Abstract
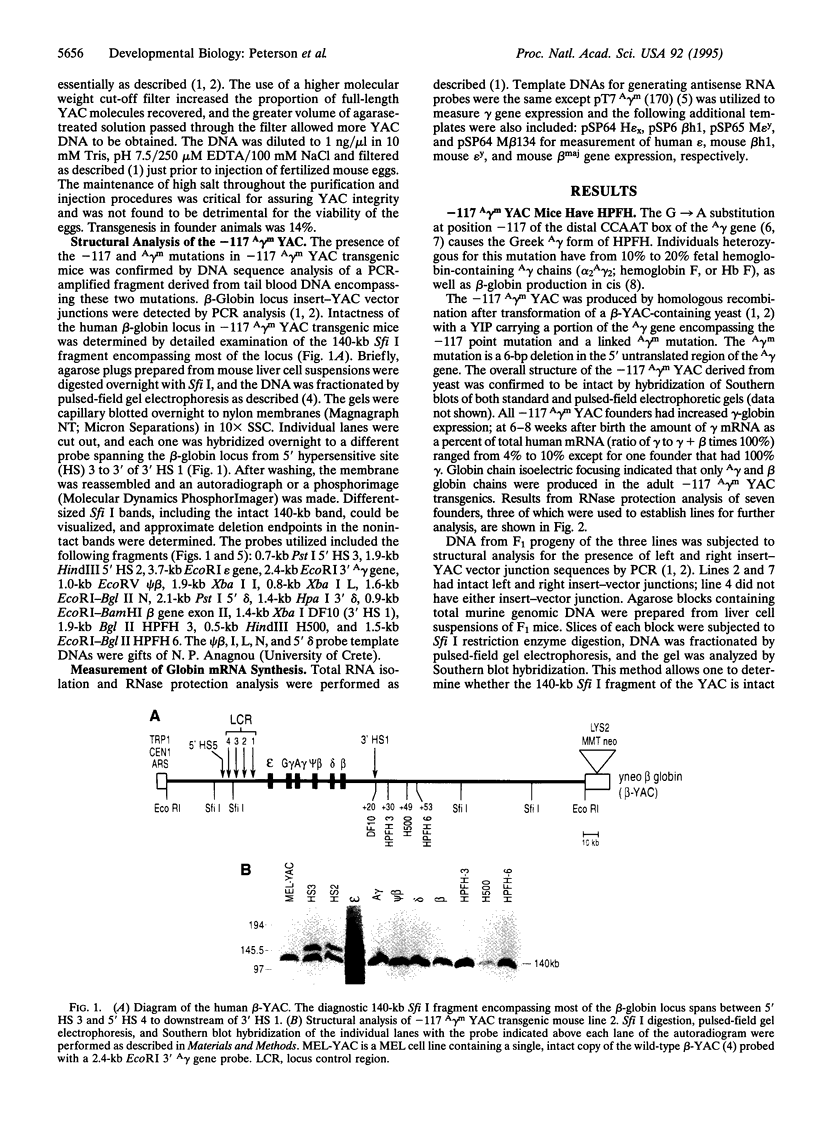
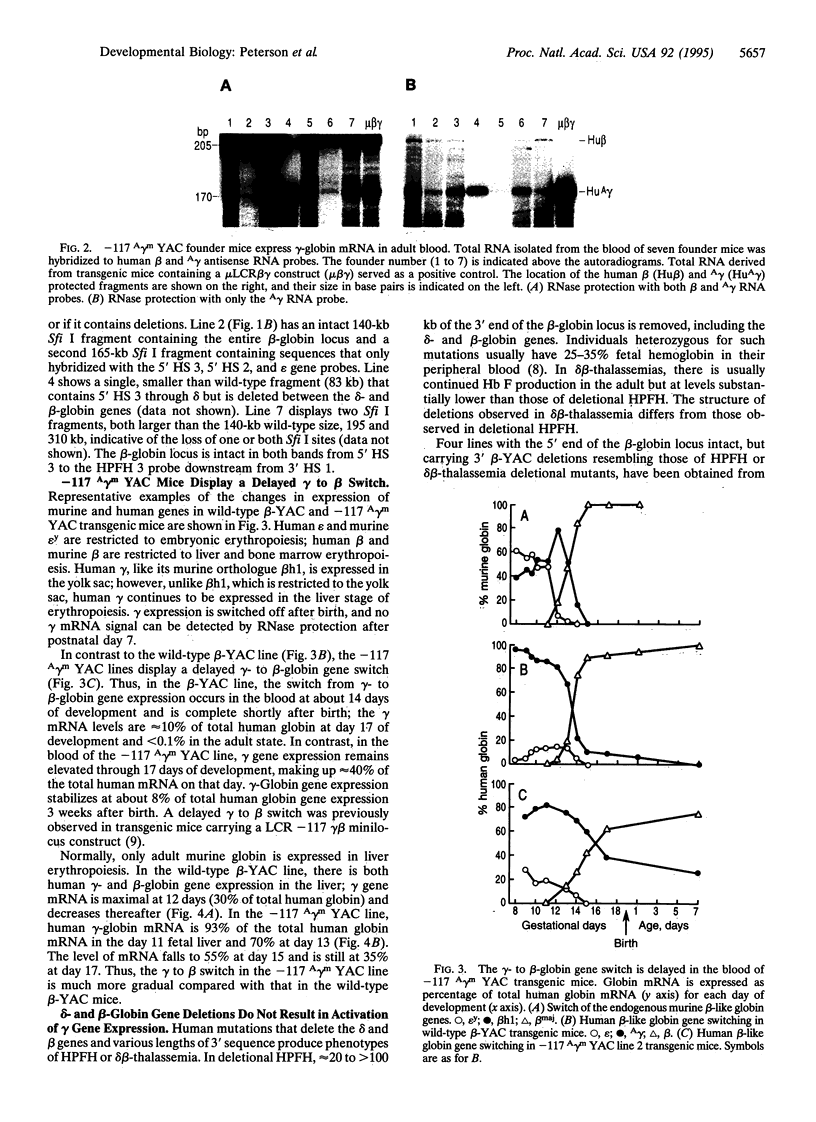
To test whether yeast artificial chromosomes (YACs) can be used in the investigation of mammalian development, we analyzed the phenotypes of transgenic mice carrying two types of beta-globin locus YAC developmental mutants: (i) mice carrying a G-->A transition at position -117 of the A gamma gene, which is responsible for the Greek A gamma form of hereditary persistence of fetal hemoglobin (HPFH), and (ii) beta-globin locus YAC transgenic lines carrying delta- and beta-globin gene deletions with 5' breakpoints similar to those of deletional HPFH and delta beta-thalassemia syndromes. The mice carrying the -117 A gamma G-->A mutation displayed a delayed gamma- to beta-globin gene switch and continued to express A gamma-globin chains in the adult stage of development as expected for carriers of Greek HPFH, indicating that the YAC/transgenic mouse system allows the analysis of the developmental role of cis-acting motifs. The analysis of mice carrying 3' deletions first provided evidence in support of the hypothesis that imported enhancers are responsible for the phenotypes of deletional HPFH and second indicated that autonomous silencing is the primary mechanism for turning off the gamma-globin genes in the adult. Collectively, our results suggest that transgenic mice carrying YAC mutations provide a useful model for the analysis of the control of gene expression during development.
Full text
PDF
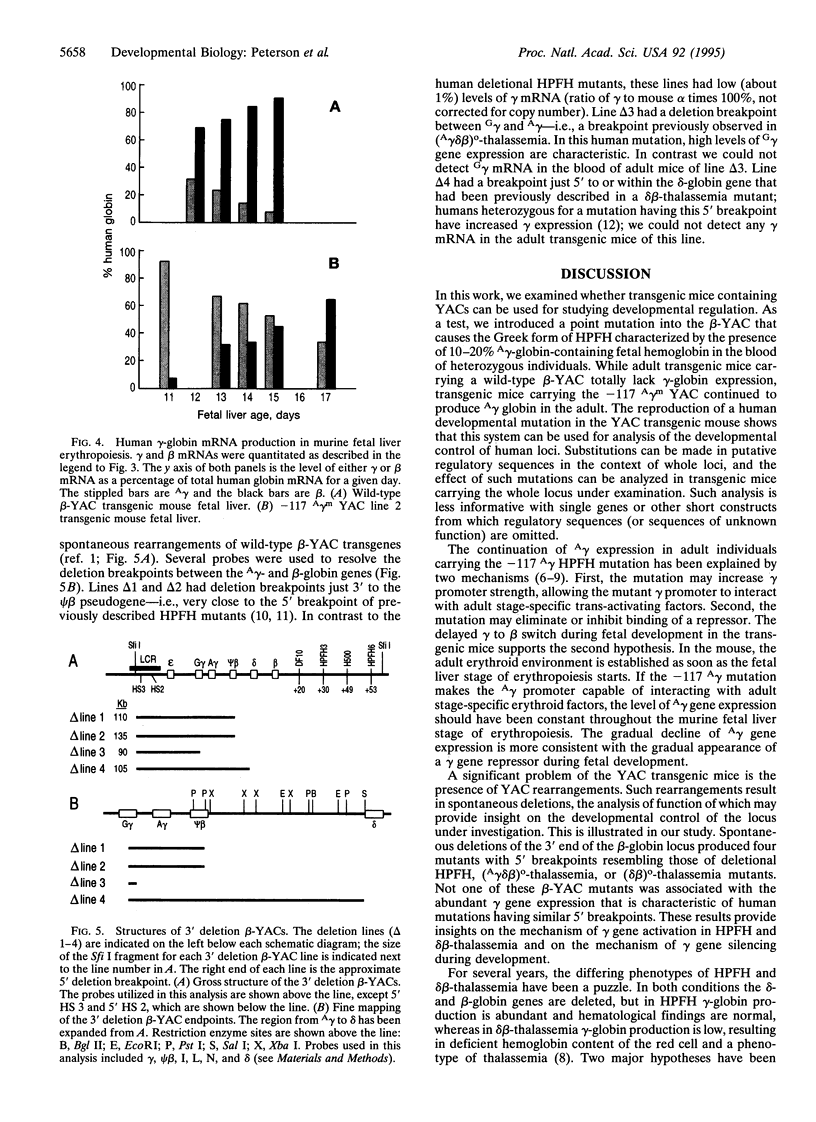

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Berry M., Grosveld F., Dillon N. A single point mutation is the cause of the Greek form of hereditary persistence of fetal haemoglobin. Nature. 1992 Aug 6;358(6386):499–502. doi: 10.1038/358499a0. [DOI] [PubMed] [Google Scholar]
- Chen L., Kosslak R., Atherly A. G. Mechanical shear of high molecular weight DNA in agarose plugs. Biotechniques. 1994 Feb;16(2):228–229. [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Metherall J. E., Yamakawa M., Pan J., Weissman S. M., Forget B. G. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991 Mar 21;350(6315):252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- Engel J. D. Developmental regulation of human beta-globin gene transcription: a switch of loyalties? Trends Genet. 1993 Sep;9(9):304–309. doi: 10.1016/0168-9525(93)90248-g. [DOI] [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Feingold E. A., Forget B. G. The breakpoint of a large deletion causing hereditary persistence of fetal hemoglobin occurs within an erythroid DNA domain remote from the beta-globin gene cluster. Blood. 1989 Nov 1;74(6):2178–2186. [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Gnirke A., Huxley C., Peterson K., Olson M. V. Microinjection of intact 200- to 500-kb fragments of YAC DNA into mammalian cells. Genomics. 1993 Mar;15(3):659–667. doi: 10.1006/geno.1993.1121. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Whyatt D., Fraser P., Yannoutsos N., Greaves D., Dillon N., Grosveld F. Importance of globin gene order for correct developmental expression. Genes Dev. 1991 Aug;5(8):1387–1394. doi: 10.1101/gad.5.8.1387. [DOI] [PubMed] [Google Scholar]
- Henthorn P. S., Smithies O., Mager D. L. Molecular analysis of deletions in the human beta-globin gene cluster: deletion junctions and locations of breakpoints. Genomics. 1990 Feb;6(2):226–237. doi: 10.1016/0888-7543(90)90561-8. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Efremov G. D., Duma H., Mladenovski B., Hyman C. B., Rachmilewitz E. A., Bouver N., Miller A., Brodie A. The present status of the heterogeneity of fetal hemoglobin in beta-thalassemia: an attempt to unify some observations in thalassemia and related conditions. Ann N Y Acad Sci. 1974;232(0):107–124. doi: 10.1111/j.1749-6632.1974.tb20576.x. [DOI] [PubMed] [Google Scholar]
- Palena A., Blau A., Stamatoyannopoulos G., Anagnou N. P. Eastern European (delta beta) zero-thalassemia: molecular characterization of a novel 9.1-kb deletion resulting in high levels of fetal hemoglobin in the adult. Blood. 1994 Jun 15;83(12):3738–3745. [PubMed] [Google Scholar]
- Peterson K. R., Clegg C. H., Huxley C., Josephson B. M., Haugen H. S., Furukawa T., Stamatoyannopoulos G. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7593–7597. doi: 10.1073/pnas.90.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Stamatoyannopoulos G. Role of gene order in developmental control of human gamma- and beta-globin gene expression. Mol Cell Biol. 1993 Aug;13(8):4836–4843. doi: 10.1128/mcb.13.8.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Zitnik G., Huxley C., Lowrey C. H., Gnirke A., Leppig K. A., Papayannopoulou T., Stamatoyannopoulos G. Use of yeast artificial chromosomes (YACs) for studying control of gene expression: correct regulation of the genes of a human beta-globin locus YAC following transfer to mouse erythroleukemia cell lines. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11207–11211. doi: 10.1073/pnas.90.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Enver T., Nakamoto B., Josephson B., Papayannopoulou T., Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990 Nov 23;250(4984):1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- Raich N., Papayannopoulou T., Stamatoyannopoulos G., Enver T. Demonstration of a human epsilon-globin gene silencer with studies in transgenic mice. Blood. 1992 Feb 15;79(4):861–864. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Josephson B., Zhang J. W., Li Q. Developmental regulation of human gamma-globin genes in transgenic mice. Mol Cell Biol. 1993 Dec;13(12):7636–7644. doi: 10.1128/mcb.13.12.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouboulis J., Dillon N., Grosveld F. Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev. 1992 Oct;6(10):1857–1864. doi: 10.1101/gad.6.10.1857. [DOI] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]