Abstract
Paclitaxel is widely used to treat cancer patients through the blocking of mitosis and result in formation of polyploidy giant cancer cells (PGCCs), which are generally believed to be non-dividing cells or in mitotic catastrophe. Here we showed that PGCCs following the treatment of paclitaxel of MCF-7 breast cancer cell line have capability to generate regular-sized progeny cells through budding. The PGCCs not only grew into well differentiated cancer cells that formed cancer organotypic structures in vitro but also trans-differentiated into multiple tumor stromal cells including myoepithelial, endothelial, and erythroid cells. PGCCs formed glandular and vessel-like cancer organotypic structures that expressed normal stem cell markers. These progeny cells generated from PGCCs showed decreased ability of proliferation, invasion and tumor growth and became more resistant to paclitaxel than parental MCF-7 cells. These results demonstrated that paclitaxel-induced PGCCs have properties of cancer stem cells that can generate both epithelial cancer cells and multi-lineage of stromal cells. PGCCs are not only the morphogenic determinant to tumor histogenesis and but also contribute to paclitaxel resistance.
Keywords: Polyploidy giant cancer cells, Paclitaxel, Cancer organotypic structure
Introduction
Human cancer is composed of epithelial cancer cells and multiple lineages of benign cells including fibroblasts, endothelium, myoepithelial cells, inflammatory cells, and red blood cells together formed stroma. These stromal cells play an important role in tumor growth, invasion and metastasis, which can supply tumor cells with nutrients and oxygen for promoting the cancer growth 1-3 although the origin of each cell type is not entirely clear 4. It has been reported that at least some stromal cells can originate from epithelial cancer cells 5, mutation in p53 has been identified in stromal fibroblasts 6-7. Cancer stem cells are known to be capable of generating multiple lineages of normal and neoplastic cells 8-9, however, it is not clear whether established cancer cell lines are capable of generating multiple benign lineages of stromal cells.
Paclitaxel is a mitotic inhibitor and is widely used to treat cancer patients by inhibiting cell mitosis and inducing cell apoptosis. Paclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division 10. Paclitaxel stabilizes the microtubule polymer, protects it from disassembly 11,12 and blocks progression of mitosis 11, which result in the formation of polyploidy giant cancer cells (PGCCs). We recently demonstrated that paclitaxel can induce ovarian cancer epithelial cells to benign fibroblasts through formation of PGCCs 13. We further show that PGCCs can generate stem cell-like cells and contribute the cisplatin resistance 14 and generate erythroid cells following the treatment of hypoxia mimick CoCl2 15. These results demonstrated PGCCs may present the cellular basis for the generation of stem cell-like cells and may contribute the drug resistance.
Here, we report that PGCCs induced by paclitaxel from an established invasive breast ductal carcinoma cell line MCF-7 16 generated multiple lineages of tumor stromal and differentiated cancer cells and formed cancer organotypic structure (COS) in vitro. Our data suggest that an established cancer cell line has all necessary information to establish major key cellular components identified in human cancer, PGCCs may represent a morphogenic factor of tumor histogenesis and thus constitute the cellular basis for the resistance to paclitaxel.
Materials and Methods
Cell Culture
MCF-7 was obtained from the American Tissue Culture Collection and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 2 m mol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Observation of Cell Morphology
When MCF-7 cells reached 90% confluency, they were exposed to 1 μM paclitaxel for 2 days, during which 40%-60% of the cells were killed. Next, the medium was replaced with one lacking paclitaxel. The surviving cells were continuously cultured in normal media without paclitaxel for more than 4 months.
Paraffin Embedding of Spheroids
Spheroids from the medium were obtained by centrifugation at 100 × g. The supernatant was removed and 70% ethanol added to fix the spheroids, which were subsequently stained with eosin. The samples were dehydrated and then the vials were infiltrated with acetone, absolute xylene and purified paraffin at 65°C for 15 min each. All of these steps were performed in the 1.5-mL vial. Finally, the spheroids were then embedded in paraffin and sectioned.
H&E Staining
Four-micrometer sections from formalin-fixed, paraffin-embedded spheroid tissue were deparaffinized, rehydrated, and then counterstained with hematoxylin for 1 min and eosin for 2 min. The sections were then dehydrated and mounted with coverslips.
Hoechst33342 Staining
After being exposed to paclitaxel, the MCF-7 cells were cultured in normal DMEM and generated daughter cells by budding. Hoechst 33342 (H342; B2261; Sigma) was added to the flask at a final concentration of 5 μg/mL. Next, the flask was rinsed with PBS twice to remove the H342 dye, wrapped with foil, and cultured with normal DMEM. Budding division patterns and DNA distribution in the cells were observed and photographed 8h later.
Immunohistochemical staining (IHC) Staining
IHC staining was performed with use of the avidin-biotin-peroxidase method as described previously 17, 18. The sections were washed with 1 × PBS and then subjected to antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) in an autoclave for 10 min. The sections were incubated with primary antibodies overnight at 4°C in a humidity chamber (for detailed antibody information, see Table S1). The signal was detected with the labeled streptavidin-biotin system in the presence of the chromogen 3,3′-diaminobenzidine or alkaline phosphatase.
In vitro Cell Proliferation Assays
Serial dilutions of cells in culture medium were prepared, and 100 μL of the dilutions (containing 5 × 103, 1 × 104, and 5 × 104 per 100 μL) was added into triplicate wells of a 96-well microtiter tissue culture plate; all cell dilutions were repeated three times. Cells were incubated for 12 h, and then 10 μL of 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) reagent was added to each well. Three control wells were incubated with only medium as a blank. When a purple precipitate was clearly visible in the cells, 100 μL of reagent with detergent was added. Three hours later, the absorbance in each well was measured at 570 nm with use of a plate reader (uQuant, BioTek Instruments, Inc.). The values from triplicate readings were averaged, and the average value for the control wells was subtracted.
Wound-Scratch Assays
Control MCF-7 and paclitaxel-treated MCF-7 cells (1 × 105) were plated in dishes for 12 h. Confluent cells were uniformly scratched by using sterile pipette tips. Then, the medium was replaced with fresh EMEM without fetal bovine serum. The cells were photographed with use of a microscope (Nikon) and counted in several pre-marked areas at 0 h, 6 h, 24 h, and 48 h.
Western Blot Analysis
Western blot analyses were done as described previously 18, 19. Cell extracts of control MCF-7 and MCF-7 after paclitaxel treatment were lysed in ice-cold buffer. The proteins separated on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (PVDF Membrane; GE Healthcare). Afterward, the membranes were blocked with 5% nonfat milk in 1 × tris buffered saline with 0.1% Tween-20 (TBST) for 1 h at room temperature, and incubated with the appropriate
primary antibody overnight at 4°C and then with the appropriate secondary antibody for 1 h at room temperature. Expression of proteins were measured by using mixed ECL Plus reagents (RPN2132OL/AK, GE Life Sciences Co.) and developed by using an X-OMAT 2000 film processor (Kodak). β-actin was used as a protein-loading control.
Paclitaxel Resistance of Progeny Cells from PGCCs or COSs
Medium with PGCCs or COSs was centrifuged, and the pellets were resuspended in complete DMEM without paclitaxel in a new flask. Some COSs reattached to the flask wall. When the confluency of the reattached cells reached 90%, 1 μM paclitaxel was added to the medium, which was allowed to stand for 2 d, and then the cells were cultured with complete DMEM without paclitaxel to compare the period of recovery following paclitaxel treatment for first time and second time. The PGGC is defined as the increased size of nucleus that is at least three times of the regular cells or cells with minimum of three nuclei. To quantify the number of PGCCs of MCF-7 before and after paclitaxel treatment, an equal number control MCF-7 and reattached cells (1 × 105 cells) were cultured in 75 mm2 flask and then stained with Hoechst 33342. 10 fields (10 ×) were randomly chosen and the number of PGCCs was scored. The average number of PGCCs in total 10 fields was used for analysis.
Tumorigenesis in Nude Mice
To determine the ability of the cells to form tumors, we administered bilateral injections of control MCF-7 and paclitaxel-treated MCF-7 cells subcutaneously into 6-week-old female athymic nude mice (National Cancer Institute). Each subcutaneous injection consisted of 1 × 106 control MCF-7 cells and paclitaxel-treated MCF-7 cells together with the mixture of 0.1 ml of PBS buffer and 0.1 ml of Matrigel. The mice were kept in a specific pathogen-free environment and checked for tumor development for 2-5 months. The mice were then euthanized by CO2 inhalation. The tumors were excised, fixed in 10% formalin overnight, and subjected to routine histological H&E staining. All mouse experiments were performed in accordance with guidelines approved by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee.
Results
Time Lapse Observation of MCF-7 after Paclitaxel Treatment
MCF-7 was treated with 1 μM paclitaxel for 2 d. As observed with phase-contrast microscopy, the control MCF-7 cells without paclitaxel treatment exhibited typical growth patterns and a smooth, flattened epithelial-like morphology (Fig. 1A-a), while 50%-70% of the cells were killed and floated in the medium following 2 d treatment with paclitaxel (Fig. 1A-b). The surviving cells were followed weekly over a period of 6 months. The period of recovery following paclitaxel treatment was defined as from the time of paclitaxel removal to the point when PGCCs began generating daughter cells. The remaining cells acquired a larger epithelial morphology, and some PGCCs continued dying when cultured with normal DMEM. PGCCs that survived the paclitaxel treatment remained in a static state for almost 3 months: no cells died and no daughter cells were generated. At the beginning of fourth month, the cells survived from paclitaxel treatment were most PGCCs and the percentage of PGCCs composed more than 80% of the total cell population which was confirmed by H342 staining (Fig. 1A-c). Unexpectedly, small daughter cells began to bud out from the large cells 4 months after paclitaxel treatment (Fig. 1A-d). With increased time in the tissue culture, many more small daughter cells were generated from the large cells (Fig. 1A-e and -f). When these cells reached 90% confluency, the cells were trysinized. Surprisingly, 12 hours after trypsinization, most of these cells formed spheroid-like structures and floated in the medium (Fig. 1A-g and -h), while portion of these spheroid-like structures attached to the wall and began proliferating after transferred into new flasks (Fig. 1A-i).
Fig. 1.
Morphologic changes of MCF-7 cells after paclitaxel treatment. A. Control MCF-7 cells were treated with 1 μM paclitaxel for 48 h and then recovered with complete DMEM. a) Morphology of control MCF-7 cells (×10). b) Morphology of control MCF-7 cells at 0 d after paclitaxel treatment (×10) and c) at 30 d after paclitaxel treatment (×10). d) MCF-7 PGCCs generated small-sized daughter cells by budding at 120 d after paclitaxel treatment (×10). e and f) Many small-sized daughter cells surrounded large cells at 120 d after paclitaxel treatment (×10). g and h) Spheroid-like structures of MCF-7 cells that formed after paclitaxel treatment can be observed at 12 h after trypsinization (×10). i) Some spheroid-like structures can reattach to the wall when cultured in complete medium without paclitaxel (×10). B. PGCC formation of MCF-7 cells after paclitaxel treatment confirmed by Hoechst33342 staining. a-c) Hoechst33342 staining shows MCF-7 cells with normal-sized nucleus (×15). d-f) PGCCs of MCF-7 after paclitaxel treatment. g-i) PGCCs generated daughter cells by budding (×15).
To directly visualize the nuclei of in PGCCs, these cells were stained with H342. In control MCF-7 cells, the frequency of PGCCs is about 1 in 10,000 - 100,000 regular diploid cancer cells. After paclitaxel treatment, most of the diploid cells were selectively killed, the remaining survival cells were most PGCCs with multinucleus (Fig. 1B, d-f), and the average size of this nucleus is 3-5 times that of control MCF-7 cells’ nucleus (Fig. 1B, a-c), while the nuclear size of daughter cells budding from PGCCs (Fig 1A d-f) is similar to that of control MCF-7 cells (Fig. 1B, g-i). To validate the above findings, cells from two additional cancer cell lines, HEY, T29H, were treated with 1 μM paclitaxel for 2 days. Similar to MCF-7, most of the cells were dead after paclitaxel treatment and the surviving cells were in a static state 5 months for T29H cells, and 6 months for HEY cells before the budding of daughter cells was observed. The morphology of PGCCs and daughter cells in HEY and T29H after paclitaxel was showed in supplementary figure 1A and 1B. After stained with H342, PGCCs have a large nucleus or multinucleus containing multiple copies of genomic DNAs (Fig. 1B, d-f), and the size of this nucleus is at least 3 times that of control MCF-7 cells’ nucleus (Fig. 1B, a-c). The nuclear size of daughter cells budding from PGCCs (Fig 1A d-f) is similar to that of control MCF-7 cells (Fig. 1B, g-i).
Formation of Cancer Organotypic Structures (COSs) of MCF-7
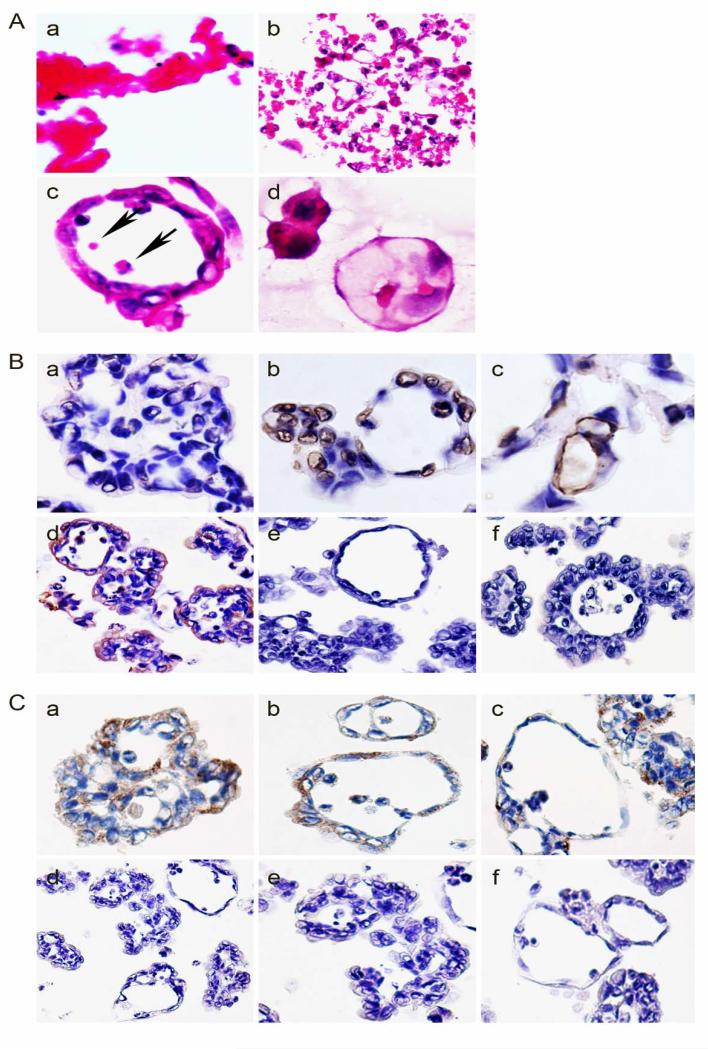
To directly examine the nature of the spheroid-like structure of MCF-7 after paclitaxel treatment, we span down them from the medium and embedded with paraffin. Histological examination of these spheroid-like structures showed multicellular structures which named cancer organotypic structures (COSs) closely mimicked the well differentiated ductual carcinoma observed in patients, which included papilla-like, adenoid, and vessel-like (Fig. 2A-a and -b). The number of papilla-like, adenoid and vessel-like structures in the H&E stained sections were counted using × 100 magnification in the same field. The average number of papilla-like, adenoid and vessel-like structures was 3 ± 1.84, 4.2 ± 2.04 and 1.9 ± 1.52 in ten randomly chosen fields respectively. Immunohistochemical staining of COSs were positive for pan-cytokeratin (Fig. 2A-c) and negative for vimentin (Fig. 2A-d), while no change of protein expression of pan-cytokeratin and vimentin by western blot analysis before and after paclitaxel treatment (Fig. 2B), confirming the epithelial nature of COSs. The morphology of the papilla-like structure was similar to that of papillary structure in human tumors (Fig. 2C-a), while the adenoid structure had lumen in the center (Fig. 2C-b) similar to the lumens in adenocarcinoma. Vessel-like structures formed by single layers of flat cells associated erythroid cells in the center of some lumens were also observed (Fig. 2C-c). Furthermore, papilla-adenoid (Fig. 2C-d), papilla-vessel-like (Fig. 2C-e), and adenoid-vessel-like structures (Fig. 2C-f) also coexisted in one single spheroid. To further confirm whether epithelial cancer cells can differentiate into the vessel-like structures, we performed immunohistochemical staining against CD31 and SMA. While only a few cells in papilla-like structures were positive for CD31 (Fig. 2D-a), abundant CD31-positive cells were observed in adenoid (Fig. 2D-b) and vessel-like structures (Fig. 2D-c). SMA-positive staining is also observed in these cells around the adenoid (Fig. 2E-a) and vessel-like structures (Fig. 2E-b) but was weakly expressed on the papilla-like structures (Fig. 2E-c), demonstrating that these epithelial cancer cells have transdifferentiated into endothelial and myoepithelial cells to form COSs following the treatment of paclitaxel.
Fig. 2.
Multiple lineage differentiations of COSs of MCF-7 after paclitaxel treatment. A. Morphology and immunohistochemical (IHC) staining of COSs. a-b) Morphologic characteristic of COSs at low magnification (×10). c) Anti-cytokeratin (AE1/AE3) IHC staining (×20). d) Anti-vimentin IHC staining (×20). B. Western blot analysis of cytokeratin (AE1/AE3) and vimentin expression in MCF-7 cells with and without paclitaxel treatment. C. COSs formation in MCF-7 cells after paclitaxel treatment. a-c) Papilla-like, adenoid, and vessel-like structures in MCF-7 cells after paclitaxel treatment. d-f) Papilla-adenoid, papilla-vessel, and adenoid-vessel structures coexist in a single COS. D. Anti-CD31 IHC staining in three types of COSs. a) Weak expression of anti-CD31 staining on papilla-like structure (×20). b) Positive anti-CD31 staining on adenoid structure (×20). c) Positive anti-CD31 staining on vessel-like structure (×20). E. Anti-SMA IHC staining in three types of COSs. a) Weak expression of anti-SMA staining on papilla-like structure (×20). b) Positive anti-SMA staining at the edge of adenoid structure (×20). c) Positive anti-SMA staining at the edge of vessel-like structure (×20).
Erythroid Differentiation in MCF-7 Induced by Paclitaxel
We have demonstrated recently that fibroblasts and cancer cell lines can generate erythroid cells following treatment of hypoxia mimetics CoCl2 15, suggesting that fibroblasts and cancer cells are capable of generating their own erythroid cells in response to hypoxia. Here we show that cancer cells can also generate erythroid cells following paclitaxel treatment. To characterize the nature of erythroid cells, thus we examined the histomorphology of these erythroid cells and the expression of different forms of hemoglobins in these COSs. As shown in Fig. 3A-a and -b, erythorid cells can be observed in clusters within these spheroid structures, erythroid cells also appeared in the lumen of the vessel-like structure (Fig. 3A-c, black arrow heads), and while others were in the cytosol of cells that formed the wall of the vessel-like structure (Fig. 2C-c, black arrow heads). A smear of floating paclitaxel-treated MCF-7 cells also confirmed the presence of erythroid cells derived from cancer cells (Fig. 3A-d). We performed IHC staining with anti-hemoglobin-α, -β/γ/σ/ε, -ζ, and fetal hemoglobin antibodies on these COSs. The papilla-like structures have a few of the cells positive for hemoglobin-α (Fig. 3B-a), and most cells within adenoid and vessel-like structures were strongly positive for hemoglobin-α (Fig. 3B-b and -c), they are also positive for hemoglobin-β/γ/σ/ε staining (Fig. 3B-a). The mean percentage of positive cells for hemoglobin-α and hemoglobin-β/γ/σ/ε expression among papilla-like, adenoid-like and vessel-like structures from five randomly chosen fields, were showed in table-1. The mean percentage of hemoglobin-α in adenoid-like and vessel-like structure was significantly higher than it in the papilla-like structures (P < 0.05). There was no statistical significance of hemoglobin-β/γ/σ/ε expression among papilla-like, adenoid-like and vessel-like structures. All of the cells in COSs were negative for fetal hemoglobin (Fig. 3B-e) and hemoglobin -ζ (Fig. 3B-f). Cells of papilla-like, adenoid, and vessel-like COSs of MCF-7 after paclitaxel treatment also expressed KP-1 (Fig. 3C-a to -c), a marker of macrophages, and were negative for PF4 IHC staining, a marker of blood platelets (Fig. 3C-d to -f).
Fig. 3.
Erythroid cell differentiation and hemoglobin generated by MCF-7 after paclitaxel treatment. A. Erythroid cells differentiation. a and b) H&E staining showed erythroid cells differentiation (×20) with spheroids. c) Erythroid cells appeared in the lumen of the vessel-like structure. d) Smear of floating COSs (×20). B. Anti-hemoglobin immunohistochemical (IHC) staining. a-c) Anti-hemoglobin-α staining of papilla-like structure (a), adenoid structure (b) and vessel-like structure (c) (×20). d). Anti-hemoglobin-β/γ/σ/ε staining of COSs (×20). e) Anti-fetal hemoglobin IHC staining. f) Anti-hemoglobin-ζ IHC staining (×20). C. Macrophage and blood platelet differentiation. a-c) Anti-KP-1 staining of papilla-like structure (a), adenoid structure (b), and vessel-like structure (c) (×20). d-f). Anti-PF-4 staining on papilla-like structure (d), adenoid structure (e), and vessel-like structure (f) (×20).
Table 1.
Comparison of the mean percentage of positive cells for hemoglobin-α and hemoglobin-β/γ/σ/ε expression among papilla-like, adenoid-like and vessel-like structures (±s)
| Name of hemoglobin | ±s | ||
|---|---|---|---|
| hemoglobin-α | papilla-like structures |
33.6 ± 5.3 | |
| adenoid -like structures |
59.8 ± 3.8 | P<0.05 | |
| vessel-like structures | 65.8 ± 13.8 | ||
| Hemoglobin-β/γ/σ/ε | papilla-like structures |
22.3 ±14.3 | P>0.05 |
| adenoid -like structures |
25.5 ± 9.47 | ||
| vessel-like structures | 29.4 ± 13.1 |
Paclitaxel-Treated MCF-7 Cells Exhibited Decreased Ability to Migrate and Proliferate
We examined the ability to migrate and proliferate in these MCF-7 cells before and after paclitaxel treatment. The MTT assay showed a decrease in the growth of MCF-7 cells after paclitaxel treatment compared with control cells (P < 0.05; Fig. 4A). Wound-scratch assays were used to compare the migratory ability of MCF-7 after paclitaxel treatment and parental MCF-7 cells (4B and 4C). At 48 h, the mean number of control MCF-7 cells that had migrated across the wound was 6.4 times higher than the number of MCF-7 cells that had migrated across the wound after paclitaxel treatment (P < 0.001; Fig. 4B and 4C).
Fig. 4.
A. Proliferative potential of reattached cells and control MCF-7 cells after plating assessed by using a modified MTT assay with different cell numbers. Reattached cells exhibited a decrease in growth compared with control MCF-7 cells (*P < 0.05). B. Quantitative results are shown as a wound-healing index, which indicated differences in the numbers of cells at 0, 6, 24, and 48 h. The Y-axis indicates the number of migratory cells. C. Wound-scratch assays were performed to compare the migratory potential of control MCF-7 and MCF-7 after paclitaxel treatment at 0, 24, and 48 h. D. Reattached cells from floating COSs showed more resistance to paclitaxel treatment than do control cells. a) Most of MCF-7 cells were dead after the first paclitaxel treatment (×10). b) A few cells died after the second paclitaxel treatment (×10). c) It took 20 d for MCF-7 cells to recover from the second paclitaxel treatment (×10). d). A few of PGCCs cells in control MCF-7 (×10). e). Multiple PGCCs can be observed after paclitaxel treatment (×10). E. Quantitative results indicated the differences of PGCCs numbers in control and reattached cells after the first paclitaxel treatment. F. Morphological characteristics of tumor derived from control MCF-7 cells and paclitaxel-treated cells.
COSs are More Resistance to Paclitaxel than that of Control MCF-7 Cells
To determine the response to drug treatment of COSs, these COSs were continued to be cultured in another flask. A portion of COSs can reattach to the wall and became attached, and these reattached cells showed more resistance to paclitaxel than did control MCF-7 cells. While it takes 4 months for control MCF-7 cells to recover from paclitaxel treatment, the reattached progeny cells from COSs started to grow only 20 days after paclitaxel treatment. Furthermore, most of control MCF-7 cells died after paclitaxel treatment (Fig. 4D-a), however, most of the re-attached cells survived (Fig. 4D-b). The number of PGCCs in the reattached cells after paclitaxel treatment is significantly higher than that in control MCF-7, which was indicated by H342 staining after counting the number of PGCCs in 10 randomly chosen fields (10 ×), (P < 0.01, Fig. 4D-e and -f and Fig. 4E).
Morphological Characteristics of Tumors Derived from Control MCF-7 and Paclitaxel-Treated MCF-7
To observe the morphologic changes and tumor growth in vivo following paclitaxel treatment, we injected MCF-7 cells into the flanks of two groups of nude mice (5 mice for each group), one group receiving a subcutaneous injection of control MCF-7 cells and the other receiving the same number of paclitaxel-treated MCF-7 cells. Two months later, tumors in the mice that had received the control MCF-7 injection had reached the average 0.8 cm in diameter. In the paclitaxel-treated MCF-7 cells group, however, only three mice had the tumor nodules with the average 0.5 cm in diameter 3 months after injection, the tumors remain in a static state for another 2 months without evidence of further growth. Histological examination of these tumors revealed that the control MCF-7 cells had abundant epithelial cancers with minimal intervening stroma (Fig. 4F-a and -b), while tumors formed from paclitaxel-treated MCF-7 cells have significantly decreased epithelial tumor cells density surrounded by rich extracellular matrix and collagen (Fig. 4F-c and -d). Our results show that the paclitaxel-treated MCF-7 cells have markedly deceased tumorigenecity than that of the control MCF-7 cells.
Expression of Wnt Signal-Related Proteins
We determined the altered expression of several signaling molecules involved in stem cells and tumor development. First, we examined whether these COSs exhibited increased expression of normal stem cell markers. Immunohistochemical staining showed that COSs were positive for SOX-2 and OCT3/4 and negative for Nanog and ABCG2 expression (Fig. 5A). Secondly, we examined several key proteins that are known to be involved in the regulation of stem cells and cancer. The c-Myc protein is known to play a critical role in controlling self-renewal versus differentiation in hematopoietic stem cells. We showed that paclitaxel-treated MCF-7 expressed more c-Myc than did control MCF-7 cells. The expression of β-catenin and E-cadherin was however higher in control than in paclitaxel-treated MCF-7 cells, similar results were confirmed by Western blot analysis and immunocytochemical staining (Fig. 5B and Fig. 5C). SMA expression is increased and thus provided further support for myoepithelial differentiation in COSs. There is no significant difference in PTEN or snail expression between paclitaxel-treated and control MCF-7 cancer cells (Fig. 5B). These results showed that these COSs gained stem cell-like properties through dysregulation signaling pathway involved in stem cell differentiation including c-Myc and Wnt-signaling pathways.
Fig. 5.
A. Immunohistochemical staining of normal stem cell markers of COSs. a). Anti-SOX-2 staining (×20). b) Anti-OCT3/4 staining (×20). c) Anti-Nanog staining (×20). d) Anti-ABCG2 staining (×20). B. Different expression of SMA, Snail, P27, PTEN, c-Myc, β-catenin, and E-cadherin on control MCF-7 cells and paclitaxel-treated MCF-7 cells. β-actin was used as a loading control. C. Immunohistochemical staining verified that expression of β-catenin and E-cadherin in paclitaxel-treated cells is lower than in control MCF-7 cells (×20).
Discussion
Cancer is generally considered to be a clonal proliferation of cancer epithelial cells, while the origin of tumor associated-stromal fibroblasts or myoepithelial cells is not entirely clear 20, which is often considered as borrow-derived or non-cancer cell-derived. We showed that paclitaxel treatment can induce PGCCs formation in MCF-7 cancer cell line, which differentiate into multiple stromal cells and differentiated cancer cells which express CD31, SMA, and erythroid cells made of different forms of hemoglobin and KP-1. These results demonstrated that cancer cells can generate multiple lineages of benign stromal cells from paclitaxel induced PGCCs. In addition, these PGCCs can form COSs with common histomorphology including papilla-like, adenoid, and vessel-like structures seen in ductal adenocarcinomas seen in human breast cancer. These findings suggest that PGCCs can also serve as a morphologic factor for glandular formation and is the cellular basis for organogenesis and tumor histogenesis similar to low organisms 21. The ability that cancer cell lines can generate stromal cells has been demonstrated in our recent studies13. SKOv3 ovarian cancer epithelial cells that are treated with high concentrations of paclitaxel can be induced to become non-tumorigenic fibroblasts 13. Thus, depending on the genetic background of cancer cell lines, the specific stromal cell lines generated in response to paclitaxel may be different.
Paclitaxel-treated MCF-7 cells had slow proliferation and low rates of invasion and tumor formation, suggesting that these treated cells are less aggressive or malignant than that of parental cells. We found that c-Myc expression in paclitaxel-treated MCF-7 cells was higher than that in control MCF-7 cells. C-Myc, an oncogene of four pluripotency genes whose activation is essential for the production of induced pluripotent stem cells22. Importantly, cells of organotypic structures express normal stem cell markers SOX-2 and OCT3/4. These results may demonstrate that paclitaxel may decrease the tumorigenicity through activation of the normal stem cell program.
Functional pharmacology has been a limiting step for new drug discovery due to lack of efficient and reliable cancer model system 23. As most of cancer cell lines were generated through multiple selections in vitro, these cell lines may invariably obtain more malignant characteristics in vitro rather than the clinical cancers from patients. In this regard, paclitaxel-induced COSs of MCF-7 may more closely resemble human cancer that is resistant to paclitaxel. Thus, due to unlimited supplies of COSs generated in vitro, the COS may serve as an in vitro model for the novel drug discovery against paclitaxel resistance.
Supplementary Material
What is new?
The origin of tumor stroma is often considered as non-cancer cell-derived. We showed that polyploid giant cancer cells (PGCCs) induced by paclitaxel, in contrast to conventional belief that these cells are non-dividing cells, can generate progeny cells through budding and form cancer organotypic structures in vitro. In addition to differentiate into well differentiated cancer cells, the budded cells can differentiate into multiple lineages of tumor stromal cells and become more resistant to the treatment of paclitaxel. Our data demonstrated that tumor stromal cells can be directly generated from cancer cells, which may constitute the cellular basis of tumor histogenesis and drug resistance.
Acknowledgments
This research was supported by an R01 grant (R01CA131183-01A2) and ovarian cancer Specialized Programs of Research Excellence (SPORE) grant (IP50CA83638) from the National Institutes of Health and Cancer Prevention and Research Institute of Texas (CPRIT) multi-investigator grant. This work was also supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CA016672).
Abbreviations used
- PGCCs
polyploidy giant cancer cells
- COS
cancer organotypic structure
- DMEM
Dulbecco’s modified Eagle’s medium
- H342
Hoechst 33342
- IHC
Immunohistochemical staining
- d
day
- h
hour
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Buchheit CL, Rayavarapu RR, Schafer ZT. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin Cell Dev Biol. 2012;23:402–11. doi: 10.1016/j.semcdb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–6. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akahane T, Hirasawa A, Tsuda H, et al. The origin of stroma surrounding epithelial ovarian cancer cells. Int J Gynecol Pathol. 2013;32:26–30. doi: 10.1097/PGP.0b013e3182518533. [DOI] [PubMed] [Google Scholar]
- 6.Kurose K, Gilley K, Matsumoto S, et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32(3):355–7. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 7.Patocs A, Zhang L, Xu Y, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357(25):2543–51. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 8.Bussolati B, Grange C, Sapino A, Camussi G. Endothelial cell differentiation of human breast tumour stem/progenitor cells. J Cell Mol Med. 2009;13:309–19. doi: 10.1111/j.1582-4934.2008.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaffidi P, Misteli T. In vitro generation of human cells with cancer stem cell properties. Nat Cell Biol. 2011;13:1051–61. doi: 10.1038/ncb2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 11.Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–27. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- 12.Brito DA, Yang Z, Rieder CL. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol. 2008;182:623–9. doi: 10.1083/jcb.200805072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia L, Zhang S, Ye Y, et al. Paclitaxel inhibits ovarian tumor growth by inducing epithelial cancer cells to benign fibroblast-like cells. Cancer Lett. 2012;326:176–82. doi: 10.1016/j.canlet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Mercado-Uribe I, Xing Z, et al. Generation of cancer stem-like cells through formation of polyploid giant cancer cells. Oncogene. 2013 Mar 25; doi: 10.1038/onc.2013.96. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Mercado-Uribe I, Liu J. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer Letters. 2013;333(2):205–12. doi: 10.1016/j.canlet.2013.01.037. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–89. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Guo X, Yang G, et al. AURKA and BRCA2 expression highly correlate with prognosis of endometrioid ovarian carcinoma. Mod Pathol. 2011 Jun;24(6):836–45. doi: 10.1038/modpathol.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang G, Chang B, Yang F, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res. 2010;16:3171–81. doi: 10.1158/1078-0432.CCR-09-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Yang G, Thompson-Lanza JA, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–63. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 20.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–97. [PMC free article] [PubMed] [Google Scholar]
- 21.Anisimov AP. Endopolyploidy as a morphogenetic factor of development. Cell Biol Int. 2005;29:993–1004. doi: 10.1016/j.cellbi.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Gopfert U, Kullmann M, Hengst L. Cell cycle-dependent translation of p27 involves a responsive element in its 5′-UTR that overlaps with a uORF. Hum Mol Genet. 2003;12:1767–79. doi: 10.1093/hmg/ddg177. [DOI] [PubMed] [Google Scholar]
- 23.Walker TBaLJG Michael J.A. Functional pharmacology: the drug discovery bottleneck? Drug Discovery Today: TARGETS. 2004;3:208–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.