Abstract
In this study, we aimed to investigate age related changes in systems implicated in top down attention and the implications of this for amygdala responses to emotional distracters. Fifty-one healthy subjects including 18 children (aged 10–14), 15 adolescents (aged 14–18), and 18 young adults (aged 18–25) completed the affective Stroop paradigm while undergoing functional MRI. While achieving comparable behavioral performance, children, relative to adolescents and adults, showed increased activation in areas including anterior cingulate gyrus and precentral gyrus in task relative to view trials. In addition, children showed increased activation within the amygdala and fusiform gyrus in response to emotional stimuli. Notably, the group difference within the amygdala was particularly pronounced during task trials. Also children showed increased connectivity between amygdala and superior frontal gyrus and bilateral postcentral gyrii in response to negative task trials. These data are consistent with previous work indicating less consolidated functional integrity in regions implicated in top down attention in children relative to older participants and extend this work by indicating that this less consolidated functional integrity leads to reduced automatic emotion regulation as a function of top down attention. Given that reduced automatic emotion regulation as a function of top down attention is considered a risk factor for the development of anxiety disorders, these data may contribute to an understanding of the increased risk for the development of these disorders at this age.
1. Introduction
Dysfunctional emotion regulation is thought to be a risk factor for the development of mood and anxiety conditions (Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Rive et al., 2013). However, emotion regulation is a broad term that subsumes a range of cognitive processes (Gyurak, Gross, & Etkin, 2011). Within this range, it has been argued that emotion regulation can engage two sets of control processes (Ochsner & Gross, 2005; Phillips, Drevets, Rauch, & Lane, 2003). The first type of emotion regulation involves ventromedial prefrontal systems that represent emotional value and/or a form of emotional conflict adaptation (Etkin et al., 2010). Some previous developmental work has investigated tasks related to this process: emotional Stroop and emotional go/no-go (Sebastian et al., 2010; Somerville, Hare, & Casey, 2011). These studies have reported increased activation of inferior frontal cortex in children, compared to adolescents and adults, during no-go relative to go trials on a go/no-go task (Somerville et al., 2011) as well as decreased activation of inferior frontal cortex in adolescents relative to adults during performance of the emotional Stroop task (Sebastian et al., 2010).
The second type of emotion regulation involves prefrontal (both dorsomedial and lateral regions) and parietal cortex. Attentional control represents one vital function of these systems: the priming of relevant representations at the expense of irrelevant ones, thereby resolving representational competition (Desimone & Duncan, 1995). Arguably, such control processes can be recruited explicitly within cognitive reappraisal paradigms, where subjects willfully attempt to alter stimulus representations by priming non-emotional features (Ochsner, Bunge, Gross, & Gabrieli, 2002) (see, for reviews, Kalisch, 2009; Ochsner & Gross, 2005). It is argued that these processes are recruited implicitly through attention distraction paradigms (e.g., the affective Stroop task; Blair et al., 2007) where subjects prime task-relevant features of a stimulus array at the expense of the representation of (and consequent emotional reaction to) emotional distracters (Blair et al., 2007; Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Pessoa, Padmala, & Morland, 2005).
It is the second form of emotion regulation that is of particular interest here. There have been reports that children, relative to adults, show reduced activation in frontal and parietal regions implicated in top down attention (Konrad et al., 2005; Vuontela et al., 2013; Wendelken, Baym, Gazzaley, & Bunge, 2011); (for reviews of this literature, see; Bunge & Wright, 2007; Hwang, Velanova, & Luna, 2010; Rubia, 2012). Similar changes have been observed with respect to working memory and interference control, with age-related improvements in performance and changes in brain activation, particularly in lateral PFC and posterior parietal cortex during task performance being documented (Casey et al., 1995; Hwang et al., 2010; Kwon, Reiss, & Menon, 2002; Olesen, Macoveanu, Tegner, & Klingberg, 2007; Somerville et al., 2011; Velanova, Wheeler, & Luna, 2009). With respect to top down attention driven emotion regulation, the suggestion is that directing attention towards task relevant stimuli will result in reduced representation of emotional distracters and consequent amygdala responses to these distracters (Blair et al., 2007; Pessoa, 2009; Pessoa, Kastner, & Ungerleider, 2002). In other words, if children or adolescents show reduced top down enhancement of task relevant stimuli then it can be predicted that they will show greater emotional (amygdala) responses to emotional distracters. However, this remains to be empirically demonstrated.
Various tasks have been used to index the role of top down attention in the automatic regulation of responding to emotional distracters (dorsomedial and lateral frontal cortices, and inferior parietal cortex; e.g., Blair et al., 2007; Mitchell et al., 2008; Mitchell et al., 2007; Mitchell, Richell, Leonard, & Blair, 2006; Pessoa, Kastner, et al., 2002; Vythilingam et al., 2007). Here we will concentrate on the affective Stroop task (aST; Blair et al., 2007). In the aST, participants are required to determine the number of numbers presented on the screen. These numbers are temporally bracketed by emotional or neutral distracters. A body of studies with the aST and its variants has shown task performance is associated with increased activity within regions implicated in top down attention (dorsomedial and lateral frontal cortices, and inferior parietal cortex; e.g., Blair et al., 2007; Mitchell et al., 2008; Mitchell et al., 2007). The amygdala shows responsiveness to emotional relative to neutral distracters and a reduction in this activity in task relative to view trials (where no task performance is required; see Figure 1; e.g., Blair et al., 2012; Blair et al., 2007; Mitchell et al., 2008; Mitchell et al., 2007). Importantly, the recruitment of top down attention systems by task demands is not thought to directly inhibit the amygdala. Rather, following Desimone and Duncan (Desimone & Duncan, 1995), priming of task relevant representations within temporal cortex augments their representation such that the representation of emotional distracters within temporal cortex is suppressed following representational competition. This reduced representation of the emotional distracters results in a reduced amygdala response to these distracters. The aST has been used not only in studies for healthy individuals but also in a series of studies with patients with a variety of anxiety conditions (Social Phobia [SP], Generalized Anxiety Disorder [GAD] and Post Traumatic Stress Disorder [PTSD]; Blair et al., 2012; Blair et al., 2013; Vythilingam et al., 2007). Patients with these conditions show a reduction during task trials in the recruitment of regions implicated in top down attention (dorsomedial [SP, GAD] and lateral frontal and parietal cortices ([SP, GAD, PTSD]); Blair et al., 2012; Blair et al., 2013). Given this evidence that patients with anxiety disorders face difficulty in automatic emotion regulation as a function of top down attention and evidence that children, relative to adults, show reduced recruitment of frontal and parietal regions implicated in top down attention (Bunge & Wright, 2007; Hwang et al., 2010; Rubia, 2012), it is possible that children may show heightened amygdala responses to emotional distracters and that this enhanced responsiveness to emotional task-irrelevant information may contribute to the enhanced risk for the development of anxiety disorders in early adolescence.
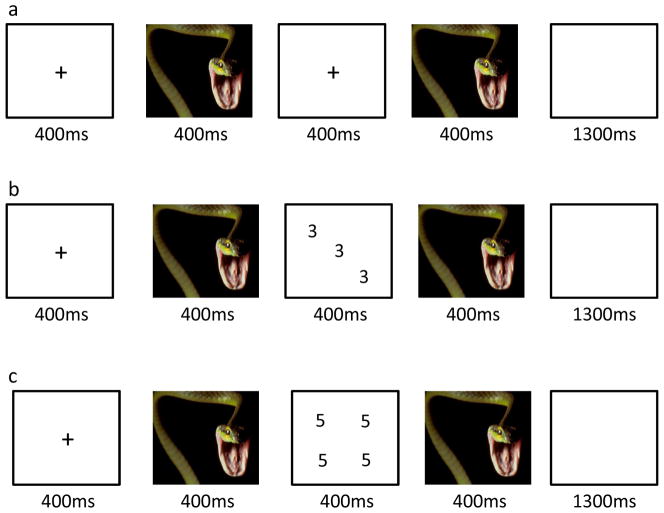
Figure 1.
Example trial sequences. (a) negative view trial; (b) negative congruent trial; (c) negative incongruent trial.
In short, we believe this study is the first to examine developmental changes in emotion regulation as a function of top down attention. Specifically, we test two predictions. First, given previous results of reduced recruitment of regions implicated in top down attention (dorsomedial, lateral frontal and parietal cortices) in children and adolescents relative to young adults on tasks requiring top down attention (Konrad et al., 2005; Vuontela et al., 2013; Wendelken et al., 2011), we predict that this will also be seen in the current study during performance of the affective Stroop task. Second, given that priming of task relevant stimulus features via top down attention control is associated with reduced amygdala responses to emotional distracters (Blair et al., 2007; Mitchell et al., 2007), potentially reduced priming of task relevant stimulus features in children/adolescents should be associated with increased amygdala responses to emotional distracters under task conditions. In addition, we examine age related changes in connectivity between the amygdala and cortical regions as a function of task performance.
Method
2.1. Subjects
Fifty two healthy participants from the Washington D.C. metropolitan area volunteered for the study and were paid for their participation. Participants were recruited from the community through advertising. One subject was excluded from the final data analysis due to movement (over 15% of this participant’s TRs were censored due to movement > 1mm within the TR). Fifty one healthy participants were included in the final analysis (twenty two females, twenty nine males; aged 10–25, average age=17.45 (5.03), forty five right handed and six left handed). Eighteen participants were children (10≤ age <14, average age=12.37 (1.11), 6 females and 12 males, 4 left handed), fifteen were adolescents (14≤ age <18, average age=15.95 (0.93), 9 females and 6 males, 2 left-handed), and eighteen were young adults (18 ≤ age ≤25, average age=23.78 (0.97), 7 females and 11 males). There were no significant group differences in gender distribution (χ2= 2.577, p=0.276). All subjects, or their legal guardians in the case of minors, gave written informed consent and assent to participate in the study, which was approved by the National Institute of Mental Health Institutional Review Board. Subjects were assessed and examined by an expert psychologist and physicians and included if they were in good health with no history of medical, psychiatric, or neurological disease. There was no significant between group difference of IQ (as indexed by the Wechsler Abbreviated Scale of Intelligence two-subtest form) among the three groups (F=2.678, p=0.079), nor was there any significant correlation between age and IQ (r=0.195, p=0.171; IQ for children = 107.83, 11.29 (mean, SD), IQ for adolescents = 102.07, 14.63; IQ for young adults = 112.44, 12.69).
Exclusion criteria were pervasive developmental disorder, Tourette’s syndrome, lifetime history of psychosis, depression, bipolar disorder, generalized, social or separation anxiety disorder, Post Traumatic Stress Disorder, neurologic disorder, history of head trauma, history of substance abuse, and IQ<70. All children/adolescents and parents completed Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) (Kaufman et al., 1997) assessments conducted by a doctoral-level clinician as part of a comprehensive psychiatric assessment. The K-SADS has demonstrated good validity and inter-rater reliability (kappa >0.75 for all diagnoses) (Kaufman et al., 1997).
2.2. The affective Stroop task
The affective Stroop task used here was an adapted version of the paradigm described in previous work (Blair et al., 2012; Blair et al., 2007). Each trial began with a fixation point presented for 400ms in the middle of the screen; see Figure 1. An image was then presented for 400 ms. For the view trials, there was then a 400 ms blank image. For the task trials, a numerical display was displayed for 400ms. For both view and task trials, there was then a second 400ms period when the same image was displayed. Following this the screen was blank for 1300ms. For task trials, the subjects made a button press corresponding to the numerosity of the numerical display, i.e., how many of the numbers were displayed, not the actual value of the numbers. For congruent trials, the numbers were displayed with the same numerosity and number value (e.g., three 3s). For incongruent trials, the numbers were displayed with different numerosities and number values (e.g., four 3s). There were an equal number of congruent and incongruent trials (48 each for a run). Participants could respond at any time from the presentation of the numerical display until the end of the blank screen. The participants made no response for view trials. Trial order was randomized across participants.
The individual numerical stimuli consisted of three, four, five, or six 3s, 4s, 5s, or 6s presented within a 9-point grid with random sequence; see Figure 1. The emotional stimuli consisted of 48 positive, 48 negative, and 48 neutral pictures selected from the IAPS. The normative mean valence and arousal values on a 9-point scale were respectively 3.35±0.77 and 5.97±1.07 for negative pictures, 7.43±0.52 and 4.99±1.10 for positive pictures, and 4.87±0.28 and 2.66±0.54 for neutral pictures. There were nine trial types: view, congruent, and incongruent trials involving negative, positive, and neutral emotional stimuli. Subjects completed two runs, generating 288 picture-trial events (32 in each 9 categories) and 96 fixation points (each of 2500 ms length) to generate a baseline. The order of presentation of trial and fixation point events was randomized across participants, and there was no regressor collinearity.
2.3. Image acquisition and analysis
Whole-brain blood oxygen level-dependent (BOLD) fMRI data were acquired using a 3-T General Motors MRI scanner. Following sagittal localization, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix of 64×64 mm, repetition time (TR) of 3000ms, echo time (TE) of 30ms, field of view (FOV) of 240 mm, and voxels of 3.75 × 3.75 × 4 mm. Images were acquired in 30 continuous 4mm axial slices per brain volume across two runs. The duration of each run was 8 min 13 s. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional Spoiled GRASS; TR = 8.1ms; TE = 3.2 ms, flip angle 20°; field of view = 240mm, 128 axial slices, thickness = 1.0mm; 256×256 acquisition matrix).
2.4. fMRI analysis
Data were analyzed within the framework of a random effects general linear model using Analysis of Functional Neuroimages (AFNI). Both individual and group-level analyses were conducted. The first 5 volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI dataset to a volume that was collected shortly before acquisition of the high-resolution anatomical dataset.
The EPI datasets for each subject were spatially smoothed (using an isotropic 6mm Gaussian kernel) to reduce the influence of anatomical variability among the individual maps in generating group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percent signal change from the mean. The model involved six motion regressors and the following task regressors: negative congruent, negative incongruent, negative view, neutral congruent, neutral incongruent, neutral view, positive congruent, positive incongruent and positive view. A regressor modeling incorrect responses was also included. All regressors were convolved with a canonical hemodynamic response function (HRF) to account for the slow hemodynamic response (with time point commencing at time of first image onset).
The participants’ anatomical scans were individually registered to the Talairach and Tournoux atlas (Talairach & Tournoux, 1988). The individuals’ functional EPI data were then registered to their Talairach anatomical scan within AFNI. Linear regression modeling was performed using the 10 regressors described earlier, plus regressors to model a first-order baseline drift function. This produced β coefficients and associated t statistics for each voxel and regressor.
The BOLD data were analyzed via a 3 (group: children, adolescents, young adults) by 3 (emotion: negative, positive, neutral) by 3 (task: congruent, incongruent, view) ANOVA. Statistical maps were created for each main effect and interaction by thresh-holding at a single-voxel p value of p<0.005 (except the main effect of task which involved an initial threshold of p<0.0001). To correct for multiple comparisons, we performed a spatial clustering operation using ClustSim with 10,000 Monte Carlo stimulations taking into account the EPI matrix covering the gray matter. This procedure yielded a minimum cluster size (43 voxels) with a map-wise false-positive probability of p<0.05, corrected for multiple comparisons. Follow-up analyses were performed to facilitate interpretations. For these analyses, average percent signal change was measured across all voxels within each region of interest (ROI) generated from the functional masks, and data were analyzed using appropriate follow-up independent t-tests within SPSS.
Given our a priori hypotheses regarding the amygdala, regions of interest (ROIs) were created for left and right amygdala using anatomical ROIs taken from the AFNI software (TT_Daemon atlas based on the Talairach-Tournoux Atlas)(Talairach & Tournoux, 1988). A small volume-corrected ROI analysis was conducted for these regions using ClustSim (initial threshold: p<0.005 corrected at p<0.02) for the number of voxels within the ROI.
2.5. Connectivity analyses
In addition, a connectivity analysis was conducted to examine differential functional connectivity between the amygdala and cortical regions as a function of age, task and emotion. A seed region was identified from the region of left amygdala that yielded a significant group-by-emotion interaction (see Figure 3). The average activation from each seed region was extracted across the time series. Interaction regressors were created by multiplying each of these average time series with nine task time course vectors (one for each task and emotion condition), which were coded: 1= task and emotion condition present and 0= task and emotion condition not present. The average activation for the amygdala seed was entered into a linear regression model along with the nine interaction regressors (one per task and emotion condition) and 6 motion regressors. The differences in correlation between task and emotion conditions were then examined in a 3 (group; children, adolescents, young adults) by 3 (task; incongruent, congruent, view) x 3 (emotion; negative, positive, neutral) whole-brain repeated measures ANOVA.
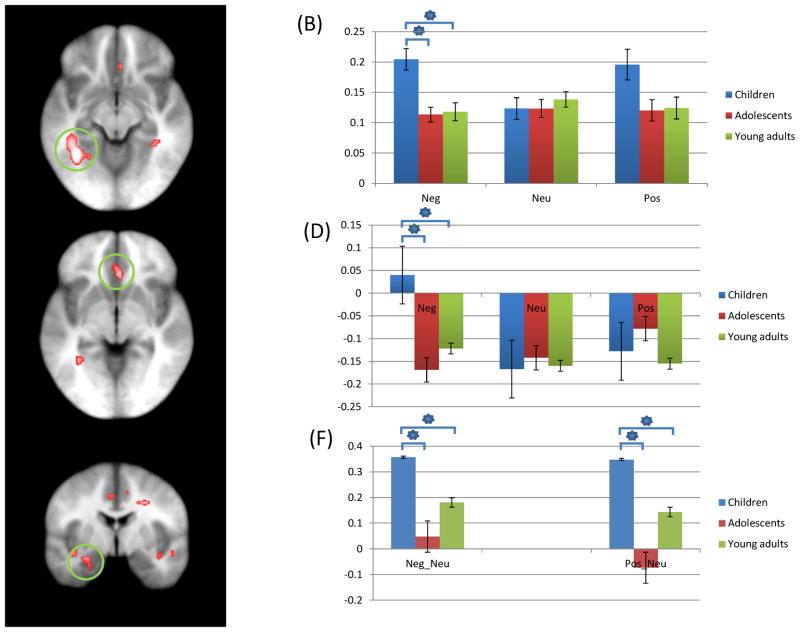
Figure 3.
Regions showing increased activation during aST performance as a significant interaction of group-by-emotion; (A) Children showed increased activation of left fusiform gyrus (coordinates; −34.5, −49.5, −9.5) in response to negative trials, relative to adolescents and young adults; (B) parameter estimates for this interaction; (C) Children showed increased activation of right anterior cingulate gyrus (coordinates; 4.5, 25.5, −3.5) in response to negative trials, relative to adolescents and young adults; (D) Parameter estimates for this interaction; (E) Children showing greater difference of activation in left amygdala anatomical ROI (coordinates; −22.5, −1.5, −21.5) between emotional (negative and positive) stimuli and neutral stimuli, relative to adolescents and young adults; (F) Parameter estimates for this region. * = significant contrasts.
Neg: Negative; Neu: Neutral; Pos: Positive.
3. Results
3.1. Behavioral Data
Two 3 (group: children, adolescents, young adults) by 2 (task: congruent, incongruent) by 3(emotion: negative, positive, neutral) ANOVAs were applied to the reaction time (RT) and accuracy data respectively; see Table 1. With respect to RT data, there was a significant main effect of task (F(1,48)=215.786, p=0.000); RTs were slower for incongruent relative to congruent trials. There was also a main effect of emotion (F(2, 47)=2.726, p=0.076); RTs were slower for negative and positive relative to neutral trials. There were no significant interactions.
Table 1.
Behavioral Data (standard deviations in brackets)
| Children | Adolescents | Young Adults | |
|---|---|---|---|
| RT (milliseconds) | |||
| Negative Congruent | 756.38 (38.26) | 818.05 (41.92) | 717.77 (38.26) |
| Negative Incongruent | 849.55 (38.89) | 907.04 (42.60) | 807.35 (38.89) |
| Neutral Congruent | 745.75 (38.44) | 802.43 (42.11) | 705.01 (38.44) |
| Neutral Incongruent | 843.24 (39.33) | 902.52 (43.09) | 783.81 (39.33) |
| Positive Congruent | 758.69 (39.21) | 821.31 (42.95) | 728.07 (39.21) |
| Positive Incongruent | 832.49 (38.88) | 896.83 (42.59) | 795.73 (38.88) |
|
| |||
| Congruent | 753.61 (37.89) | 813.93 (41.51) | 716.95 (37.89) |
| Incongruent | 841.76 (38.19) | 902.13 (41.83) | 795.63 (38.19) |
|
| |||
| Accuracy (percent) | |||
| Negative Congruent | 69.4 (3.5) | 72.3 (3.8) | 77.4 (3.5) |
| Negative Incongruent | 61.6 (3.4) | 71.7 (3.8) | 66.7 (3.4) |
| Neutral Congruent | 72.2 (2.9) | 71.0 (3.2) | 74.3 (2.9) |
| Neutral Incongruent | 68.2 (3.1) | 66.0 (3.4) | 67.2 (3.1) |
| Positive Congruent | 71.7 (3.1) | 74.0 (3.4) | 77.1 (3.1) |
| Positive Incongruent | 68.2 (3.1) | 62.7 (3.4) | 66.3 (3.1) |
|
| |||
| Negative* | 65.5 (3.0)a | 72.0 (3.3)b | 72.0 (3.0)b |
| Neutral | 70.2 (2.4) | 68.5 (2.7) | 70.7 (2.4) |
| Positive | 70.0 (2.5) | 68.3 (2.3) | 71.7 (2.5) |
p<0.05 (difference between a and b)
With respect to accuracy, there was a main effect of task; responses were significantly less accurate for incongruent relative to congruent trials. [F(1,48)=16.490, p<.001]. There was also a significant group-by-emotion interaction [F(4,96)=4.420, p=0.003]. Children were less accurate for negative (but not positive or neutral) trials relative to adolescents and young adults [F(2,48)=4.420 & 6.838; p<0.05 respectively] who did not differ [F(1, 31)=1.258, p=0.271]. There was no main effect of emotion [F(2, 47)=0.019, p=0.981] or significant group-by-congruency interaction [F(2, 48)=0.746, p=0.479]. There were no group differences for accuracy for positive or neutral trials.
3.2. Functional MRI Data
Whole brain analysis
A whole-brain 3(group: children, adolescents, young adults) by 3 (task: congruent, incongruent, view) by 3 (emotion: negative, positive, neutral) ANOVA was applied to the BOLD data. With respect to our predictions, this revealed significant group-by-task, group-by-emotion and group-by-task-by-emotion interactions. In addition, there were main effects of both task and emotion (see Appendix).
3.2.1. Interaction between group and task
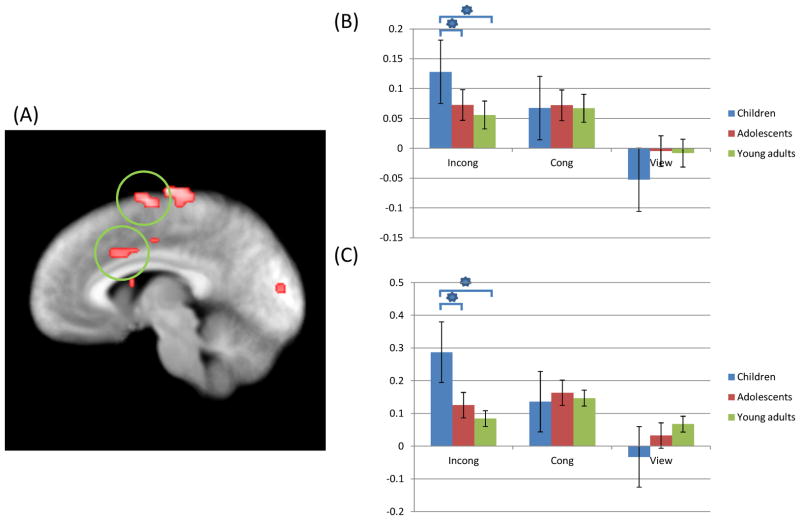
Regions showing significant interactions between group and task included right anterior cingulate gyrus, bilateral precentral gyrus, left middle temporal gyrus and right superior temporal gyrus; see Table 2. Within all these regions follow-up analyses on the BOLD responses within the identified regions using SPSS showed that the groups differed in the response to incongruent trials with children showing greater activity relative to older comparison groups, particularly young adults [t=3.09–8.34; p<0.05]; see Figure 2. The groups did not differ in BOLD responses within these regions for congruent or view trials. Adolescents and young adults did not differ in their responses within these regions.
Table 2.
Brain Regions showing significant group-by-task and group-by-emotion interaction
| Coordinates of Peak Activation
|
|||||||
|---|---|---|---|---|---|---|---|
| Regiona | Left/Right | BA | x | y | z | F | Voxels |
| Group by task | |||||||
| Medial frontal gyrus | Left | 6 | −4.5 | −4.5 | 62.5 | 6.2302 | 42* |
| Anterior cingulate gyrus | Right | 24 | 7.5 | 13.5 | 29.5 | 6.5005 | 49 |
| Precentral gyrus | Left | 4 | −16.5 | −25.5 | 56.5 | 7.7947 | 146 |
| Precentral gyrus | Right | 24 | 19.5 | −16.5 | 47.5 | 6.6979 | 71 |
| Middle temporal gyrus | Left | 22 | −49.5 | −37.5 | 5.5 | 8.9204 | 797 |
| Superior temporal gyrus | Right | 41 | 40.5 | −34.5 | 14.5 | 7.1974 | 170 |
| Cuneus | Right | 19 | 7.5 | −88.5 | 23.5 | 8.8698 | 140 |
| Culmen | Right | 13.5 | −49.5 | −6.5 | 6.8019 | 74 | |
| Pyramis | Right | 28.5 | −70.5 | −33.5 | 7.3246 | 43 | |
| Group by emotion | |||||||
| Anterior cingulate gyrus | Right | 24 | 4.5 | 25.5 | −3.5 | 6.6906 | 21* |
| Fusiform gyrus | Left | 37 | −34.5 | −49.5 | −9.5 | 9.7344 | 67 |
| Middle occipital gyrus | Left | 18 | −31.5 | −88.5 | 5.5 | 7.3602 | 43 |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon).
below the ClusterSim cluster size (43 voxels)
Figure 2.
Regions showing increased activation during aST performance (p=0.005) as a significant interaction of group-by-task; (A) Children showed increased activation of right anterior cingulate (coordinates; 7.5, 13.5, 29.5) and left medial frontal gyrus (coordinates; −4.5, −4.5, 62.5) during incongruent trials relative to adolescents and young adults; (B), (C) Parameter estimates for these regions. * = significant contrasts.
Incong: Incongruent; Cong: Congruent.
3.2.2. Interaction between group and emotion
Regions showing a significant group-by-emotion interaction included left fusiform gyrus and within the left amygdala anatomical ROI; see Table 2. Given its relevance to models of emotional processing, we also note that there was a region of ventral anterior cingulate cortex that showed a significant group-by-emotion interaction albeit at an extent threshold (k=21) that did not survive correction for multiple comparisons. For left fusiform gyrus (and right ventral anterior cingulate cortex), follow-up analyses on the BOLD responses within the identified regions using SPSS showed that the groups differed in BOLD response to negative trials [t=3.86 & 3.93 respectively; p<0.05] with children showing greater activity relative to older comparison groups; see Figure 3. Within the amygdala anatomical ROI, there was a region showing group differences in response to negative and positive trials relative to neutral trials. Emotional stimuli trials (negative and positive trials) produced increased BOLD responses compared to neutral trials [t=3.82 & 10.70 respectively; p<0.05], and these differences were greater for the children than older comparison groups.
3.2.3. Interaction between group, task, and emotion
There was a significant group-by-task-by-emotion interaction within the right amygdala anatomical ROI; see Figure 4. Within this region, follow-up analyses revealed that children relative to older comparison groups showed greater activity during task trials in the presence of negative distracters relative to neutral distracters [t=5.198, p=0.009]. In addition, they showed greater activity, relative to comparison groups, during positive view trials relative to neutral view trials [t=4.639, p=0.014].
Figure 4.
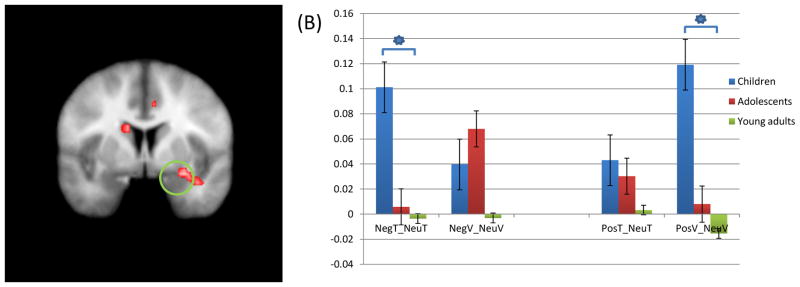
(A) Children showed increased activation of right amygdala anatomical ROI (coordinates; 25.5, −1.5, −21.5)in response to negative task trials compared to neutral task trials, and positive view trials compared to neutral view trials, relative to adolescents and young adults; (B) Parameter estimate for this region. * = significant contrasts.
NegT: Negative task trial; NeuT: Neutral task trial; NegV: Negative view trial; NeuV: Neutral view trial; PosT: Positive task trial; PosV: Positive view trial.
3.2.4. Connectivity analyses
There was a significant group-by-task-by-emotion interaction of connectivity between left amygdala seed and right superior frontal gyrus, bilateral postcentral gyrus, bilateral angular gyrus, left superior temporal gyrus, and left precuneus; see Table 3 and Figure 5. Between all these regions and the amygdala, there was significantly greater connectivity for task trials in the presence of negative distracters relative to neutral distracters in children relative to adults and, to a lesser extent, adolescents (particularly negative congruent trials relative to neutral congruent trials; t=2.693–2.747, p=0.011–0.012).
Table 3.
Brain regions showing significant group-by-task-by emotion interaction for the connectivity data (left amygdala seed)
| Coordinates of Peak Activation
|
|||||||
|---|---|---|---|---|---|---|---|
| Regiona | Left/Right | BA | x | y | z | F | Voxels |
| Left amygdala anatomical ROI seed | |||||||
| Group by task by emotion | |||||||
| Superior frontal gyrus | Right | 6 | 1.5 | 4.5 | 50.5 | 3.5690 | 29* |
| Postcentral gyrus | Right | 3 | 19.5 | −34.5 | 53.5 | 4.2838 | 53 |
| Postcentral gyrus | Left | 3 | −13.5 | −40.5 | 65.5 | 4.6120 | 39* |
| Angular gyrus | Right | 39 | 31.5 | −61.5 | 35.5 | 4.0497 | 40* |
| Angular gyrus | Left | 39 | −28.5 | −55.5 | 35.5 | 4.1314 | 49 |
| Superior temporal gyrus | Left | 41 | −46.5 | −31.5 | 14.5 | 4.7512 | 35* |
| Precuneus | Left | 7 | −10.5 | −64.5 | 38.5 | 3.9454 | 34* |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon).
below the ClusterSim cluster size (43 voxels)
Figure 5.
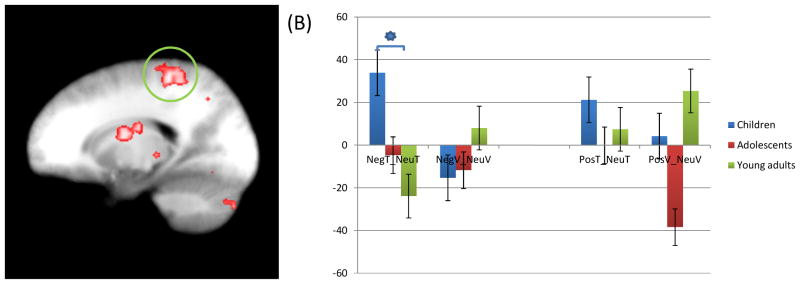
(A) Children showed significantly increased connectivity of right postcentral gyrus (coordinates; 19.5, −34.5, 53.5) with left amygdala anatomical ROI seed (coordinates; −22.5, −1.5, −21.5) in response to negative task trials compared to neutral task trials, relative to adolescents and young adults; (B) average activation of this region. * = significant contrasts.
NegT: Negative task trial; NeuT: Neutral task trial; NegV: Negative view trial; NeuV: Neutral view trial; PosT: Positive task trial; PosV: Positive view trial.
4. Discussion
The current study investigated the developmental changes in the responsiveness of regions implicated in top down control and emotional responding from 10 to 25 years of age. There were four main results: First, compared to adolescents and young adults, children showed increased recruitment of regions including right anterior cingulate gyrus and middle and superior temporal gyrus during task performance (specifically during incongruent task trials). Second, children showed increased recruitment of left fusiform gyrus and left amygdala in response to negative trials relative to adolescents and young adults. Third, compared to adolescents and young adults, children showed increased recruitment of right amygdala during task trials in the presence of negative relative to neutral distracters. Fourth, children showed increased connectivity, relative to adults and to a lesser extent adolescents, between left amygdala and right superior frontal gyrus, bilateral postcentral gyrus, bilateral angular gyrus, left superior temporal gyrus, and left precuneus for task trials in the presence of negative distracters relative to neutral distracters.
Previous work has indicated developmental differences in responding within regions implicated in top down attention, particularly dorsal anterior cingulate cortex. In the current study, children, compared to adolescents and young adults, showed increased recruitment of regions including right anterior cingulate gyrus during incongruent trials. Previous studies have reported both positive correlations between dACC activity and age (during executive function tasks, error monitoring and when making high risk choices on a decision making paradigm; Eshel, Nelson, Blair, Pine, & Ernst, 2007; Rubia, Hyde, Halari, Giampietro, & Smith, 2010; Rubia, Smith, Taylor, & Brammer, 2007) and decreased dACC activity in older participants relative to younger participants (Christakou et al., 2009; Marsh et al., 2006; van Leijenhorst, Crone, & Bunge, 2006). These latter results are seen when making high risk choices in decision making paradigms (van Leijenhorst et al., 2006), during performance on the Switch (Christakou et al., 2009) and notably the Stroop tasks (Marsh et al., 2006). In line with previous suggestions (Christakou et al., 2009; Marsh et al., 2006; van Leijenhorst et al., 2006), we suggest that this reflects compensatory activity for the weaker functional integrity of the region at this age. It is notable that the there was no group-by-task interaction for either the RT or accuracy data. In other words, the children were not deficient in task performance. We speculate that in order to achieve the same behavioral results as the older participants, the children required greater dACC activation. We predict that increasing the difficulty of the behavioral task sufficiently to induce group differences in behavior might be reflected in reduced dACC activity in youth at this age.
Considerable previous work has demonstrated that increased attentional control on task relevant stimuli leads to reduced amygdala responses to emotional distracters (Mitchell et al., 2008; Mitchell et al., 2007; Pessoa, 2009; Pessoa, Kastner, et al., 2002). Increased representation of task relevant stimuli as a function of top down attention is thought, following representational competition (Desimone & Duncan, 1995), to result in reduced representation of emotional distracters and thus reduced amygdala responses to these distracters. The current study extends this work by showing that for children, where there are indications of reduced functional integrity of regions implicated in top down attention (as indexed by the current dACC findings considered above), there are also increased amygdala responses to emotional distracters. This was seen in the current study where the group-by-task-by-emotion interaction revealed that children showed greater activity within right amygdala, relative to older comparison groups, during task trials in the presence of negative distracters relative to neutral distracters. We suggest that this reflected less efficient priming of task relevant representations in the children leading to less competitive suppression of representations of negative emotional distracters and consequent greater amygdala responses to these emotional distracters. There were also indications though of generally increased left amygdala responsiveness to emotional stimuli in the children. This was identified by the group by emotion interaction and was seen in the children, relative to adolescents and adults, for both negative and positive distracter stimuli. This is consistent with some previous reports of increased amygdala responsiveness to emotional stimuli in younger age groups (Gee et al., 2013; Guyer et al., 2008).
While the group-by-task-by-emotion interaction identified by the main ANOVA indicated that children, relative to older individuals, were showing greater amygdala responses to negative distracters during task relative to view trials, the group-by-task-by-emotion on the connectivity data indicated increased connectivity, relative to adults and to a lesser extent adolescents, between left amygdala and a series of cortical regions for task trials in the presence of negative distracters relative to neutral distracters. These regions included right superior frontal gyrus, bilateral postcentral gyrus, bilateral angular gyrus, left superior temporal gyrus, and left precuneus. It is important to note though that with few exceptions (e.g. superior temporal gyrus) these regions do not show clear anatomical connections with the amygdala. We therefore speculate that this apparent connectivity reflects a correlated, compensatory response within these regions. In other words, the greater the amygdala response to the negative distracter, the greater the recruitment of these regions was necessary in the children to enable successful performance.
It has been argued that reduced top down attention may be a risk factor for the development of anxiety conditions (Bishop, 2007; Blair et al., 2012; Blair et al., 2013). The individual will be more likely to be distracted by and consequently process aversive emotional stimuli leading to heightened anxiety levels. Previous work has shown that the period between 10–14 years of age is marked by the greatest increase in cases of anxiety (social and other phobias and generalized anxiety disorder; Beesdo, Knappe, & Pine, 2009; Kendall et al., 2010; Merikangas et al., 2010; Moffitt et al., 2007). Interestingly, this is the age range in the current study where participants showed evidence of decreased functional integrity in dACC. This is particularly noteworthy as adult patients with anxiety (social phobia and generalized anxiety disorder) show a markedly reduced ability to recruit a proximal region of dACC in the context of the aST (coordinates in current study: 7.5, 13.5, 29.5; coordinates showing greates group differential in adult patients with anxiety: −5, 26, 24; Blair et al., 2012). We suggest that the functional integrity of dACC may serve to protect individuals from the development in anxiety disorders. The current data suggest that the age range at which this functional integrity is weaker is also the age range at greatest risk for the development of anxiety. Our previous data indicate that patients who have developed anxiety disorders show impairment in the ability to recruit this region in the context of tasks assessing automatic emotion regulation as a function of top down attention. Interestingly, it is possible to hypothesize that dACC functional integrity at age 10 might predict resilience with respect to the development of anxiety disorders by age 14.
Three caveats should be considered with respect to the current study. First, we did not assess the physical developmental stage of the subjects, especially their puberty status. Given previous findings of the impact of puberty on relevant brain structures (especially amygdala; Goddings et al., 2014; Killgore, Oki, & Yurgelun-Todd, 2001), it would be useful to know to what extent pubertal status may have contributed to the current results. Second, contrary to our hypothesis, we did not find any significant difference in lateral frontal or parietal cortices, that are known by previous studies to be related to top-down attention control (Blair et al., 2007). This may indicate that the greater dACC activation in the children to achieve comparable behavioral results to adolescents and adults may be sufficient to generate a “normalized” activity profile in the children in these other regions. However, this is of course highly speculative. Third, the group-by-task-by-emotion interaction was particularly notable for negative distracters; the children showed significantly greater amygdala responses to negative distracters relative to neutral distracters under task conditions. This could imply particular sensitivity to negative distracters. However, it is important to remember that we could not match the negative and positive distracters for arousal. As such, differential effects between negative and positive distracters may be attributable to differences in arousal. Future research might use negative and positive stimuli matched for arousal.
5. Conclusion
In this study, we investigated developmental changes in the recruitment of systems implicated in top down attention and emotional responding. In line with previous work, children showed evidence of deficient functional integrity of regions implicated in top down attention, particularly dorsal anterior cingulate cortex. In addition, and also in line with previous work (Gee et al., 2013; Guyer et al., 2008), children showed significantly increased amygdala responses to emotional stimuli. Notably, group differences in the response to negative stimuli were particularly pronounced during task trials. In addition, children showed increased connectivity between the amygdala and several cortical regions including superior frontal gyrus, bilateral postcentral gyrus, bilateral angular gyrus, and left precuneus relative to adults for task trials in the presence of negative distracters relative to neutral distracters. We argue that these data are consistent with the suggestion that deficient functional integrity of regions implicated in top down attention results in deficient automatic emotion regulation as a function of top down attention. We suggest that this leads to enhanced amygdala responses in the children relative to the adults, particularly during task trials. This may at least partly explain the increased risk for the development of anxiety conditions before the age of 14 years. Children prior to this age may be more sensitive to aversive emotional stimulation and therefore more likely to develop anxiety disorders from it. Interestingly, the connectivity data do suggest a degree of compensatory activity to this heightened emotional responding to negative distractors in the children relative to the adults. Future work suggested by the current studies is the determination of whether dACC functional integrity at age 10 predicts resilience from the development of anxiety disorders by age 14.
Supplementary Material
Highlights.
Investigates age related changes in top down attention and amygdala emotional responding
The affective stroop task examines representational interference of emotion on task stimuli
Task performance required greater ACC activity in children relative to adolescents/adults
Children showed greater amygdala responses to emotion particularly during task performance
This suggests reduced automatic top down attention based emotion regulation in children
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. S0193-953X(09)00056-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. S1364-6613(07)00132-5 [pii] [DOI] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M, Pine DS. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72(6):476–482. doi: 10.1016/j.biopsych.2012.04.013. S0006-3223(12)00364-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. S1053-8119(06)01117-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, Blair RJ. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2013;43(1):85–95. doi: 10.1017/S0033291712000840. S0033291712000840 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. S0959-4388(07)00029-3 [pii] [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. S1053-8119(85)71029-4 [pii] [DOI] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48(1):223–236. doi: 10.1016/j.neuroimage.2009.06.070. S1053-8119(09)00710-1 [pii] [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. S0028-3932(06)00398-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. appi.ajp.2009.09070931 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. 33/10/4584 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. S1053-8119(13)01009-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. 933887834 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. 30/46/15535 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. S0149-7634(09)00083-9 [pii] [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS- PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Compton SN, Walkup JT, Birmaher B, Albano AM, Sherrill J, Piacentini J. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–365. doi: 10.1016/j.janxdis.2010.01.009. S0887-6185(10)00026-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Fink GR. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28(2):429–439. doi: 10.1016/j.neuroimage.2005.06.065. S1053-8119(05)00423-4 [pii] [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci U S A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. S0890-8567(10)00476-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Luo Q, Mondillo K, Vythilingam M, Finger EC, Blair RJ. The interference of operant task performance by emotional distracters: an antagonistic relationship between the amygdala and frontoparietal cortices. Neuroimage. 2008;40(2):859–868. doi: 10.1016/j.neuroimage.2007.08.002. S1053-8119(07)00701-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. S1053-8119(06)01038-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Richell RA, Leonard A, Blair RJ. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. J Abnorm Psychol. 2006;115(3):559–566. doi: 10.1037/0021-843X.115.3.559. 2006-09167-017 [pii] [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64(6):651–660. doi: 10.1001/archpsyc.64.6.651. 64/6/651 [pii] [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. S1364-6613(05)00090-2 [pii] [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex. 2007;17(5):1047–1054. doi: 10.1093/cercor/bhl014. bhl014 [pii] [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. S1364-6613(09)00046-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15(1):31–45. doi: 10.1016/s0926-6410(02)00214-8. S0926641002002148. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences U S A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. S1053-8119(05)00363-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. S0006322303001719 [pii] [DOI] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhe HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.07.018. S0149-7634(13)00189-9 [pii] [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. European child & adolescent psychiatry. 2012 doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage. 2010;51(2):817–827. doi: 10.1016/j.neuroimage.2010.02.058. S1053-8119(10)00226-0 [pii] [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, Roiser JP, Tan GC, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain Behav. 2010;9(6):628–637. doi: 10.1111/j.1601-183X.2010.00596.x. GBB596 [pii] [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach, & Tournoux. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44(11):2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. S0028-3932(06)00049-2 [pii] [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J Neurosci. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. 29/40/12558 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuontela V, Jiang P, Tokariev M, Savolainen P, Ma Y, Aronen ET, Carlson S. Regulation of brain activity in the fusiform face and parahippocampal place areas in 7-11-year-old children. Brain Cogn. 2013;81(2):203–214. doi: 10.1016/j.bandc.2012.11.003. S0278-2626(12)00156-X [pii] [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Blair KS, McCaffrey D, Scaramozza M, Jones M, Nakic M, Blair RJ. Biased emotional attention in post-traumatic stress disorder: a help as well as a hindrance? Psychological Medicine. 2007;37(10):1445–1455. doi: 10.1017/S003329170700092X. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Baym CL, Gazzaley A, Bunge SA. Neural indices of improved attentional modulation over middle childhood. Dev Cogn Neurosci. 2011;1(2):175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.