Abstract
Some patients with chronic constipation may undergo colectomy yielding tissue appropriate to diagnosis of underlying neuromuscular pathology. The analysis of such tissue has, over the past 40 years, fuelled research that has explored the presence of neuropathy, myopathy and more recently changes in interstitial cells of Cajal (ICC). In this chapter, the data from these studies have been critically reviewed in the context of the significant methodological and interpretative issues that beset the field of gastrointestinal neuromuscular pathology. On this basis, reductions in ICC appear to a consistent finding but one whose role as a primary cause of slow transit constipation requires further evaluation. Findings indicative of significant neuropathy or myopathy are variable and in many studies subject to considerable methodological bias. Methods with practical diagnostic utility in the individual patient have rarely been employed and require further validation in respect of normative data.
Keywords: chronic constipation, enteric nervous system, histopathology, enteric neuropathy, enteric myopathy, interstial cells of Cajal, slow-transit constipation
Introduction
The fundamental question of whether chronic constipation might be associated with a histopathologically detectable abnormality of the neuromuscular apparatus of the colon is not new with early studies of idiopathic megacolon and megarectum [1-3] following those of Hirschsprung disease [4,5]. The past 100 years have seen the evolution of surgery performed with the intent of treating chronic constipation with [6, 7] or without [8-10] megacolon. The examination of tissue thus derived has paralleled changes in popularity of colectomy as a surgical approach. Many histological studies thus date from the 1980s and 1990s when colectomy was most popular [10-14] but have declined in number as surgeons began to appreciate that, aside from potential for serious complications, long-term functional outcomes were highly variable [15,16]. The question however remains pertinent as newer minimally invasive approaches continue to be developed [17-20] with the possibility in the future that histopathology could guide management.
The interpretation of data on histological abnormalities in chronic constipation raises several methodological and ontological issues:
Are tissues studied from slow transit constipation representative of the ‘average’ patient with idiopathic chronic constipation?
Are histological methods used technically valid?
Do findings actually deviate from normality (i.e. are there qualitative or quantitative differences compared to adequately studied controls)?
Do findings have a causal relationship with the observed clinical phenotype?
The reader may not be surprised to discover that these issues have only rarely been addressed by studies of chronic constipation to date. Indeed, the vast majority of studies described in this review fail on most counts. These points must be addressed to facilitate understanding of the results of this review.
Patient selection
The data provided are necessarily those from patients undergoing colectomy. Thus patients with other forms of chronic constipation e.g. evacuation disorder have rarely been studied. The evolution of colectomy to date has progressed from the early abdominal surgery era when colectomy was performed for a variety of oft spurious indications [8], through unselected cases of severe constipation (a mix of megacolon, pseudo-Hirshsprung's disease, cathartic and normal caliber colons) [10, 21], to the modern era where the procedure is only performed on a highly selected population of patients with physiologically defined idiopathic STC [13,22]. Thus it must be appreciated that historical studies suffer not only from having outdated histologic methodologies but also of having a case mix that is probably not representative of current chronic constipation patients. Furthermore, even now, there can be no doubt that patients submitting themselves for surgery de facto have medically refractory slow transit constipation and may thus not represent the ‘average’ idiopathic constipation patient. This selection bias must be appreciated in the interpretation of review data.
Methodological considerations
The delineation of techniques and reporting for the histopathological evaluation of GI neuropathology has been the subject of recent international consensus with guidelines published that are applicable to the general pathologist [23]. Of relevance to the constipation literature, and particularly in relation to the diagnosis of neuropathy, these guidelines make recommendations on the acquisition, processing and staining of tissue sections. These are discussed where pertinent to the review data.
Delineation of abnormality
The recent publication of the London Classification of GINMP delivered a comprehensive list of histological phenotypes which, on the basis of robust diagnostic criteria, can be safely made by pathologists (using technical standards from the above guidelines)[24] for a spectrum of GINMD. For idiopathic STC, these are:
-
Neuropathy
Hypoganglionosis ± degenerative neuropathy
Intestinal neuronal dysplasia (IND)-type B
Lymphocytic ganglionitis
Abnormal neurochemical coding
-
Myopathy
Amphophilic inclusion bodies
Abnormal ICC networks
It should be noted that the diagnostic criteria for defining these histological phenotypes were necessarily highly conservative being based on the ability to diagnose abnormality in the individual rather than observe group differences (as in many research studies). Indeed, the technical guidelines [23] while defining the qualitative criteria of several conditions necessarily deferred a detailed discussion of normal ranges of different cell types that might allow for more subtle quantitative diagnoses due to the perception that published normative data were inadequate. This perception has been confirmed by systematic review of published quantitative data for elements of the human enteric nervous system. The classification also omitted some findings that have been reported in GINMD (including in chronic constipation) on the basis that the findings had not been corroborated by 2 research groups. The results of this review include some histological phenotypes omitted from the London Classification.
Relationship to disease
The London classification allocated classified histopathological phenotypes to one of two basic categories reflecting their relationship to recognised clinical entities. These were: AETIOLOGIC: observed GINMP finding(s) is(are) diagnostic of a well-characterised disease with established cause and/or natural history and thus provide strong evidence of a pathogenic mechanism, and MORPHOLOGIC (ASSOCIATED) CHANGES: observed GINMP findings can be considered definite morphological abnormalities, i.e. findings are clearly identifiable and are not seen in normal tissue, but provide only weak evidence of pathogenic mechanism. The findings may or may not be causally related to observed clinical entities. For STC, all the above listed histological phenotypes were allocated to the MORPHOLOGIC (ASSOCIATED) category [24] reflecting consensus that causation could not be implied on current evidence.
Methods & Scope
The review is focused on human pathology as evident using histological techniques but includes, where supportive, those data from in-vitro functional studies using diseased human tissues. A systematic review is not possible in this field due to the paucity of quality studies. The review has however utilized standard key word and MeSH literature searches (Pubmed 1947 to date inc. MEDLINE & OLDMEDLINE) with these supplemented by an in depth bibliography compiled by the authors and other experts in the field (from the international working group). One author was contacted regarding duplication of data. To remain comprehensive, this review includes all studies in the English Language that document histological data from patients with chronic constipation regardless of patient numbers. The review addresses paediatric and adult idiopathic chronic constipation i.e. that without a defined systemic cause and has excluded studies of patients with secondary constipation such as Parkinson's disease which has its own distinct literature [25]. It also excludes those studies in which the patients are primarily those with idiopathic megacolon, megarectum or Hirschprung disease. The results have been organized in a manner that permits the reader to clearly identify the quality of study, particularly in terms of fulfilling selection, methodological and diagnostic criteria outlined above.
Neuropathy
Neuropathy can be defined as the presence of ‘an abnormal number or phenotype of neurons’. With reference to the GI tract this necessarily implies an abnormality of intrinsic neurons of the enteric nervous system (ENS). Such enteric neuropathy occurring in the colon has been suggested as a primary cause of chronic constipation since 1969 [26].
Hypoganglionosis ± degenerative neuropathy (London Classification 1.2.1 and 1.4)
Patient selection
The majority of data in this area relate to idiopathic slow transit constipation although earlier studies include less selected groups of patients including those who may have suffered neuronal damage from now outdated neurotoxic laxatives [27].
Methodological considerations
A reduction in number of neurons with or without degenerative forms should now only be made on the basis of a combination of H&E staining and immunohistochemistry using well established antibodies to several neuronally associated antigens [23,24]. Few studies meet these criteria with many historical studies relying on impractical or capricious methods. Silver staining requires specific mention in this respect because the received wisdom that a primary neuropathy is the cause of chronic constipation has largely been based on studies using this technique. Neurons contain several types of filamentous proteins serving as cytoskeleton or as part of the intracellular transport system of the cell. One method to histologically study the neurofibrillar system uses silver impregnation, which was pioneered by Golgi and further developed by Bodian and others [28-30]. It was introduced into the neuropathology of the GI tract in 1967 by Smith [31]. From the early 1980s, several reports were published on neuronal damage of the enteric nervous system based on Smith's method mainly by Schuffler's group at the Mayo Clinic [32-37] but also from some other centres [38]. 50 μm thick frozen tangential / horizontal sections, prepared with a ‘sledge’ microtome that is not generally available in most general pathology laboratories, were stained with silver nitrate revealing two types of neurons: argyrophilic and argyrophobic. The staining gives good cytomorphology of perikarya, axons and dendrites but, as with other silver impregnations, it is very susceptible to precipitation of the silver. Despite its undoubted advantages in terms of qualitative and quantitative analysis of neurons, silver staining is a capricious method even in experienced hands that is very susceptible to artefacts, labour intensive and requires a large tissue sample. Other methods that require enzyme histochemistry on frozen sections are also no longer recommended because of the impracticality and expertise required for this approach [23].
Diagnostic criteria
Central to the diagnosis of hypoganglionosis in an individual patient is the ability of the pathologist faced with appropriately stained sections to determine if the number of neurons or ganglia fall outside (below) the normal range. Despite 40 papers in the published literature to date, insufficient data exist regarding the normal density and distribution of submucosal or myenteric ganglion cells across the age spectrum to diagnose partial deficiency of neurons except in severe cases in which the majority of ganglion cells are missing (as seen in the distal transition zone of HSCR resections). Robust normative data are lacking for either H&E or immunohistochemically-stained sections. Thus comment can only be made on severe qualitative reductions in neurons or ganglia. Several studies have however demonstrated more subtle group differences in numbers of neurons or ganglia and these are reviewed.
In contrast to hypoganglionosis, the diagnosis of degenerative neuropathy can be made in an individual but requires considerable experience. Abnormal appearances can include hypertrophy, central chromatolysis, (swollen/ballooning degeneration), hypoxic alterations, inclusions or coarse granules and occasionally, oxyphilic change. The most seriously damaged nerve cells may undergo programmed cell death (e.g. apoptosis) and necrosis. Distinguishing artefactual changes from subtle degenerative features requires experience, and a definitive diagnosis should not be made unless unequivocal degenerative features are present. Enteric ganglionitis (below) is a common accompaniment.
Studies using H&E and immunohistochemistry (IHC)
In colonic tissue from patients with STC, routine light microscopy using only H&E staining has failed to identify a persistent abnormality other than melanosis coli [10,21,39] with only 2 studies finding variable or non-specific abnormalities in small proportions of those studied [40,41]. Table 1 shows those studies in which both H&E and IHC methods have been used. Pan-neuronal markers used in studies of the colon in patients with STC include neuron-specific enolase (NSE), protein gene product 9.5 (PGP 9.5), the satellite cell marker S100 protein and neurofilament stains. Early reports did not note decreases in numbers of neurons or ganglia. This is in contrast to some later studies (representing about 50% of the whole) that suggest that group differences indicative of oligoneuronia ± hypoganglionosis were present. It should be noted however that the seminal studies in this regard used methods that are not practical for clinical use. For instance, the two papers by Wedel et al., used microdissected whole-mounts with morphometric analyses [42, 43] whereas others have used counts per area based on epifluorescence microscopy [44, 45] or volume reconstructions [46]. In contrast, using standard methods, Knowles et al., reported that when cases were viewed individually there were no evident changes in neuronal number or convincing evidence of degeneration in any of 36 patients with STC [47].
Table 1. Controlled studies using immunostaining for neuronal associated antigens in the colon of patients with STC.
| Author | Year | N | Immunostain | Number of neurons | Degeneration |
|---|---|---|---|---|---|
| Benson et al. [48] | 1992 | 12 | S100 / NSE / NF2F11 | Normal | Not stated |
| Park et al. [49] | 1995 | 14 | PGP 9.5 / S100 | Normal | No |
| Porter et al. [44] | 1998 | 15 | NSE | Normal | No |
| Schouten et al. [50] | 1993 | 39 | NF2F11 | Normal | No |
| Romanska et al. [51] | 1996 | 6 | NCAM | Normal | No |
| F-Pellegrini et al. [52] | 1999 | 16 | NSE / S100 | ↓ neurons | No |
| Wedel et al. [43] | 2001 | 10 | PGP9.5 | ↓ neurons & ganglia | Not stated |
| Knowles et al. [47] | 2001 | 36 | NSE, PGP9.5, S100 | Normal | No |
| Wedel et al. [42] | 2002 | 11 | PGP9.5 * | ↓ neurons & ganglia | Not stated |
| Yu et al. [53] | 2002 | 14 | NF2F11 | ↓ neurons & ganglia | Not stated |
| Bassotti et al. [54] | 2006 | 26 | NSE / S100 | ↓ neurons | apoptosis |
| Wattchow et al. [45] | 2008 | 4 | anti-Hu C/D | Normal † | No |
KEY: PGP9.5 = protein gene product 9.5, NF = neurofilament, NSE = neuron specific enolase,
included 9 patients from earlier publication (REF 2001),
non significant reductions noted in neurons and ganglia.
Silver staining and other methods
Several studies have reported morphological abnormalities of the colonic innervation using silver staining following the description in 1969 of a single patient with childhood onset constipation and inflammatory and degenerative neuropathic changes [26]. Several studies of patients with severe idiopathic constipation without proven STC report argyrophilic degeneration of the myenteric plexus, with or without associated Schwann cell hyperplasia [37, 55, 56]. Notably, Krishnamurthy et al. [37] in their widely-cited, semi-blinded and controlled study of 12 patients noted a reduction in the total number of argyrophilic neurones coupled with morphological abnormalities. Preston et al. [10] suggested that there was a loss of the argyrophil plexus in 9 / 10 patients with proven STC, with associated Schwann cell hyperplasia. Two subsequent studies have confirmed Krishnamurthy's findings using silver staining in un-blinded studies of 12 and 25 colons respectively [57,58]. Oligoneuronal hypoganglionosis has also been shown using enzyme histochemical techniques on whole mount frozen preparations with morphometric measurements. Two studies of 7 children [59] and 12 adults [60] with STC who were already deemed by ‘histopathological diagnosis’ to be hypoganglionic were confirmed to have reduced numbers of neurons and ganglia.
Intestinal neuronal dysplasia type IIB (IND type IIB) (London classification: 1.3.2)
IND type IIB refers to the diagnosis of submucosal hyperganglionosis and is currently based on the finding of greater than 8 nerve cells in ≥20% of at least 25 ganglia [61, 62]. It has only been established with enzyme-histochemically stained (lactate dehydrogenase), 15 μm-thick frozen sections and no analogous quantitative criteria exist for the recognition of ‘giant’ submucosal ganglia in H&E or immunohistochemically stained paraffin sections. The diagnosis is most relevant to young children with infantile onset of chronic constipation where it may represent a developmental delay of ENS maturation limited to the submucosal plexus [63,64]. The diagnosis should however not be rendered in patients under the age of 1 year because giant ganglia and clinical findings often disappear as infants age. The overall contribution of this histological finding to pediatric and adult forms of chronic constipation remains highly contentious.
Lymphocytic ganglionitis (London Classification 1.5.1)
Inflammatory infiltrates in the myenteric plexus have proven significance in a number of acquired primary and secondary GINMD [65-67]. Gross infiltrates can be diagnosed from H&E sections but smaller quantitative estimations require IHC. On the basis of contemporary methods and defined cut-offs [23], significantly increased numbers of lymphocytes in or around ganglia have only been reported in one controlled study of 28 full thickness jejunal biopsies from patients of whom only 2 had STC [68].
Abnormal neurochemical coding (London Classification 1.7)
There are no firm current diagnostic criteria based on IHC to define abnormalities of neurochemically-defined subsets of neurons in individuals with GINMD [23]. Nevertheless, in relation to the putative effects of an imbalance of neurotransmitters, numerous studies have used a variety of methods (staining and assay) to demonstrate group alterations in the expression of one or more neurotransmitters (the majority of which are neuropeptides) or their enzyme markers in the large intestine of patients with constipation. Studies have been performed on neurotransmitters thought to be predominantly inhibitory: NO [44,45,52,69,70], VIP [51,69,71-74]; NPY [44,71-73]; or excitatory: SP [44,51,71-75], ACh [44,45]. Table 2 demonstrates the wide inconsistency of results with the most frequently studied neuropeptides. For example, considering only studies with physiological selection for STC, decreased [51], increased [74], and unchanged [73] levels of VIP have been demonstrated by immunoassay, and similarly, decreased [51], increased [69] or unchanged [44, 71] VIP immunoreactivities have been shown by immunostaining. Further, many of these findings are limited to changes in numbers of nerve fibers rather than neurons and thence only in certain colonic regions.
Table 2.
Immunohistochemical (IHC) and immunoassay (IA) studies of nurotransmitters in colon tissue from patients with either severe idiopathic constipation or proven STC.
| Author | Year | N | STC | Specimen | IHC | IA | |
|---|---|---|---|---|---|---|---|
| Substance P | |||||||
| Goldin et al.[75] | 1989 | 24 | n.s | rectal biopsy | NP | ↓ | |
| Milner et al.[72] | 1990 | 8 | n.s | sigmoid colon | → | → | |
| Dolk et al.[71] | 1990 | 7 | Y | colon levels | → | NP | |
| Tzavella et al.[73] | 1996 | 22 | Y | rectal biopsies | NP | ↓ | |
| Hutson et al.[17] | 1996 | 10P | Y | colon levels | ↓† | NP | |
| Sjolund et al.[74] | 1997 | 18 | Y | colon levels | ↑* | ↑ (AC only) | |
| Porter et al.[44] | 1998 | 15 | Y | colon levels & ileum | ↓ | NP | |
| King et al.[70] | 2010 | 51P | Y | colon levels | ↓† (AC only) | NP | |
| Choline acetyltransferase (ChAT) | |||||||
| Porter et al.[44] | 1998 | 15 | Y | colon levels & ileum | →† | NP | |
| Wattchow et al.[45] | 2008 | 4 | Y | colon levels | ↓ | NP | |
| Nitric oxide synthase (NOS) | |||||||
| Faussone-Pellegrini et al.[52] | 1999 | 24 | Y | caecocolonic | ↓ | NP | |
| Wattchow et al.[45] | 2008 | 4 | Y | colon levels | ↑ | NP | |
| King et al.[70] | 2010 | 51P | Y | colon levels | →† | NP | |
| VIP | |||||||
| Koch et al.[79] | 1988 | 4 | Y | descending colon | ↓ † | ↓ † | |
| Milner et al.[72] | 1990 | 8 | n.s | sigmoid colon | → | ↓ † | |
| Dolk et al.[71] | 1990 | 7 | Y | colon levels | → | NP | |
| Cortisini et al.[69] | 1995 | 5 | Y | colon levels | ↑ ↓ ‡ | NP | |
| Tzavella et al. | 1996 | 22 | Y | rectal biopsies | NP | → | |
| Hutson et al.[17] | 1996 | 10P | Y | colon levels | ↓ † | NP | |
| Sjolund et al.[74] | 1997 | 18 | Y | colon levels | ↑ (TC only) | ↑ (AC only) | |
| Porter et al.[44] | 1998 | 15 | Y | colon levels & ileum | → | NP | |
| King et al.[70] | 2010 | 51P | Y | colon levels | ↓ † | NP | |
| NPY | |||||||
| Milner et al.[72] | 1990 | 8 | n.s | sigmoid colon | → | → | |
| Dolk et al.[71] | 1990 | 7 | Y | colon levels | → | NP | |
| Sjolund et al.[74] | 1997 | 18 | Y | colon levels | ↑* | NP | |
| Porter et al.[44] | 1998 | 15 | Y | colon levels & ileum | → | NP | |
| 5 hydroxy-tryptamine (5HT) | |||||||
| Dolk et al.[71] | 1990 | 7 | Y | colon levels | → | NP | |
| Lincoln et al.[80] | 1990 | 9 | Y | sigmoid colon | → | ↑ † | |
| Sjolund et al.[74] | 1997 | 18 | Y | colon levels | ↑ $ | NP | |
Key: n.s: not stated, NP: not performed, AC: ascending colon, TC: transverse colon,
: descending colonic myenteric plexus only,
: changes in muscularis propria (fibres or assay levels),
: decreased in myenteric plexus, increased in circular muscle,
: endothelial cells of mucosa of descending colon only.
In those studies that focus on neuronal staining, the interpretation of such changes is challenging without the concurrent use of a pan neuronal marker to determine whether observed changes represent an absolute reduction in number of a particular neurochemically defined subset of neurons or plasticity in a stable neuronal population. On this note, the Flinders group used methods that are well established in their laboratory [76] to detail significant reductions in cholinergic and increases in nitrinergic neuron subpopulations [45]. Such findings corroborate with in-vitro studies showing release reduced release of acetylcholine (ACh)[77], and increased release of inhibitory neurotransmitters (NO, ATP) [78] to electrical field stimulation.
Other histological findings
There are several reports of histological findings with potential biological rationale that are not considered currently to be valid diagnostically. The first, numbers of nerve fibres in the muscularis propria, has relevance to some of the studies of neurochemical coding (table 2) and others using either neuronal associated antigens or silver staining [37]. There are several reports of significantly decreased [46, 50,81,82]; or increased [49] immunohistochemical densities of nerve structures (variably described as nerve fibres, enteric neurofilaments and small axonal fibres) as determined by subjective or automated counting methods. While these changes may have potential pathophysiological importance [83], aside from the contradictory nature of findings, there are no current standardized methods or robust normative data to render diagnostic utility to such measurements.
Schwann cell hyperplasia has been reported by silver staining in some patients with degenerative neuropathy [10,26]. Now better termed reactive gliosis, excessive focal accumulation of glial cells around degenerating neurons may be considered as an adjunct to diagnosis of neuropathy [23]. However, normative data for number of glial cells are sparse in the literature with only 3 studies of the colon, which used different methods of immunostaining, counting, and data presentation. Further, counting glia is not easy by H&E staining alone and other more complex methods are not generally applicable. In relation to STC, findings vary from those using silver staining (increased) to those showing significant decreases [54] or increased ratio to neurons [43] using immunostaining.
Practice points
The diagnosis of enteric neuropathy is subject to methodological issues that negate the findings of many research studies;
Neuropathy based on current diagnostic criteria is probably not a common finding in chronic constipation;
Changes in functional subsets of enteric neurons or glia may have biological relevance but have little current diagnostic utility.
Research agenda
Studies that utilize agreed contemporary techniques and diagnostic criteria are required;
Normative data are lacking and are urgently required to diagnose quantitative changes.
Myopathy
Several hereditary and systemic disorders are associated with gastrointestinal smooth muscle abnormalities and constipation. These include familial visceral myopathy, hereditary visceral myopathy, megacystis microcolon and scleroderma. There is therefore an existing association of abnormalities in smooth muscle and constipation. This is not surprising, given that smooth muscle cells are the final effectors of propulsive activity in the gastrointestinal tract. It is therefore reasonable to assume that abnormalities in the smooth muscle layers of the gastrointestinal tract could also contribute to chronic idiopathic constipation. This assumption is also supported by physiological data in-vivo where ambulatory 24 hour colonic manometry showed features suggestive of a myopathy in 24% of select patients with STC [84]. It is supported by some in-vitro data from organ bath studies of colonic muscle strips from patients with constipation [78, 83] but not by others [85]. Despite this, there is remarkably little known about myopathies in chronic idiopathic constipation and slow transit constipation at a tissue level.
Methodological issues & diagnostic criteria
Gross developmental alterations in muscle layering, degenerative and inflammatory changes are usually evident on H&E staining with supplemental information provided by other tinctorial (e.g. trichrome stain or van Gieson preparations) or immunohistochemical (e.g. alpha smooth muscle actin) techniques. Transmission electron microscopy (TEM) represents an adjunctive investigation. Experience, particularly in relation to distinguishing artifactual changes, is essential. A major hurdle to research in this area is the lack of a well accepted, sensitive and specific marker for gastrointestinal smooth muscle cell abnormalities. The most rigorous study to date is from Wedel et al [86]. They studied Hirschsprung's disease, idiopathic megacolon and 13 patients with slow transit constipation was well as 12 controls and stained the tissue for smooth muscle α-actin, smooth muscle myosin heavy chain, smoothelin and histone deactylase-8. Transmission electron microscopy showed smooth muscle abnormalities in all 13 patients. Smooth muscle α-actin was abnormal in the longitudinal smooth muscle layer in 2/13, smooth muscle myosin heavy chain in 7/13, smoothelin in 8/13, and histone deactylase-8 in 7/13. In those labeled as abnormal, there were differences between the markers in involvement of circular muscle, longitudinal muscle or both. These data suggest that the most commonly used marker, smooth muscle α-actin may lack sufficient sensitivity to detect subtle alterations that could be indicative of myopathy and that these might be better sought by more rigorous immunohistochemical profiling and TEM.
Most histological series report no evidence of the well established degenerative or inflammatory forms of myopathy in patients with chronic idiopathic constipation with or without defined STC with only one study showing a minority (4 / 36) patients with degenerative changes ± fibrosis [47]. Accepting that many phenotypes included within the London Classification [24] may not have been systematically sought in many studies, the only finding currently documented in idiopathic STC is that of one of the 3 recognized forms of myocyte inclusions.
Amphophilic inclusion bodies (London Classification 2.4.2.2)
Intestinal smooth muscle inclusion bodies have been reported in a variety of systemic conditions and have also been reported in slow transit constipation [47]. In that paper, inclusion bodies were detected in ascending and sigmoid colonic and ileal specimens from normal subjects and the frequency of patients with inclusions increased with age. Of 25 patients with idiopathic slow transit constipation studied, inclusions were found more often than in controls (ascending colon 50% versus 19% in controls and descending colon 43% versus 20%). Interestingly, similar findings were also found in the ileum, with more inclusion bodies in the slow transit constipation group. The authors suggested that the inclusions were a result of denervation but this hypothesis was not directly tested and the exact makeup of the inclusion bodies has not yet been established.
Other histological findings
Two other reported changes merit some discussion. The first, thickening of the circular muscle layer has been reported in chronic constipation [49] but is subject to enormous methodological variability. The second, ‘atrophic desmosis’ - a total or focal lack of the mesh network of collagen tissue in particular between the circular and longitudinal muscle layer is rendered by the detailed study of connective tissue of the muscularis propria and particularly that surrounding the myenteric plexus. The studies require use of a connective tissue tinctorial stain such as picrosirius red and have thus far been performed on 15μm cryostat sections. The condition has been described in the colon of children with chronic constipation with or withour megacolon, the former termed ‘hypoperistalsis syndrome’ [87, 88] and has reasonable biological rationale based on clinical [89] and animal studies [90].
Practice points
Well established developmental, degenerative and inflammatory myopathic phenotypes are at best an uncommon finding in colonic tissues from patients with chronic constipation.
Research agenda
There are remarkably few data on myopathy in chronic constipation and this should be an area of future research;
More sensitive immunolabels require evaluation with normal values and the physiological correlates of such changes determined.
Abnormal Interstitial Cells of Cajal
Interstitial cells of Cajal (ICC) are increasingly recognized as important for the control of normal gastrointestinal function, including colonic motility. Since the original paper describing a significant loss of ICC in patients with STC in 2000 [46], there have been many reports confirming this finding.
Patient selection
In contrast to neuropathy, all studies have been performed on well characterized patients with physiologically defined STC.
Methodological and diagnostic considerations
A diagnosis of abnormal ICC can only be made using receptor tyrosine kinase c-Kit (CD117) or Ano1 (DOG1) immunostaining. Current standard formalin fixation methods, even when coupled with optimal antigen retrieval techniques, underestimate the number of ICC present in a section, and the number of ICC visualized varies according to the anti-kit (CD117) antibody used. For instance, a recent study compared ICC numbers in the human colon when counted using visible light on formalin fixed and paraffin embedded sections compared to immunofluorescence on frozen, paraformaldehyde fixed sections [91]. Even with optimal antigen retrieval, immunohistochemistry on formalin fixed and paraffin embedded sections detected 42% of ICC detected by immunofluorescence in the circular muscle layer of the colon, 38% in the longitudinal muscle and 42% in the myenteric plexus region. This is problematic in a diagnostic sense since these more complex methods investigated in a research context, e.g. thick cryostat sections examined using fluorescent microscopy or whole mount preparations examined using confocal microscopy and other antibodies will be difficult for most to replicate in routine diagnosis. They highlight also that extra care needs to be taken when using immunohistochemistry on formalin fixed and paraffin embedded sections, including using the same antigen retrieval process and labeling controls and diseased sections at the same time. On this basis, current guidelines [23, 24] advise that given the wide variation in normal values, when considering an individual patient, a reduction in ICC numbers should only be reported if more than 50%. Qualitative changes in ICC morphology, whilst not-diagnostic in isolation may accompany ICC loss as may loss of enteric nerves. Another important variable to account for is age. ICC numbers decreased markedly with age with an average of a 13% loss per decade of life in adults [92]. Therefore care must also be taken to find the appropriate control for patients with slow transit constipation. This can be a significant challenge given that most patients with slow transit constipation are young and female while most controls come from resections from colon cancer, usually from an older age group.
Abnormal ICC networks (London Classification 3.1)
Abnormalities in ICC, including mutations in Kit [93, 94], the receptor tyrosine kinase expressed in ICC, have been reported in chronic constipation for both children and adults. Although one study [95], perhaps in relation to methodology, did not report reduced ICC, there is overwhelming evidence for significant reductions in ICC based on group comparisons with controls (Table 3).
Table 3. Studies of ICC in colonic tissue from patients with slow-transit constipation.
| Author | Year | N | Methods | Findings |
|---|---|---|---|---|
| He et al.[46] | 2000 | 6 | c-kit 3D laser CFM | ↓ ICC all layers |
| Lyford et al.[96] | 2002 | 6 | c-kit 3D laser CFM | ↓ ICC all layers |
| Wedel et al.[97] | 2002 | 11 | c-kit + morphometry | ↓ ICC all layers exc. LM |
| Yu et al.[53] | 2002 | 14 | c-kit | ↓ ICC all layers |
| Lee et al.[81] | 2005 | 10 | c-kit | ↓ ICC all layers |
| Tong et al.[98] | 2005 | 12 | c-kit RT-PCR / IHC | ↓ ICC all layers |
| Bassotti et al.[54] | 2006 | 26 | c-kit | ↓ IC-MY & IC-SM |
| Toman et al.[95] | 2006 | 13 | c-kit / CD34 | No differences |
| Shafik et al.[99] | 2006 | 28 | c-kit | absent ICC |
| Wang et al.[82] | 2008 | 15 | c-kit | ↓ ICC all layers |
| Van den berg et al.[100] | 2009 | 8P | c-kit | ↓ or focally absent |
Key: ICC = interstitial cells of Cajal, P = paediatric, LM = longitudinal muscle, IC-MY = ICC myenteric plexus, IC-SM = submucosal ICC, CFO confocal microscopy
A review of the studies published to date suggests that loss of ICC is usually not the only defect seen in slow transit constipation [46,81,82] and that there is frequently a concomitant loss of enteric nerves. This suggests that there is either a common unknown factor that targets both cell types or that loss of one cell type influences the survival of the other. Survival and proliferation of adult ICC is dependent on several factors including Kit, 5HT (serotonin) signaling through the 5HT2B receptor, Ano1, heme oxygenase 1 and neuronal nitric oxide synthase [101]. There is fairly rapid turnover of ICC populations in the gastrointestinal tract with both apoptosis [102] and proliferation of adult ICC seen [103]. Furthermore, stem cells that generate ICC are present in the adult [104]. Loss of any of these factors would affect ICC numbers. Kit is one of the factors that ICC are particularly dependent on for survival and enteric nerves express stem cell factor, the ligand for Kit. However enteric nerve derived stem cell factor does not seem to be required for ICC survival [105] and other sources of stem cell factor, including smooth muscle cells appear to be more critical to ICC survival [106]. Conversely, ICC may themselves releases key messengers that are required for enteric nerve survival. The relationship between loss of ICC and of enteric nerves deserves further study as it may point towards the original insult as well as potential therapies.
Relationship to disease
Decreased ICC numbers and abnormal networks have been described not only in constipation but also in several other motility disorders. Such a widespread association raises issues. One is whether ICC loss is part of the disease or is secondary to a subsequent insult such as inflammation or obstruction. Inflammation [107] and obstruction [108] have both been shown in animal models to be associated with reversible loss of Kit-positive ICC. Whether this reflects actual death of the cell remains unclear and probably unlikely. In humans, ICC loss has been well described in conditions including slow transit constipation where dilatation is not part of the clinical picture. Also in conditions associated with dilation, such as intestinal pseudo-obstruction, subclasses of ICC are reported to be selectively affected arguing against a simple dilation effect. However dilation should be kept in mind when assessing ICC and other cell types especially in cases associated with marked dilation. ICC are also susceptible to ischaemia and care must be taken to minimize ischemia when collecting human specimens [101]. This is particularly relevant with laparoscopic surgery where the blood supply is often cut off a substantial time before the tissue is extracted. Close coordination with the surgeon is essential, as is use of appropriate controls. Another issue is whether the ICC defect is in the primary target in slow transit constipation or is bystander. This issue cannot currently be resolved as to do so we need a clear understanding of aetiology of slow transit constipation and the ability to obtain colonic tissue at longitudinal time points. While this is an area requiring more research it is perhaps of less clinical relevance as a loss of ICC or disrupted networks will lead to colonic dysfunction irrespective of the exact sequence of events that lead to their loss.
Practice points
Reduction of ICC is the most consistent histological finding in STC to date.
Standardized approaches are required to determine significant ICC loss, particularly for diagnosis in individual patients as several variables such as age and fixation method can markedly alter the results.
Research agenda
Little to nothing is known about ICC numbers in normal transit constipation as these patients rarely come to surgery – these would provide an interesting comparison group;
Determination of electrophysiological correlates between loss of ICC and neuronal and smooth muscle function in-vitro and in-vivo;
Mechanistic studies to determine whether ICC loss is predominantly due to decreased replacement versus increased loss;
Studies of the triggers for ICC loss (primary or secondary) in relation to enteric nerves and smooth muscle.
Summary
The available data on histological abnormalities in patients with chronic constipation are limited in the main to group comparisons with controls using research techniques. These data give consistent evidence for an association of STC with reduced numbers of ICC. In contrast, the evidence for neuropathy is variable using contemporary techniques and that for myopathy un-compelling. The findings of reduced ICC and neuropathy, which may be interdependent, have good biological rationale in respect of causation although this remains to be proven. In contrast, due to methodological considerations (especially control data), there are few (with the possible exception of ICC) if any findings at the current time that have sufficient practical diagnostic utility to function as biomarkers of chronic constipation in individual patients [100]. This situation may however change with international efforts to rationalize techniques and reporting in the field of GINMP and the publication of more robust normative data. Whether such findings, if determined in a relatively non-invasive manner, can guide specific medical or surgical intervention remains attractive but speculative.
Figure 1.
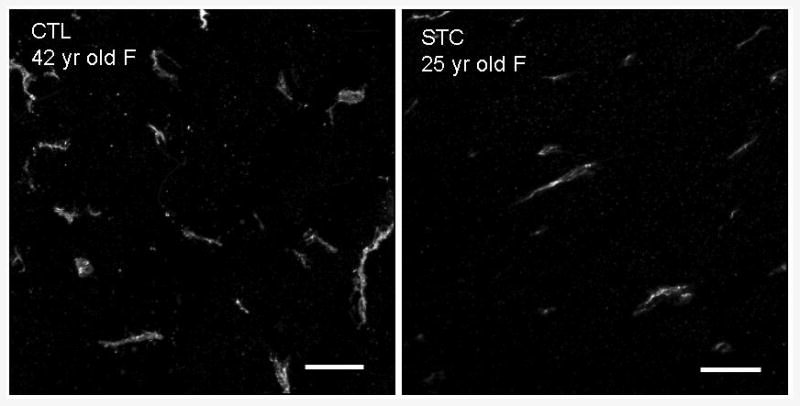
Colonic sections from patient with STC and control immunostained using antibody to CD117 (c-kit). There is a significant (>50%) reduction in ICC within the circular muscle in the patient with STC" Scale bar = 25 μm. Images were collected on the LSM 510 using a 40X Zeiss water immersion objective
Acknowledgments
Grant support: CHK is supported by HEFCE; GF is supported by NIH DK57061, DK52766 and DK68055
Footnotes
Conflict of Interest: None
References
- 1.Bill AH, Jr, Creighton SA, Stevenson JK. The selection of infants and children for the surgical treatment of Hirschsprung's disease. Surg Gynecol Obstet. 1957;104:151–6. [PubMed] [Google Scholar]
- 2.Ravitch MM. Pseudo Hirschsprung's disease. Ann Surg. 1958;147:781–95. [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenpreis T. Pseudo-Hirschsprung's Disease. Arch Dis Child. 1965;40:177–9. doi: 10.1136/adc.40.210.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehouse FR, Kernohan JW. Myenteric plexus in congenital megacolon; study of 11 cases. Arch Intern Med (Chic) 1948;82:75–111. doi: 10.1001/archinte.1948.00220250085005. [DOI] [PubMed] [Google Scholar]
- 5.Bodian M, Stephens FD, Ward BC. Hirschsprung's disease and idiopathic megacolon. Lancet. 1949;1:6–11. doi: 10.1016/s0140-6736(49)90340-2. [DOI] [PubMed] [Google Scholar]
- 6.Lane RH, Todd IP. Idiopathic megacolon: a review of 42 cases. Br J Surg. 1977;64:307–10. doi: 10.1002/bjs.1800640502. [DOI] [PubMed] [Google Scholar]
- 7.Stabile G, Kamm MA. Surgery for idiopathic megarectum and megacolon. Int J Colorectal Dis. 1991;6:171–4. doi: 10.1007/BF00341241. [DOI] [PubMed] [Google Scholar]
- 8.Lane WA. Remarks ON THE RESULTS OF THE OPERATIVE TREATMENT OF CHRONIC CONSTIPATION. Br Med J. 1908;1:126–30. doi: 10.1136/bmj.1.2455.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertrand P, Girard M, Denis M. Long term results of total colectomy for chronic, inveterate constipation. Arch Mal Appar Dig Mal Nutr. 1951;40:1251–9. [PubMed] [Google Scholar]
- 10.Preston DM, Hawley PR, Lennard-Jones JE, Todd IP. Results of colectomy for severe idiopathic constipation in women (Arbuthnot Lane's disease) Br J Surg. 1984;71:547–52. doi: 10.1002/bjs.1800710726. [DOI] [PubMed] [Google Scholar]
- 11.Hughes ES, McDermott FT, Johnson WR, Polglase AL. Surgery for constipation. Aust N Z J Surg. 1981;51:144–8. doi: 10.1111/j.1445-2197.1981.tb05926.x. [DOI] [PubMed] [Google Scholar]
- 12.Platell C, Scache D, Mumme G, Stitz R. A long-term follow-up of patients undergoing colectomy for chronic idiopathic constipation. Aust N Z J Surg. 1996;66:525–9. doi: 10.1111/j.1445-2197.1996.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 13.Pemberton JH, Drelichman ER. Quality of life after subtotal colectomy for constipation: selection of the right patient, operation, and tools to measure outcome. Dis Colon Rectum. 2003;46:1720–1. doi: 10.1007/BF02660786. author reply 1. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo MF, Reissman P, Wexner SD. Colectomy as treatment for constipation in selected patients. Br J Surg. 1995;82:898–901. doi: 10.1002/bjs.1800820713. [DOI] [PubMed] [Google Scholar]
- 15.Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg. 1999;230:627–38. doi: 10.1097/00000658-199911000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles CH, Dinning PG, Pescatori M, Rintala R, Rosen H. Surgical management of constipation. Neurogastroenterol Motil. 2009;21(Suppl 2):62–71. doi: 10.1111/j.1365-2982.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 17.Hutson JM, Chow CW, Borg J. Intractable constipation with a decrease in substance P-immunoreactive fibres: is it a variant of intestinal neuronal dysplasia? J Pediatr Surg. 1996;31:580–3. doi: 10.1016/s0022-3468(96)90501-1. [DOI] [PubMed] [Google Scholar]
- 18.King SK, Sutcliffe JR, Hutson JM. Laparoscopic seromuscular colonic biopsies: a surgeon's experience. J Pediatr Surg. 2005;40:381–4. doi: 10.1016/j.jpedsurg.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Rajan E, Gostout CJ, Lurken MS, Talley NJ, Locke GR, Szarka LA, et al. Endoscopic “no hole” full-thickness biopsy of the stomach to detect myenteric ganglia. Gastrointest Endosc. 2008;68:301–7. doi: 10.1016/j.gie.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebouvier T, Coron E, Chaumette T, Paillusson S, Bruley des Varannes S, Neunlist M, et al. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol Motil. 2010;22:e11–4. doi: 10.1111/j.1365-2982.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamm MA, Hawley PR, Lennard-Jones JE. Outcome of colectomy for severe idiopathic constipation. Gut. 1988;29:969–73. doi: 10.1136/gut.29.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wexner SD, Daniel N, Jagelman DG. Colectomy for constipation: physiologic investigation is the key to success. Dis Colon Rectum. 1991;34:851–6. doi: 10.1007/BF02049695. [DOI] [PubMed] [Google Scholar]
- 23.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 24.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 2010;59:882–7. doi: 10.1136/gut.2009.200444. [DOI] [PubMed] [Google Scholar]
- 25.Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N'Guyen JM, et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS One. 2010;5:e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyer NH, Dawson AM, Smith BF, Todd IP. Obstruction of bowel due to lesion in the myenteric plexus. Br Med J. 1969;1:686–9. doi: 10.1136/bmj.1.5645.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller-Lissner S. Laxatives in chronic constipation. Med Monatsschr Pharm. 1996;19:337–8. [PubMed] [Google Scholar]
- 28.Bielschowsky M. Die Silberimprägnation der Achsencylinder. Neurologisches Zentralblatt. 1902;21 [Google Scholar]
- 29.Bodian D. A new method for staining nerve fibers and nerve endings in mounted paraffin sections. Anat Rec. 65:89–97. [Google Scholar]
- 30.Da Fano C, Ingleby H. Demonstration of Preparations from Cases of Encephalitis Lethargica. Proc R Soc Med. 1919;12:42–5. doi: 10.1177/003591571901201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith B. The myenteric plexus in Hrischsprung's disease. Proc R Soc Med. 1967;60:803. doi: 10.1177/003591576706000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuffler MD, Jonak Z. Chronic idiopathic intestinal pseudo-obstruction caused by a degenerative disorder of the myenteric plexus: the use of Smith's method to define the neuropathology. Gastroenterology. 1982;82:476–86. [PubMed] [Google Scholar]
- 33.Schuffler MD, Leon SH, Krishnamurthy S. Intestinal pseudoobstruction caused by a new form of visceral neuropathy: palliation by radical small bowel resection. Gastroenterology. 1985;89:1152–6. doi: 10.1016/0016-5085(85)90223-9. [DOI] [PubMed] [Google Scholar]
- 34.McDonald GB, Schuffler MD, Kadin ME, Tytgat GN. Intestinal pseudoobstruction caused by diffuse lymphoid infiltration of the small intestine. Gastroenterology. 1985;89:882–9. doi: 10.1016/0016-5085(85)90587-6. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamurthy S, Schuffler MD, Belic L, Schweid AI. An inflammatory axonopathy of the myenteric plexus producing a rapidly progressive intestinal pseudoobstruction. Gastroenterology. 1986;90:754–8. doi: 10.1016/0016-5085(86)91134-0. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida MM, Krishnamurthy S, Wattchow DA, Furness JB, Schuffler MD. Megacolon in myotonic dystrophy caused by a degenerative neuropathy of the myenteric plexus. Gastroenterology. 1988;95:820–7. doi: 10.1016/s0016-5085(88)80034-9. [DOI] [PubMed] [Google Scholar]
- 37.Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE., 2nd Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology. 1985;88:26–34. doi: 10.1016/s0016-5085(85)80128-1. [DOI] [PubMed] [Google Scholar]
- 38.Navarro J, Sonsino E, Boige N, Nabarra B, Ferkadji L, Mashako LM, et al. Visceral neuropathies responsible for chronic intestinal pseudo-obstruction syndrome in pediatric practice: analysis of 26 cases. J Pediatr Gastroenterol Nutr. 1990;11:179–95. doi: 10.1097/00005176-199008000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Redmond JM, Smith GW, Barofsky I, Ratych RE, Goldsborough DC, Schuster MM. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995;90:748–53. [PubMed] [Google Scholar]
- 40.Watier A, Devroede G, Duranceau A, Abdel-Rahman M, Duguay C, Forand MD, et al. Constipation with colonic inertia. A manifestation of systemic disease? Dig Dis Sci. 1983;28:1025–33. doi: 10.1007/BF01311732. [DOI] [PubMed] [Google Scholar]
- 41.Pluta H, Bowes KL, Jewell LD. Long-term results of total abdominal colectomy for chronic idiopathic constipation. Value of preoperative assessment. Dis Colon Rectum. 1996;39:160–6. doi: 10.1007/BF02068070. [DOI] [PubMed] [Google Scholar]
- 42.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–67. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 43.Wedel T, Roblick UJ, Ott V, Eggers R, Schiedeck TH, Krammer HJ, et al. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54–62. doi: 10.1007/s10350-004-6114-3. [DOI] [PubMed] [Google Scholar]
- 44.Porter AJ, Wattchow DA, Hunter A, Costa M. Abnormalities of nerve fibers in the circular muscle of patients with slow transit constipation. Int J Colorectal Dis. 1998;13:208–16. doi: 10.1007/s003840050163. [DOI] [PubMed] [Google Scholar]
- 45.Wattchow D, Brookes S, Murphy E, Carbone S, de Fontgalland D, Costa M. Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterol Motil. 2008;20:1298–305. doi: 10.1111/j.1365-2982.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- 46.He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 47.Knowles CH, Nickols CD, Scott SM, Bennett NI, de Oliveira RB, Chimelli L, et al. Smooth muscle inclusion bodies in slow transit constipation. J Pathol. 2001;193:390–7. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH797>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 48.Benson MJK, D, Roberts J, Martin JE, Swash M, Wingate DL, Williams NS. Colonic neural and smooth muscle abnormalities in slow transit constipation (STC) Gastroenterology. 1992;102:A424. [Google Scholar]
- 49.Park HJ, Kamm MA, Abbasi AM, Talbot IC. Immunohistochemical study of the colonic muscle and innervation in idiopathic chronic constipation. Dis Colon Rectum. 1995;38:509–13. doi: 10.1007/BF02148851. [DOI] [PubMed] [Google Scholar]
- 50.Schouten WR, ten Kate FJ, de Graaf EJ, Gilberts EC, Simons JL, Kluck P. Visceral neuropathy in slow transit constipation: an immunohistochemical investigation with monoclonal antibodies against neurofilament. Dis Colon Rectum. 1993;36:1112–7. doi: 10.1007/BF02052258. [DOI] [PubMed] [Google Scholar]
- 51.Romanska HM, Bishop AE, Lee JC, Walsh FS, Spitz L, Polak JM. Idiopathic constipation is not associated with increased NCAM expression on intestinal muscle. Dig Dis Sci. 1996;41:1298–302. doi: 10.1007/BF02088550. [DOI] [PubMed] [Google Scholar]
- 52.Faussone-Pellegrini MS, Infantino A, Matini P, Masin A, Mayer B, Lise M. Neuronal anomalies and normal muscle morphology at the hypomotile ileocecocolonic region of patients affected by idiopathic chronic constipation. Histol Histopathol. 1999;14:1119–34. doi: 10.14670/HH-14.1119. [DOI] [PubMed] [Google Scholar]
- 53.Yu CS, Kim HC, Hong HK, Chung DH, Kim HJ, Kang GH, et al. Evaluation of myenteric ganglion cells and interstitial cells of Cajal in patients with chronic idiopathic constipation. Int J Colorectal Dis. 2002;17:253–8. doi: 10.1007/s00384-001-0380-5. [DOI] [PubMed] [Google Scholar]
- 54.Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41–6. doi: 10.1136/gut.2005.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith B, Grace RH, Todd IP. Organic constipation in adults. Br J Surg. 1977;64:313–4. doi: 10.1002/bjs.1800640504. [DOI] [PubMed] [Google Scholar]
- 56.Yoshioka K, Keighley MR. Clinical results of colectomy for severe constipation. Br J Surg. 1989;76:600–4. doi: 10.1002/bjs.1800760625. [DOI] [PubMed] [Google Scholar]
- 57.Zenilman ME, Dunnegan DL, Soper NJ, Becker JM. Successful surgical treatment of idiopathic colonic dysmotility. The role of preoperative evaluation of coloanal motor function. Arch Surg. 1989;124:947–51. doi: 10.1001/archsurg.1989.01410080083013. [DOI] [PubMed] [Google Scholar]
- 58.Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg. 1991;214:403–11. doi: 10.1097/00000658-199110000-00005. discussion 11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier-Ruge WA, Longo-Bauer CH. Morphometric determination of the methodological criteria for the diagnosis of intestinal neuronal dysplasia (IND B) Pathol Res Pract. 1997;193:465–9. doi: 10.1016/s0344-0338(97)80098-2. [DOI] [PubMed] [Google Scholar]
- 60.Stoss F, Meier-Ruge WA, Knecht NA, Muller-Lobeck H, Ammann K. Atrophic hypoganglionosis in the colon of adults with slow-transit constipation: a morphometric histopathological investigation. Eur Surg. 2005;37:87–93. [Google Scholar]
- 61.Meier-Ruge WA, Bronnimann PB, Gambazzi F, Schmid PC, Schmidt CP, Stoss F. Histopathological criteria for intestinal neuronal dysplasia of the submucosal plexus (type B) Virchows Arch. 1995;426:549–56. doi: 10.1007/BF00192108. [DOI] [PubMed] [Google Scholar]
- 62.Meier-Ruge WA, Bruder E, Kapur RP. Intestinal neuronal dysplasia type B: one giant ganglion is not good enough. Pediatr Dev Pathol. 2006;9:444–52. doi: 10.2350/06-06-0109.1. [DOI] [PubMed] [Google Scholar]
- 63.Imaji R, Kubota Y, Hengel P, Hutson JM, Chow CW. Rectal mucosal biopsy compared with laparoscopic seromuscular biopsy in the diagnosis of intestinal neuronal dysplasia in children with slow-transit constipation. J Pediatr Surg. 2000;35:1724–7. doi: 10.1053/jpsu.2000.19228. [DOI] [PubMed] [Google Scholar]
- 64.Meier-Ruge WA, Bruder E. Pathology of chronic constipation in pediatric and adult coloproctology. Pathobiology. 2005;72:1–102. doi: 10.1159/000082310. [DOI] [PubMed] [Google Scholar]
- 65.Clark SB, Rice TW, Tubbs RR, Richter JE, Goldblum JR. The nature of the myenteric infiltrate in achalasia: an immunohistochemical analysis. Am J Surg Pathol. 2000;24:1153–8. doi: 10.1097/00000478-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 66.Lindberg G, Tornblom H, Iwarzon M, Nyberg B, Martin JE, Veress B. Full-thickness biopsy findings in chronic intestinal pseudo-obstruction and enteric dysmotility. Gut. 2009;58:1084–90. doi: 10.1136/gut.2008.148296. [DOI] [PubMed] [Google Scholar]
- 67.Lennon VA, Sas DF, Busk MF, Scheithauer B, Malagelada JR, Camilleri M, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology. 1991;100:137–42. doi: 10.1016/0016-5085(91)90593-a. [DOI] [PubMed] [Google Scholar]
- 68.Veress B, Nyberg B, Tornblom H, Lindberg G. Intestinal lymphocytic epithelioganglionitis: a unique combination of inflammation in bowel dysmotility: a histopathological and immunohistochemical analysis of 28 cases. Histopathology. 2009;54:539–49. doi: 10.1111/j.1365-2559.2009.03265.x. [DOI] [PubMed] [Google Scholar]
- 69.Cortesini C, Cianchi F, Infantino A, Lise M. Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig Dis Sci. 1995;40:2450–5. doi: 10.1007/BF02063253. [DOI] [PubMed] [Google Scholar]
- 70.King SK, Sutcliffe JR, Ong SY, Lee M, Koh TL, Wong SQ, et al. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol Motil. 2010;22:883–92. e234. doi: 10.1111/j.1365-2982.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- 71.Dolk A, Broden G, Holmstrom B, Johansson C, Schultzberg M. Slow transit chronic constipation (Arbuthnot Lane's disease). An immunohistochemical study of neuropeptide-containing nerves in resected specimens from the large bowel. Int J Colorectal Dis. 1990;5:181–7. doi: 10.1007/BF00303272. [DOI] [PubMed] [Google Scholar]
- 72.Milner P, Crowe R, Kamm MA, Lennard-Jones JE, Burnstock G. Vasoactive intestinal polypeptide levels in sigmoid colon in idiopathic constipation and diverticular disease. Gastroenterology. 1990;99:666–75. doi: 10.1016/0016-5085(90)90953-x. [DOI] [PubMed] [Google Scholar]
- 73.Tzavella K, Riepl RL, Klauser AG, Voderholzer WA, Schindlbeck NE, Muller-Lissner SA. Decreased substance P levels in rectal biopsies from patients with slow transit constipation. Eur J Gastroenterol Hepatol. 1996;8:1207–11. doi: 10.1097/00042737-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Sjolund K, Fasth S, Ekman R, Hulten L, Jiborn H, Nordgren S, et al. Neuropeptides in idiopathic chronic constipation (slow transit constipation) Neurogastroenterol Motil. 1997;9:143–50. doi: 10.1046/j.1365-2982.1997.d01-46.x. [DOI] [PubMed] [Google Scholar]
- 75.Goldin E, Karmeli F, Selinger Z, Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci. 1989;34:754–7. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- 76.Murphy EM, Defontgalland D, Costa M, Brookes SJ, Wattchow DA. Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil. 2007;19:126–34. doi: 10.1111/j.1365-2982.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 77.Burleigh DE. Evidence for a functional cholinergic deficit in human colonic tissue resected for constipation. J Pharm Pharmacol. 1988;40:55–7. doi: 10.1111/j.2042-7158.1988.tb05151.x. [DOI] [PubMed] [Google Scholar]
- 78.Mitolo-Chieppa D, Mansi G, Rinaldi R, Montagnani M, Potenza MA, Genualdo M, et al. Cholinergic stimulation and nonadrenergic, noncholinergic relaxation of human colonic circular muscle in idiopathic chronic constipation. Dig Dis Sci. 1998;43:2719–26. doi: 10.1023/a:1026615730533. [DOI] [PubMed] [Google Scholar]
- 79.Koch TR, Carney JA, Go L, Go VL. Idiopathic chronic constipation is associated with decreased colonic vasoactive intestinal peptide. Gastroenterology. 1988;94:300–10. doi: 10.1016/0016-5085(88)90416-7. [DOI] [PubMed] [Google Scholar]
- 80.Lincoln J, Crowe R, Kamm MA, Burnstock G, Lennard-Jones JE. Serotonin and 5-hydroxyindoleacetic acid are increased in the sigmoid colon in severe idiopathic constipation. Gastroenterology. 1990;98:1219–25. doi: 10.1016/0016-5085(90)90336-y. [DOI] [PubMed] [Google Scholar]
- 81.Lee JI, Park H, Kamm MA, Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 2005;20:1292–8. doi: 10.1111/j.1440-1746.2005.03809.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang LM, McNally M, Hyland J, Sheahan K. Assessing interstitial cells of Cajal in slow transit constipation using CD117 is a useful diagnostic test. Am J Surg Pathol. 2008;32:980–5. doi: 10.1097/PAS.0b013e318164e469. [DOI] [PubMed] [Google Scholar]
- 83.Slater BJ, Varma JS, Gillespie JI. Abnormalities in the contractile properties of colonic smooth muscle in idiopathic slow transit constipation. Br J Surg. 1997;84:181–4. [PubMed] [Google Scholar]
- 84.Rao SS, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405–16. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 85.Hoyle CH, Kamm MA, Lennard-Jones JE, Burnstock G. An in vitro electrophysiological study of the colon from patients with idiopathic chronic constipation. Clin Auton Res. 1992;2:327–33. doi: 10.1007/BF01824303. [DOI] [PubMed] [Google Scholar]
- 86.Wedel T, Van Eys GJ, Waltregny D, Glenisson W, Castronovo V, Vanderwinden JM. Novel smooth muscle markers reveal abnormalities of the intestinal musculature in severe colorectal motility disorders. Neurogastroenterol Motil. 2006;18:526–38. doi: 10.1111/j.1365-2982.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 87.Meier-Ruge WA, Holschneider AM, Scharli AF. New pathogenetic aspects of gut dysmotility in aplastic and hypoplastic desmosis of early childhood. Pediatr Surg Int. 2001;17:140–3. doi: 10.1007/s003830000475. [DOI] [PubMed] [Google Scholar]
- 88.Meier-Ruge WA. Desmosis of the colon: a working hypothesis of primary chronic constipation. Eur J Pediatr Surg. 1998;8:299–303. doi: 10.1055/s-2008-1071218. [DOI] [PubMed] [Google Scholar]
- 89.Chase JW, Stillman BC, Gibb SM, Clarke MC, Robertson VJ, Catto-Smith AG, et al. Trunk strength and mobility changes in children with slow transit constipation. J Gastroenterol Hepatol. 2009;24:1876–84. doi: 10.1111/j.1440-1746.2009.05940.x. [DOI] [PubMed] [Google Scholar]
- 90.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Colonic elongation inhibits pellet propulsion and migrating motor complexes in the murine large bowel. J Physiol. 2010;588:2919–34. doi: 10.1113/jphysiol.2010.191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garrity MM, Gibbons SJ, Smyrk TC, Vanderwinden JM, Gomez-Pinilla PJ, Nehra A, et al. Diagnostic challenges of motility disorders: optimal detection of CD117+ interstitial cells of Cajal. Histopathology. 2009;54:286–94. doi: 10.1111/j.1365-2559.2008.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Robert Shen K, Cima RR, et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2010 doi: 10.1111/j.1365-2982.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tong WD, Liu BH, Zhang LY, Zhang SB. Analysis of the c-kit gene in patients with slow transit constipation. Gut. 2006;55:1207–8. doi: 10.1136/gut.2006.094953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Breuer C, Oh J, Molderings GJ, Schemann M, Kuch B, Mayatepek E, et al. Therapy-refractory gastrointestinal motility disorder in a child with c-kit mutations. World J Gastroenterol. 2010;16:4363–6. doi: 10.3748/wjg.v16.i34.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toman J, Turina M, Ray M, Petras RE, Stromberg AJ, Galandiuk S. Slow transit colon constipation is not related to the number of interstitial cells of Cajal. Int J Colorectal Dis. 2006;21:527–32. doi: 10.1007/s00384-005-0041-1. [DOI] [PubMed] [Google Scholar]
- 96.Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, et al. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496–501. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wedel T, Bottner M, Krammer HJ. The enteric nervous system and interstitial cells of Cajal. Changes in chronic constipation in adults. Pathologe. 2007;28:143–8. doi: 10.1007/s00292-007-0900-3. [DOI] [PubMed] [Google Scholar]
- 98.Tong WD, Liu BH, Zhang LY, Xiong RP, Liu P, Zhang SB. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2005;20:363–7. doi: 10.1007/s00384-004-0679-0. [DOI] [PubMed] [Google Scholar]
- 99.Shafik A, Shafik AA, El-Sibai O, Shafik IA. Interstitial cells of cajal in patients with constipation due to total colonic inertia. J Invest Surg. 2006;19:147–53. doi: 10.1080/08941930600674637. [DOI] [PubMed] [Google Scholar]
- 100.van den Berg MM, Di Lorenzo C, Mousa HM, Benninga MA, Boeckxstaens GE, Luquette M. Morphological changes of the enteric nervous system, interstitial cells of cajal, and smooth muscle in children with colonic motility disorders. J Pediatr Gastroenterol Nutr. 2009;48:22–9. doi: 10.1097/MPG.0b013e318173293b. [DOI] [PubMed] [Google Scholar]
- 101.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroent Motil. 2008;20:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 102.Gibbons SJ, De Giorgio R, Pellegrini MSF, Garrity-Park MM, Miller SM, Schmalz PF, et al. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroent Motil. 2009;21:85–93. doi: 10.1111/j.1365-2982.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tharayil VS, Wouters MM, Stanich JE, Roeder JL, Lei S, Beyder A, et al. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil. 2010;22:462–9. e109–10. doi: 10.1111/j.1365-2982.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lorincz A, Bardsley MR, Rumessen JJ, Strege PR, Horvath VJ, Redelman D, et al. Irreversible Dedifferentiation of Interstitial Cells of Cajal (ICC) in the Absence of Adequate Kit Signaling. Gastroenterology. 2009;136:A52–A. [Google Scholar]
- 105.Ward SM, Ordog T, Bayguinov JR, Horowitz B, Epperson A, Shen LY, et al. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology. 1999;117:584–94. doi: 10.1016/s0016-5085(99)70451-8. [DOI] [PubMed] [Google Scholar]
- 106.Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 107.Der T, Bercik P, Donnelly G, Jackson T, Berezin I, Collins SM, et al. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology. 2000;119:1590–9. doi: 10.1053/gast.2000.20221. [DOI] [PubMed] [Google Scholar]
- 108.Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–68. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]


