Abstract
Task-induced deactivation of the default-mode network (DMN) has been associated in adults with successful episodic memory formation, possibly as a mechanism to focus allocation of mental resources for successful encoding of external stimuli. We investigated developmental changes of deactivation of the DMN (posterior cingulate, medial prefrontal, and bilateral lateral parietal cortices) during episodic memory formation in children, adolescents, and young adults (ages 8–24), who studied scenes during functional magnetic resonance imaging (fMRI). Recognition memory improved with age. We defined DMN regions of interest from a different sample of participants with the same age range, using resting-state fMRI. In adults, there was greater deactivation of the DMN for scenes that were later remembered than scenes that were later forgotten. In children, deactivation of the default-network did not differ reliably between scenes that were later remembered or forgotten. Adolescents exhibited a pattern of activation intermediate to that of children and adults. The hippocampal region, often considered part of the DMN, showed a functional dissociation with the rest of the DMN by exhibiting increased activation for later remembered than later forgotten scene that was similar across age groups. These findings suggest that development of memory ability from childhood through adulthood may involve increased deactivation of the neocortical DMN during learning.
Keywords: fMRI, children, task suppression, memory encoding, resting-state fMRI
Introduction
The ability to form detailed memories for facts and events is essential for education and for everyday life, and increases from childhood to adulthood (Cycowicz et al., 2001; Ghetti and Angelini, 2008; Mandler and Robinson, 1978). Successful memory formation in adults is correlated with activations in a number of brain regions, including the prefrontal cortex (PFC) and the medial temporal lobe (MTL) (Brewer et al., 1998; Wagner et al., 1998). Activations in these regions are also correlated with successful memory formation in children (Chai et al., 2010; Ghetti et al., 2010; Ofen, 2012; Ofen et al., 2007). Activations in these regions are greater during encoding of items that are subsequently remembered compared to those that are subsequently forgotten. In adults, deactivations of a different set of brain regions, including midline regions such as posterior cingulate cortex (PCC) and lateral parietal cortices, are also associated with successful memory encoding (Daselaar et al., 2004). The amplitude of deactivation in these regions is greater for items that are later remembered than for items that are later forgotten. Here we asked whether deactivation or suppression of those brain regions during memory formation undergoes maturation between childhood and adulthood.
Brain regions exhibiting deactivation during successful memory encoding in adults overlap with regions of the default-mode network (DMN), a network of brain regions commonly deactivated during tasks that demand external attention (Raichle et al., 2001). The DMN is consistently comprised of the PCC, medial prefrontal cortex (MPFC), and left and right lateral parietal cortices (LLP and RLP)(Raichle et al., 2001), and also frequently extends to the hippocampal region bilaterally (Buckner et al., 2008) The DMN may be activated in internal- and self-oriented processing (Buckner et al., 2008). Suppression of the DMN, on the other hand, appears to be functionally important for successful operation of cognitive processes that demand attention to the environment. For example, better sustained attention is associated with more deactivation of the DMN (Lawrence et al., 2003), whereas momentary lapses in attention are associated with reduced task-induced deactivation of the DMN (Lawrence et al., 2003; Weissman et al., 2006). Greater working memory demands provoke both increased activation in cognitive control regions (e.g., PFC) and also increased deactivation in the DMN (McKiernan et al., 2003). Task-induced deactivation of the DMN may signal the suppression of attention to one’s own thoughts or feelings and promote the allocation of mental and neural resources to tasks involving external stimuli (Anticevic et al., 2012; Whitfield-Gabrieli et al.). In the case of episodic memory formation, more deactivation of the DMN may enhance resources allocated to memory encoding of external stimuli and thus better long-term memory.
Development of the DMN has been studied using resting-state fMRI, and although there is considerable evidence that the DMN develops from childhood through adulthood, methodological issues have made uncertain the specific nature of that development. Some studies suggest that long-range correlations among the DMN components grow markedly from childhood through young adulthood (Barber et al., 2013; Fair et al., 2008; Fair et al., 2007; Supekar et al.; Supekar et al., 2010). Other studies, noting evidence that differences in head movement have major influences on the analysis of resting-state connectivity (Power et al., 2012; Van Dijk et al., 2012; Yan et al., 2013) and that such movement declines precipitously with age, have controlled for such movement and have reported far smaller developmental effect of DMN correlations (Satterthwaite et al., 2012; Chai et al., submitted). Developmental effects for DMN may be more robust when anticorrelations between the DMN and cortical areas involved in cognitive control are considered (Barber et al., 2013; Chai et al., submitted).
Based on evidence of DMN deactivation during memory encoding in adults and the maturation of DMN in resting-state, here we examined whether or not there were developmental changes related to deactivation of the DMN during memory encoding that predicted subsequent memory. Prior studies of such development in children and adolescents relative to adults have focused exclusively on activations related to successful memory formation, and not deactivations. For scenes, there were developmental increases in PFC and parietal activations for the successful encoding of well-remembered scenes (Ofen et al., 2007), and a similar finding for the successful retrieval of memory for scenes (Ofen et al., 2012). MTL activations were associated with successful encoding and retrieval, but did not change with age (Ofen et al., 2012; Ofen et al., 2007). Other studies, however, have reported developmental differences in MTL activation related to memory formation for specifically complex scenes (Chai et al., 2010) or contextual information (Ghetti et al., 2010). Thus, there are findings of both early maturation in which memory-related activations are adult-like in childhood, and also late maturation in which memory-related activations grow through young adulthood. Here we investigated the development of task-induced deactivation of the DMN during memory formation in a reanalysis of previously published data (Ofen et al., 2007) that examined the normal development of activations related to successful memory formation, in healthy children, adolescents and adults from age 8 to 24.
Methods
Participants
Fifty-two volunteers, ages 8 to 24 years, were recruited from the Stanford University community and provided informed consent as indicated by a Stanford University IRB-approved protocol. All participants were right-handed, had normal or corrected-to-normal vision, with no history of psychiatric or neurological disorder. Two participants were excluded as a result of motion artifacts during scan (maximum head movement during the fMRI task exceeded 3mm). In addition, two participants were excluded due to incomplete data. We present data from the remaining 48 participants (mean age = 15.7 ± 4.5, 25 females). Analyses were performed on three age groups: children (ages 8–12, N = 16), adolescents (ages 13–17, N = 18) and adults (ages 18–24, N = 14).). All participants were tested on a standardized speed of processing (SOP) test (Visual Matching, Woodcock-Johnson III (Woodcock et al., 2001)). Age-normed scores on that test did not differ among the groups (F(2,45) = 2.45, p > .1), suggesting the validity of cross-sectional comparison in this sample.
Memory task
Participants viewed 125 indoor and 125 outdoor scenes during a scanned study phase that was followed by a recognition memory test. During scanning, each picture was presented for 3 s with 1 s of inter-trial interval. Participants made “indoor” or “outdoor” judgments to each scene by pressing a button on the button box. Trials with incorrect or no responses were excluded from the analyses (error trials). The study phase was divided into five sessions, each with 50 scenes. After the scanning session, participants were given a self-paced recognition test of the 250 scenes studied during the scanning session and 250 new scenes. If the participant responded “old” to a scene, they were further asked to indicate if they “actually remembered” the scene (R) or if the scene “just looks familiar” (Know, K). Adjusted memory accuracy was calculated by subtracting the false alarm rate (“old” responses to new pictures) from the hit rate (“old” responses to studied pictures). In addition to the over all accuracy (Hits − FA), accuracy for “R” and “K” trial types were calculated separately, by subtracting the corresponding false alarm rate from the hit rate for R or K trial types (R accuracy: R − FAR; K accuracy: K/(1−R) − FAK, adjusted for being mathematically constrained by R responses). If a “new” response was given to a studied scene, the trial was classified as a “forgotten” trial (F).
Imaging procedure
MRI data were acquired in a 1.5 T GE scanner. T1-weighted whole-brain anatomy images (256 × 256 voxels, 0.86-mm in-plane resolution, 1.2-mm slice thickness) were acquired prior to the functional scans. Functional images were acquired using T2*-sensitive two-dimensional gradient-echo sequence in 24 contiguous, 6-mm slices parallel to the line connecting anterior and posterior commissures, with 2 s repetition time, 60 degree flip angle, 64 × 64 voxels, and 3.75 mm in-plane resolution. The first two volumes of each run were discarded.
fMRI analysis
Functional imaging data were analyzed in SPM8 (Department of Imaging Neuroscience, London, UK). Functional images were slice-time corrected and motion corrected. The anatomical image was coregistered to the mean functional image that was created during motion correction. Functional images were then spatially normalized to the T2 Montreal Neurological Institute (MNI) template, and smoothed with a 6-mm Gaussian kernel. Data were inspected for artifacts and motion using custom software (http://www.nitrc.org/projects/artifact_detect/). First-level analysis was performed with a general linear model (GLM) with regressors for R, K, and F and error trials. Additional regressors accounted for head movement (3 translation, 3 rotation parameters) and outlier scans (images in which average intensity deviated more than 3 SD from the mean intensity in the session or in which movement exceeded 0.5mm in translation or 0.01 degree in rotation from the previous image). Each outlier scan was represented by a single regressor in the GLM, with a 1 for the outlier time point and 0s elsewhere. There was a significant age-group difference in the number of outlier images (F(2,47) = 5.3, p = .009). Children had more outliers (mean = 15.9 ± 11.7) than both adults (5.7 ± 10.5) and adolescents (7.3 ± 6.4) (children vs. adults: t(28) = 2.6, p = .016; children vs. adolescents t(32) = 2.7, p = .011). Adolescents and adults did not differ in the number of outliers (t(30) = .6, p > .5).
DMN Region of Interest (ROI) analysis
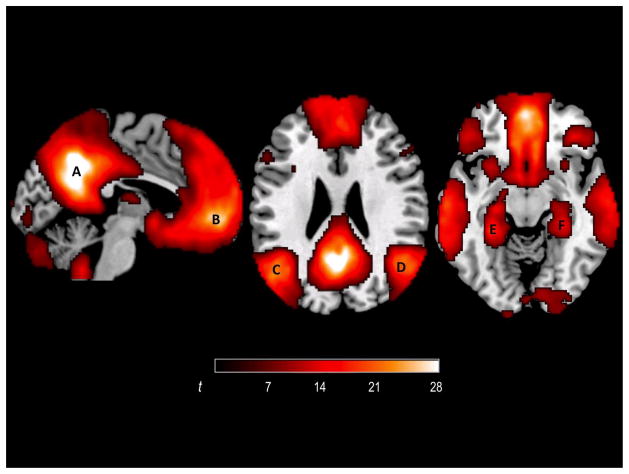
We examined activations during R and F conditions in four independently defined neocortical default-mode regions of interests: MPFC, PCC, LLP, and RLP created as 15mm spheres around peak coordinates from an independent developmental resting-state fMRI study (Chai et al., under review) in 82 participants of the same age range (8–24 years) as in the present study. In that study, first-level correlation maps for each of the four DMN seeds (created around coordinates from literature (Fox et al., 2005)) were produced by computing Pearson’s correlation coefficients between the seed time course and the time course of all other voxels. Average time courses from the four DMN seeds were used to produce a DMN correlation map for each participant. A group-level correlation map was produced from fisher z transformed first-level DMN correlation maps (Figure 1). The peaks of the group level correlation map were: PCC (−2, −54, 38), MPFC (2, 56, −4), LLP (−48, −70, 34) and RLP (48, −68, 40). These coordinates were then used to create sphere ROIs for the present study. We also explored activations during memory encoding in bilateral hippocampal regions. The hippocampal region ROIs were created as 10mm spheres around the peak coordinates from the resting-state fMRI study described above (left: −28, −38, −10, right: 30, −30, −14). The hippocampal-region spheres were smaller than the neocortical spheres so as to better approximate the smaller extent of MTL structures and not extend into lateral temporal neocortex.
Figure 1.
DMN in 82 participants of 8–24 years of age, defined from resting-state connectivity data in an independent sample of participants. A = PCC; B = MPFC; C = LLP; D = RLP; E = left hippocampal region; F = right hippocampal region
Activations for R and F trial types in each of the four neocortical DMN ROIs defined above were extracted from the memory task fMRI data. We focused on the R trial type because there was no developmental difference for the K trial type. Because there are potentially different activation patterns for R and F trial types in different regions in different age groups, we constructed a mixed-effect analysis of covariance (ANCOVA), with memory outcome (R or F), region (MPFC, PCC, LLP and RLP) as repeated measures and group (adults, adolescents, children) as the between-group measure. The number of outliers was included as the covariate to account for group differences in outlier images. Post-hoc t-tests were conducted to determine if there was significant deactivation for R minus F trial type in each of the four DMN ROIs.
We performed the same ANCOVA for the hippocampal-region ROIs, with memory outcome (R or F), region (left or right hippocampal-region ROI) as repeated measures and group (adults, adolescents, children) as the between-group measure. The number of outliers was included as the covariate to account for group differences in outlier images.
To visualize subsequent-memory related deactivation in the DMN regions, we also created group-level activation maps for R < F. In each age group, single-subject level R < F contrasts were entered into a second-level group analysis using a random-effects model. Group contrasts were constructed using a one-sample t-test and thresholded at voxel-level p < .001 (uncorrected), and cluster-level FWE corrected at p < .05. These group activation maps for R < F were intersected with the 15mm spherical DMN ROIs described above to show subsequent memory deactivation within the DMN regions.
Results
Behavioral
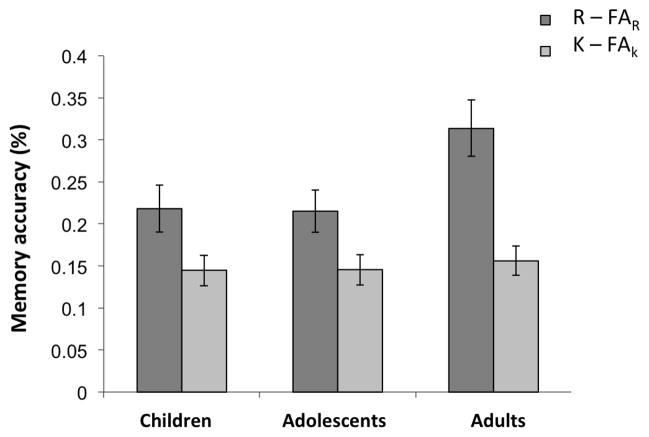
There was a significant age group effect for recognition memory accuracy for “Remembered” items (R − FAR, F(2,45) = 3.57, p = .037), but not for “Know” items (K − FAK, F(2,45) = .30, p = .7) (Figure 2; Table 1). There was no group effect for overall accuracy (Hits − FA) (F(2,45) = 1.38, p = .26). Post-hoc tests showed that adults had better accuracy for “Remembered” items (R−FAR) than children (t(28) = 2.22, p = .034) and adolescents (t(30) = 2.42, p = .022). Children and adolescents did not differ in accuracy for R or K trials (ps > .5). All three age group were highly accurate on the encoding task (making indoor/outdoor judgments) during scanning and there was no significant group difference (F(2,47) = 1.44, p = .25; Children: 96.2% ± 2.5, Adolescents: 97.9% ± 1.8, Adults: 97.8% ± 4.6). Moreover, only studied items that elicited correct indoor/outdoor responses were used in the imaging analysis. This prevented the small influence of age on accuracy in the encoding phase from influencing the subsequent memory analyses.
Figure 2.
Recognition memory accuracy. Accuracy for “Remembered” (R) and “Know” (K) trial types were calculated by subtracting the corresponding false alarm rate from the hit rate for R or K trial types (R accuracy: R − FAR; K accuracy: K/(1−R) − FAK, adjusted for being mathematically constrained by R responses).
Table 1.
Mean proportions of “Remembered” (R), “Know” (K) responses and of false alarms categorized as R (FAR) and K (FAK) in each group. Standard deviations are shown in parenthesis.
| R | K | FAR | FAk | |
|---|---|---|---|---|
| Children | .27(.12) | .24(.09) | .05(.06) | .19(.13) |
| Adolescents | .25(.11) | .22(.08) | .04(.03) | .16(.11) |
| Adults | .34(.16) | .20(.05) | .05(.06) | .17(.08) |
fMRI
We examined activations during memory encoding in the DMN ROIs defined from resting-state fMRI data from an independent sample described above. The 3-way ANCOVA with memory outcome (R or F), region (MPFC, PCC, LLP or RLP), and group (adults, adolescents, children) as factors showed significant main effects of memory outcome (F(1,44) = 6.6, p = .01), region (F(3,132) = 5.3, p = .002) and group (F(1,44) = 18.3, p < .001). There was a significant memory outcome by region by age group interaction (F(6,132) = 3.1, p = .006). To understand the source of the interaction, we examined the activations for R versus F trial types in each age group in each of the four DMN ROIs (Figure 3). We assessed the magnitude of subsequent memory deactivations (R < F) in DMN ROIs across age groups.
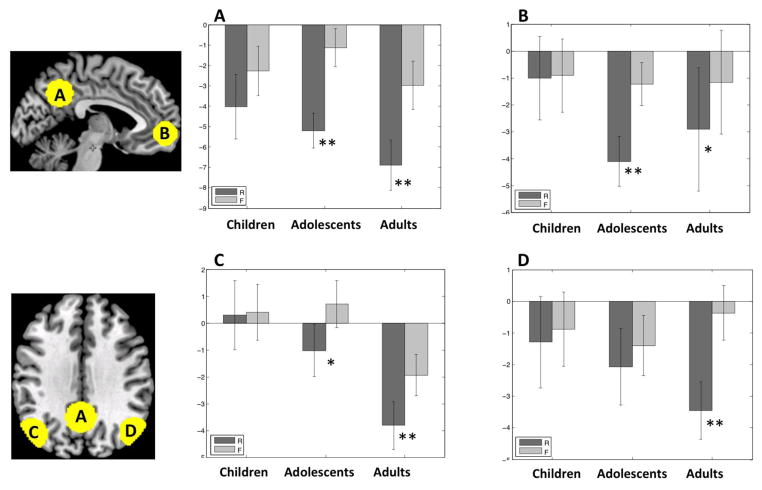
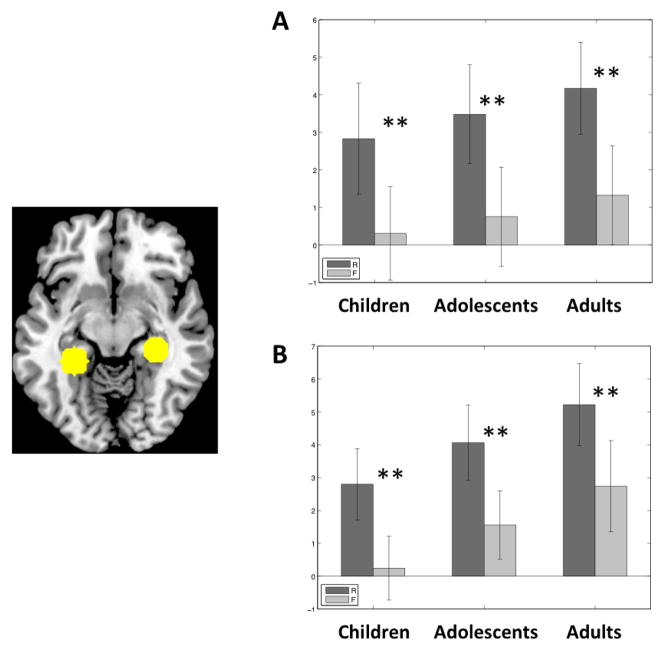
Figure 3.
Subsequent memory deactivations in the DMN in each age group. A = PCC; B = MPFC; C = LLP; D = RLP. Dark grey bars represent trials in which a scene was later remembered (R). Light grey bars represent trials in which a scene was later forgotten (F). Error bars are standard errors of the mean. ** p < .01 for R < F. * p < .05 for R < F.
Adults exhibited significant subsequent memory deactivations (R < F) in all four DMN regions (PCC: t(13) = 6.72, p < .001; LLP: t(13) = 4.20, p = .001; RLP: t(13) = 6.01, p < .001; MPFC: t(13) = 2.74, p = .017). Adolescents exhibited significant subsequent memory deactivations in PCC (t(17) = 5.27, p < .001), MPFC (t(17) = 3.62, p = .003) and LLP (t(17) = 2.55 p = .02), but not in RLP. Children did not exhibit any subsequent memory effects in any of these regions (PCC: p = .07; LLP: p = .90; RLP: p = .4; MPFC: p = .9). A subset of children (N = 11) who did not differ from adults on the number of outliers (t(23) = 1.10, p = .29) also exhibited the same lack of subsequent memory deactivation in all four DMN ROIs (PCC: p = .13; LLP: p = .92; RLP: p = .71; MPFC: p = .72).
Further, in direct comparison between groups (one-tailed t tests), adults compared to children had more subsequent memory deactivation (R < F) in RLP (t(28) = 3.49, p = .001), PCC (t(28) = 2.03, p = .03), and LLP (t(28) = 1.82, p = .04), and a trend for more subsequent memory deactivations in MPFC (t(28) = 1.68, p = .055). Adolescents compared to children had more subsequent memory deactivation (R < F) in PCC (t(32) = 1.97, p = .03) and MPFC (t(32) = 2.54, p = .01), whereas adolescents compared to adults had less subsequent memory deactivation in RLP (t(30) = 2.45, p = .01).
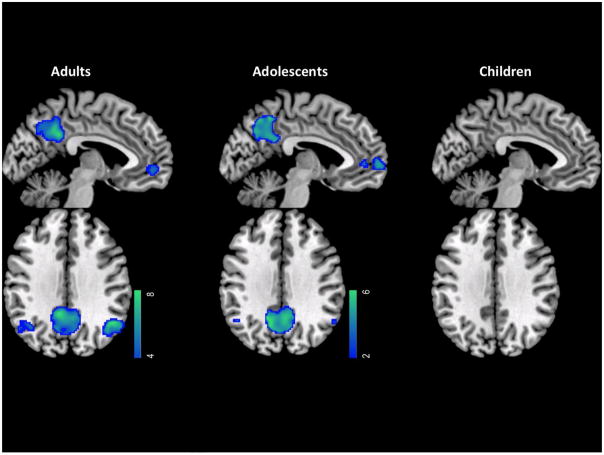
To visualize subsequent memory deactivation of DMN we created group-level t-maps for R < F in each of the three age groups, and restricted the results within the independently-defined DMN ROIs (Figure 4). Adults exhibited significant subsequent memory deactivations during encoding of scenes in all four DMN ROIs. Adolescents exhibited subsequent memory deactivations similar to adults in MPFC and PCC, but far smaller subsequent memory deactivations in LLP and RLP. Children failed to exhibit significant subsequent memory deactivations in any DMN ROI.
Figure 4.
Regions within DMN ROIs that showed deactivations for remembered trials compared to forgotten trials for each age group.
The 3-way ANCOVA for the hippocampal regions showed a main effect of memory outcome (R or F) (F(1, 44) = 39.61, p < .001), but no significant memory outcome by age group by region interaction (p = .93) or memory outcome by age group interaction (p = .85). There was a trend towards significance for effect of age group (p = .1), reflecting a growth of overall activation with age across. The pattern of activation in the hippocampal region was the opposite of the other nodes of the DMN: remembered trials elicited higher positive activation compared to forgotten trials. All three groups exhibited significant subsequent memory activations (R > F) in bilateral hippocampal regions (Figure 5; left hippocampal region: children, t(15) = 3.14, p =.007, adolescents: t(16) = 3.87, p =.001, adults, t(13) = 4.27, p =.001; right hippocampal region: children: t(15) = 3.14, p =.007, adolescents: t(16) = 5.14, p < .001, adults t(13) = 5.94, p < .001).
Figure 5.
Subsequent memory activations in hippocampal regions. A = left hippocampus; B = right hippocampus. Dark grey bars represent trials in which a scene was later remembered (R). Light grey bars represent trials in which a scene was later forgotten (F). Error bars are standard errors of the mean. ** p < .01 for R > F.
Discussion
In adults, there were greater DMN deactivations during successful versus unsuccessful memory encoding in all four major neocortical components of the network, whereas no such deactivations were evident in children. Adolescents, intermediate in age, also showed an intermediate pattern, with subsequent memory deactivation in three of four DMN regions. These findings indicate that the development of memory abilities is supported not only by increases in PFC activations related to successful memory formation (Ofen, 2012; Ofen et al.), but also by increases in DMN deactivations related to successful memory formation.
The absence of DMN subsequent memory deactivation in children is noteworthy. First, it occurred in the contrast between successful and unsuccessful memory formation in each individual, so it cannot be accounted for simply by lower overall accuracy in the children. Second, although increased movement and artifacts in children are challenges in developmental neuroimaging (Power et al., 2012; Satterthwaite et al., 2012), the findings were identical in a subgroup of children matched to adults on these measures. Third, prior neuroimaging studies finding developmental differences in activation associated with memory have reported differences in the magnitude of activations in some regions across age (Ofen et al., 2007, 2012; Chai et al., 2010; Ghetti et al., 2010), but not the absence of such activations in any age group. The children in the present study failed to exhibit any reliable DMN deactivation associated with memory formation.
The task-induced deactivation of the DMN in adults observed in the present study is consistent with previous reports using similar subsequent memory tasks (Daselaar et al., 2004; Miller et al., 2008; Otten and Rugg, 2001). In the present study, we restricted analyses to the DMN, as defined by an independent group of similarly aged participants. The finding that deactivation specifically in the DMN is associated with successful memory formation is in accord with a prior study demonstrating strong overlap between the DMN (defined as brain regions activated at rest relative to task) and deactivations during successful memory formation (Daselaar et al., 2009). Prior studies have most consistently reported such deactivation in the PCC, and often in the LLP and the RLP, but rarely in the MPFC as was found in the present study. This may reflect increased statistical sensitivity from our approach of interrogating the MPFC ROI, whereas other studies employed whole-brain analyses. Indeed, the weakest activations in adults in the present study occurred in the MPFC.
An exception to this pattern of findings occurred in the hippocampal region. The hippocampal region often exhibits resting-state fluctuations that are correlated with the major neocortical components of the DMN, and is therefore often considered another component of the DMN. Indeed, we also found the hippocampal region to be functionally connected with the neocortical DMN during rest. Despite this resting-state relation with the DMN, prior studies with adults have found that the hippocampal region is positively activated for stimuli during encoding, and more activated for subsequently remembered than forgotten stimuli (Daselaar et al., 2009; Huijbers et al., 2012). We observed the same pattern of activation not only in adults, but also in children and adolescents. This parallels the prior findings of similar MTL activation in children, adolescents, and adults associated with successful memory encoding (Ofen et al., 2007 with the same participants, but with the MTL ROI defined by activations or anatomy, not resting-state correlations) and successful memory retrieval (Ofen et al., 2012).
The age-related increase of subsequent memory deactivations in DMN mirrors the age-related decline of DMN deactivation in older adults (de Chastelaine et al., 2011; Duverne et al., 2009; Miller et al., 2008). Across several studies of successful memory formation, young adults exhibited deactivations in PCC, whereas older adults (around 70 years of age) exhibited an absence or even reversal of such deactivations (de Chastelaine et al., 2011; Duverne et al., 2009; Miller et al., 2008). It thus appears that DMN deactivation is highly sensitive to both developmental growth and decline in memory ability.
The age-related development of DMN deactivation in association with successful memory formation was clear-cut, but interpretation of the memory mechanism mediated by the DMN deactivation is less certain. In broad terms, successful memory encoding demands that attention be paid to a stimulus or event; dividing attention during learning greatly reduces successful episodic memory encoding (Fisk and Schneider, 1984; Moray, 1959). In this regard, suppression of the DMN during memory formation may be another example of a wide range of cognitive tasks, including working memory tasks, in which greater suppression of the DMN is associated with more demanding performance across conditions or better performance across individuals or trials (Lawrence et al., 2003; McKiernan et al., 2003; Weissman et al., 2006; Whitfield-Gabrieli et al., 2009). DMN deactivation may reflect allocation of resources to other neural systems that are important for cognition about the environment. There is widespread and substantial growth of attentional and executive functions from ages 8–24, and the deactivation of the DMN for successful memory formation could simply be another expression of this broad growth of cognitive control and/or resources that characterizes typical development.
Alternatively, the DMN may be a substrate of specific mnemonic processes that influence successful memory encoding. In young adults some DMN regions, and especially the PCC, show greater activation for successful than unsuccessful retrieval of memories (what has been termed the “encoding/retrieval flip”) (Buckner et al., 1996; Daselaar et al., 2009; Huijbers et al., 2013). This reversal of the relation between activation and memory success across encoding and retrieval may reflect specific competition between resources for encoding information from the environment versus retrieving information from the mind and brain. Independent of memory encoding, the DMN has been associated with internal (versus external) orientation (reviewed in Nakao et al., 2012) and self-reference (versus reference to others) (reviewed in Northoff et al., 2006), and memory encoding for scenes would benefit from suppression of processes focused on internal and self-referential processes. By this perspective, the DMN may mediate specific processes that are disadvantageous for encoding, rather than processes that simply reduce attentional resources for memory formation. It is unknown at present whether or not DMN regions undergo a functional maturation for successful retrieval of memories that parallels the functional maturation of successful encoding of memories.
Three limitations of this study are salient. First, the absence of any significant difference in DMN deactivation between subsequently remembered or forgotten scenes in children may reflect limited statistical power. Second, it is somewhat surprising that although the adolescents appeared to exhibit a pattern of deactivation that was intermediate to that of children and adults, the adolescents performed no better on the recognition memory test than did the children. Third, the present study cannot shed light on what specific cognitive mechanism that is correlated with age may be most related to the reduced deactivations, such as age-associated development of cognitive control or working memory capacities.
What is clear from the present study is that typical functional brain development associated with successful memory formation occurs not only for activations in prefrontal, parietal, and sometimes MTL regions (Ghetti and Bunge, 2012; Ofen, 2012), but also for deactivations in all four major components of the neocortical DMN. Most strikingly, DMN suppression during encoding exhibited no apparent relation to memory formation in children, and grew to have a strong relation to memory formation in adults.
Highlights.
Children, adolescents and adults studied scenes during fMRI
Default-mode network (DMN) deactivation was examined during memory encoding
DMN deactivation was associated with successfully memory encoding in adults
In Children, deactivation of the DMN did not predict memory outcome
Acknowledgments
We thank the Richard M. Lucas Center for Imaging at Stanford University for help with the memory fMRI scans, and the Athinoula A. Martinos Imaging Center at MIT for help with resting-state fMRI scans. We thank Kelly Halverson for help with scoring the behavioral tests. This research was supported by RO1-MH-080344 to JDEG
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Jacobs LF, Gabrieli JD. Scene complexity: influence on perception, memory, and development in the medial temporal lobe. Front Hum Neurosci. 2010;4:21. doi: 10.3389/fnhum.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG, Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39:255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 2011;21:2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk AD, Schneider W. Memory as a function of attention, level of processing, and automatization. J Exp Psychol Learn Mem Cogn. 1984;10:181–197. doi: 10.1037//0278-7393.10.2.181. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. 2008;79:339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. J Neurosci. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Schultz AP, Vannini P, McLaren DG, Wigman SE, Ward AM, Hedden T, Sperling RA. The Encoding/Retrieval Flip: Interactions between Memory Performance and Memory Stage and Relationship to Intrinsic Cortical Networks. J Cogn Neurosci. 2013;25:1163–1179. doi: 10.1162/jocn_a_00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Mandler JM, Robinson CA. Developmental changes in picture recognition. J Exp Child Psychol. 1978;26:122–136. doi: 10.1016/0022-0965(78)90114-5. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moray N. Attention in dichotic listening: Affective cues and the influence of instructions. Quarterly Journal of Experimental Psychology. 1959;11:56–60. [Google Scholar]
- Nakao T, Bai Y, Nashiwa H, Northoff G. Resting-state EEG power predicts conflict-related brain activity in internally guided but not in externally guided decision-making. Neuroimage. 2012;66C:9–21. doi: 10.1016/j.neuroimage.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ofen N. The development of neural correlates for memory formation. Neurosci Biobehav Rev. 2012;36:1708–1717. doi: 10.1016/j.neubiorev.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Chai XJ, Schuil KD, Whitfield-Gabrieli S, Gabrieli JD. The development of brain systems associated with successful memory retrieval of scenes. J Neurosci. 2012;32:10012–10020. doi: 10.1523/JNEUROSCI.1082-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nat Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]