Abstract
Voltage-sensitive dye (VSD) imaging is a powerful technique that can provide, in single experiments, a large-scale view of network activity unobtainable with traditional sharp electrode recording methods. Here we review recent work using VSDs to study small networks and highlight several results from this approach. Topics covered include circuit mapping, network multifunctionality, the network basis of decision making, and the presence of variably participating neurons in networks. Analytical tools being developed and applied to large-scale VSD imaging data sets are discussed, and the future prospects for this exciting field are considered.
How do nervous systems produce behaviors? One fruitful approach to this question has been to focus on circuits containing relatively few neurons (e.g., 30–2000), usually in invertebrate ganglia, that produce well-characterized behaviors. Until recently, such networks were studied with sharp electrodes, limiting our view to the activity of two to four neurons at a time, but nonetheless allowing researchers to piece together circuit diagrams for various behaviors, including reflex withdrawals, feeding, and various escape responses. These networks have in turn been used to investigate mechanisms mediating higher-order processing such as decision making, pattern generation, and modulation (Getting 1989; Pittenger and Kandel 2003; Friesen and Kristan 2007; Marder and Bucher 2007; Briggman and Kristan 2008).
A watershed moment occurred with the development of voltage-sensitive dyes (VSDs) that allow neuronal activity to be recorded with light rather than with electrodes (Davila et al. 1973; Salzberg et al. 1973). Initially, this was accomplished by physically aligning multiple single photodiodes with selected neurons (Salzberg et al. 1977), but investigators soon began focusing the light from entire ganglia onto manufactured photodiode arrays (PDAs), opening the way for the simultaneous recording of dozens, and eventually, hundreds of neurons. Because PDAs achieve maximum signal-to-noise ratios at high light levels and high pass filter the signals before amplifying and digitizing them, they are typically used with fast absorbance VSDs to record action potentials rather than slower synaptic events or absolute membrane potential. The other type of recording platform in wide use, camera-based imaging systems, is used mainly with fluorescent VSDs to record both action potentials and synaptic potentials. The relative merits of these two recording platforms have been discussed elsewhere (Frost et al. 2010).
Much of the early work on VSDs was conducted by Larry Cohen and several others (Cohen 2010), who developed and screened large numbers of dyes to identify several having sufficient signal-to-noise ratios and low toxicity. One of the best of these, the absorbance dye RH155, was then used by their group to explore several issues in the marine mollusks Navanax (London et al. 1987) and Aplysia (Zecevic et al. 1989; Wu et al. 1994). One such study, which assessed the degree to which neurons in Aplysia are dedicated versus multifunctional with respect to different features of the animal's gill withdrawal reflex, concluded that both organizational schemes play a role. For example, although as expected many neurons responded to siphon stimulation, these were differentiable into a group whose firing correlated with reflex amplitude, another with reflex duration, and a third that correlated with both reflex components (Zochowski et al. 2000). Their finding was consistent with a prior realistic modeling study of the animal's siphon withdrawal reflex network constructed from sharp-electrode data (Lieb and Frost 1997), which suggested that some neurons and synaptic connections primarily mediate reflex amplitude, others reflex duration, and still others contribute to both.
Although Cohen subsequently shifted his attention to vertebrate preparations, several laboratories have since begun using VSDs to study small neuronal networks. Here we review recent work using VSDs to study such networks and highlight several of the advances that have been gained using this approach. Further we discuss tools being developed that can aid in extracting useful information from the enormous data sets produced by large-scale VSD imaging.
Leech segmental ganglia
Over four decades of research on the leech has led to a good understanding of the neuronal network distributed along the segmental ganglia that produce the animal's crawling and swimming behaviors (Friesen and Kristan 2007). During most of this time, this work was done with sharp electrodes, two to four neurons at a time. In 1999, William Kristan and colleagues introduced fluorescence resonance energy transfer (FRET) VSD imaging with a CCD camera to study this network (Cacciatore et al. 1999). This approach quickly led to the discovery of three neurons that were monosynaptic followers of a neuron known to halt swimming (Tr2). Two of these new cells, neurons 256 and 54, were then penetrated with intracellular electrodes and were found to stop the swimming motor program when stimulated, identifying them as intermediaries in the control of the leech swimming behavior (Taylor et al. 2003). This example of the use of VSD imaging demonstrates how it can speed the discovery of new neurons in known networks.
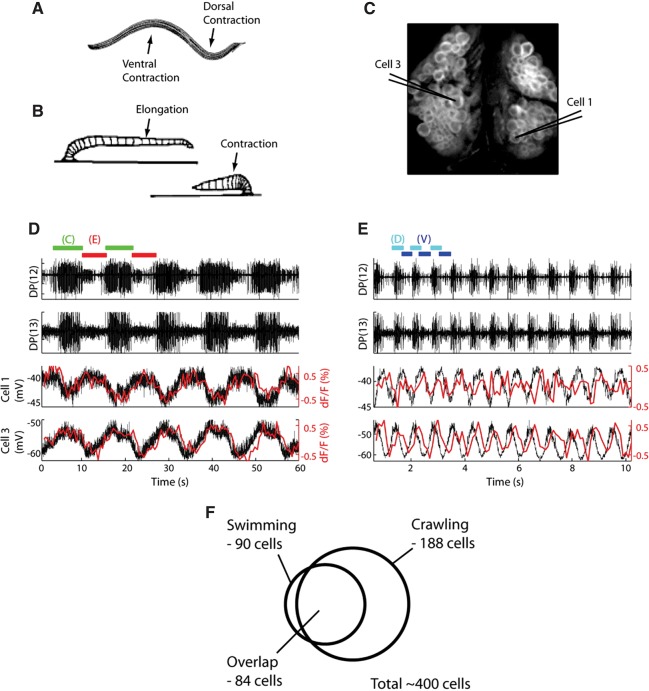
Kristan and colleagues subsequently used VSD imaging to explore the degree to which the leech swimming and crawling networks are organized according to a dedicated or multifunctional scheme (Briggman and Kristan 2006). Figure 1 shows their finding that 84 neurons in ganglion 10 are members of both networks. This large-scale imaging approach is consistent with findings obtained with traditional recording methods that many networks contain both dedicated and multifunctional neurons (Briggman and Kristan 2008).
Figure 1.
Imaging showed a high degree of overlap between the leech swimming and crawling networks. (A) The leech swimming behavior consists of alternating dorsal/ventral contractions of longitudinal muscles. (B) The crawling behavior is produced by contraction of circular muscles causing elongation followed by activation of longitudinal muscles causing contraction. (C) Image of the dorsal surface of ganglion 10, stained with a VSD. Cells 1 and 3 were impaled with sharp electrodes for simultaneous intracellular recording. (D) Simultaneous extracellular, intracellular, and optical recording during a crawling episode with alternating bouts of contraction (green bars) and elongation (red bars). Optical signals in cells 1 and 3 are shown in red overlaying the intracellularly recorded activity (black). Cells 1 and 3 oscillated 180° out of phase with each other during crawling. (E) Simultaneous extracellular, intracellular, and optical recording during a swimming episode with alternating dorsal/ventral flexions (dorsal—cyan bars, ventral—blue bars). Again, intracellular (black) and optical (red) signals for cells 1 and 3 are overlaid. Cells 1 and 3 oscillated 90° out of phase with each other during swimming. (F) Venn diagram showing the total number of cells in ganglion 10 that oscillated with swimming (90), with crawling (188), and the total number of cells that oscillated during both behaviors (84). (Adapted from Briggman and Kristan 2006 with permission from the Society for Neuroscience.)
Next, a landmark imaging study by Kristan and colleagues focused on the neuronal mechanisms underlying decision making (Briggman et al. 2005). Prior sharp-electrode studies had identified separate individual command neurons that can drive swimming versus crawling. Traditional thinking in the field considered such command neurons as the decision-makers for behavioral choices. However, the ability to record a large proportion of the total network provided an opportunity to test whether single neurons or groups of neurons mediate decision making. By treating the network as a dynamical system and applying principal component analysis followed by linear discriminant analysis, they identified a small group of neurons that collectively are the earliest predictors of what the animal will choose to do in response to a stimulus, firing even ahead of the command neurons. See below for further detail on this study, which exemplifies the power of imaging combined with sophisticated analytical techniques to reveal higher-level cognitive features of network function.
Recently, Kristan and colleagues developed a new type of fast VSD that combines the best properties of electrochromic and FRET dyes (Miller et al. 2012). This new fluorescent dye uses a process known as photon-induced electron transfer, and has been shown capable of resolving action potentials in both rat hippocampal neurons and leech neurons (Miller et al. 2012). Continuing development of new VSDs with high signal-to-noise ratios and response speeds fast enough to resolve action potentials is important for the goal of imaging as many neurons as possible in the effort to understand how neuronal circuits produce behavior.
Tritonia escape swim network
In our laboratory we have adopted Cohen's method of using a fast absorbance VSD and a photodiode array, with which we are able to record action potential activity in up to 200 neurons simultaneously in central ganglia of the marine mollusks Tritonia and Aplysia. Raw data from the 464 diodes are spike-sorted into single neuron traces using independent component analysis (ICA) (Brown et al. 2001). ICA is a fully automated, blind source separation procedure that transforms data so that the resulting independent components are maximally statistically independent. We recently confirmed the accuracy of ICA spike-sorting in experiments where intracellular and optical recording were performed together. In all cases, each intracellular trace was found to match, spike-for-spike, one of the ICA spike-sorted traces (Hill et al. 2010).
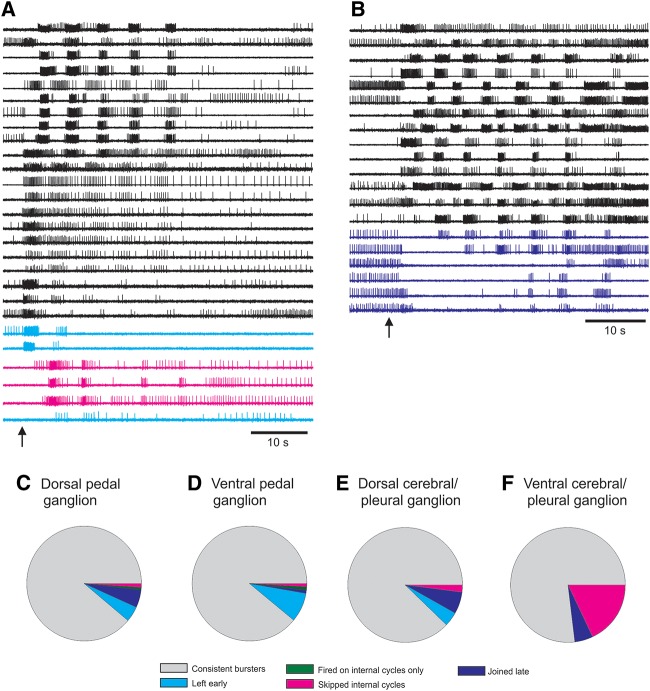
Using this approach, we quickly made the unexpected discovery that many neurons participate quite variably in otherwise stereotypic, rhythmic motor programs in three molluscan species. Figure 2, A and B, shows examples of Tritonia pedal ganglion neurons skipping cycles of the escape swim motor program (SMP). Such variably participating neurons were found in the cerebral, pleural, and pedal ganglia of Tritonia (Fig. 2C–F). Further, these loosely committed neurons varied their level of participation from motor program episode to episode (Hill et al. 2012b). This finding is consistent with studies in vertebrates that report trial-to-trial variability in neurons participating in what appear to be unvarying behaviors (Carmena et al. 2005; Ziv et al. 2013). Taken together, these studies support the idea that networks, even for very stereotypic behaviors, may be more fluid in their moment-to-moment functional structure than generally envisioned.
Figure 2.
VSD imaging revealed that surprising network fluidity can underlie stereotyped motor programs. (A,B) Examples of optically recorded Tritonia SMPs in which some pedal ganglion neurons skipped cycles of the motor program. Note: The majority of pedal neurons fired bursts on every cycle of the motor program. (Arrows) Stimuli given to pedal nerve 3 to elicit motor programs. (C–F) Neurons that skipped cycles of the Tritonia SMP belonged to four categories: those that left early (cyan), fired on internal cycles only (green), skipped internal cycles (magenta), joined late (blue). Neurons that skipped cycles of the SMP were found in all Tritonia central ganglia (cerebral, pleural, and pedal).
As in the leech, VSD imaging has proven useful for identifying new neurons in the Tritonia swim network (Frost and Wu 2014). One tool that has facilitated this is our construction of a hybrid microscope having two lens systems, one for imaging and another for providing stereopsis for depth perception when using sharp electrodes to penetrate neurons of interest identified by optical recording (Frost et al. 2007). The ability of large-scale imaging to accelerate circuit mapping has the potential to stimulate comparative studies of neuronal networks, which, as emphasized in a recent NSF workshop, are key routes to the discovery of general principles of brain function (Striedter et al. 2014).
Aplysia locomotion network
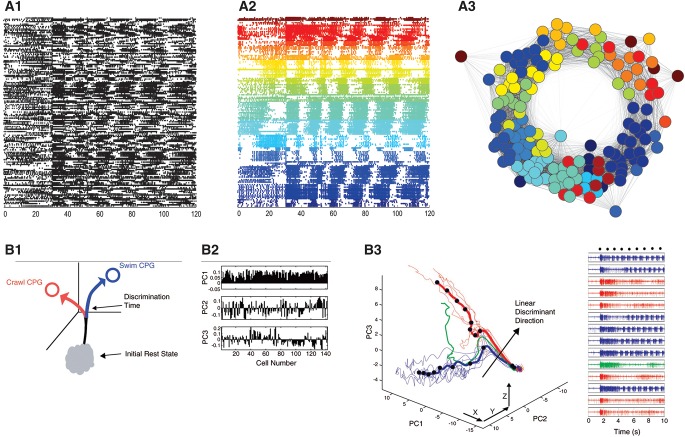
Aversive tail stimuli elicit escape crawling in Aplysia, which is driven by a rhythmic series of muscular contraction cycles that travel repeatedly head-to-tail down the length of the animal, propelling it forward. Sharp electrode studies of the network mediating this behavior have identified a large number of neurons in the pedal ganglion that burst with each muscle contraction cycle; some of these have been identified as motor neurons, with others suggested to be modulatory neurons (Jahan-Parwar and Fredman 1978; Hening et al. 1979; Fredman and Jahan-Parwar 1980, 1983; McPherson and Blankenship 1991; Romanova et al. 2007). However, little is known about the functional organization of these neurons. Our recent imaging studies reveal that during the crawling motor program, firing progresses through a series of neuronal ensembles in a continuous, repeating manner (Fig. 3A; AM Bruno, WN Frost, MD Humphries, in prep.), presumably driving the orderly sequence of muscle contractions that pass head-to-tail down the animal on each cycle of the motor program.
Figure 3.
Examples of analytic tools used in small network imaging studies. (A) Use of graph theoretic-based clustering to derive the ensemble organization of the Aplysia locomotion motor program. (A1) Action potential recording of 155 neurons. At 30 sec into the 2-min recording, a nerve stimulus was applied to trigger the rhythmic locomotion motor program. (A2) Unsupervised consensus clustering yielded 22 significant ensembles by their correlated firing. (A3) Visualization of the degree of correlation of all recorded neurons, revealing the order of firing of the ensembles. Distance between neurons indicates their degree of correlated firing. The ensembles clearly fire in an orderly, repeating sequence. (B) Use of PCA and LDA to study decision-making in the leech. (B1) Abstract representation of behavioral state as a trajectory phase space following a stimulus. The divergence point would represent a time at or near the decision point. (B2) Contributions of all 143 recorded neurons to each of the first three principal components. (B3) Trajectories of a 14 trial experiment. Right panel shows a nerve recording from each trial. The same stimulus led to a decision to swim in the blue trials, and to crawl in the red trials. The green trial was one in which the brain changed its mind, and switched from a swimming to a crawling motor program in midstream. (Adapted from Briggman et al. 2005 with permission from the American Association for the Advancement of Science.)
Our imaging work in Aplysia has also reinforced some of the findings described above from Tritonia. First, combined optical and sharp-electrode recordings in the Aplysia buccal ganglion have confirmed the accuracy of automated ICA spike-sorting of raw PDA data into single neuron traces (Hill et al. 2012a). Second, optical recordings of the Aplysia locomotion motor program revealed the presence of variably participating neurons that burst on some but not all cycles, extending the generality of this finding (Hill et al. 2012b).
Crustacean stomatogastric ganglion
The crustacean stomatogastric ganglion (STG) is a small neuronal network consisting of some 30 cells, which produces chewing and filtering behaviors. Decades of work with sharp electrodes has provided a detailed understanding of the connectivity between STG neurons, as well as the numerous modulators that affect different synapses among STG neurons (Marder and Bucher 2007). Fluorescent VSD imaging has recently been used to image the activity of neurons in both the lobster and crab STG (Stadele et al. 2013). The authors showed that they could resolve action potentials as well as excitatory and inhibitory synaptic potentials, and could stably image neuronal activity for many hours. The ability to record synaptic potentials from many STG neurons for extended periods offers a great opportunity to examine the long-term effects of neuromodulators on subthreshold potentials, a difficult feat with sharp electrodes due to electrode drift (Stadele et al. 2013). Given its potential for recording all STG neurons simultaneously, VSD imaging may significantly extend our understanding of how this small network operates.
Vertebrate respiratory networks
VSD imaging, although without single-neuron resolution, has also recently been applied to the vertebrate pre-Bötzinger complex and other areas of the ventral medulla involved in respiration. The pre-Bötzinger complex contains ∼1000 neurons, and plays a key role in the genesis of the respiratory rhythm (Feldman et al. 2013). The neurons of the pre-Bötzinger complex drive inspiration via projections to neurons in premotor areas of the medulla, which in turn drive inspiratory muscles such as the diaphragm and the external intercostals (Feldman et al. 2013). Recently, it has been shown that stimulation of only three to nine inspiratory phase neurons can initiate respiration network activity, which then rapidly “percolates” through the network (Kam et al. 2013). Researchers have begun using VSD imaging to gain insights into how this small network operates. For example, imaging of the pre-Bötzinger complex has shown that areas not active during eupnea are recruited with gasping (Potts and Paton 2006). Further, VSD imaging revealed that an area of the ventral medulla localized ventrolateral to the facial nucleus (the pFRG) shows preinspiratory population activity. Lesioning of this area leads to a reduction in respiratory frequency and to a change in the spatiotemporal pattern of respiratory neuron activity, suggesting that the pFRG is involved with respiratory rhythm generation (Onimaru and Homma 2003). Thus, VSD imaging has led to the identification an area of the ventral medulla involved in respiratory rhythm generation and has also revealed how network activity in the pre-Bötzinger complex is altered in different circumstances. These findings illustrate the usefulness of VSD imaging for efforts to understand how networks in the mammalian ventral medulla produce respiratory rhythms.
Vertebrate enteric ganglia
The mammalian enteric nervous system (ENS) is a self-contained network of neurons that produces the complex behaviors that together comprise normal gastrointestinal function. ENS neurons range in size from 10 to 25 microns and are arranged in plexuses located in the wall of the gut. The myenteric plexus is located between the longitudinal and circular muscle layers, and the submucosal plexus is located between the circular muscle layer and the mucosa. Since the ENS plexuses are organized essentially in two dimensions, they are particularly attractive for optical imaging studies. In fact, the first optical recordings of electrical activity in the mammalian nervous system with single-cell resolution were performed in the submucosal plexus of the guinea-pig small intestine (Obaid et al. 1999). In that study, the authors found that nicotinic acetylcholine receptors (nAChRs) that reversibly desensitize following nicotine exposure may be responsible for the enhancement of neuronal activity observed following nicotine application (Obaid et al. 1999). The same group then used VSD imaging to show that nAChRs may be capable of regulating the activity of both excitatory and inhibitory pathways (Obaid et al. 2005). Similarly, another group used VSD imaging to examine the activity of neurons in the guinea-pig and mouse ENS (Neunlist et al. 1999), and have gone on to use this technique to record the activity of cultured human myenteric neurons, recording spike discharge following nicotine application (Vignali et al. 2010). VSD imaging, with its ability to record the activity of many neurons simultaneously in the two-dimensionally arranged ENS, promises to further increase our understanding of how this complex, distributed network of neurons functions to produce healthy gastrointestinal function.
Special analysis tools are needed to mine the data obtained from large-scale imaging
Optical recording, with its ability to simultaneously record large numbers of neurons, is ideal for exploring network-level issues such as how circuits reorganize to store memory, or whether decision making involves top down or more consensus-like mechanisms. Although some topics can be addressed through simple visual inspection of the data, in most cases more sophisticated approaches are necessary. As many have noted, the next frontier in network neuroscience depends on the development and use of analytical methods for revealing signatures of network organization and the corresponding computations performed by those network structures (Briggman et al. 2006; Koch 2012; Alivisatos et al. 2013; O'Leary and Marder 2014).
For example, learning studies can benefit from methods for tracking changes in the network ensemble structure with experience. Many methods have been developed to partition data sets into ensembles of neurons having significantly correlated firing patterns (Feldt et al. 2009; Humphries 2011; Lopes-dos-Santos et al. 2011; Lyttle and Fellous 2011; Gerstein et al. 2012). Our laboratory has used an unsupervised graph theoretic-based clustering approach to reveal the existence of physically segregated ensembles of neurons that are re-identifiable across preparations during the Aplysia locomotion motor program (Fig. 3A; Bruno et al. 2013; AM Bruno, WN Frost, MD Humphries, in prep.). These can then be tracked across training trials to identify ensembles involved in learning and to characterize the changes in network organization that encode memory. The same approach could be used to investigate how networks functionally reorganize in response to modulation, injury, and disease.
More complex analysis tools are needed to investigate higher-order features of network function, such as those related to cognitive processing. For example, are behavioral decisions based on top-down or consensus-like, deliberative network mechanisms? As described earlier, Kristan and colleagues (Briggman et al. 2005) combined PCA with linear discriminant analysis (LDA) to test whether single neurons or groups of neurons mediate decision making in the leech. PCA was used as a first step to reduce the dimensionality of the data set, which helped distinguish between the crawling versus swimming motor programs and visualize the divergence point associated with the brain's decision regarding which behavior to choose (Fig. 3B). Such PCA-based dynamical portraits are increasingly used to provide useful insights into computations performed by both vertebrate and invertebrate networks (Niessing and Friedrich 2010; Churchland et al. 2012). The leech study then used LDA to identify the time point when the trajectories for swimming and crawling significantly diverged. After finding that decision making was correlated with activity in a defined group of neurons, they then used sharp electrodes to find one that acted to bias the decision to swim or crawl. Their work implicated that the earliest moment of decision making involves consensus-like correlated activity in a group of neurons, which occurs earlier than the activity in the previously identified individual command-type neurons in this system (Kristan 2008).
Logistic regression, which is similar to LDA, has been applied to multi-electrode array data from primate cortex by Newsome and colleagues to discriminate the neural correlate of changes of mind during decision making in primates (Kiani et al. 2014). The study involved the simultaneous recording of nearly 200 units from a circuit containing millions of neurons. This work was not concerned with identifying the contributions of individual neurons, but rather with the deliberative process of the recorded sub-network during changes of mind. Interestingly, in the leech study discussed above, a subset of trials found that the much simpler invertebrate preparation could also start to make a decision and then subsequently change to a different behavioral choice, raising the opportunity to explore whether changes in mind during decision making may involve similar underlying principles in simple and complex brains.
Although it is beyond the scope of this review to cover the array of analytical tools available (see Briggman et al. 2006), the few studies highlighted here show how their application to large-scale recordings can expose fundamental features of cognitive processing and predict behavioral outcomes. The future of brain network analysis depends critically on the development and application of such tools (Alivisatos et al. 2013). Their use in a variety of preparations, from vertebrate to invertebrate, will be key to uncovering general principles of network function.
What lies ahead for VSD imaging of small neuronal networks?
Small neuronal networks remain attractive model systems for discovering general principles of nervous system function (Selverston 1999; Pittenger and Kandel 2003; Clarac and Pearlstein 2007; Katz et al. 2013). Such networks offer the advantage that a significant fraction of the total network can be recorded simultaneously. Further, the behavioral relevance of specific neurons and ensemble types can be evaluated. These features also make small networks useful for bench-testing analysis tools that can be applied equally well to vertebrate networks (Humphries 2013; AM Bruno, WN Frost, MD Humphries, in prep.).
Although many of the imaging findings reviewed here confirm insights already gained from sharp-electrode studies, optical recording greatly increases the speed with which such advances can be made. For example, a single imaging experiment can reveal the basic organizational features of a given network, e.g., how many neurons and ensemble types participate in a motor program (Fig. 3A), orders of magnitude faster than can be accomplished with sharp electrodes. As mentioned earlier, this may encourage comparative studies, which are necessary for establishing whether given phenomena are general features of neuronal networks.
Given the rapidly expanding array of large-scale recording methods and analysis tools, this is an exciting time in systems neuroscience. In addition to the topics reviewed here, several areas are increasingly open for exploration. For example, what organizing features of network function are common across species? How flexible are network structures moment-to-moment and across longer intervals of time? What is the role of spontaneous activity in determining how inputs are interpreted and processed? How do damaged networks regain function after injury? Small, typically invertebrate, neuronal networks remain ideal preparations in which to explore such fundamental issues of brain function.
Acknowledgments
Work by the authors was supported by National Institutes of Health (NIH) grants NS060921 (to W.F.) and NS079036 (to A.B.) and National Science Foundation (NSF) grant 1257923 (to W.F.). We also thank Mark Humphries for collaborative work contributing to Figure 3A and Lise Eliot for useful comments on the manuscript.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.035964.114.
References
- Alivisatos AP, Chun M, Church GM, Deisseroth K, Donoghue JP, Greenspan RJ, McEuen PL, Roukes ML, Sejnowski TJ, Weiss PS, et al. 2013. Neuroscience. The brain activity map. Science 339: 1284–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB Jr 2006. Imaging dedicated and multifunctional neural circuits generating distinct behaviors. J Neurosci 26: 10925–10933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB 2008. Multifunctional pattern-generating circuits. Annu Rev Neurosci 31: 271–294 [DOI] [PubMed] [Google Scholar]
- Briggman KL, Abarbanel HD, Kristan WB Jr 2005. Optical imaging of neuronal populations during decision-making. Science 307: 896–901 [DOI] [PubMed] [Google Scholar]
- Briggman KL, Abarbanel HD, Kristan WB Jr 2006. From crawling to cognition: analyzing the dynamical interactions among populations of neurons. Curr Opin Neurobiol 16: 135–144 [DOI] [PubMed] [Google Scholar]
- Brown GD, Yamada S, Sejnowski TJ 2001. Independent component analysis at the neural cocktail party. Trends Neurosci 24: 54–63 [DOI] [PubMed] [Google Scholar]
- Bruno AM, Frost WN, Humphries MD 2013. Evolution of a motor program in dynamical and physical space. In 2013 Neuroscience meeting planner, abstract program, Vol. 39 Society for Neuroscience, San Diego [Google Scholar]
- Cacciatore TW, Brodfuehrer PD, Gonzalez JE, Jiang T, Adams SR, Tsien RY, Kristan WB Jr, Kleinfeld D 1999. Identification of neural circuits by imaging coherent electrical activity with FRET-based dyes. Neuron 23: 449–459 [DOI] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, Henriquez CS, Nicolelis MA 2005. Stable ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci 25: 10712–10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV 2012. Neural population dynamics during reaching. Nature 487: 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Pearlstein E 2007. Invertebrate preparations and their contribution to neurobiology in the second half of the 20th century. Brain Res Rev 54: 113–161 [DOI] [PubMed] [Google Scholar]
- Cohen LB 2010. Historical overview and general methods of membrane potential imaging. In Membrane potential imaging in the nervous system: methods and applications (ed. Canepari M, Zecevic D), pp. 1–11 Springer, New York [Google Scholar]
- Davila HV, Salzberg BM, Cohen LB, Waggoner AS 1973. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol 241: 159–160 [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA 2013. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt S, Waddell J, Hetrick VL, Berke JD, Zochowski M 2009. Functional clustering algorithm for the analysis of dynamic network data. Phys Rev 79: 056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman SM, Jahan-Parwar B 1980. Role of pedal ganglia motor neurons in pedal wave generation in Aplysia. Brain Res Bull 5: 179–193 [DOI] [PubMed] [Google Scholar]
- Fredman SM, Jahan-Parwar B 1983. Command neurons for locomotion in Aplysia. J Neurophysiol 49: 1092–1117 [DOI] [PubMed] [Google Scholar]
- Friesen WO, Kristan WB 2007. Leech locomotion: swimming, crawling, and decisions. Curr Opin Neurobiol 17: 704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Wu J-Y 2014. Voltage-sensitive dye imaging. In Basic electrophysiological methods (ed. Covey E, Carter M). Oxford University Press, New York: (in press) [Google Scholar]
- Frost WN, Wang J, Brandon CJ 2007. A stereo-compound hybrid microscope for combined intracellular and optical recording of invertebrate neural network activity. J Neurosci Methods 162: 148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Wang J, Brandon CJ, Moore-Kochlacs C, Sejnowski TJ, Hill ES 2010. Use of fast-responding voltage-sensitive dyes for large-scale recording of neuronal spiking activity with single-cell resolution. In Membrane potential imaging in the nervous system: methods and applications (ed. Canepari M, Zecevic D), pp. 53–60, Springer, New York [Google Scholar]
- Gerstein GL, Williams ER, Diesmann M, Grun S, Trengove C 2012. Detecting synfire chains in parallel spike data. J Neurosci Methods 206: 54–64 [DOI] [PubMed] [Google Scholar]
- Getting PA 1989. Emerging principles governing the operation of neural networks. Annu Rev Neurosci 12: 185–204 [DOI] [PubMed] [Google Scholar]
- Hening WA, Walters ET, Carew TJ, Kandel ER 1979. Motorneuronal control of locomotion in Aplysia. Brain Res 179: 231–253 [DOI] [PubMed] [Google Scholar]
- Hill ES, Moore-Kochlacs C, Vasireddi SK, Sejnowski TJ, Frost WN 2010. Validation of independent component analysis for rapid spike sorting of optical recording data. J Neurophysiol 104: 3721–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill ES, Bruno AM, Vasireddi SK, Frost WN 2012a. ICA applied to VSD imaging of invertebrate neuronal networks. In Independent component analysis for audio and biosignal applications (ed. Naik G), pp. 235–246 InTech, Rijeka, Croatia [Google Scholar]
- Hill ES, Vasireddi SK, Bruno AM, Wang J, Frost WN 2012b. Variable neuronal participation in stereotypic motor programs. PLoS One 7: e40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD 2011. Spike-train communities: finding groups of similar spike trains. J Neurosci 31: 2321–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD 2013. Modularity of multi-neuron recordings: decoding neural ensembles and population dynamics. In 2013 Neuroscience meeting planner, abstract program, Vol. 39 Society for Neuroscience, San Diego [Google Scholar]
- Jahan-Parwar B, Fredman SM 1978. Control of pedal and parapodial movements in Aplysia. I. Proprioceptive and tactile reflexes. J Neurophysiol 41: 600–608 [DOI] [PubMed] [Google Scholar]
- Kam K, Worrell JW, Ventalon C, Emiliani V, Feldman JL 2013. Emergence of population bursts from simultaneous activation of small subsets of preBotzinger complex inspiratory neurons. J Neurosci 33: 3332–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P, Grillner S, Wilson R, Borst A, Greenspan R, Buzsaki G, Martin K, Marder E, Kristan W, Friedrich R, et al. 2013. Vertebrate versus invertebrate neural circuits. Curr Biol 23: R504–R506 [DOI] [PubMed] [Google Scholar]
- Kiani R, Cueva CJ, Reppas JB, Newsome WT 2014. Dynamics of neural population responses in prefrontal cortex indicate changes of mind on single trials. Curr Biol 247: 1542–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C 2012. Modular biological complexity. Science 337: 531–532 [DOI] [PubMed] [Google Scholar]
- Kristan WB 2008. Neuronal decision-making circuits. Curr Biol 18: R928–R932 [DOI] [PubMed] [Google Scholar]
- Lieb JR Jr, Frost WN 1997. Realistic simulation of the Aplysia siphon-withdrawal reflex circuit: roles of circuit elements in producing motor output. J Neurophysiol 77: 1249–1268 [DOI] [PubMed] [Google Scholar]
- London JA, Zecevic D, Cohen LB 1987. Simultaneous optical recording of activity from many neurons during feeding in Navanax. J Neurosci 7: 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-dos-Santos V, Conde-Ocazionez S, Nicolelis MA, Ribeiro ST, Tort AB 2011. Neuronal assembly detection and cell membership specification by principal component analysis. PLoS One 6: e20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttle D, Fellous JM 2011. A new similarity measure for spike trains: sensitivity to bursts and periods of inhibition. J Neurosci Methods 199: 296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D 2007. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316 [DOI] [PubMed] [Google Scholar]
- McPherson DR, Blankenship JE 1991. Neural control of swimming in Aplysia brasiliana. II. Organization of pedal motoneurons and parapodial motor fields. J Neurophysiol 66: 1352–1365 [DOI] [PubMed] [Google Scholar]
- Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB Jr, Tsien RY 2012. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci 109: 2114–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Peters S, Schemann M 1999. Multisite optical recording of excitability in the enteric nervous system. Neurogastroenterol Motil 11: 393–402 [DOI] [PubMed] [Google Scholar]
- Niessing J, Friedrich RW 2010. Olfactory pattern classification by discrete neuronal network states. Nature 465: 47–52 [DOI] [PubMed] [Google Scholar]
- Obaid AL, Koyano T, Lindstrom J, Sakai T, Salzberg BM 1999. Spatiotemporal patterns of activity in an intact mammalian network with single-cell resolution: optical studies of nicotinic activity in an enteric plexus. J Neurosci 19: 3073–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Nelson ME, Lindstrom J, Salzberg BM 2005. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. J Exp Biol 208: 2981–3001 [DOI] [PubMed] [Google Scholar]
- O'Leary T, Marder E 2014. Neuroscience. Mapping neural activation onto behavior in an entire animal. Science 344: 372–373 [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I 2003. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Kandel ER 2003. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci 358: 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Paton JF 2006. Optical imaging of medullary ventral respiratory network during eupnea and gasping in situ. Eur J Neurosci 23: 3025–3033 [DOI] [PubMed] [Google Scholar]
- Romanova EV, McKay N, Weiss KR, Sweedler JV, Koester J 2007. Autonomic control network active in Aplysia during locomotion includes neurons that express splice variants of R15-neuropeptides. J Neurophysiol 97: 481–491 [DOI] [PubMed] [Google Scholar]
- Salzberg BM, Davila HV, Cohen LB 1973. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature 246: 508–509 [DOI] [PubMed] [Google Scholar]
- Salzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN 1977. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol 40: 1281–1291 [DOI] [PubMed] [Google Scholar]
- Selverston A 1999. What invertebrate circuits have taught us about the brain. Brain Res Bull 50: 439–440 [DOI] [PubMed] [Google Scholar]
- Stadele C, Andras P, Stein W 2013. Simultaneous measurement of membrane potential changes in multiple pattern generating neurons using voltage sensitive dye imaging. J Neurosci Methods 203: 78–88 [DOI] [PubMed] [Google Scholar]
- Striedter GF, Belgard TG, Chen CC, Davis FP, Finlay BL, Gunturkun O, Hale ME, Harris JA, Hecht EE, Hof PR, et al. 2014. NSF workshop report: discovering general principles of nervous system organization by comparing brain maps across species. Brain Behav Evol 83: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Cottrell GW, Kleinfeld D, Kristan WB Jr 2003. Imaging reveals synaptic targets of a swim-terminating neuron in the leech CNS. J Neurosci 23: 11402–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali S, Peter N, Ceyhan G, Demir IE, Zeller F, Senseman D, Michel K, Schemann M 2010. Recordings from human myenteric neurons using voltage-sensitive dyes. J Neurosci Methods 192: 240–248 [DOI] [PubMed] [Google Scholar]
- Wu JY, Tsau Y, Hopp HP, Cohen LB, Tang AC, Falk CX 1994. Consistency in nervous systems: trial-to-trial and animal-to-animal variations in the responses to repeated applications of a sensory stimulus in Aplysia. J Neurosci 14: 1366–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic D, Wu JY, Cohen LB, London JA, Hopp HP, Falk CX 1989. Hundreds of neurons in the Aplysia abdominal ganglion are active during the gill-withdrawal reflex. J Neurosci 9: 3681–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ 2013. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16: 264–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochowski M, Cohen LB, Fuhrmann G, Kleinfeld D 2000. Distributed and partially separate pools of neurons are correlated with two different components of the gill-withdrawal reflex in Aplysia. J Neurosci 20: 8485–8492 [DOI] [PMC free article] [PubMed] [Google Scholar]