Summary
Downregulation of the miR-143/145 microRNA (miRNA) cluster has been repeatedly reported in colon cancer and other epithelial tumors. In addition, overexpression of these miRNAs inhibits tumorigenesis, leading to broad consensus that they function as cell-autonomous epithelial tumor suppressors. We generated mice with deletion of miR-143/145 to investigate the functions of these miRNAs in intestinal physiology and disease in vivo. While intestinal development proceeded normally in the absence of these miRNAs, epithelial regeneration after injury was dramatically impaired. Surprisingly, we found that miR-143/145 are expressed and function exclusively within the mesenchymal compartment of intestine. Defective epithelial regeneration in miR-143/145-deficient mice resulted from dysfunction of smooth muscle and myofibroblasts and was associated with de-repression of the novel miR-143 target Igfbp5, which impaired IGF signaling after epithelial injury. These results provide important insights into the regulation of epithelial wound healing and argue against a cell-autonomous tumor suppressor role for miR-143/145 in colon cancer.
Introduction
MicroRNAs (miRNAs) represent a broad class of 18–22 nucleotide RNAs that negatively regulate the stability and translation of target messenger RNAs. Although initially identified over two decades ago as developmental regulators in invertebrates (Lee et al., 1993; Reinhart et al., 2000; Wightman et al., 1993), subsequent evaluation of numerous miRNAs in worms, flies, and mice has demonstrated that these transcripts rarely provide essential functions during animal development (Miska et al., 2007; van Rooij et al., 2007). Instead, miRNA deletion has repeatedly been associated with latent pathological defects exposed only upon stressing or taxing an organ system (Leung and Sharp, 2010; Mendell and Olson, 2012). These experimental findings have led to the suggestion that mammalian miRNAs commonly function in vivo to buffer cells and tissues against stressors and thereby maintain homeostasis.
In keeping with their roles in stress-activated pathways, miRNAs have repeatedly emerged as important diagnostic markers and therapeutic targets in human disease states. A particularly important role for miRNAs in cancer pathogenesis has been uncovered through the examination of human tumor samples. Virtually all examined tumor types are characterized by globally abnormal miRNA expression patterns and profiles of miRNA expression are highly informative for tumor classification, prognosis, and response to therapy (Kong et al., 2012; Lu et al., 2005; Lujambio and Lowe, 2012). Moreover, numerous reports have documented a functional contribution of specific miRNAs to cellular transformation and tumorigenesis (He et al., 2005; Medina et al., 2010). Among the first reported examples of abnormal miRNA expression in human cancer was downregulation of miR-143 and miR-145, two co-transcribed miRNAs, in human colorectal adenocarcinoma (Michael et al., 2003). This observation has been reproduced in numerous subsequent studies (Bandres et al., 2006; Motoyama et al., 2009; Schepeler et al., 2008; Slaby et al., 2007) and similar findings have been reported in breast cancer, pancreatic cancer, and other solid tumors of epithelial origin (Iorio et al., 2005; Papaconstantinou et al., 2013; Takagi et al., 2009). In addition, functional studies have demonstrated that ectopic expression of these miRNAs inhibits proliferation, induces apoptosis, and/or suppresses anchorage-independent growth and tumor-forming ability of diverse cancer cell types in vitro and in vivo (Chen et al., 2009; Clapé et al., 2009; Kent et al., 2010; Sachdeva et al., 2009). These effects are mediated, at least in part, by the direct repression of oncogenes such as MYC and KRAS. Based on these data, it is broadly accepted that miR-143/145 function as cell autonomous tumor suppressors in colon cancer and other epithelial tumor types. Nevertheless, the natural functions of miR-143/145 in the mammalian intestine and other epithelial tissues that underlie their presumed tumor suppressor activity have not been investigated.
The intestine is unique among mammalian tissues in the rate and number of ongoing mitotic divisions it requires for homeostasis. The rapid turnover of the intestinal epithelium depends on the presence of renewing stem cells that can provide a continuous supply of epithelial cells under basal conditions as well as additional cells to re-establish the epithelial barrier after mucosal injury (Barker et al., 2007; Sangiorgi and Capecchi, 2008; Takeda et al., 2011; Tian et al., 2011; Yan et al., 2012). The delicate balance between self-renewal, proliferation, and differentiation, as well as robust responses to various insults, relies both on intrinsic epithelial mechanisms and extrinsic signals from several sources, including local mesenchymal cells and immune cells. Among the most important regulators of the intestinal epithelium are pericryptal mesenchymal cells, sometimes referred to as intestinal subepithelial myofibroblasts (ISEMFs), which occupy a position just below the basement membrane of the epithelium. ISEMFs provide important paracrine regulatory signals to intestinal epithelial cells during normal physiologic turnover and in the setting of wound repair (Madison et al., 2005; Otte et al., 2003; Powell et al., 2011; Shao et al., 2006). The precise origin of these cells is ill-defined, but may include activation of resident fibroblasts, dedifferentiation of local smooth muscle cells, or even migration of bone marrow resident progenitors (Powell et al., 2011). A comprehensive understanding of the mechanisms that regulate myofibroblast behavior after injury and the paracrine factors that these and other stromal cells secrete to regulate epithelial proliferation and repair is of broad interest because it may allow the co-option of these pathways for therapeutic benefit.
To better understand the functions of miR-143/145 in intestinal physiology and disease in vivo, we generated mice with constitutional or tissue-restricted deletion of these miRNAs. While miR-143/145 loss-of-function had no overt effect on intestinal development, we uncovered an essential role for these miRNAs in the epithelial regenerative response induced by intestinal injury. Surprisingly, this phenotype was entirely attributable to a mesenchymal function, as demonstrated by a complete absence of miR-143/145 expression in the intestinal epithelium and lineage-specific deletion studies. Furthermore, we provide evidence that the observed defect in epithelial regeneration is mediated by aberrant paracrine signaling by smooth muscle and myofibroblasts. These findings provide important insight into the functions miR-143/145 in intestinal physiology and tumorigenesis and contribute to our understanding of the mechanisms that govern epithelial repair in this tissue.
Results
Normal baseline intestinal architecture and turnover in miR-143/145−/− mice
To study the physiologic functions of miR-143/145 in the intestinal epithelium in vivo, we generated mice harboring miR-143/145flox (miRflox) and miR-143/145null (miR−) alleles using standard homologous recombination techniques (Figure S1A). As expected, miR-143/145 were undetectable in miR−/− animals whereas miRflox/flox animals exhibited normal levels of the miRNAs in the absence of Cre (Figures 1A and S1B). As reported elsewhere, germline deletion of these miRNAs resulted in no overt developmental defects and the targeted alleles were transmitted at the expected Mendelian ratios (data not shown) (Boettger et al., 2009; Xin et al., 2009).
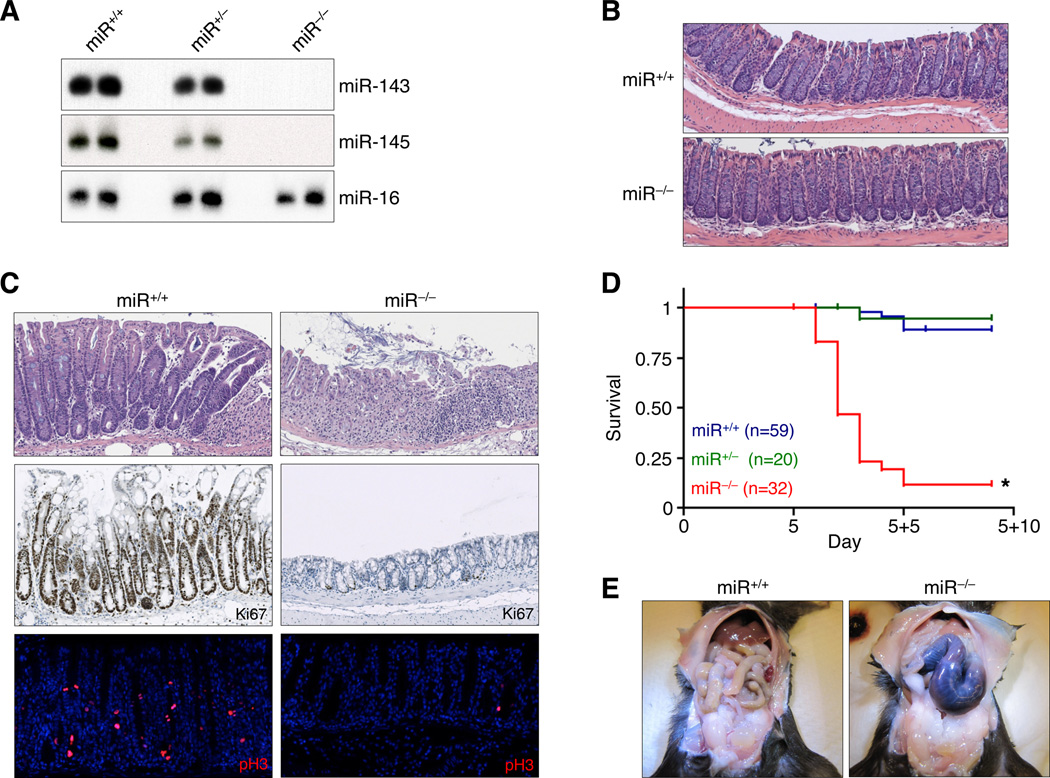
Figure 1. Lethal failure of intestinal regeneration in miR-143/145−/− mice.
(A) Northern blot analysis of bladder miRNA expression in mice of indicated genotypes.
(B) H&E-stained colon sections of adult miR+/+ and miR−/− mice. Representative images from at least 5 mice of each genotype are depicted.
(C) Proliferation of ulcer-adjacent crypts assessed by H&E (upper panels), Ki67, and pH3 staining. Histology (H&E) was examined in over 20 mice of each genotype while a subset of sections (n ≥ 3) were further stained for Ki67 and pH3.
(D) Kaplan-Meier survival curves of miR+/+, miR+/−, and miR−/− mice, administered 3.5% DSS in drinking water for 5 days. *, p<0.0001, log rank test.
(E) miR+/+ and miR−/− animals dissected 2 days after completion of DSS treatment. Images are representative of over 20 mice examined per genotype.
See also Figures S1–S3.
Detailed histologic examination of adult (8–10 weeks of age) wild-type (miR+/+) and miR−/− mice did not reveal any overt abnormalities in the architecture of the intestinal crypt or villus compartments in small and large intestines (Figure 1B and data not shown). Bromodeoxyuridine (BrdU) pulse-chase experiments demonstrated equivalent BrdU uptake and epithelial turnover rates in miR+/+ and miR−/− intestines (Figure S2). Thus miR-143/145 are dispensable for normal small and large intestinal development in the mouse and do not appreciably regulate the baseline rate of epithelial turnover in this tissue.
Lethal failure of intestinal regeneration in miR-143/145−/− mice
To assess whether a latent stress-induced defect exists in the intestines of miR−/− mice, we administered dextran sulfate sodium (DSS), which induces a well-tolerated epithelial injury-regeneration sequence in wild-type animals (Clapper et al., 2007). Following 5 days of DSS administration via drinking water, control mice exhibited transient weight loss and severe acute colitis, leaving patches of completely de-epithelialized mucosa (Figures S3A–B). This was followed by a robust regenerative response, evident by 2 days after removal of DSS (experimental day 5+2, hereafter referred to as D5+2), characterized by the presence of elongated, hyperproliferative crypts in ulcer-adjacent areas that stained positively for Ki67 and phosphorylated histone 3 (pH3, a mitotic marker) throughout their lengths, but lacked differentiated goblet cells (Figure 1C). Near complete restoration of the intestinal mucosal architecture was achieved by 9 days after DSS injury (data not shown). Surprisingly, when miR−/− animals were subjected to the identical DSS treatment, a majority succumbed to fulminant disease by D5+2 (Figure 1D). Grossly, the colons of these animals uniformly appeared distended, hemorrhagic, and necrotic (Figure 1E).
The mortality of miR−/− mice following exposure to DSS could result from more extensive epithelial injury or a failure to regenerate the damaged epithelium. Weight loss of wild-type and knockout mice after DSS treatment was indistinguishable, suggesting equivalent injury (Figure S3A). Similarly, histologic evidence of tissue injury and apoptosis on experimental day 5 were comparable between miR+/+ and miR−/− animals (Figure S3B). However, despite the similar appearance of wild-type and knockout colons immediately after exposure to DSS, miR−/− mice exhibited a striking deficiency of regenerating crypts after injury (D5+2) (Figure 1C). In miR−/− mice, ulcer-adjacent crypts remained exclusively in the non-regenerative state with numerous goblet cells and proliferation limited to the base of the crypt. Together, these data indicate that mortality following DSS treatment in miR−/− mice is specifically attributable to a failure of the intestinal epithelium to mount a regenerative response following injury.
miR-143/145 are exclusively expressed in the intestinal mesenchyme
The regenerative failure in miR−/− mice could result either from an intrinsic epithelial proliferative defect or from a non-cell-autonomous abnormality in a supporting cell population that produces one or more critical paracrine factors. In order to distinguish between these possibilities, we first examined the miR-143/145 expression domain in mouse colon using in situ hybridization (ISH) (Figures 2A–B). Robust miR-143/145 expression was detected in the mesenchymal cells of the intestine, especially in smooth muscle cells as reported elsewhere (Boettger et al., 2009; Xin et al., 2009) and in pericryptal cells located in a position consistent with lamina propria myofibroblasts. In contrast, expression of these miRNAs was not detectable within intestinal epithelial cells, contrary to some previously reported ISH studies (Zhu et al., 2011).
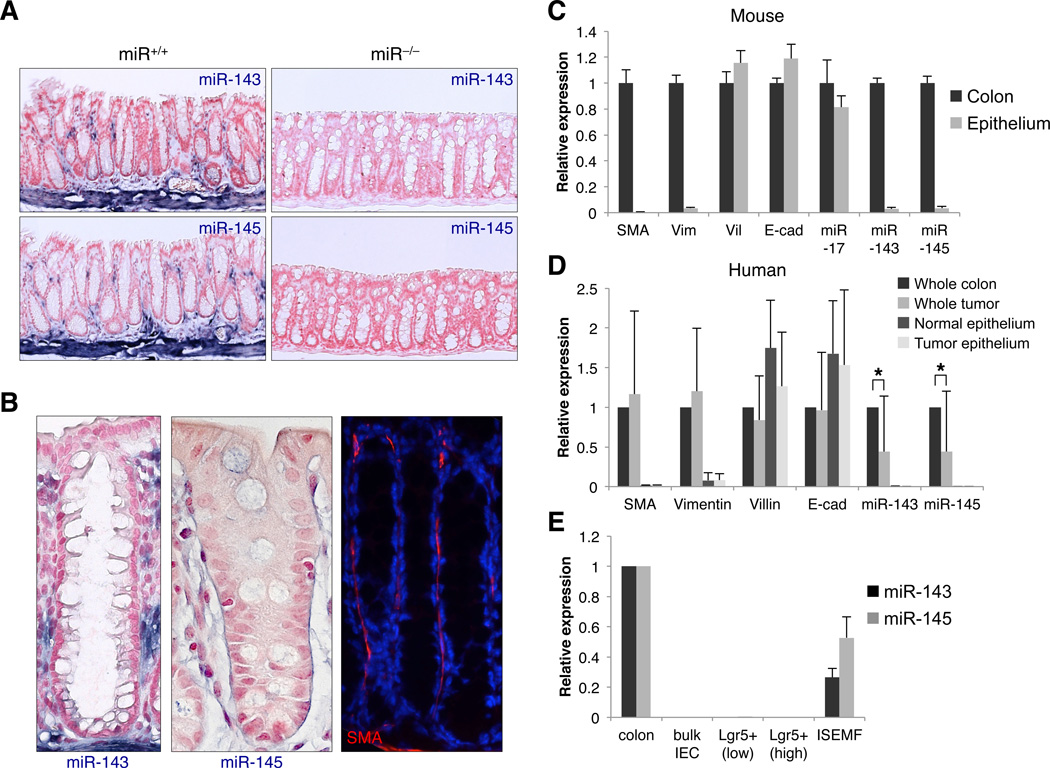
Figure 2. miR-143/145 are exclusively expressed in the intestinal mesenchyme.
(A) In situ hybridization analysis of miR-143 and miR-145 expression in mouse colon. Representative images from 3 animals per genotype shown. Blue, miRNA; red, nuclear fast red counterstain.
(B) In situ hybridization (left panels) showing miR-143/145-expressing pericryptal cells located in a position consistent with SMA+ lamina propria myofibroblasts (right panel).
(C) Quantitative RT-PCR analysis of mRNA and miRNA expression in full-thickness mouse colon specimens and purified epithelium. The purity of epithelial preparations was demonstrated by enrichment of the epithelial markers Villin (Vil) and E-cadherin (Cdh1) and near complete depletion of the mesenchymal transcripts smooth muscle actin (Sma) and vimentin (Vim). n ≥ 3 samples per condition. For this and all subsequent figures, error bars represent standard deviations.
(D) mRNA and miRNA expression in human colorectal tumor and paired normal colon specimens and in corresponding purified epithelium. n=10 samples per condition. *, p<0.05 (Student’s t-test).
(E) Quantitative RT-PCR analysis of miR-143/145 expression in purified intestinal epithelial cells (IEC), isolated epithelial stem cells (Lgr5+), and ISEMFs, compared to full-thickness colon specimens.
See also Figure S4.
Since these data were at odds with a large body of published literature that implicates miR-143/145 as cell-autonomous tumor suppressors in the intestine, we measured miRNA expression in purified epithelial cells using a more sensitive quantitative reverse transcription PCR (qRT-PCR) assay. Consistent with our ISH findings, miR-143 and miR-145 were easily detectable in whole colon but nearly undetectable by PCR in mouse (Figure 2C) and human (Figure 2D) epithelial preparations. We also considered the possibility that miR-143/145 might be selectively induced in epithelial cells following injury, but crypt isolation during and after DSS treatment revealed an identical non-epithelial expression pattern at all time-points (Figure S4A).
A low level of amplification of miR-143/145 in purified epithelium was most likely due to residual contaminating mesenchymal cells in these preparations. To address this possibility directly, we took advantage of our conditional miRflox allele which can be deleted in specific lineages using appropriate Cre driver lines. Previously characterized Villin-Cre (Madison et al., 2002) and Twist2+/Cre (Geske et al., 2008; Sosic et al., 2003) mice were obtained and crossed to miRflox animals. The Villin-Cre transgene directs recombination in all cells of the GI tract epithelium, whereas the Twist2Cre allele drives Cre expression in early mesenchymal cells but, importantly, spares bone marrow cells (Yu et al., 2003). We confirmed the specificity of both Cre lines by crossing to ROSA26LSL-LacZ reporter mice (Figure 3A) and directly examining recombination of the miR-143/145 locus in small and large intestine (Figure S5A). Additionally, a low level of recombination in total bone marrow cells, hematopoietic stem cells, and major hematopoietic lineages was confirmed in Twist2Cre/+; miRflox/flox animals (Figure S5B). miR-143 and miR-145 levels in whole colon were unchanged in Villin-Cre; miRflox/flox animals compared to control littermates, indicating that the detectable miR-143/145 expression in this tissue does not derive from the epithelial compartment (Figure S5C). In contrast, Twist2Cre; miRflox/flox animals displayed greatly reduced miR-143/145 expression in whole colon and undetectable expression in purified epithelium, supporting a mesenchymal-restricted expression domain.
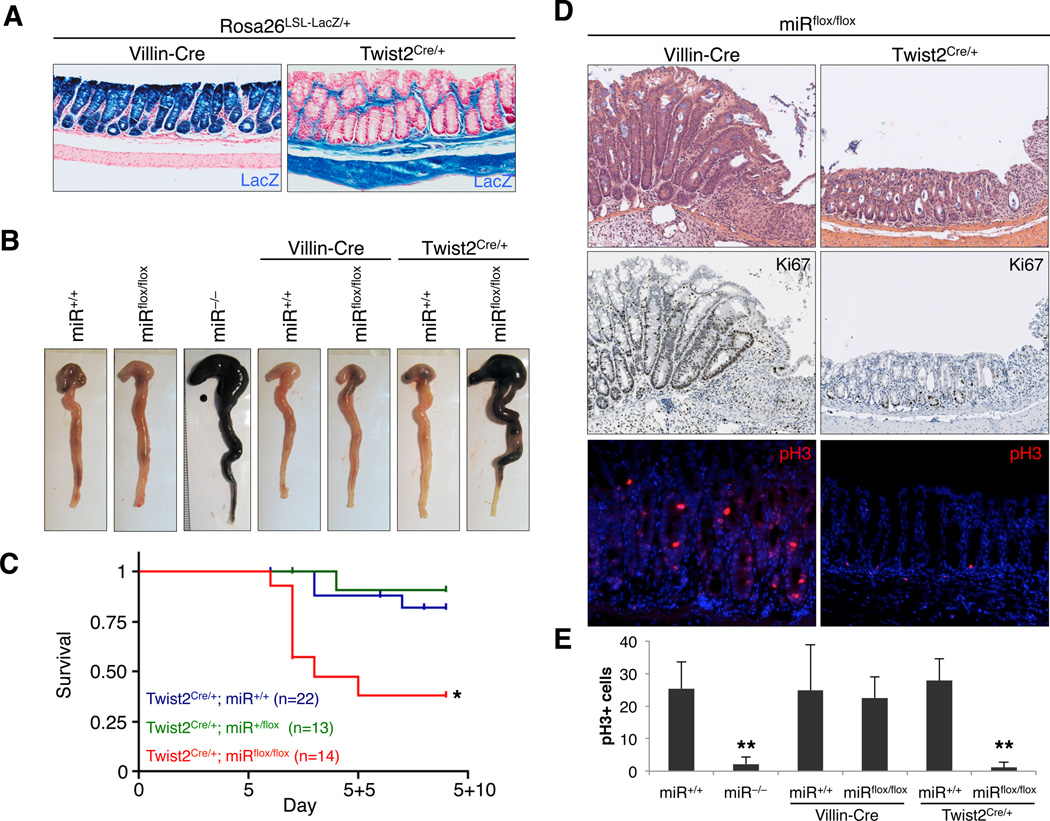
Figure 3. Mesenchymal, but not epithelial, miR-143/145 deletion phenocopies germline deletion.
(A) X-gal-stained colon sections demonstrating domains of Cre-mediated recombination in mice of the indicated genotypes. Blue, LacZ expression; red, nuclear fast red counterstain.
(B) Colons from mice of the indicated genotypes, dissected 2 days after completion of DSS treatment. Representative images from 5–10 examined mice per genotype.
(C) Kaplan-Meier survival curves of mice of indicated genotypes, administered 3.5% DSS in drinking water for 5 days. *, p=0.001, log rank test.
(D) Proliferation of ulcer-adjacent crypts assessed by H&E (upper panels), Ki67, and pH3 staining. Histology (H&E) was examined in 5–10 mice of each genotype while a subset of sections (n ≥ 3) were further stained for Ki67 and pH3.
(E) Quantification of the average number of pH3+ cells within 500 µm of deepthelialized zones. Multiple ulcer-adjacent areas in at least 3 mice per genotype were quantified. **, p<0.01 (Student’s t-test).
See also Figure S5.
We further considered the possibility that miR-143/145 might be expressed exclusively in rare intestinal stem cells (ISCs) and that analysis of bulk epithelial preparations might therefore be insufficiently sensitive to detect them. Fluorescence-activated cell sorting (FACS) was used to purify ISCs from Lgr5+/eGFP mice (Barker et al., 2007), revealing undetectable levels of miR-143/145 in this epithelial-derived population (Figure 2E). On the contrary, miR-143/145 were readily expressed in primary intestinal subepithelial myofibroblasts (ISEMFs) isolated from newborn mouse colon (Shaker et al., 2010). Collectively, the data from ISH, purified epithelial preparations, sorted ISCs, and cultured ISEMFs conclusively demonstrate that miR-143/145 expression is restricted to the intestinal mesenchyme in human and mouse.
miR-143/145 are not expressed in colorectal tumor epithelium
These data suggest that the apparent downregulation of miR-143/145 that has been repeatedly observed in colorectal cancer is the result of a sampling artifact due to depletion of mesenchymal cells in tumors relative to normal mucosal biopsies typically used as a basis of comparison. To examine this possibility, we obtained biopsies of human colorectal cancers and paired normal colon and measured miR-143/145 levels in the unfractionated tissue as well as in purified epithelium. As reported, miR-143/145 levels were significantly lower in whole tumors compared to whole colon samples (Figure 2D). miR-143/145 in purified epithelial cells from the same biopsies, however, were detectable only at background levels. Thus, the reduced levels of these miRNAs that is observed when comparing tumors to normal tissue is not due to their downregulation in tumor epithelial cells.
Lastly, we addressed the possibility that miR-143/145 are induced in intestinal epithelium as a protective mechanism against transformation following an initial oncogenic hit and then later silenced as tumorigenesis progresses. Among the earliest and most common oncogenic lesions in colorectal cancer is loss of the APC tumor suppressor gene and consequent constitutive activation of the Wnt/β-catenin pathway. Apcmin/+ mice harbor a heterozygous truncating mutation in Apc and, as a result, develop numerous intestinal adenomas due to bi-allelic Apc loss-of-function (Haigis et al., 2002; Lamlum et al., 2000). As observed in human tumors, whole Apcmin adenomas exhibit lower levels of miR-143/145 compared to wild-type intestine (Figure S4B). Importantly, levels of miR-143/145 remain at background levels in purified epithelial cells from these adenomas. Thus, while it remains formally possible that other oncogenic pathways activate expression of these miRNAs in epithelial cells, this is not a feature of the Wnt/β-catenin pathway that is hyperactive in the majority of human colorectal cancers.
Mesenchymal, but not epithelial, miR-143/145 deletion phenocopies germline deletion
Despite the extensive evidence that miR-143/145 expression is absent in the intestinal epithelial compartment, it remained formally possible that levels of these miRNAs below the limit of detection were sufficient to support a function within epithelial cells. To address this possibility, Villin-Cre and Twist2+/Cre animals were used to induce miR-143/145 loss-of-function specifically within epithelial or mesenchymal lineages, respectively. Following DSS injury, colons of Villin-Cre; miRflox/flox mice grossly resembled wild-type and Villin-Cre; miR+/+ colons (Figure 3B) and histologic evidence of robust epithelial regeneration was present including numerous elongated, hyperproliferative crypts flanking ulcerated areas (Figure 3D). Quantification of pH3+ cells in ulcer-adjacent zones demonstrated equivalent numbers in miR+/+, Villin-Cre; miR+/+, and Villin-Cre; miRflox/flox mice (Figure 3E). Thus, epithelial deletion of miR-143/145 is fully compatible with normal regeneration. Conversely, Twist2+/Cre; miRflox/flox, but not Twist2+/Cre;miR+/+, animals displayed hemorrhagic and necrotic colons upon gross inspection and exhibited elevated mortality after DSS treatment with kinetics similar to miR−/− mice (Figures 3B–3C). Hyperproliferative crypts were absent in Twist2+/Cre; miRflox/flox animals and pH3+ cells were restricted to the crypt base and reduced in number (Figure 3D–E). We conclude from these results that the functions of these miRNAs are exclusively performed within the mesenchymal compartment of the intestine.
Evidence for smooth muscle and myofibroblast dysfunction in miR-143/145−/− mice
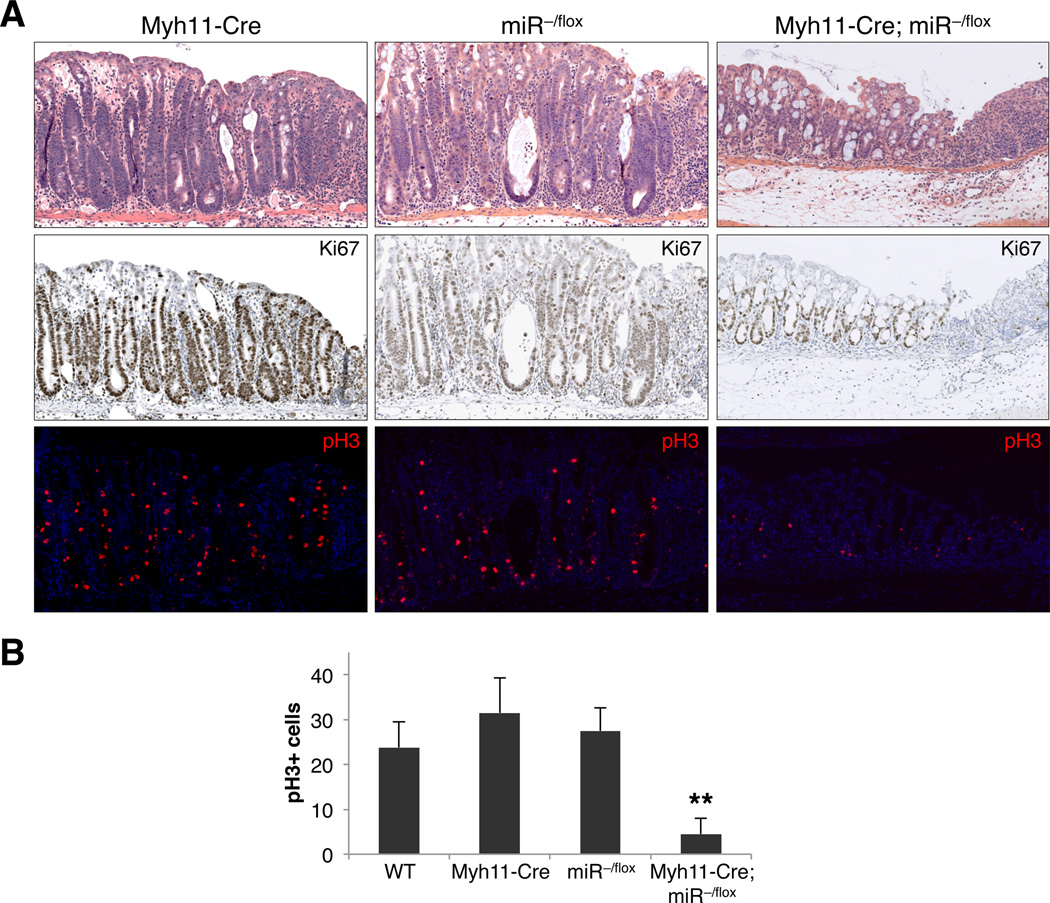
A role for smooth muscle in the regulation of intestinal epithelial regeneration has not previously been documented. Yet given the prominent smooth muscle expression of miR-143/145 as reported elsewhere (Boettger et al., 2009; Xin et al., 2009) and confirmed by our ISH studies (Figure 2A), we sought to determine whether dysfunction of this cell type underlies the regenerative failure in miR−/− mice. To this end, miRflox mice were crossed to transgenic Myh11-Cre/eGFP mice (Xin et al., 2002), which direct Cre and eGFP expression under the control of the murine smooth muscle myosin heavy chain (smMHC) promoter. In colon tissues from these mice, transgene expression was restricted to the muscularis propria, muscularis mucosae, and rare lamina propria cells (see Figure 5D).
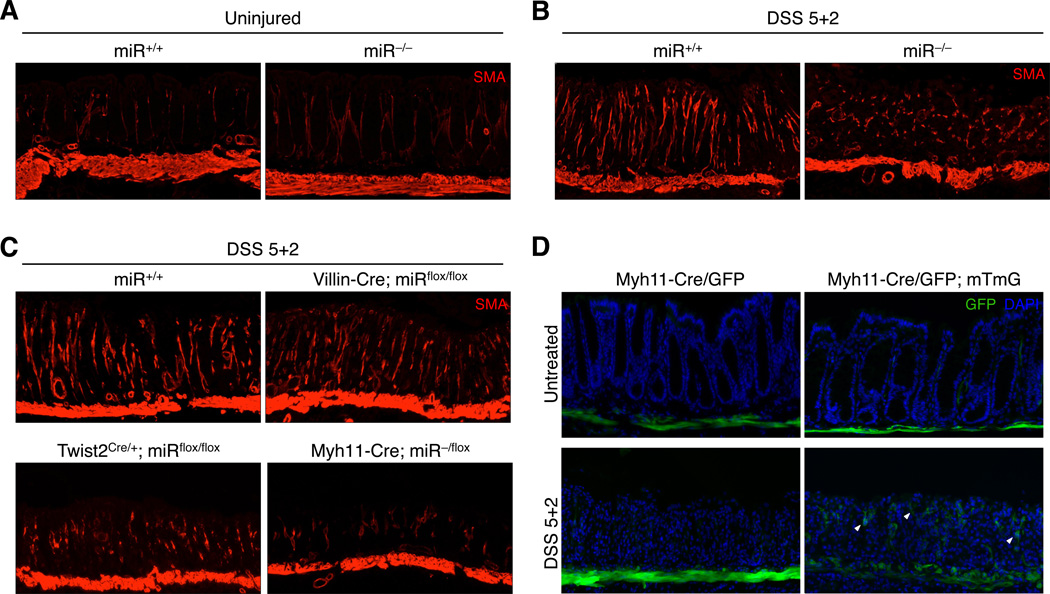
Figure 5. Disorganized myofibroblasts are associated with defective epithelial regeneration.
(A) SMA immunofluorescence showing muscularis mucosa and pericryptal myofibroblasts in uninjured miR+/+ and miR−/− colons. Images representative of ≥10 animals of each genotype.
(B–C) SMA immunofluorescence showing muscularis mucosa and pericryptal myofibroblasts in ulcerated regions of colon from mice of the indicated genotypes 2 days after completion of DSS treatment. Images representative of ≥10 miR+/+ and miR−/− animals and ≥3 animals of the other genotypes.
(D) GFP fluorescence showing labeled smooth muscle cells and their progeny in Myh11-Cre/eGFP; mTmG mice (right panels) before and after DSS injury, compared to Myh11-Cre/eGFP alone (left panels). White arrowheads show representative smooth muscle-derived cells that have migrated into the lamina propria. Images representative of 3 animals per genotype.
See also Figure S6.
Colons of compound Myh11-Cre/eGFP; miR−/flox mice were grossly abnormal after DSS treatment (Figure S6A), although the hemorrhage and necrosis did not reach the full extent characteristic of miR−/− and Twist2+/Cre; miRflox/flox colons after injury. Histological analysis of Myh11-Cre/eGFP; miR−/flox intestines confirmed significantly impaired epithelial regeneration compared to Myh11-Cre/eGFP; miR+/+ and miR−/flox mice, as measured by H&E staining, Ki67 expression, and enumeration of pH3+ cells in ulcer-adjacent zones (Figures 4A–4B). These results demonstrate that dysfunction of intestinal smooth muscle measurably contributes to the defective regenerative response of miR−/−mice, while the reduced severity of disease in Myh11-Cre/eGFP; miR−/flox compared to miR−/− and Twist2+/Cre; miRflox/flox animals suggests a possible contribution of additional non-smooth muscle lineages in the mesenchyme.
Figure 4. Defective epithelial regeneration of Myh11-Cre/eGFP; miR−/flox mice.
(A) Proliferation of ulcer-adjacent crypts in mice of the indicated genotypes assessed by H&E (upper panels), Ki67, and pH3 staining. Histology (H&E) was examined in 8–24 mice of each genotype while a subset of sections (n ≥ 3) were further stained for Ki67 and pH3.
(B) Quantification of the average number of pH3+ cells within 500 µm of deepthelialized zones. Multiple ulcer-adjacent areas in at least 3 mice per genotype were quantified. **, p<0.01 (Student’s t-test).
See also Figure S6.
In addition to smooth muscle, ISH documented strong expression of miR-143/145 in pericryptal cells of the lamina propria, located in a position consistent with intestinal subepithelial myofibroblasts (ISEMFs) (Figure 2B). Accordingly, we observed robust miR-143/145 expression in cultured primary ISEMFs (Figure 2E). Given that myofibroblasts are known to act as major regulators of epithelial cell proliferation in normal physiology and during wound repair (Powell et al., 2011), we hypothesized that dysfunction of this cell type contributed to regenerative failure in miR−/− mice. The morphology of lamina propria myofibroblasts was identical in miR+/+ and miR−/− colons prior to DSS treatment, as documented by SMA staining (Figure 5A). After injury, myofibroblasts in wild-type colons upregulated SMA and underwent a dramatic reorganization within ulcerated zones, adopting an elongated configuration with highly parallel basal-luminal orientation (Figure 5B). Strikingly, myofibroblasts within ulcers of miR−/− mice were highly disorganized and failed to elongate along the basal-luminal axis. Identical morphologic abnormalities were present in Twist2+/Cre; miRflox/flox colons, while myofibroblasts in Villin-Cre; miRflox/flox mice resembled those in wild-type (Figure 5C). To our surprise, we also observed morphologically abnormal myofibroblasts in the ulcerated regions of Myh11-Cre/eGFP; miR−/flox colons, despite restriction of Cre expression to smooth muscle. Importantly, the presence of the Villin-Cre, Twist2Cre, and Myh11-Cre/GFP alleles alone did not result in myofibroblast disorganization after injury (Figure S6B). Thus, in every assayed genotype, regenerative failure was associated with abnormal myofibroblast morphology, suggesting a role for this cell type in the miR−/− DSS phenotype.
Smooth muscle gives rise to a population of lamina propria myofibroblasts following colonic epithelial injury
The precise origin of the organized myofibroblasts in ulcers of wild-type mice after DSS injury is unclear, as they have not been reported previously. In other settings, myofibroblasts have been reported to arise from pre-existing myofibroblasts, activated fibroblasts, smooth muscle cells, and even bone marrow-derived cells (Powell et al., 2011). Given the surprising finding that smooth muscle ablation of miR-143/145 led to abnormal myofibroblasts in the lamina propria, we tested whether myofibroblasts in DSS ulcers are derived from smooth muscle. Myh11-Cre/eGFP; Rosa26mTmG/+ mice (Muzumdar et al., 2007) were generated in order to label smooth muscle cells and their descendants with permanent GFP expression, even after inactivation of the Myh11 promoter. Indeed, we found a significant number of smooth muscle-derived lamina propria cells present within DSS-induced ulcers (Figure 5D). All the GFP+ cells co-expressed SMA (Figure S6C), consistent with a myofibroblast identity. Interestingly, a subset of myofibroblasts did not express GFP, suggesting that these cells arise from preexisting myofibroblasts or other non-smooth muscle lineages. Taken together, these results support a model whereby smooth muscle and other mesenchymal lineages contribute to the myofibroblast pool, which in turn regulates epithelial regeneration following injury.
Derepression of Igfbp5 in miR-143/145−/− myofibroblasts correlates with intestinal regenerative failure
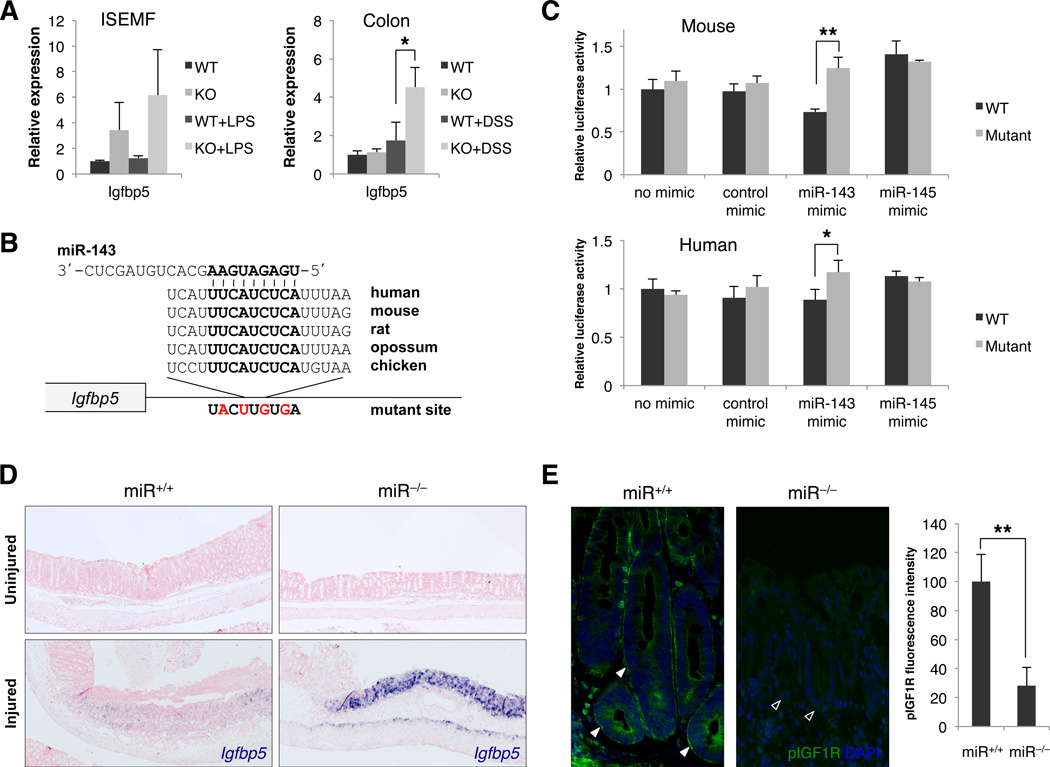
The abnormal morphology of lamina propria myofibroblasts after deletion of miR-143/145 suggests that dysfunction of this cell type contributes to the observed regenerative failure. We hypothesized that dysregulation of one or more critical paracrine signaling molecules produced by these cells contributes to the DSS phenotype. To identify such factors, we performed global mRNA expression profiling of miR+/+ and miR−/− primary ISEMFs under standard culture conditions and, to model inflammatory stress, after exposure to bacterial lipopolysaccharide (LPS). Among transcripts upregulated by 2-fold or more in miR−/− cells in at least one of the assayed conditions was Insulin-like growth factor binding protein 5 (Igfbp5) (Table S1), which is predicted to be a target of miR-143 by the Targetscan algorithm (Friedman et al., 2009). IGFBP5 is a secreted negative regulator of IGF signaling that binds and sequesters IGF ligands (Pollak, 2008). IGF signaling, in turn, is known to stimulate intestinal epithelial proliferation and promotes recovery after DSS treatment (Howarth et al., 1998). qRT-PCR validated the upregulation of Igfbp5 in miR−/− ISEMFs and, notably, in whole colons exclusively after DSS injury (Figure 6A). Although no other predicted miR-143/145 targets were upregulated >2-fold in miR−/− ISEMFs, microarray analysis did reveal upregulation of Bone morphogenic protein 4 (Bmp4), a known inhibitor of intestinal epithelial proliferation (He et al., 2004), and Insulin-like growth factor binding protein 4 (Igfbp4), a negative regulator of both the IGF and Wnt pathways (Zhu et al., 2008) (Table S1). While qRT-PCR validated the upregulation of these transcripts in ISEMFs (Figure S7A), they were not differentially expressed in healthy or DSS-injured miR+/+ and miR−/− colons (Figure S7B) and therefore were not studied further.
Figure 6. De-repression of Igfbp5 in miR-143/145−/− myofibroblasts in vitro and in vivo.
(A) Quantitative RT-PCR analysis of Igfbp5 expression in cultured ISEMFs with or without LPS treatment and in whole mouse colons with or without DSS administration. n= 3 independently-derived WT or KO ISEMF cell lines or animals per condition. *, p<0.05 (Student’s t-test).
(B) Sequence and evolutionary conservation of the miR-143 binding site in the 3’ UTR of Igfbp5. Mutations introduced into luciferase reporters (panel C) are indicated in red.
(C) Relative firefly luciferase activity of reporter constructs containing the miR-143 binding site or its mutated version following transfection into 293T cells alone or with control or miR-143 or miR-145 synthetic miRNA mimics. n=3 replicates per condition. *, p<0.05; **, p<0.01 (Student’s t-test).
(D) In situ hybridization analysis of Igfbp5 expression in DSS-treated miR+/+ and miR−/− colon sections. Upper panels, uninjured regions; lower panels, ulcerated regions with residual crypts on the left. n=7–8 mice per genotype examined. Blue, Igfbp5; red, nuclear fast red counterstain.
(E) Phospho-Igf1r (pIGF1R) immunofluorescence of ulcer-adjacent crypts in DSS-treated miR+/+ and miR−/− colons. Quantification of membrane fluorescence from n=4 mice of each genotype is shown in graph on right. Arrowheads, baso-lateral membranes of ulcer-adjacent crypts with (solid) or without (open) activated Igf1r. **, p<0.01 (Student’s t-test).
See also Figure S7.
To further investigate the role of Igfbp5 in the regenerative failure characteristic of miR−/− mice, we next validated the direct regulation of this transcript by miR-143. Reporter assays, in which the highly conserved predicted miR-143 binding sites from the human and mouse 3′ UTRs of Igfbp5 (Figure 6B) were cloned downstream of luciferase, demonstrated that these sequences are sufficient to confer direct repression by this miRNA (Figure 6C). Colonic Igfbp5 expression was then directly assessed by ISH before and after DSS injury. Irrespective of genotype, Igfbp5 was minimally expressed under basal conditions and in histologically uninjured regions of colon after DSS treatment (Figure 6D, upper panels). In injured wild-type colons, ISH revealed detectable but modest induction of Igfbp5 in the lamina propria, muscularis mucosae and muscularis propria specifically in ulcerated regions of the colon. In miR−/− animals, however, Igfbp5 was dramatically upregulated within these domains, consistent with the qRT-PCR data and confirming robust de-repression of this target in the absence of miR-143/145 in vivo. The high concentration of IGFBP5 in ulcerated regions of miR−/− colons would be predicted to compete strongly for soluble IGF ligand. Indeed, we observed dramatically reduced IGF1 receptor (Igf1r) activation on the baso-lateral membranes of ulcer-adjacent crypts in miR−/− mice, as revealed by staining for the phosphorylated receptor (Figure 6E). miR-143/145 deletion therefore directly de-repressed Igfbp5 expression in vitro and in vivo, resulting in impaired IGF signaling which likely contributed to regenerative failure after DSS-mediated injury.
Discussion
Our in vivo analyses of miR-143/145 functions in intestinal biology have yielded several surprising findings. First, loss of miR-143/145 causes a profound defect in intestinal epithelial regeneration. Second, lineage-specific deletion establishes that this phenotype is entirely attributable to mesenchymal, rather than epithelial, loss of function. Third, miR-143/145 are expressed solely in the mesenchymal cells of the intestine and, therefore, are unlikely to execute cell-autonomous epithelial functions as previously proposed. Last, we present evidence that the observed phenotype is associated with myofibroblast-dependent paracrine inhibition of the IGF signaling pathway. Together, these data establish a novel role for miR-143/145 in intestinal homeostasis and, for the first time, implicate smooth muscle-derived cells as critical regulators of intestinal regeneration in the mouse.
These results have clear implications for the functions of miR-143/145 in intestinal physiology and disease. These miRNAs, owing to their mesenchymal-restricted expression domain, clearly do not function as cell autonomous regulators of epithelial proliferation nor are they likely to function as cell autonomous tumor suppressors in intestinal cancers. Importantly, despite recent reports from the study of other tissues (Hergenreider et al., 2012), we find no evidence of transfer of mature miR-143 or miR-145 from mesenchymal cells to epithelial cells, as this scenario would result in detectable levels of the mature miRNAs in recipient cells. Moreover, although downregulation of miR-143/145 in colorectal tumors was one of the earliest, and since most reproduced, observations of abnormal miRNA expression in cancer, our data argue that these findings were confounded by sampling bias during tissue isolation. Since tumor samples are depleted in smooth muscle and mesenchyme compared to mucosal biopsies, tumor specimens could artifactually appear to have downregulated miR-143/145 levels when compared to normal tissue. Indeed, by purifying epithelial cells from a set of tumors that appeared to exhibit miR-143/145 downregulation, we showed that the miRNAs are not expressed in the normal or tumor epithelial compartment and thus are not downregulated in the cancer cells themselves. We further demonstrated that an early, common oncogenic lesion in colorectal cancer, loss of the APC gene, does not result in an induction of miR-143/145 in epithelial cells. Although it remains formally possible that other oncogenic pathways result in de novo activation of miR-143/145 expression in tumor cells, this scenario is not supported by existing data. Of note, our data do not contradict the potent anti-tumor activities that these miRNAs have been shown to exhibit upon enforced ectopic expression in epithelial cancer cells. Indeed, the expression of developmental lineage “inappropriate” miRNAs might represent a therapeutic avenue deserving of further study.
In addition to their potential role as tumor suppressors, miR-143/145 have been extensively studied in the context of vascular smooth muscle biology. A large number of reports have documented abundant expression of miR-143/145 in vascular smooth muscle cells (VSMCs) and have demonstrated a functional role for these miRNAs in the phenotypic responses of VSMCs to blood vessel injury (Boettger et al., 2009; Cheng et al., 2009; Cordes et al., 2009; Xin et al., 2009). In the setting of vascular injury, smooth muscle cells undergo phenotypic switching from a contractile to a migratory and secretory state, after which they contribute to neointima formation. Our analyses of myofibroblast origin and function after intestinal injury have revealed interesting parallels to the vasculature. We have shown for the first time that during wound healing, intestinal smooth muscle gives rise to a population of myofibroblasts that migrate into the lamina propria where they may be optimally positioned to signal to regenerating epithelial cells. Indeed, the muscularis mucosa, a classic histologic structure which has been historically assumed to exclusively provide contractile functions during digestion such as villus movement and micro-peristalsis, is ideally located to provide a reservoir of myofibroblasts available for mobilization after injury and may itself act as a source of paracrine signals. Once activated, myofibroblasts, and possibly smooth muscle cells within the muscularis mucosa, secrete an array of factors that promote reepithelialization including Wnt ligands, prostaglandin E2 (PGE2), and cytokines such as IL-6 (Andoh et al., 2007; Powell et al., 2011).
A role for miR-143/145 in the regulation of smooth muscle and myofibroblast-derived paracrine signaling has not previously been demonstrated in mammals. A related function, however, has been proposed for miR-145 in the zebrafish gut where this miRNA indirectly regulates intestinal smooth muscle secretion of BMP4 and is therefore essential for normal epithelial morphogenesis during embryonic development (Zeng and Childs, 2012). This function is clearly divergent from functions of miR-143/145 in mouse where, as shown here, deletion of these miRNAs is compatible with normal intestinal development and does not affect BMP4 expression (Figure S7B) nor BMP signaling at baseline or after injury, as assessed by pSMAD1/5/8 staining of intestinal epithelium (data not shown). Thus, in different vertebrate species, miR-143/145 appear to regulate distinct aspects of smooth muscle physiology.
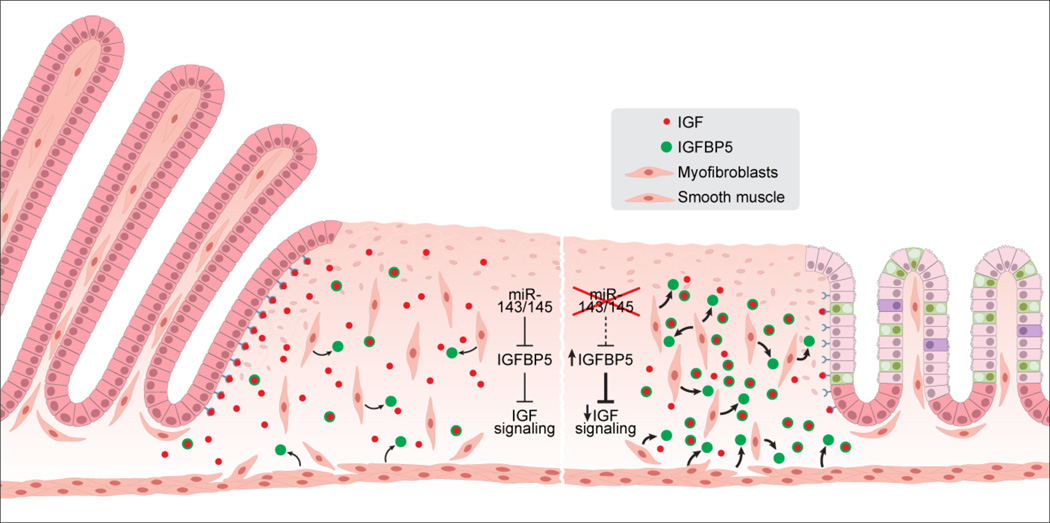
We identified and validated one likely contributor to the intestinal regenerative defect of miR-143/145-deficient mice in the IGF binding protein IGFBP5. IGF signaling is recognized as a potent stimulant for intestinal growth and repair in a variety of settings, including glucocorticoid treatment, intestinal resection, radiation injury, and DSS colitis (Howarth et al., 1998). IGF binding proteins (IGFBPs) typically function to competitively inhibit IGF signaling and, accordingly, decreased phosphorylation of epithelial Igf1r was observed in miR-143/145−/− mice. These findings suggest a model in which Igfbp5 de-repression in smooth muscle and myofibroblasts within zones of intestinal injury results in a net decrease in regeneration-promoting IGF signaling (Figure 7). Nevertheless, future studies will be necessary to determine the consequences of loss-of-function of Igfbp5 in the context of miR-143/145 deletion to definitively establish the necessity of the IGFBP5-IGF axis in this phenotype.
Figure 7. Model of miR-143/145 action in intestinal epithelial regeneration.
Upon intestinal injury, lamina propria myofibroblasts derived from multiple sources including smooth muscle participate in the wound healing response. In the absence of miR-143/145 (right side of figure), excessive secretion of IGFBP5 by myofibroblasts results in sequestration of IGF ligand and diminished IGF pathway activation within epithelial cells, likely contributing to the failure of epithelial cells to activate the regenerative proliferation program.
Given the diversity of pathways that participate in intestinal epithelial regeneration, Igfbp5 de-repression is likely only one component of the perturbed signaling milieu generated by miR-143/145 deletion. In addition, it is possible that the morphologic abnormalities of miR-143/145−/− myofibroblasts directly contribute to a signaling deficiency. Dynamic regulation of stromal cell positioning in the lamina propria after DSS-mediated injury has been shown to stimulate regeneration via more efficient delivery of paracrine secreted factors to epithelial cells (Brown et al., 2007). It is therefore plausible that the myofibroblast disorganization characteristic of knockout animals reduces effective communication with regenerating cells. Taken together, our observations support a multi-faceted contribution of structural and functional abnormalities of smooth muscle and myofibroblasts to regenerative failure in miR-143/145 deficient mice. Elucidation of the full complement of targets of these miRNAs that are responsible for these effects will provide further insight into the control of mammalian epithelial repair by the mesenchyme and uncover novel therapeutic strategies for human diseases related to epithelial proliferative dysfunction.
Experimental Procedures
Mouse strains
miR-143/145Neo/+ mice were generated using homologous recombination in ES cells in the Johns Hopkins Transgenic Core and subsequently crossed to CMV-Cre and Actb-Flp mice (Jackson Laboratory) to generate miR+/− and miRflox/+ mice, respectively. miR+/− and miRflox/+ mice were backcrossed to C57BL6/J for over 10 generations prior to performing the experiments described here. All mouse experiments were approved by Institutional Animal Care and Use Committees of the Johns Hopkins University School of Medicine and The University of Texas Southwestern Medical Center.
DSS treatment
Mice were treated with 3.5% w/v dextran sulfate sodium (DSS) of molecular weight 36,000 – 50,000 (MP Biochemicals) in drinking water for 5 days. Solutions of DSS were prepared in autoclaved tap water and passed through 0.45 µm cellulose acetate filters.
Histology, immunofluorescence, and immunohistochemistry
Intestinal segments were dissected, flushed with ice-cold saline, and opened longitudinally. Samples were fixed for 24 hours in 10% neutral buffered formalin and wrapped into “Swiss rolls” before standard tissue processing and sectioning. Routine H&E staining was performed for general histology. Immunostaining was performed as previously described (Shi et al., 2009).
Isolation of intestinal crypts
Mouse and human crypts were isolated as previously described (Sato et al., 2009) with minor modifications (see Supplemental Information).
Isolation and culture of ISEMFs
ISEMFs were isolated as previously described (Shaker et al., 2010) and maintained in high glucose DMEM media supplemented with 10% fetal bovine serum (Invitrogen), 1× ITS supplement (Sigma), and 1× Antibiotic/Antimycotic (Invitrogen). Treatment with 1 µg/mL LPS (Sigma) for 24 hours was used for microarray and qRT-PCR experiments.
In situ hybridization
In situ hybridization detection of miRNAs was performed using the miRCURY LNA miRNA ISH Kit (Exiqon), with slight modifications to the manufacturer’s protocol (see Supplemental Information). Igfbp5 ISH was performed as previously described (Acharya et al., 2012). The template for in vitro transcription of the ISH probe directed against the Igfbp5 3’UTR was generated by PCR with primers specified in Table S2.
Statistical analysis
Two-tailed Student’s t tests were performed to calculate p values, unless specified otherwise. Error bars represent standard deviations.
Supplementary Material
Highlights.
miR-143/145 are essential for mouse intestinal epithelial regeneration after injury
miR-143/145 expression and function is restricted to the intestinal mesenchyme
Upregulated IGFBP5 and reduced IGF signaling correlate with regenerative failure
Results challenge a cell-autonomous tumor suppressor role for miR-143/145 in colon
Acknowledgements
We thank Chip Hawkins in the Johns Hopkins Transgenic Core for assistance with generation of miR-143/145Neo/+ mice, John Shelton in the UT Southwestern Molecular Pathology Core for assistance with histopathology, Cheryl Lewis in the UT Southwestern Tissue Resource Core for assistance with human tissue specimens, Jose Cabrera for assistance with figures, and E. Olson and K. O’Donnell for helpful suggestions on the manuscript. This work was supported by grants from CPRIT (R1008 to JTM) and the NIH (R01CA120185 to JTM, P01CA134292 to JTM and AM, 5P30CA142543 to the UTSW Simmons Cancer Center, and 5T32GM007309 to RRC, LRZ, and EM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Number
Microarray data were deposited in the GEO repository under accession number GSE55651.
Author Contributions
RRC, GS, AM and JTM designed experiments. RRC, GS, AA, EM and LRZ performed experiments. JLA, AAA, GB, JCM and ACY collected human colon cancer specimens. RRC, GS, AM and JTM interpreted results. RRC, GS and JTM wrote the manuscript.
References
- Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacology & Therapeutics. 2007;114:94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzo M, et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Molecular Cancer. 2006;5 doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Boettger T, Beetz N, Kostin S, Schneider J, Kr xFc, ger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. The Journal of Clinical Investigation. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. The Journal of Clinical Investigation. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, Cai X, Wang K, Wang G, Ba Y, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Liu X, Yang J, Lin Y, Xu D-Z, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a Novel Smooth Muscle Cell Phenotypic Marker and Modulator, Controls Vascular Neointimal Lesion Formation. Circulation Research. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapé C, Fritz V, Henriquet C, Apparailly F, Fernandez PL, Iborra F, Avancès C, Villalba M, Culine S, Fajas L. miR-143 Interferes with ERK5 Signaling, and Abrogates Prostate Cancer Progression in Mice. PLoS ONE. 2009;4:e7542. doi: 10.1371/journal.pone.0007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper ML, Cooper HS, Chang W-CL. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee T-H, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geske MJ, Zhang X, Patel KK, Ornitz DM, Stappenbeck TS. Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development. 2008;135:2959–2968. doi: 10.1242/dev.020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis KM, Caya JG, Reichelderfer M, Dove WF. Intestinal adenomas can develop with a stable karyotype and stable microsatellites. Proceedings of the National Academy of Sciences. 2002;99:8927–8931. doi: 10.1073/pnas.132275099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong W-G, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-[beta]-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- Howarth GS, Xian CJ, Read LC. Insulin-like Growth Factor-I Partially Attenuates Colonic Damage in Rats with Experimental Colitis Induced by Oral Dextran Sulphate Sodium. Scandinavian Journal of Gastroenterology. 1998;33:180–190. doi: 10.1080/00365529850166923. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Research. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes & Development. 2010;24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. The Lancet Oncology. 2012;13:e249–e258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- Lamlum H, Papadopoulou A, Ilyas M, Rowan A, Gillet C, Hanby A, Talbot I, Bodmer W, Tomlinson I. APC mutations are sufficient for the growth of early colorectal adenomas. Proceedings of the National Academy of Sciences. 2000;97:2225–2228. doi: 10.1073/pnas.040564697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Leung AKL, Sharp PA. MicroRNA Functions in Stress Responses. Molecular cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. cis Elements of the Villin Gene Control Expression in Restricted Domains of the Vertical (Crypt) and Horizontal (Duodenum, Cecum) Axes of the Intestine. Journal of Biological Chemistry. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- Mendell Joshua T, Olson Eric N. MicroRNAs in Stress Signaling and Human Disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MZ, O' Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced Accumulation of Specific MicroRNAs in Colorectal Neoplasia11Note: Susan M. O' Connor and Nicholas G. van Holst Pellekaan contributed equally to this work. Molecular Cancer Research. 2003;1:882–891. [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Abbott AL, Lau NC, Hellman AB, McGonagle SM, Bartel DP, Ambros VR, Horvitz HR. Most Caenorhabditis elegans microRNAs Are Individually Not Essential for Development or Viability. PLoS Genet. 2007;3:e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama K, Inoue H, Takatsuno Y, Tanaka F, Mimori K, Uetake H, Sugihara K, Mori M. Over- and under-expressed microRNAs in human colorectal cancer. International Journal of Oncology. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Otte J-M, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou IG, Manta A, Gazouli M, Lyberopoulou A, Lykoudis PM, Polymeneas G, Voros D. Expression of MicroRNAs in Patients With Pancreatic Cancer and Its Prognostic Significance. Pancreas. 2013;42:67–71. doi: 10.1097/MPA.0b013e3182592ba7. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal Cells of the Intestinal Lamina Propria. Annual Review of Physiology. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo Y-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt– villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjøt L, Wiuf C, Sørensen FJ, Kruhøffer M, Laurberg S, et al. Diagnostic and Prognostic MicroRNAs in Stage II Colon Cancer. Cancer Research. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. The Journal of Clinical Investigation. 2010;120:2081–2093. doi: 10.1172/JCI40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Roles of Myofibroblasts in Prostaglandin E2–Stimulated Intestinal Epithelial Proliferation and Angiogenesis. Cancer Research. 2006;66:846–855. doi: 10.1158/0008-5472.CAN-05-2606. [DOI] [PubMed] [Google Scholar]
- Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the Acinar-Restricted Transcription Factor Mist1 Accelerates Kras-Induced Pancreatic Intraepithelial Neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered Expression of miR-21, miR-31, miR-143 and miR-145 Is Related to Clinicopathologic Features of Colorectal Cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist Regulates Cytokine Gene Expression through a Negative Feedback Loop that Represses NF-[kappa]B Activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased Expression of MicroRNA-143 and-145 in Human Gastric Cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion Between Intestinal Stem Cell Populations in Distinct Niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of Stress-Dependent Cardiac Growth and Gene Expression by a MicroRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Xin H-B, Deng K-Y, Rishniw M, Ji G, Kotlikoff MI. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiological Genomics. 2002;10:211–215. doi: 10.1152/physiolgenomics.00054.2002. [DOI] [PubMed] [Google Scholar]
- Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes & Development. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proceedings of the National Academy of Sciences. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zeng L, Childs SJ. The smooth muscle microRNA miR-145 regulates gut epithelial development via a paracrine mechanism. Developmental Biology. 2012;367:178–186. doi: 10.1016/j.ydbio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Zhu H, Dougherty U, Robinson V, Mustafi R, Pekow J, Kupfer S, Li Y-C, Hart J, Goss K, Fichera A, et al. EGFR Signals Downregulate Tumor Suppressors miR-143 and miR-145 in Western Diet–Promoted Murine Colon Cancer: Role of G1 Regulators. Molecular Cancer Research. 2011;9:960–975. doi: 10.1158/1541-7786.MCR-10-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J-i, Ueno H, Umezawa A, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.