplored. Our data also show that expressing TPβ in the endothelium from TP null mice inhibits migration and differentiation in vitro, and transgenic mice overexpressing TPβ in endothelial cells display reduced angiogenesis in matrigel plug models[89, 90]; however, only humans have TPβ with both rat and mouse TP similar to the human TPα isoform (78% homology). Thus, the small animal models upon which the pro-angiogenic properties of TXA2 are based are flawed as they do not account for TPβ making comparisons difficult. More importantly, our data indicate that the anti-angiogenic properties of TPβ dominate in EC expressing both isoforms [89, 90]. The primary deficit in the original report of the TPβ transgenic mouse was decreased placental size with microscopic evidence of ischemia [101]. Our seminal observations extend these findings and suggest that vessel formation in the developing placenta was most likely suppressed by TPβ. Further, the amplified signaling of TPα-TPβ heterodimers in response to isoprostanes [102] may be the reason for the highly potent nature of these TP agonists as anti-angiogenic compounds [97]. Thus, the existence of two TP isoforms in humans may have implications for the role of TXA2 in vascular remodeling in angiogenic diseases such as cancer.
The mechanisms by which the two TP isoforms regulate angiogenesis are still somewhat unclear. TPα does not share the same angioregulatory activity as TPβ indicating that the divergent tail residues are the source of these properties. The tail of TPβ contains multiple sites that could regulate receptor signaling but few are well characterized. Residues TPβ355–TPβ366 and TPβ337–TPβ344 are important for ligand-induced [103] and tonic [104] internalization and mediate interactions with Nm23-H2 [105] and Rab11 [106]. These data indicate the protein interactions of the two tails are different and have binding sites for signaling molecules that may regulate angiogenesis. Further, Kinsella and colleagues recently reported PRK1 and angio-associated migratory cell protein as the first non-G-protein signaling proteins that couple to TP isoforms that have direct effects on cell motility [51, 107]. These data highlight the role of non-G-protein-mediated mechanisms in the regulation of angiogenesis by TP isoforms and further strengthen the notion of divergent pathological roles for TPα and TPβ in disease.
3 Summary and future directions
In summary, these findings suggest TXA2S and TP not only play an important role in cardiovascular disorders but also in cancer progression and metastasis. Over the past decade, several advances have been made in the field of TXA2 research that have significantly increased our knowledge and understanding of the underlying mechanisms of TXA2S and TP signaling. COX-2 expression has been found to be upregulated in a variety of cancers and TXA2S and TXA2 being a downstream metabolite of COX-2 is also over expressed in various cancers. Cancer progression involves several key steps such as angiogenesis, cell survival, cell migration, cell invasion, and metastasis; studies have shown that both TXA2S and TP play important roles in one or more of these key process. It is of great importance to further determine whether and how prostanoids, such as TXA2, mediate the effects of COX-2 in cancer, potentially leading to a more targeted approach for cancer prevention and treatment. The failure of existing TXA2S inhibitors and TXA2 antagonists to show robust clinical benefit in treatment of cardiovascular disorders has largely been due to co-incident use of NSAIDs, such as aspirin, as part of the standard of care. Conversely, current therapeutic regimens for cancer incorporate few agents in this class making TXA2S/TP antagonism an attractive target with potentially significant therapeutic benefit. Moreover, in contrast to NSAIDs, TXA2S/TP inhibitors do not prevent other COX-derived anti-tumor products, such as PGI2, with beneficial effects from being synthesized [108, 109]. Hence, future work should focus on the advantages of directly targeting TXA2S and/or antagonizing TP for its functional role in cancer progression.
Acknowledgment
This work was supported by grants from the United States National Institutes of Health (KVH [1R01 CA114051-01A1]), and National Health and Medical Research Council of Australia (AWA [512154]). The work was also supported by a Biomedical Career Development Award from the National Health and Medical Research Council of Australia (AWA [402847]).
Contributor Information
Prasanna Ekambara, Department of Oncology, School of Medicine, Wayne State University, Detroit, MI 48202, USA; Bioactive Lipids Research Program (BLRP), Department of Pathology, School of Medicine, Wayne State University, Detroit, MI 48202, USA.
Wanyu Lambiv, Bioactive Lipids Research Program (BLRP), Department of Pathology, School of Medicine, Wayne State University, Detroit, MI 48202, USA.
Rosanna Cazzolli, Division of Perinatal Research, Kolling Institute for Medical Research, University of Sydney, Sydney, NSW, Australia.
Anthony W. Ashton, Division of Perinatal Research, Kolling Institute for Medical Research, University of Sydney, Sydney, NSW, Australia
Kenneth V. Honn, Email: k.v.honn@wayne.edu, Department of Oncology, School of Medicine, Wayne State University, Detroit, MI 48202, USA; Bioactive Lipids Research Program (BLRP), Department of Pathology, School of Medicine, Wayne State University, Detroit, MI 48202, USA.
References
- 1.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moncada S, Needleman P, Bunting S, Vane JR. Prostaglandin endoperoxide and thromboxane generating systems and their selective inhibition. Prostaglandins. 1976;12(3):323–335. doi: 10.1016/0090-6980(76)90014-9. [DOI] [PubMed] [Google Scholar]
- 3.Smyth EM. Thromboxane and the thromboxane receptor in cardiovascular disease. Clinical Lipidology. 2010;5(2):209–219. doi: 10.2217/CLP.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needleman P, Minkes M, Raz A. Thromboxanes: Selective biosynthesis and distinct biological properties. Science. 1976;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- 5.Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976;261(5561):558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- 6.Halushka PV, Allan CJ, Davis-Bruno KL. Thromboxane A2 receptors. Journal of Lipid Mediators and Cell Signalling. 1995;12(2–3):361–378. doi: 10.1016/0929-7855(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 7.Jones RL, Wilson NH, Armstrong RA. Characterization of thromboxane receptors in human platelets. Advances in Experimental Medicine and Biology. 1985;192:67–81. doi: 10.1007/978-1-4615-9442-0_6. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. The New England Journal of Medicine. 1986;315(16):983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 9.Katugampola SD, Davenport AP. Thromboxane receptor density is increased in human cardiovascular disease with evidence for inhibition at therapeutic concentrations by the AT(1) receptor antagonist losartan. British Journal of Pharmacology. 2001;134(7):1385–1392. doi: 10.1038/sj.bjp.0704416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neri Serneri GG, Gensini GF, Abbate R, Mugnaini C, Favilla S, Brunelli C, et al. Increased fibrinopeptide A formation and thromboxane A2 production in patients with ischemic heart disease: Relationships to coronary pathoanatomy, risk factors, and clinical manifestations. American Heart Journal. 1981;101(2):185–194. doi: 10.1016/0002-8703(81)90665-7. [DOI] [PubMed] [Google Scholar]
- 11.Fuse S, Kamiya T. Plasma thromboxane B2 concentration in pulmonary hypertension associated with congenital heart disease. Circulation. 1994;90(6):2952–2955. doi: 10.1161/01.cir.90.6.2952. [DOI] [PubMed] [Google Scholar]
- 12.Gresele P, Deckmyn H, Nenci GG, Vermylen J. Thromboxane synthase inhibitors, thromboxane receptor antagonists and dual blockers in thrombotic disorders. Trends in Pharmacological Sciences. 1991;12(4):158–163. doi: 10.1016/0165-6147(91)90533-x. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. The Journal of Clinical Investigation. 2004;114(6):784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta JL, Lawson D, Mehta P, Saldeen T. Increased prostacyclin and thromboxane A2 biosynthesis in atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(12):4511–4515. doi: 10.1073/pnas.85.12.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willerson JT, Yao SK, Ferguson JJ, Anderson HV, Golino P, Buja LM. Unstable angina pectoris and the progression to acute myocardial infarction. Role of platelets and platelet-derived mediators. Texas Heart Institute Journal. 1991;18(4):243–247. [PMC free article] [PubMed] [Google Scholar]
- 16.Lariviere R, Moreau C, Rodrigue ME, Lebel M. Thromboxane blockade reduces blood pressure and progression of renal failure independent of endothelin-1 in uremic rats. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2004;71(2):103–109. doi: 10.1016/j.plefa.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Willerson JT, Buja LM. Potential of combined thromboxane A2 and serotonin antagonists to prevent the development of unstable angina and acute myocardial infarction. Texas Heart Institute Journal. 1990;17(3):157–164. [PMC free article] [PubMed] [Google Scholar]
- 18.Willerson JT, Golino P, Eidt J, Yao SK, Buja LM. Potential usefulness of combined thromboxane A2 and serotonin receptor blockade for preventing the conversion from chronic to acute coronary artery disease syndromes. The American Journal of Cardiology. 1990;66(16):48G–53G. doi: 10.1016/0002-9149(90)90396-i. [DOI] [PubMed] [Google Scholar]
- 19.Haurand M, Ullrich V. Isolation and characterization of thromboxane synthase from human platelets as a cytochrome P-450 enzyme. Journal of Biological Chemistry. 1985;260(28):15059–15067. [PubMed] [Google Scholar]
- 20.Ohashi K, Ruan KH, Kulmacz RJ, Wu KK, Wang LH. Primary structure of human thromboxane synthase determined from the cDNA sequence. Journal of Biological Chemistry. 1992;267(2):789–793. [PubMed] [Google Scholar]
- 21.Shen RF, Tai HH. Thromboxanes: Synthase and receptors. Journal of Biomedical Science. 1998;5(3):153–172. doi: 10.1007/BF02253465. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama C, Miyata A, Ihara H, Ullrich V, Tanabe T. Molecular cloning of human platelet thromboxane A synthase. Biochemical and Biophysical Research Communications. 1991;178(3):1479–1484. doi: 10.1016/0006-291x(91)91060-p. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe T, Ullrich V. Prostacyclin and thromboxane synthases. Journal of Lipid Mediators and Cell Signalling. 1995;12(2–3):243–255. doi: 10.1016/0929-7855(95)00031-k. [DOI] [PubMed] [Google Scholar]
- 24.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, et al. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 25.Raychowdhury MK, Yukawa M, Collins LJ, McGrail SH, Kent KC, Ware JA. Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. Journal of Biological Chemistry. 1994;269(30):19256–19261. [Comparative Study]. [PubMed] [Google Scholar]
- 26.Kinsella BT. Thromboxane A2 signalling in humans: A 'Tail' of two receptors. Biochemical Society Transactions. 2001;29(Pt 6):641–654. doi: 10.1042/0300-5127:0290641. [DOI] [PubMed] [Google Scholar]
- 27.Miggin SM, Kinsella BT. Expression and tissue distribution of the mRNAs encoding the human thromboxane A2 receptor (TP) alpha and beta isoforms. Biochimica et Biophysica Acta. 1998;1425(3):543–559. doi: 10.1016/s0304-4165(98)00109-3. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 28.Nakahata N. Thromboxane A2: Physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacology and Therapeutics. 2008;118(1):18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Namba T, Narumiya S. Thromboxane A2 receptor; structure, function and tissue distribution. Nihon Rinsho. 1993;51(1):233–240. [PubMed] [Google Scholar]
- 30.Honn KV, Bockman RS, Marnett LJ. Prostaglandins and cancer: A review of tumor initiation through tumor metastasis. Prostaglandins. 1981;21(5):833–864. doi: 10.1016/0090-6980(81)90240-9. [DOI] [PubMed] [Google Scholar]
- 31.Honn KV, Busse WD, Sloane BF. Prostacyclin and thromboxanes. Implications for their role in tumor cell metastasis. Biochemical Pharmacology. 1983;32(1):1–11. doi: 10.1016/0006-2952(83)90644-5. [DOI] [PubMed] [Google Scholar]
- 32.Menter DG, Neagos J, Dunn R, Pallazo TT, Chen T, Taylor JD, et al. Tumor Cell induced Platelet aggregration: Inhibition by prostacylin, thromboxane A2 and phosphodiesterase inhibitors. In: Powles TJ, Bockman RS, Honn KV, Ramwell PW, editors. Prostaglandins and cancer. New York: Alan Liss, Inc.; 1982. pp. 369–374. [Google Scholar]
- 33.Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cellular and Molecular Life Sciences. 2002;59(5):799–807. doi: 10.1007/s00018-002-8468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honn KV, Meyer J. Thromboxanes and prostacyclin: Positive and negative modulators of tumor growth. Biochemical and Biophysical Research Communications. 1981;102(4):1122–1129. doi: 10.1016/s0006-291x(81)80128-3. [DOI] [PubMed] [Google Scholar]
- 35.U.S.C.S.W.Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Google Scholar]
- 36.Bairati I, Meyer F, Fradet Y, Moore L. Dietary fat and advanced prostate cancer. The Journal of Urology. 1998;159(4):1271–1275. [Research Support, Non-U.S. Gov't]. [PubMed] [Google Scholar]
- 37.Rose DP, Connolly JM. Dietary fat, fatty acids and prostate cancer. Lipids. 1992;27(10):798–803. doi: 10.1007/BF02535853. [Review]. [DOI] [PubMed] [Google Scholar]
- 38.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: A case-control study with special emphasis on aggressive tumors. Cancer Causes & Control. 1991;2(2):85–94. doi: 10.1007/BF00053126. [Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is over expressed in prostate adenocarcinoma. Cancer. 2001;91(4):737–743. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 40.Nie D, Che M, Grignon D, Tang K, Honn KV. Role of eicosanoids in prostate cancer progression. Cancer Metastasis Reviews. 2001;20(3–4):195–206. doi: 10.1023/a:1015579209850. [DOI] [PubMed] [Google Scholar]
- 41.Nie D, Hillman GG, Geddes T, Tang K, Pierson C, Grignon DJ, et al. Platelet-type 12-lipoxygenase regulates angiogenesis in human prostate carcinoma. Advances in Experimental Medicine and Biology. 1999;469:623–630. doi: 10.1007/978-1-4615-4793-8_90. [DOI] [PubMed] [Google Scholar]
- 42.Nie D, Nemeth J, Qiao Y, Zacharek A, Li L, Hanna K, et al. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clinical & Experimental Metastasis. 2003;20(7):657–663. doi: 10.1023/a:1027302408187. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. The Prostate. 2000;42(1):73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Lee LM, Pan CC, Cheng CJ, Chi CW, Liu TY. Expression of cyclooxygenase-2 in prostate adenocarcinoma and benign prostatic hyperplasia. Anticancer Research. 2001;21:1291–1294. [PubMed] [Google Scholar]
- 45.Nie D, Che M, Zacharek A, Qiao Y, Li L, Li X, et al. Differential expression of thromboxane synthase in prostate carcinoma: Role in tumor cell motility. American Journal of Pathology. 2004;164(2):429–439. doi: 10.1016/S0002-9440(10)63133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dassesse T, de Leval X, de Leval L, Pirotte B, Castronovo V, Waltregny D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor Gleason score and pathologic stage. Eur Urol. 2006;50(5):1021–1031. doi: 10.1016/j.eururo.2006.01.036. discussion 1031. [DOI] [PubMed] [Google Scholar]
- 47.Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. Journal of Urology. 1989;142(3):763–768. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- 48.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nature reviews. Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [Review] [DOI] [PubMed] [Google Scholar]
- 49.Nie D, Guo Y, Yang D, Tang Y, Chen Y, Wang MT, et al. Thromboxane A2 receptors in prostate carcinoma: Expression and its role in regulating cell motility via small GTPase Rho. Cancer Research. 2008;68(1):115–121. doi: 10.1158/0008-5472.CAN-07-1018. [DOI] [PubMed] [Google Scholar]
- 50.Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. Journal of Biological Chemistry. 2006;281(36):26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- 51.Turner EC, Kavanagh DJ, Mulvaney EP, McLean C, Wikstrom K, Reid HM, et al. Identification of an interaction between the TPalpha and TPbeta isoforms of the human thromboxane A2 receptor with protein kinase C-related kinase (PRK) 1: Implications for prostate cancer. Journal of Biological Chemistry. 2011;286(17):15440–15457. doi: 10.1074/jbc.M110.181180. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Expression of thromboxane synthase, TBXAS1 and the thromboxane A2 receptor, TBXA2R, in human breast cancer. International Seminars in Surgical Oncology. 2005;2:23. doi: 10.1186/1477-7800-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abraham JE, Harrington P, Driver KE, Tyrer J, Easton DF, Dunning AM, et al. Common polymorphisms in the prostaglandin pathway genes and their association with breast cancer susceptibility and survival. Clinical Cancer Research. 2009;15(6):2181–2191. doi: 10.1158/1078-0432.CCR-08-0716. [DOI] [PubMed] [Google Scholar]
- 55.Ermert L, Dierkes C, Ermert M. Immunohistochemical expression of cyclooxygenase isoenzymes and downstream enzymes in human lung tumors. Clinical Cancer Research. 2003;9(5):1604–1610. [PubMed] [Google Scholar]
- 56.Kreutzer M, Fauti T, Kaddatz K, Seifart C, Neubauer A, Schweer H, et al. Specific components of prostanoid-signaling pathways are present in non-small cell lung cancer cells. Oncology Reports. 2007;18(2):497–501. [PubMed] [Google Scholar]
- 57.Yoshimoto A, Kasahara K, Kawashima A, Fujimura M, Nakao S. Characterization of the prostaglandin biosynthetic pathway in non-small cell lung cancer: A comparison with small cell lung cancer and correlation with angiogenesis, angiogenic factors and metastases. Oncology Reports. 2005;13(6):1049–1057. [PubMed] [Google Scholar]
- 58.Wei J, Yan W, Li X, Ding Y, Tai HH. Thromboxane receptor alpha mediates tumor growth and angiogenesis via induction of vascular endothelial growth factor expression in human lung cancer cells. Lung Cancer. 2010;69(1):26–32. doi: 10.1016/j.lungcan.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Wei J, Yan W, Li X, Chang WC, Tai HH. Activation of thromboxane receptor alpha induces expression of cyclooxygenase-2 through multiple signaling pathways in A549 human lung adenocarcinoma cells. Biochemical Pharmacology. 2007;74(5):787–800. doi: 10.1016/j.bcp.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Tai HH. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis. 2009;30(9):1606–1613. doi: 10.1093/carcin/bgp161. [DOI] [PubMed] [Google Scholar]
- 61.Nie D, Lamberti M, Zacharek A, Li L, Szekeres K, Tang K, et al. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochemical and Biophysical Research Communications. 2000;267(1):245–251. doi: 10.1006/bbrc.1999.1840. [DOI] [PubMed] [Google Scholar]
- 62.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [Review]. [DOI] [PubMed] [Google Scholar]
- 63.Fujimura M, Kasahara K, Shirasaki H, Heki U, Iwasa K, Ueda A, et al. Up-regulation of ICH-1 L protein by thromboxane A2 antagonists enhances cisplatin-induced apoptosis in non-small-cell lung-cancer cell lines. Journal of Cancer Research and Clinical Oncology. 1999;125(7):389–394. doi: 10.1007/s004320050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leung KC, Hsin MK, Chan JS, Yip JH, Li M, Leung BC, et al. Inhibition of thromboxane synthase induces lung cancer cell death via increasing the nuclear p27. Experimental cell research. 2009;315(17):2974–2981. doi: 10.1016/j.yexcr.2009.06.025. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 65.Leung KC, Li MY, Leung BC, Hsin MK, Mok TS, Underwood MJ, et al. Thromboxane synthase suppression induces lung cancer cell apoptosis via inhibiting NF-kappaB. Experimental Cell Research. 2010;316(20):3468–3477. doi: 10.1016/j.yexcr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 66.McLemore TL, Hubbard WC, Litterst CL, Liu MC, Miller S, McMahon NA, et al. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Research. 1988;48(11):3140–3147. [PubMed] [Google Scholar]
- 67.Huang RY, Li MY, Hsin MK, Underwood MJ, Ma LT, Mok TS, et al. 4-Methylnitrosamino-1-3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane A2 and its receptor. Oncogene. 2011;30(1):106–116. doi: 10.1038/onc.2010.390. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 68.Cathcart MC, Gately K, Cummins R, Kay E, O'Byrne KJ, Pidgeon GP. Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer. Molecular Cancer. 2011;10:25. doi: 10.1186/1476-4598-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAfee AJ, McSorley EM, Cuskelly GJ, Moss BW, Wallace JM, Bonham MP, et al. Red meat consumption: An overview of the risks and benefits. Meat science. 2010;84(1):1–13. doi: 10.1016/j.meatsci.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 70.Bing RJ, Miyataka M, Rich KA, Hanson N, Wang X, Slosser HD, et al. Nitric oxide, prostanoids, cyclooxygenase, and angiogenesis in colon and breast cancer. Clinical Cancer Research. 2001;7(11):3385–3392. [PubMed] [Google Scholar]
- 71.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(7):3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pradono P, Tazawa R, Maemondo M, Tanaka M, Usui K, Saijo Y, et al. Gene transfer of thromboxane A(2) synthase and prostaglandin I(2) synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Research. 2002;62(1):63–66. [PubMed] [Google Scholar]
- 73.Gustafsson A, Hansson E, Kressner U, Nordgren S, Andersson M, Lonnroth C, et al. Prostanoid receptor expression in colorectal cancer related to tumor stage, differentiation and progression. Acta Oncologica. 2007;46(8):1107–1112. doi: 10.1080/02841860701403061. [DOI] [PubMed] [Google Scholar]
- 74.Sakai H, Suzuki T, Takahashi Y, Ukai M, Tauchi K, Fujii T, et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Letters. 2006;580(14):3368–3374. doi: 10.1016/j.febslet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Review of Anticancer Therapy. 2006;6(7):1087–1104. doi: 10.1586/14737140.6.7.1087. [Review]. [DOI] [PubMed] [Google Scholar]
- 76.McDonough W, Tran N, Giese A, Norman SA, Berens ME. Altered gene expression in human astrocytoma cells selected for migration: I. Thromboxane synthase. Journal of Neuropathology and Experimental Neurology. 1998;57(5):449–455. doi: 10.1097/00005072-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Giese A, Hagel C, Kim EL, Zapf S, Djawaheri J, Berens ME, et al. Thromboxane synthase regulates the migratory phenotype of human glioma cells. Neuro-Oncology. 1999;1(1):3–13. doi: 10.1093/neuonc/1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schauff AK, Kim EL, Leppert J, Nadrowitz R, Wuestenberg R, Brockmann MA, et al. Inhibition of invasion-associated thromboxane synthase sensitizes experimental gliomas to gamma-radiation. Journal of Neuro-Oncology. 2009;91(3):241–249. doi: 10.1007/s11060-008-9708-0. [DOI] [PubMed] [Google Scholar]
- 79.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt NO, Ziu M, Cargioli T, Westphal M, Giese A, Black PM, et al. Inhibition of thromboxane synthase activity improves glioblastoma response to alkylation chemotherapy. Translational Oncology. 2010;3(1):43–49. doi: 10.1593/tlo.09238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England Journal of Medicine. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 82.Gangwar R, Mandhani A, Mittal RD. Functional polymorphisms of cyclooxygenase-2 (COX-2) gene and risk for urinary bladder cancer in North India. Surgery. 2011;149(1):126–134. doi: 10.1016/j.surg.2010.04.004. [Comparative Study]. [DOI] [PubMed] [Google Scholar]
- 83.Danon A, Zenser TV, Thomasson DL, Davis BB. Eicosanoid synthesis by cultured human urothelial cells: Potential role in bladder cancer. Cancer Research. 1986;46(11):5676–5681. [PubMed] [Google Scholar]
- 84.Moussa O, Riker JM, Klein J, Fraig M, Halushka PV, Watson DK. Inhibition of thromboxane synthase activity modulates bladder cancer cell responses to chemotherapeutic agents. Oncogene. 2008;27(1):55–62. doi: 10.1038/sj.onc.1210629. [DOI] [PubMed] [Google Scholar]
- 85.Moussa O, Yordy JS, Abol-Enein H, Sinha D, Bissada NK, Halushka PV, et al. Prognostic and functional significance of thromboxane synthase gene overexpression in invasive bladder cancer. Cancer Research. 2005;65(24):11581–11587. doi: 10.1158/0008-5472.CAN-05-1622. [DOI] [PubMed] [Google Scholar]
- 86.Moussa O, Ashton AW, Fraig M, Garrett-Mayer E, Ghoneim MA, Halushka PV, et al. Novel role of thromboxane receptors beta isoformin bladder cancer pathogenesis. Cancer Research. 2008;68(11):4097–4104. doi: 10.1158/0008-5472.CAN-07-6560. [DOI] [PubMed] [Google Scholar]
- 87.Patan S. Vasculogenesis and angiogenesis. Cancer Treatment and Research. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- 88.Ashton AW, Yokota R, John G, Zhao S, Suadicani SO, Spray DC, et al. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2) Journal of Biological Chemistry. 1999;274(50):35562–35570. doi: 10.1074/jbc.274.50.35562. [DOI] [PubMed] [Google Scholar]
- 89.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor–induced endothelial cell differentiation andmigration. Circulation Research. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- 90.Ashton AW, Cheng Y, Helisch A, Ware JA. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and αvβ3. Circulation Research. 2004;94:735–742. doi: 10.1161/01.RES.0000122043.11286.57. [DOI] [PubMed] [Google Scholar]
- 91.Gao Y, Yokota R, Tang S, Ashton AW, Ware JA. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2) Circulation Research. 2000;87(9):739–745. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- 92.Beauchamp MH, Martinez-Bermudez AK, Gobeil F, Jr, Marrache AM, Hou X, Speranza G, et al. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. Journal of Applied Physiology. 2001;90(6):2279–2288. doi: 10.1152/jappl.2001.90.6.2279. [DOI] [PubMed] [Google Scholar]
- 93.De La Cruz JP, Moreno A, Ruiz-Ruiz MI, Sanchez De La Cuesta F. Effect of DT-TX 30, a combined thromboxane synthase inhibitor and thromboxane receptor antagonist, on retinal vascularity in experimental diabetes mellitus. Thrombosis Research. 2000;97(3):125–131. doi: 10.1016/s0049-3848(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 94.Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51(1):198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- 95.Kishi Y, Numano F. In vitro study of vascular endothelial injury by activated platelets and its prevention. Atherosclerosis. 1989;76(2–3):95–101. doi: 10.1016/0021-9150(89)90092-0. [DOI] [PubMed] [Google Scholar]
- 96.Pal S, Wu J, Murray JK, Gellman SH, Wozniak MA, Keely PJ, et al. An antiangiogenic neurokinin-B/ thromboxane A2 regulatory axis. The Journal of Cell Biology. 2006;174(7):1047–1058. doi: 10.1083/jcb.200603152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didié M, et al. Isoprostanes inhibit vascular endothelial growth factor–induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A2 receptor. A potential link between oxidative stress and impaired angiogenesis. Circulation Research. 2008;103:1037–1046. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
- 98.Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Research. 1999;59(18):4574–4577. [PubMed] [Google Scholar]
- 99.Sakurai T, Tamura K, Kogo H. Stimulatory effects of eicosanoids on ovarian angiogenesis in early luteal phase in cyclooxygenase-2 inhibitor-treated rats. European Journal of Pharmacology. 2005;516(2):158–164. doi: 10.1016/j.ejphar.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 100.de Leval X, Dassesse T, Dogne JM, Waltregny D, Bellahcene A, Benoit V, et al. Evaluation of original dual thromboxane A2 modulators as antiangiogenic agents. Journal of Pharmacology and Experimental Therapeutics. 2006;318(3):1057–1067. doi: 10.1124/jpet.106.101188. [DOI] [PubMed] [Google Scholar]
- 101.Rocca B, Loeb AL, Strauss JF, 3rd, Vezza R, Habib A, Li H, et al. Directed vascular expression of the thromboxane A2 receptor results in intrauterine growth retardation. Nature Medicine. 2000;6(2):219–221. doi: 10.1038/72334. [DOI] [PubMed] [Google Scholar]
- 102.Wilson SJ, McGinley K, Huang AJ, Smyth EM. Heterodimerization of the alpha and beta isoforms of the human thromboxane receptor enhances isoprostane signaling. Biochemical and Biophysical Research Communications. 2007;352(2):397–403. doi: 10.1016/j.bbrc.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parent JL, Labrecque P, Orsini MJ, Benovic JL. Internalization of the TXA2 receptor alpha and beta isoforms. Role of the differentially spliced cooh terminus in agonist-promoted receptor internalization. Journal of Biological Chemistry. 1999;274(13):8941–8948. doi: 10.1074/jbc.274.13.8941. [DOI] [PubMed] [Google Scholar]
- 104.Parent JL, Labrecque P, Driss Rochdi M, Benovic JL. Role of the differentially spliced carboxyl terminus in thromboxane A2 receptor trafficking: Identification of a distinct motif for tonic internalization. Journal of Biological Chemistry. 2001;276(10):7079–7085. doi: 10.1074/jbc.M009375200. [DOI] [PubMed] [Google Scholar]
- 105.Rochdi MD, Laroche G, Dupre E, Giguere P, Lebel A, Watier V, et al. Nm23-H2 interacts with a G protein-coupled receptor to regulate its endocytosis through an Rac1-dependent mechanism. Journal of Biological Chemistry. 2004;279(18):18981–18989. doi: 10.1074/jbc.M312621200. [DOI] [PubMed] [Google Scholar]
- 106.Theriault C, Rochdi MD, Parent JL. Role of the Rab11-associated intracellular pool of receptors formed by constitutive endocytosis of the beta isoform of the thromboxane A2 receptor (TP beta) Biochemistry. 2004;43(19):5600–5607. doi: 10.1021/bi036268v. [DOI] [PubMed] [Google Scholar]
- 107.Reid HM, Wikstrom K, Kavanagh DJ, Mulvaney EP, Kinsella BT. Interaction of angio-associated migratory cell protein with the TPalpha and TPbeta isoforms of the human thromboxane A receptor. Cellular Signalling. 2011;23(4):700–717. doi: 10.1016/j.cellsig.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Honn KV, Cicone B, Skoff A. Prostacyclin: A potent antimetastatic agent. Science. 1981;212(4500):1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- 109.Menter DG, Onoda JM, Taylor JD, Honn KV. Effects of prostacyclin on tumor cell-induced platelet aggregation. Cancer Research. 1984;44(2):450–456. [PubMed] [Google Scholar]