Abstract
New approaches, techniques and tools invented over the last decade and a half have revolutionized the functional dissection of neural circuitry underlying Drosophila learning. The new methodologies have been used aggressively by researchers attempting to answer three critical questions about olfactory memories formed with appetitive and aversive reinforcers: (1) Which neurons within the olfactory nervous system mediate the acquisition of memory? (2) What is the complete neural circuitry extending from the site(s) of acquisition to the site(s) controlling memory expression? (3) How is information processed across this circuit to consolidate early-forming, disruptable memories to stable, late memories? Much progress has been made and a few strong conclusions have emerged: (1) Acquisition occurs at multiple sites within the olfactory nervous system but is mediated predominantly by the γ mushroom body neurons. (2) The expression of long-term memory is completely dependent on the synaptic output of α/β mushroom body neurons. (3) Consolidation occurs, in part, through circuit interactions between mushroom body and dorsal paired medial neurons. Despite this progress, a complete and unified model that details the pathway from acquisition to memory expression remains elusive.
Dissecting the neural circuitry and molecular players that shape memory acquisition, consolidation, forgetting, and retrieval are of fundamental interest for understanding how the brain processes information about external stimuli in normal and diseased individuals. Although Drosophila melanogaster exhibit numerous types of learning including visual and place learning (Kashai and Zars 2011), olfactory learning has proven, so far, to be the most robust and valuable type of learning for dissecting these issues. In an olfactory learning and memory paradigm used for Drosophila, flies learn to associate an odor (conditioned stimulus: CS+) with the negative reinforcement of mild, electric shock or with the positive reinforcement of a food reward (unconditioned stimulus: US). Memory of this learned association is tested in a T-maze, in which trained flies avoid or approach the punished or rewarded odor, respectively (Tully and Quinn 1985; Davis 2005).
Aversive and appetitive reinforcements produce mechanistically different forms and quantitatively different levels of performance measured as the strength of memory expression at defined times after conditioning. For instance, a single conditioning trial with nutritious sucrose forms stable, protein synthesis-dependent appetitive memory that lasts for days. Appetitive memory is resistant to a brief episode of cold shock amnesia applied at 2 h after conditioning (Tempel et al. 1983; Krashes and Waddell 2008). In contrast, a single aversive conditioning trial generates decaying memory composed of two forms measured at intermediate times (intermediate-term memory, ITM, ∼0.5–5 h after conditioning), including anesthesia resistant memory (ARM), which is a consolidated form of memory by definition given its resistance to cold anesthesia, and anesthesia sensitive memory (ASM), which is sensitive to cold shock treatment (Tully et al. 1994). Long-term memory (LTM, >6 h after conditioning) has been dissected into two distinct forms (Tully et al. 1994): protein synthesis-dependent LTM (PSD-LTM) and protein synthesis-independent LTM(PSI-LTM). PSD-LTM generated with the aversive stimuli of electric shocks requires multiple conditioning trials with intervening rest intervals (spaced conditioning). PSI-LTM can be generated after aversive conditioning with multiple training trials without intervening rest periods (massed conditioning). This form of LTM has also been referred to as ARM (Tully et al. 1994). Although PSD-LTM formation with aversive and appetitive stimuli require different training protocols, they are both dependent on the normal activity of the transcription factor CREB (Yin et al. 1994; Krashes and Waddell 2008).
Experimental approaches used in functional neuroanatomy have evolved enormously from older techniques that revolved around pharmacological and surgical ablations and genetic lesions. In Drosophila, several critical tools have been developed that are used widely for the dissection of circuit function. The available toolset includes thermogenetic tools that allow the stimulation or inhibition of specific neurons using neuron-specific gal4 driver lines. Transgenic expression of the temperature-sensitive ion channel TrpA1 activates specific sets of neurons at high temperatures (Hamada et al. 2008). The shibirets (shits) transgene can be used for inhibition. It rapidly blocks neurotransmission at high temperatures but not low (Kitamoto 2001). This is due to the fact that the transgene encodes a dominant negative, temperature-sensitive dynamin that blocks neurotransmitter reuptake, such that the readily releasable pool becomes diminished. Functional cellular imaging has literally provided a new window into the changes in response properties to the CS+ that occur in living flies due to conditioning (Davis 2011). These memory traces are visualized using transgenically supplied reporters like synapto-pHluorin or G-CaMP, to record how specific neurons respond to odors before and after conditioning. These tools, along with optogenetics and the more standard techniques of rescuing mutants by spatially restricted expression of wild-type transgenes or inactivating gene products by spatially restricted expression of RNAi, have yielded a myriad of approaches for the functional dissection of circuits (Venken et al. 2011). Understanding how different neuron types contribute to encoding and storing aversive and appetitive memories in the Drosophila brain using this arsenal of functional neuroanatomical approaches has been a major focus of researchers over the last 15 yr.
Neural circuit model for olfactory memory formation
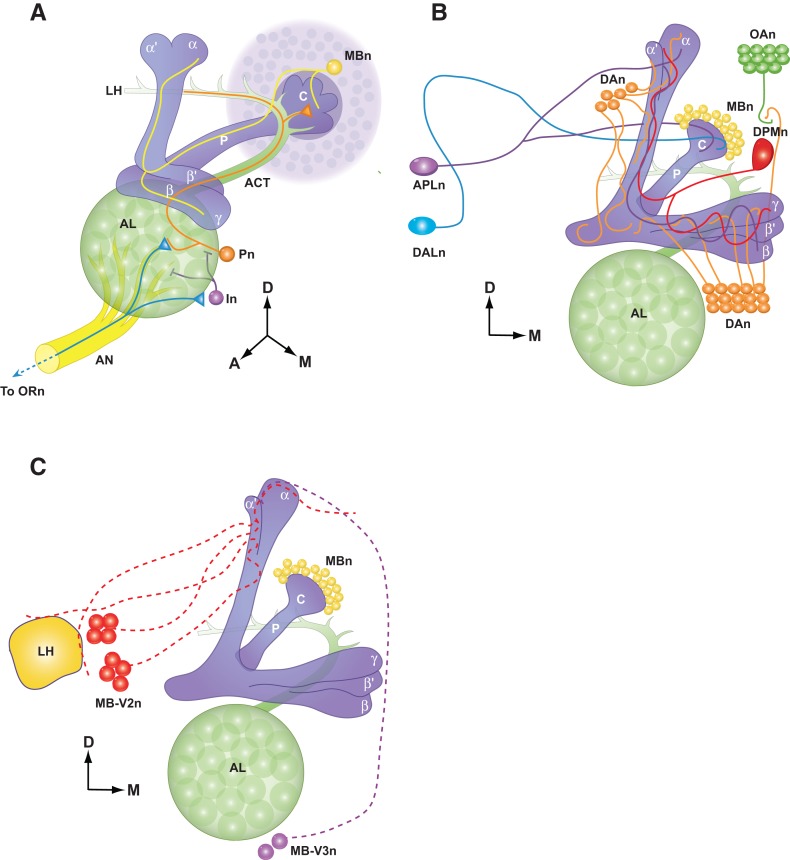
Olfactory memory formation is mediated principally by the olfactory nervous system (Davis 2004, 2005). Drosophila receive olfactory input through olfactory receptor neurons located in the antennae and maxillary palps and transmit this information to the antennal lobe (AL). Odor information is further processed by local interneurons in the AL and projection neurons (Pn) then convey the information to the mushroom body neuron (MBn) dendrites and to an area of the brain known as the lateral horn (Fig. 1A). Numerous lines of evidence have pointed to MBn as critical centers for olfactory memory formation (Heisenberg et al. 1985; Davis 1993; de Belle et al. 1994).
Figure 1.
Olfactory nervous system of Drosophila. A schematic diagram of the olfactory nervous system components within the fly's right brain hemisphere viewed from an anterior and slightly dorsal perspective. (D) Dorsal, (A) anterior, (M) medial. (A) Olfactory information is conveyed from olfactory receptors neurons (ORn) to the antennal lobe (AL) via the antennal nerve (AN). The ORn axons synapse with projection neurons (Pn) in discrete glomeruli as well as interneurons (In). Pn carry this information to the mushroom body neurons (MBn), forming synapses with the MBn in the calyx (C) at the posterior edge of the brain, as well as the lateral horn (LH). Each MBn sends a single axon anterior through the peduncle (P). Near the anterior face of the brain, MBn neurites turns to form one or more lobes (vertical: α or α′; horizontal: β, β′, or γ) according to the MBn cell type (α/β, α′/β′, or γ). (B) MB extrinsic neurons that are involved in learning and memory. (APLn) Anterior paired lateral neuron, (DPMn) dorsal paired medial neuron, (DAn) dopaminergic neurons, (DALn) dorsal anterior lateral neurons, (OAn) octopaminergic neurons. (C) Output neurons of the MBn. (MB-V2n) Mushroom body ventral lobe arborizing neuron 2, (MB-V3) mushroom body ventral lobe arborizing neuron 3. (Adapted with permission from Davis 2011.)
Odors are used as the conditioned stimulus (CS+) for olfactory associative conditioning in flies and are conveyed and processed through the circuitry described in Figure 1. That neuromodulatory neurons, such as dopamine neurons (DAn), might convey the US signal was first presented as a model by Davis (1993; Figs. 1, 2). The subsequent isolation of dopamine (DA) and octopamine (OA) receptor genes and mutants (Han et al. 1996, 1998; Kim et al. 2003, 2007, 2013) along with other reagents allowed this specific hypothesis to be tested (Schwaerzel et al. 2003), which produced results consistent with the broad model that the US pathway for aversive conditioning is mediated by G-protein-coupled DA receptors expressed by the MBn and that the US pathway for appetitive conditioning is mediated by both G-protein coupled OA and DA receptors expressed by the MBn (Connolly et al. 1996; Schwaerzel et al. 2003; Kim et al. 2007, 2013). The CS and US coincidence integration in the MBn occurs, at least in part, through the activity of an adenylyl cyclase encoded by the rutabaga (rut) gene (Tomchik and Davis 2009). Although experimental results obtained since the introduction of this minimal model have confirmed its general features, many new circuit elements have been added that add significant complexity to the model. Newer studies have shown that there are multiple types of MBn, and that information presented to the MBn is further modified by anterior paired lateral neurons (APLn), dorsal paired medial neurons (DPMn), and subsets of DAn. Other new MB extrinsic neurons with proposed roles in Drosophila olfactory memory include MB-V2, MB-V3, and dorsal anterior lateral (DAL) neurons (Fig. 1B,C). The contributions of these and other neuron types to this original model of olfactory memory formation are discussed below.
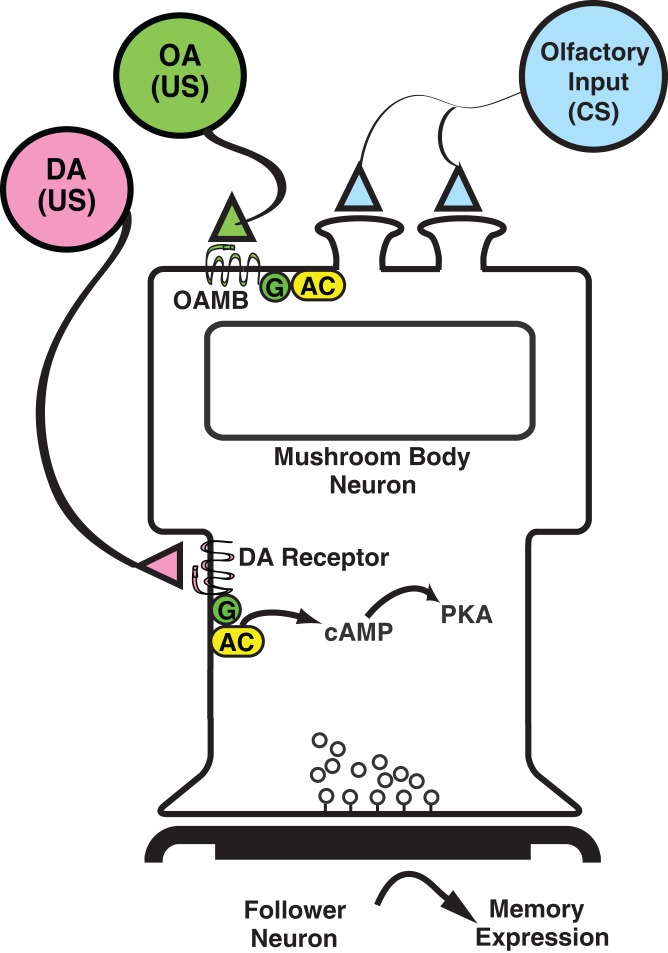
Figure 2.
Original neural circuit model for CS and US integration. Model for CS and US integration in the MBn after olfactory classical conditioning as originally proposed (Davis 1993; Han et al. 1996, 1998). Olfactory information (CS) is transmitted to the dendrites of MBn where it is integrated with information about negative or positive reinforcers. CS information is modified by the simultaneous activation of the G-protein (G)-coupled, octopamine receptor (OAMB) from octopaminertic inputs to MBn dendrites for appetitive learning. Increases in cAMP that occur through the stimulation of the rutabaga-encoded, adenylyl cyclase (AC) modify the processing of CS information in the MBn dendrites. Information representing aversive US stimuli is modified in the MBn by the activation of dopaminergic (DA) receptors on the axon tracks of the MBn. DA inputs modify CS information presented simultaneously to the MBn through the activation of AC. The elevated cAMP and activation of protein kinase A (PKA) modulates the synaptic output of MBn to downstream motor circuits. (Adapted with permission from Han et al. 1998.)
The role for projection neurons (Pn) in olfactory memory formation
Several lines of evidence indicate that Pn do not simply convey olfactory information to the MBn but are themselves, actively involved in memory formation. First, functional cellular imaging experiments monitoring synaptic transmission from Pn showed that conditioning induces neural plasticity for ∼5 min after conditioning by recruiting new synaptic activity into the representation of the learned odor (Yu et al. 2004). This change in how the conditioned odor is represented by synaptic activity constitutes a memory trace, defined as a change in the response properties to the CS+ odor instilled by conditioning and observable after the conditioning event. Second, expression of a rut cDNA in Pn rescues the appetitive memory deficit of rut mutants but not the aversive memory deficit (Thum et al. 2007). This discovery, by itself, suggests that the neural circuitry for appetitive learning is extended, requiring some processing by the rut-encoded AC in Pn that is not required for aversive learning. Third, RNAi knockdown of polyglutamine tract-binding protein-1 in Pn impairs aversive memory, potentially by reducing the levels of NMDA receptor subunit 1 (Tamura et al. 2010). Fourth, the induction of PSD-LTM by spaced conditioning increases the translation of calcium:calmodulin-dependent protein kinase II (CaMKII) in Pn synapses in the antennal lobes (Ashraf et al. 2006). Together, the results from these experiments argue for a functional involvement of Pn in olfactory memory formation, outside of the central involvement of MBn. This involvement is suggested by aforementioned memory trace to be limited to early memories for aversive conditioning, whereas the increased translation of CaMKII suggests an involvement in late memories. More studies are required to disentangle these observations and obtain a better understanding of Pn involvement in olfactory memory formation.
MBs are a primary brain center for Drosophila olfactory memory formation
MBs are a primary olfactory learning center in Drosophila with ∼2500 Kenyon cells per hemisphere (Fig. 1; Technau et al. 1982; Davis 1993). They integrate olfactory input with punishment or reward and are thought to be part of the driving force for the behavioral response. The activity of these neurons contributes to different temporal phases of memory. Blocking synaptic transmission from the MBn impairs the expression of olfactory memory, consistent with the model that many of the plastic events underlying the representation of olfactory memories occur within the MBs themselves or at prior nodes of information flow within the olfactory nervous system (Dubnau et al. 2001; McGuire et al. 2001).
MB intrinsic neurons (Kenyon cells) are now classified into three major subtypes, α/β, α′/β′, and γ MBn, based on the trajectory and final destination of their axons into different brain neuropil (lobes) (Fig. 1). The axons of α/β and α′/β′ neurons bifurcate into vertical α and α′ lobes, and horizontal β and β′ lobes, whereas the γ neurons form only horizontal lobes (Crittenden et al. 1998). Functional neuroanatomical studies that use transient blocks in synaptic transmission from MBn subtypes utilizing shits, and/or rescue of the memory impairment of rut mutation, suggest that the MBn subtypes perform distinct roles at different times and durations after conditioning.
MB γ neurons
There is good evidence that coincidence detection of the CS and US takes place at least in part, within the γ MBn. The dumb mutants that are impaired in the function of the dDA1 DA receptor express no memory after aversive olfactory conditioning, consistent with the model that the US never arrives in a functional state at the neurons that integrate the temporal activity of the CS and US. Expression of this receptor only in the γ MBn of otherwise mutant dumb flies restores all phases of memory following aversive training, indicating that dDA1 expression within γ MBn is sufficient for coincidence detection underlying all temporal forms of memory (Kim et al. 2007; Qin et al. 2012). In addition, stimulated conditioning of flies with odor and thermogenetic activation of DAn revealed that neuronal plasticity, revealed through functional imaging using G-CaMP of subsequent calcium responses to odors, occurs primarily in the γ MBn (Boto et al. 2014). In an independent study, the presynaptic activity of γ MBn was shown to be inhibited by G(o) signaling using synapto-pHluorin imaging (Zhang and Roman 2013). Moreover, expression in the γ MBn of the rut AC, a coincidence detector, provides partial to complete rescue of memory immediately after conditioning, depending on the γ MBn driver used (Zars et al. 2000; Akalal et al. 2006; Blum et al. 2009; Xie et al. 2013). Furthermore, blocking γ neurons’ synaptic transmission specifically during retrieval at 15 min after conditioning impairs both aversive and appetitive memory expression (Cervantes-Sandoval et al. 2013). These observations are consistent with a primary role for the γ MBn in the process of acquisition and expression of very early memories. However, other lines of evidence point to possible roles for other brain areas in the process of acquisition and memory expression.
MB α′/β′ neurons
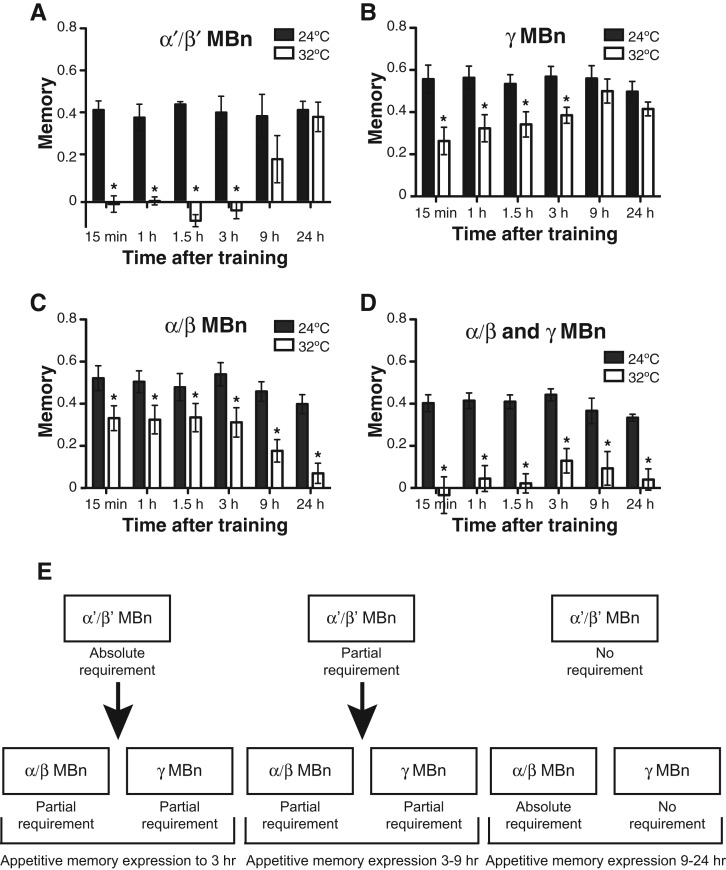
Blocking neurotransmission from α′/β′ MBn during aversive or appetitive training or at anytime up to ∼90 min after conditioning impairs memory performance (Krashes et al. 2007; Cervantes-Sandoval et al. 2013). One interpretation of this observation is that these neurons must communicate with their postsynaptic partners for normal levels of memory acquisition and expression. There are no direct experimental results that attempt to integrate this observation with the model that γ MBn serve a primary role for acquisition (above), but it is possible that the α′/β′ MBn are direct or indirect postsynaptic partners that receive information from the γ MBn or that they represent a parallel and distinct site for acquisition. Strikingly, the most detailed analysis using shits showed that disrupting synaptic activity from these neurons completely abolishes appetitive memory expression at any time within the first 3 h after conditioning, yet disruptions up to 90 min after aversive conditioning impair, but do not abolish, memory expression (Fig. 3; Cervantes-Sandoval et al. 2013). This study also adopted an important and alternative strategy for such blocking experiments. Prior studies (Krashes et al. 2007) using shits to block synaptic output would impose a block over a certain time window, for instance 1 h, and then measure memory expression at some later time point without the synaptic block, such as 3 h. Thus, memory expression measured in these prior experiments represents the combined effects of imposing a synaptic blockade over a limited time window and then allowing normal synaptic transmission across a second time window before measuring memory. The experiments of Cervantes-Sandoval et al. (2013) indicate that active communication from α′/β′ MBn to their postsynaptic partners is absolutely required for the expression of appetitive memories up to 3 h, but only partially required for the expression of aversive memories to 90 min. It may be that appetitive odor memories are held in these neurons for 3 h post-conditioning, or that synaptic transmission from these neurons is required for memory expression through some other set of neurons. The partial effect of the synaptic block after aversive conditioning may signal that there exists a parallel neural circuit for this type of memory outside of the α′/β′ MBn. Functional imaging of odor-evoked Ca2+ responses of α′/β′ neurons immediately after learning elicits an early memory trace in these neurons that persists for at least 1 h after either aversive or appetitive conditioning (Wang et al. 2008). We prefer the hypothesis that these neurons hold early memories for some window of time after conditioning, as reflected by the memory trace, as opposed to the alternative hypothesis that these neurons provide essential synaptic input for the expression of early memory from an alternative site. Thus, early behavioral performance may be driven largely from the output of the memory trace in α′/β′ MBn (Davis 2011).
Figure 3.
Roles for various subsets of MBn for the expression of appetitive olfactory memory. Flies expressing shits in various MBn were conditioned using an appetitive US at 24°C and shifted to 32°C 10 min prior to a retrieval test at the time indicated. (A) Synaptic blockade of α′/β′ MBn abolished expression up to 3 h after training. (B) Synaptic blockade of γ MBn significantly reduced appetitive olfactory memory expression from 15 min to 3 h after training, but was without effect after 9 h. (C) Synaptic blockade α/β MBn significantly reduced appetitive olfactory memory expression from 15 min to 3 h after training, and essentially abolished expression at times after 9 h. (D) Synaptic blockade of α/β and γ MBn eliminated appetitive olfactory memory expression at all times tested. (E) A schematic summary of the data, showing the relevance of synaptic activity of various subsets of MBn across three time intervals, 0–3 h, 3–9 h, and 9–24 h. The middle panel of 3–9 h is inferred from the data, given that no specific timepoint within this interval was tested. (Adapted with permission from Cervantes-Sandoval et al. 2013.)
MB αβ neurons
A clear function for α/β MBn is for the expression of memory, also referred to in some publications as retrieval. Blocking the synaptic output of these neurons after appetitive conditioning using shits strongly diminishes performance at all times tested after acquisition, from immediate memory expression to PSD-LTM tested at 24 h (Fig. 3; Dubnau et al. 2001; McGuire et al. 2001; Cervantes-Sandoval et al. 2013; Xie et al. 2013). However, the impairment is graded, with the expression being ∼60% of control values at early times tested to little or no expression after 9 h (Fig. 3; Cervantes-Sandoval et al. 2013). These results indicate that α/β MBn are required for the expression of all temporal forms of memory but that expression of PSD-LTM is completely dependent on these neurons. A similar shift in the requirement for these neurons occurs for the expression of temporally different forms of aversive memory (Cervantes-Sandoval et al. 2013). Strikingly, blocking both α/β and γ MBn simultaneously after appetitive conditioning blocks all performance across all time points (Fig. 3; Cervantes-Sandoval et al. 2013; Xie et al. 2013). This is an important observation, given that blocking each type of neuron individually has only a partial effect over the first 9 h. This indicates that there are two channels for memory expression up to 24 h after conditioning, one through the α/β MBn and the other through the γ MBn. This conclusion, combined with the aforementioned discovery that the synaptic output of α′/β′ MBn is an absolute but temporary requirement for memory expression up to 3 h after conditioning, fits with a model positioning α′/β′ MBn function over the first 3 h as upstream of the combined actions of α/β and γ MBν (Fig. 3). That early memories are dependent on α′/β′ MBn function, while LTM in the same fly are independent, was shown nicely in a unique experiment testing the integrity of LTM for one odor, and the disruptability of STM of another after insult to α′/β′ MBn, in flies trained to learn both odors (Cervantes-Sandoval et al. 2013).This shift in overall dependence to different neuron sets for the expression of early vs. late memories has strong similarities with systems consolidation in mammalian systems (Dudai 2004; Cervantes-Sandoval et al. 2013).
Transgenic rescue experiments using rut+ provide additional evidence for a dual pathway for memory expression. Expressing UAS-rut in α/β neurons rescues the deficits in STM, ITM, PSD-LTM, and PSI-LTM of rut mutants only partially. However, expressing UAS-rut in α/β together with γ neurons fully rescues all temporal forms of memory (Akalal et al. 2006; Blum et al. 2009). Taken together, these results are consistent with dual, parallel pathways for memory expression to between 9 and 24 h. With time, the dependence on γ neurons fades and LTM becomes completely dependent on α/β neurons (Cervantes-Sandoval et al. 2013).
The analyses of brain structure mutants and functional imaging experiments have provided strong evidence that the vertical processes of the α/β MBn are critical to the expression of PSD-LTM. The mutant “alpha-lobes-absent (ala),” which lacks the vertical lobes of the MBn, is unable to form PSD-LTM after spaced conditioning, indicating that the vertical axonal projections of α/β or α′/β′ MBn are required for the expression of PSD-LTM (Pascual and Preat 2001). Functional imaging experiments narrowed this requirement to the vertical branches of the α/β MBn. The α branch of the α/β MBn forms a memory trace 9–24 h after spaced conditioning, shown by increased G-CaMP fluorescence to the conditioned stimulus (Yu et al. 2006). This memory trace is dependent on protein synthesis, normal activity of calcium:calmodulin-dependent protein kinase II, and the transcription factor, CREB. Furthermore, 26 different mutants in PSD-LTM fail to form this memory trace (Akalal et al. 2011). These observations provide strong evidence that the α/β MBn participate in PSD-LTM storage and expression.
Some Gal4 drivers that divide α/β MBn into subsets have been used to further dissect the functional anatomy of olfactory learning. One subdivision that has come to light in recent years is between the surface, core, and posterior areas of the α/β lobes. Huang et al. (2012) proposed a distinct function for the core: to gate the consolidation of both appetitive and aversive LTM (Huang et al. 2012). This proposal is based principally on the discovery that a single training trial is capable of producing PSD-LTM when “highwire” gene function (E3 ubiquitin ligase activity) is removed from the α/β MBn that comprise the core of the α/β lobes. The α/β MBn that project to the surface and posterior regions mediate the memory expression function (Huang et al. 2013; Perisse et al. 2013).
DPM neurons
Dorsal paired medial neurons (DPMn) are MB extrinsic neurons that modulate memory (Waddell et al. 2000). There is but one DPMn within each brain hemisphere consisting of a large cell body extending a single neurite that branches into two, one of which ramifies throughout the vertical lobes of the ipsilateral MB with the other ramifying throughout the horizontal lobes (Fig. 1B). Blocking neurotransmission from DPMs during acquisition or retrieval using shits for conditioning is without effect, but blocking neurotransmission from these neurons after learning but before retrieval impairs memory expression tested 1 or 3 h later (Keene et al. 2004; Yu et al. 2005). This observation was interpreted initially as impairing the consolidation of memory (Keene et al. 2004), since the time window for the disruptive effects overlap with the time window during which memory consolidation is thought to occur. The neuroanatomy of the DPMn lends itself to this interpretation; early studies hypothesized that DPMn participate in “reciprocal interactions” with the MBn, with some DPMn neurites functioning as postsynaptic partners to receive olfactory information from the MBn, and other neurites as presynaptic partners, returning it to the MBn in some processed, and perhaps consolidated form (Yu et al. 2005). Blocking neurotransmission primarily between the DPMn and α′/β′ MBn 1 h after training disrupts the stability of aversive and appetitive memories, mapping some of the interactions between this pair of neurons (Krashes et al. 2007). The DPMn are serotonergic and were discovered to provide input to the α/β MBn through the d5HT1A receptor for the formation of ARM (Lee et al. 2011). Thus, there appears to exist an α′/β′ MBn→DPMn→α/β MBn circuit for mediating the formation of this type of consolidated memory.
Earlier studies suggested that DPMn might be peptidergic, releasing a neuropeptide similar in sequence to pituitary adenylyl cyclase activating peptide (PACAP) on the basis of sequence similarity of PACAP to the predicted product of the amnesiac (amn) gene (Feany and Quinn 1995). This possibility has been awaiting verification for nearly 20 yr without additional supportive evidence.
The involvement of DPMn at intermediate times after conditioning was also shown by the existence of a memory trace that forms between 30 and 70 min after aversive conditioning in the DPMn neurites that innervate the vertical lobes of the MB (Yu et al. 2005; Davis 2011; Cervantes-Sandoval and Davis 2012). The essence of this observation is that challenging trained flies with the learned odor elicits increased calcium influx into the neurites innervating the vertical lobes across the 30–70 min time window after conditioning compared with the vertical lobe neurites of untrained flies, and the horizontal lobe neurites of trained flies (Yu et al. 2005). Thus, there is a change in response properties that occurs likely within the DPMn vertical lobe neurites themselves that enhances the calcium response to the learned odor. This memory trace requires the normal activity of the amn gene product (Yu et al. 2005). Of particular interest is the contrast of the aversive DPMn memory trace with one that forms after appetitive conditioning. The appetitive trace is not restricted to the vertical lobe neurites, but forms also in the horizontal lobe neurites (Cervantes-Sandoval and Davis 2012). In addition, the appetitive memory trace is more durable, persisting to ∼150 min after conditioning. The importance of this longer-lasting memory trace was tested by blocking neurotransmission from these neurons after appetitive conditioning only during the time window of its extended persistence. The results demonstrated that the increased persistence is essential for complete levels of appetitive memory expression at later time points (Cervantes-Sandoval and Davis 2012). Although it seems likely that the physiological changes registered as memory traces represent intrinsic changes within the neurons being monitored, at least in part, it may be that other, extrinsic neurons are contributing to the measured physiological changes after learning.
The emerging model for DPMn function is that these neurons receive input about olfactory cues through their broad innervation of the MB lobes. The learning associated with specific odors registered in MBn could lead to increased excitability changes in the DPMn, manifested as the delayed memory trace, which feed back onto the MBn conveying in learned odor to function in the consolidation of ARM. Given the requirement for normal amn function in DPMn for normal PSD-LTM (Yu et al. 2006), it seems probable that these neurons also participate in consolidating PSD-LTM.
APL neurons
The anterior paired lateral neuron (APLn) cell body is located in the lateral protocerebrum and innervates the MB lobes and calyx (Liu and Davis 2009; Fig. 1B). The two APLn, one in each brain hemisphere, function to suppress olfactory learning by activating the GABAA receptor, Rdl, expressed by the MBn (Liu et al. 2007). Learning, in turn, suppresses APLn Ca2+ responses, and presumably thus inhibits the inhibitory input to the MBn, facilitating acquisition and/or memory stability (Liu and Davis 2009; Liu et al. 2007). More recently, the APLn have been suggested to exert inhibitory effects on MBn in a feedback loop to maintain sparse odor coding, a mechanism to discriminate similar odors to retrieve distinct responses (Lin et al. 2014).
Blocking synaptic transmission of APLn after aversive or appetitive training impairs 3 h memory, similar to a requirement for DPMn and MBn α′/β′ output (Pitman et al. 2011; Wu et al. 2013). Interestingly, APLn were reported to be octopaminergic (Wu et al. 2013) as well as GABAergic (Liu and Davis 2009) and coupled to DPMn through gap junctions (Wu et al. 2011). GRASP experiments suggest that the APLn connect to both MBn and to DPMn within the MB lobe neuropil (Pitman et al. 2011), consistent with APLn modulating the degree of inhibition on both types of neurons.
Dopamine neurons
DAn mediate punishment and reward reinforcement during associative learning by acting directly on MBn (Schwaerzel et al. 2003; Riemensperger et al. 2005; Kim et al. 2007). There are eight clusters of DAns in Drosophila. Neurons in three of these clusters (PPL1, PPL2ab, and PAM) extend processes into the MB neuropil in distinct zones, suggesting different functions for specific groups of DAn (Mao and Davis 2009).
DA was initially proposed to reinforce the formation of aversive memories and octopamine reinforce the formation of appetitive memories by functioning as distinct US signals (Fig. 2; Han et al. 1996, 1998, Schwaerzel et al. 2003). Optogenetic stimulation of subsets of DAn from the PPL1 and PAM clusters while pairing with odor drives learning and avoidance behaviors mimicking aversive learning in the absence of shock reinforcement (Schroll et al. 2006; Claridge-Chang et al. 2009). This observation, along with the impaired learning observed by blocking DAn or mutating the dDA1 receptor, fit nicely with the model that DA provides aversive reinforcement to the MBn through the dDA1 receptor (Fig. 2; Schwaerzel et al. 2003; Kim et al. 2007). Similarly, opto- or thermogenetic stimulation of octopaminergic neurons (OAn) is sufficient for appetitive learning (Schroll et al. 2006; Burke et al. 2012), and mutants for the expression of the octopamine receptor, OAMB, in the α/β and γ MBn are impaired in reward learning (Kim et al. 2013). These observations are all consistent with the original idea that the rewarding US is conveyed through the octopaminergic system and OAMB (Fig. 2). However, rewarding stimuli also require the DA system, since flies mutant in the dDA1 type DA receptor are impaired in both aversive and appetitive memory formation (Kim et al. 2007). In addition, thermogenetic stimulation of a group of DAn is sufficient to act as an appetitive US (Liu et al. 2012), and thermogenetic stimulation of OAn is insufficient as the US when flies are mutant for the dDA1 receptor (Burke et al. 2012). The model that ensues from these observations is that rewarding stimuli engage the octopaminergic system, which then signals through DAn to the MBn (Fig. 1B).
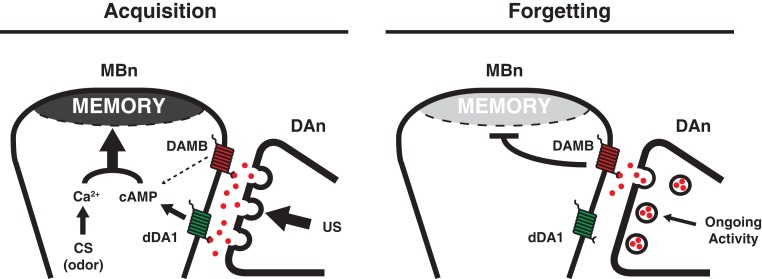
Do DA neurons have functions in memory besides in acquisition? Remarkably, blocking the synaptic output of PPL1 DAn to the MB neuropil after conditioning for as little as 40 min across any time window between training and testing enhances 3 h memory (Berry et al. 2012; Placais et al. 2012). The enhanced memory is labile and exists in a non-consolidated form, given its sensitivity to cold shock. The reciprocal experiment of activating these DAn after conditioning abolishes the expression of both labile ASM and consolidated ARM. This bidirectional modulation of memory expression with this manipulation is consistent with the model that DAn activity after conditioning normally weakens memory, or in other words, causes forgetting. This model predicts that there should exist a DA receptor to receive the forgetting signal for weakening of memories. Functional tests of mutants in the two known DA receptors, dDA1 and DAMB, showed that loss of function of dDA1 impairs memory acquisition (see above), whereas loss of function of DAMB improves memory expression, consistent with its role in initiating a forgetting signal within the MBn. In addition, the model predicts that there might be chronic DAn activity in normal flies both before and after conditioning. This was tested by functional cellular imaging and showed that indeed, DAn that innervate the MV1 and MP1 regions of the MB neuropil exhibit “ongoing” activity. Thus, DAn have a bimodal role in memory formation, one for the acquisition of memories by acting as a US, and another for forgetting memories through chronic activity after acquisition (Fig. 4).
Figure 4.
Model for DAn function in acquisition and forgetting. (Left) DAn-mediated acquisition. A US signal is delivered when DAn release large amounts of DA (red spheres) onto the MBn. Binding of DA to the dDA1 receptor (green) leads to increased cAMP while the CS odor stimuli results in increased Ca2+ signaling. Coincident Ca2+ and cAMP signaling leads to strong memory formation within the MBn (Tomchik and Davis 2009; Boto et al. 2014). (Right) DAn-mediated forgetting. After acquisition, a low level of ongoing DA release is sensed by the DAMB receptor (red) and weakens the memory over time. Differences in affinity of the two receptors for ligand, subcellular compartmentalization, coupling to downstream signaling pathways, or signaling kinetics may account for the dominant action of the dDA1 receptor for acquisition and DAMB in forgetting. (Adapted with permission from Berry et al. 2012.)
DAL neurons
A search for neurons that function in PSD-LTM through the targeted expression of a ribosome-inactivating toxin identified the dorsal anterior lateral DAL neurons (DALn) (Fig. 1B; Chen et al. 2012). These neurons, like APLn and DPMn, have only one cell body in each brain hemisphere. They extend dendritic processes primarily into the superior dorsofrontal protocerebrum and axonal processes into a few different regions of the brain, including the calyx of the MB, and specifically a region of the calyx that houses dendrites of the α/β MBn. Thus, the circuitry of these neurons suggests that they provide input to the dendrites of the α/β MBn.
Protein synthesis was reported to be required in DALn for normal PSD-LTM. In addition, synaptic activity of these neurons is also required for the retrieval of PSD-LTM but not for the acquisition or consolidation. This same study also challenged the model that MBn are required for PSD-LTM. However, a more recent study showed a requirement for protein synthesis in the MBn through the activity of the transcription factor, CREB (Hirano et al. 2013). Consistent with PSD-LTM forming in the MBn, functional imaging experiments identified a memory trace that forms only in the vertical lobes after spaced conditioning (Yu et al. 2006). This memory trace requires the normal activity of CREB, CaMKII, and the functions of 26 other mutants that disrupt PSD-LTM (Akalal et al. 2011). These observations fortify a role for protein synthesis within MBn for PSD-LTM.
MB output neurons
A screen for neurons that connect the MB lobes to other brain regions and which therefore may be “output neurons” conveying learned information to downstream motor pathways identified MB-V2 neurons (Sejourne et al. 2011). These neurons innervate a small region of the vertical lobes of the MB and connect this region with the lateral horn and the middle superiormedial protocerebrum. Blocking the synaptic output of these neurons during retrieval impairs the expression of aversive short-term and PSD-LTM (Sejourne et al. 2011).
The MB-V3 extrinsic neurons were also identified through a search for neurons that function in PSD-LTM through the targeted expression of a ribosome-inactivating toxin (Pai et al. 2013). The dendrites of the MB-V3n connect the α tip of MB with the superior dorsofrontal protocerebrum. Given that the dendrites of the DALn innervate this same region, it seems possible that synaptic connections between the α/β MBn→MB-V3n→DALn→α/β MBn provide a circuit for the consolidation of PSD-LTM, with the eventual memory expression coming from the α/β MBn, in ways similar to the hypothetical α′/β′ MBn→DPMn→α/β MBn circuit for the consolidation of ARM. Protein synthesis is required in the MB-V3n for normal PSD-LTM and the synaptic activity of these neurons is required for PSD-LTM consolidation and retrieval. MB-V3 synaptic function is not required for learning, ITM, or 24-h memory after massed training. A second study of MB-V3n reported that their synaptic function is required for the retrieval of appetitive PSD-LTM as well, although this study failed to observe a requirement for the retrieval of aversive PSD-LTM (Placais et al. 2013).
Summary
The combined evidence presented above, although currently inadequate to have a complete picture of the process of acquisition, consolidation, forgetting, and memory retrieval from neurons within the olfactory nervous system, points to four major conclusions. First, acquisition can occur within multiple types of neurons within the olfactory nervous system, including Pn, γ MBn, and perhaps α′/β′ and α/β MBn. Second, DAn are essential for acquisition of both aversive and appetitive olfactory memories, as well as forgetting. Third, consolidation appears to involve information transfer, processing, and a return of the processed information between the MBn and the DPMn for ARM, with perhaps other circuits also being involved including those with the APLn. Fourth, retrieval processes are distributed among several different types of neurons, depending on the temporal phase of memory and the valence of the memory as well, although certain neurons dominate in the retrieval process at distinct temporal phases. Despite this progress, the circuitry and circuit interactions required for forming and expressing olfactory memories is now dazzlingly complex, far beyond what was originally viewed as a relatively simple circuit (Fig. 2). Clearly, much more work is needed to solve the major issues posed at the beginning of this review.
Acknowledgments
Research in the Davis laboratory is supported by grants 2R37NS19904-30 and 5R01NS052351-07. We thank Drs. Seth Tomchik, Jacob Berry, and Isaac Cervantes-Sandoval for their helpful comments and suggestions.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.034363.114.
References
- Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL 2006. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem 13: 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalal DB, Yu D, Davis RL 2011. The long-term memory trace formed in the Drosophila α/β mushroom body neurons is abolished in long-term memory mutants. J Neurosci 31: 5643–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S 2006. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124: 191–205 [DOI] [PubMed] [Google Scholar]
- Berry JA, Cervantes-Sandoval I, Nicholas EP, Davis RL 2012. Dopamine is required for learning and forgetting in Drosophila. Neuron 74: 530–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AL, Li W, Cressy M, Dubnau J 2009. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol 19: 1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto T, Louis T, Jindachomthong K, Jalink K, Tomchik SM 2014. Dopaminergic modulation of cAMP drives nonlinear plasticity across the Drosophila mushroom body lobes. Curr Biol 24: 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S 2012. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492: 433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I, Davis RL 2012. Distinct traces for appetitive versus aversive olfactory memories in DPM neurons of Drosophila. Curr Biol 22: 1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I, Martin-Pena A, Berry JA, Davis RL 2013. System-like consolidation of olfactory memories in Drosophila. J Neurosci 33: 9846–9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wu JK, Lin HW, Pai TP, Fu TF, Wu CL, Tully T, Chiang AS 2012. Visualizing long-term memory formation in two neurons of the Drosophila brain. Science 335: 678–685 [DOI] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tullly T, O'Kane CJ 1996. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 274: 2104–2107 [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL 1998. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem 5: 38–51 [PMC free article] [PubMed] [Google Scholar]
- Davis RL 1993. Mushroom bodies and Drosophila learning. Neuron 11: 1–14 [DOI] [PubMed] [Google Scholar]
- Davis RL 2004. Olfactory learning. Neuron 44: 31–48 [DOI] [PubMed] [Google Scholar]
- Davis RL 2005. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci 28: 275–302 [DOI] [PubMed] [Google Scholar]
- Davis RL 2011. Traces of Drosophila memory. Neuron 70: 8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M 1994. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263: 692–695 [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T 2001. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 411: 476–480 [DOI] [PubMed] [Google Scholar]
- Dudai Y 2004. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55: 51–86 [DOI] [PubMed] [Google Scholar]
- Feany MB, Quinn WG 1995. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 268: 869–873 [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA 2008. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454: 217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL 1996. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron 16: 1127–1135 [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL 1998. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci 18: 3650–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M, Borst A, Wagner S, Byers D 1985. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet 1: 1–30 [DOI] [PubMed] [Google Scholar]
- Hirano Y, Masuda T, Naganos S, Matsuno M, Ueno K, Miyashita T, Horiuchi J, Saitoe M 2013. Fasting launches CRTC to facilitate long-term memory formation in Drosophila. Science 339: 443–446 [DOI] [PubMed] [Google Scholar]
- Huang C, Zheng X, Zhao H, Li M, Wang P, Xie Z, Wang L, Zhong Y 2012. A permissive role of mushroom body α/β core neurons in long-term memory consolidation in Drosophila. Curr Biol 22: 1981–1989 [DOI] [PubMed] [Google Scholar]
- Huang C, Wang P, Xie Z, Wang L, Zhong Y 2013. The differential requirement of mushroom body α/β subdivisions in long-term memory retrieval in Drosophila. Protein Cell 4: 512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashai L, Zars T 2011. Learning and memory in Drosophila: behavior, genetics and neural systems. Int Rev Neurobiol 99: 139–167 [DOI] [PubMed] [Google Scholar]
- Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S 2004. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron 44: 521–533 [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Seong CS, Han KA 2003. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns 2: 237–245 [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA 2007. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 27: 7640–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Lim J, Han KA 2013. Appetitive learning requires the α1-like octopamine receptor OAMB in the Drosophila mushroom body neurons. J Neurosci 33: 1672–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T 2001. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol 47: 81–92 [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S 2008. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci 28: 3103–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S 2007. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PT, Lin HW, Chang YH, Fu TF, Dubnau J, Hirsh J, Lee T, Chiang AS 2011. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci 108: 13794–13799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Bygrave AM, de Calignon A, Lee T, Miesenbock G 2014. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat Neurosci 4: 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Davis RL 2009. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci 12: 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Krause WC, Davis RL 2007. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron 56: 1090–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H 2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488: 512–516 [DOI] [PubMed] [Google Scholar]
- Mao Z, Davis RL 2009. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL 2001. The role of Drosophila mushroom body signaling in olfactory memory. Science 293: 1330–1333 [DOI] [PubMed] [Google Scholar]
- Pai TP, Chen CC, Lin HH, Chin AL, Lai JS, Lee PT, Tully T, Chiang AS 2013. Drosophila ORB protein in two mushroom body output neurons is necessary for long-term memory formation. Proc Natl Acad Sci 110: 7898–7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Preat T 2001. Localization of long-term memory within the Drosophila mushroom body. Science 294: 1115–1117 [DOI] [PubMed] [Google Scholar]
- Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, Waddell S 2013. Different kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron 79: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman JL, Huetteroth W, Burke CJ, Krashes MJ, Lai SL, Lee T, Waddell S 2011. A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol 21: 855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placais PY, Trannoy S, Isabel G, Aso Y, Siwanowicz I, Belliart-Guerin G, Vernier P, Birman S, Tanimoto H, Preat T 2012. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat Neurosci 15: 592–599 [DOI] [PubMed] [Google Scholar]
- Placais PY, Trannoy S, Friedrich AB, Tanimoto H, Preat T 2013. Two pairs of mushroom body efferent neurons are required for appetitive long-term memory retrieval in Drosophila. Cell Rep 5: 769–780 [DOI] [PubMed] [Google Scholar]
- Qin H, Cressy M, Li W, Coravos JS, Izzi SA, Dubnau J 2012. Gamma neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr Biol 22: 608–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A 2005. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol 15: 1953–1960 [DOI] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, et al. 2006. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16: 1741–1747 [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23: 10495–10502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejourne J, Placais PY, Aso Y, Siwanowicz I, Trannoy S, Thoma V, Tedjakumala SR, Rubin GM, Tchenio P, Ito K, et al. 2011. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci 14: 903–910 [DOI] [PubMed] [Google Scholar]
- Tamura T, Horiuchi D, Chen YC, Sone M, Miyashita T, Saitoe M, Yoshimura N, Chiang AS, Okazawa H 2010. Drosophila PQBP1 regulates learning acquisition at projection neurons in aversive olfactory conditioning. J Neurosci 30: 14091–14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau G, Heisenberg M 1982. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 295: 405–407 [DOI] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG 1983. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci 80: 1482–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum AS, Jenett A, Ito K, Heisenberg M, Tanimoto H 2007. Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci 27: 11132–11138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL 2009. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron 64: 510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Quinn WG 1985. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 157: 263–277 [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M 1994. Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47 [DOI] [PubMed] [Google Scholar]
- Venken KJ, Simpson JH, Bellen HJ 2011. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72: 202–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG 2000. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 103: 805–813 [DOI] [PubMed] [Google Scholar]
- Wang Y, Mamiya A, Chiang AS, Zhong Y 2008. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci 28: 4368–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Shih MF, Lai JS, Yang HT, Turner GC, Chen L, Chiang AS 2011. Heterotypic gap junctions between two neurons in the Drosophila brain are critical for memory. Curr Biol 21: 848–854 [DOI] [PubMed] [Google Scholar]
- Wu CL, Shih MF, Lee PT, Chiang AS 2013. An octopamine-mushroom body circuit modulates the formation of anesthesia-resistant memory in Drosophila. Curr Biol 23: 2346–2354 [DOI] [PubMed] [Google Scholar]
- Xie Z, Huang C, Ci B, Wang L, Zhong Y 2013. Requirement of the combination of mushroom body gamma lobe and α/β lobes for the retrieval of both aversive and appetitive early memories in Drosophila. Learn Mem 20: 474–481 [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49–58 [DOI] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL 2004. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron 42: 437–449 [DOI] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL 2005. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell 123: 945–957 [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL 2006. Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron 52: 845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M 2000. Localization of a short-term memory in Drosophila. Science 288: 672–675 [DOI] [PubMed] [Google Scholar]
- Zhang S, Roman G 2013. Presynaptic inhibition of gamma lobe neurons is required for olfactory learning in Drosophila. Curr Biol 23: 2519–2527 [DOI] [PubMed] [Google Scholar]