Abstract
Despite advances in surgical technology, as well as generally good outcomes, repairs of full-thickness rotator cuff tears show a retear rate of 25% to 57% and may fail to provide full return of function. The repairs tend to fail at the suture-tendon junction, which is due to several factors, including tension at the repair site, quality of the tendon, and defective tissue repair. One strategy to augment repair of large to massive rotator cuff tears is the development of biological scaffold materials, composed of extracellular matrix (ECM). The goal is to strengthen and evenly distribute the mechanical load across the repair site, thus minimizing the rupture risk of the native tendon while providing the biological elements needed for healing. The promising results of ECM-derived materials and their commercial availability have increased their popularity among shoulder surgeons. In contrast to a traditional open or arthroscopically assisted mini-open approach, this completely arthroscopic technique offers the full advantages warranted by the use of a minimally invasive approach. This technical guide describes arthroscopic rotator cuff repair using an ECM graft technique.
Rotator cuff tears remain one of the challenging yet very common conditions in orthopaedic practice. They affect as much as 40% of the population aged older than 60 years.1,2 They have a high rate of recurrence and retear, with a rate as high as 25% that more than doubles, to 57%, in patients aged older than 65 years.1-3 A better understanding of tendon healing and the degenerative nature of rotator cuff tears has led to the quest for a solution that addresses not only the mechanical factors of healing but also the biological aspects of healing. Such a solution would serve as a scaffold for repair and regeneration.2,3 The goal is to strengthen and evenly distribute the mechanical load across the repair site, thus minimizing the rupture risk of the native tendon while providing the biological elements needed for healing, such as platelet-derived growth factor, vascular endothelial growth factor, fibroblast growth factor, transforming growth factor β, and structural proteins.1,2,4 The promising results of extracellular matrix (ECM)–derived materials and their commercial availability have increased their popularity among shoulder surgeons. In contrast to a traditional open or arthroscopically assisted mini-open approach, this completely arthroscopic technique offers the full advantages warranted by the use of a minimally invasive approach such as lower morbidity and its general acceptance by patients.3,5,6
Patient selection plays an integral role in the success of the procedure. It is a preoperative as well as an intraoperative decision. The patients who would most benefit from this procedure are those with failed prior rotator cuff repairs and revisions; older patients; young and active patients; patients with tears larger than 2 cm; patients with poor tissue quality; patients with poor tissue mobilization; and patients with comorbidities increasing the risk of repair failure, such as smoking and diabetes.5,7
Technique
The rotator cuff repair is performed with the patient under general anesthesia, with an intrascalene block and subacromial bupivacaine hydrochloride (Video 1 and Table 1). The technique requires the standard shoulder arthroscopy instruments plus an arthroscopic 8.25-mm notched cannula (Arthrex, Naples, FL); Bio-Corkscrew anchor (Arthrex); Bio-Corkscrew anchor, Triple Play (Arthrex); Bio-SwiveLock anchor (Arthrex); 35-mm × 35-mm reconstructive tissue matrix; Gabbay-Frater suture guide (Teleflex Medical, Research Triangle Park, NC); and free tapered needles.
Table 1.
Tips, Pearls, Pitfalls, Key Points, Indications, Contraindications, and Risks
| Tips |
| The tear needs to be marked with a PDS suture for later visualization in the acromial space. |
| After bursectomy, debridement of the nonviable edges of the rotator cuff needs to be performed. |
| The defect (tear) is measured in the sagittal and coronal planes using a calibrated probe. |
| The suture-bridge technique is used to secure the graft-incorporated repair and ensure adequate tension either through the lateral edge of the graft or 1 to 2 mm laterally to the edge of the graft. |
| Pearls |
| The technique requires the standard shoulder arthroscopy instruments plus an arthroscopic 8.25-mm notched cannula. |
| Suture anchors are positioned at the articular margin, and by use of a combination of simple and mattress sutures, the medial row needs to be passed through the cuff. |
| The most anterior and posterior simple sutures are pulled as far laterally as possible, tied, and cut. |
| Pitfalls |
| The innermost mattress sutures are left free and need to be labeled in a methodical fashion and placed on the suture guide. |
| Key points |
| Removal of scar tissue and interval slides needs to be performed to permit satisfactory mobility anterolateral to the prepared greater tuberosity. |
| The limbs of the sutures need to be passed through the medial 3- to 5-mm edge of the graft using a free needle according to the spacing of the mattress sutures and orientation of the defect. |
| Through the use of a blunt trocar, the folded graft is advanced into the clear cannula and through its bladder. |
| The remaining sutures are tied in a load-sharing fashion. |
| Indications |
| Large to massive rotator cuff tears |
| Contraindications |
| Allergy to ECM products |
| Risks |
| The risks are similar to those of arthroscopic rotator cuff repairs. |
NOTE. Rotator cuff repair with an ECM graft is an arthroscopic surgical technique offering the full advantages warranted by the use of a minimally invasive approach.
The procedure begins with routine sterile preparation and draping of the operative upper extremity. The rotator cuff tear is visualized by intra-articular and subacromial diagnostic arthroscopy through a standard posterior portal. The tear is then marked with a PDS suture (Ethicon, Somerville, NJ) for later visualization in the acromial space (Table 2).
Table 2.
Surgical Technique Phases
| 1. Perform tear pattern recognition. |
| 2. Perform releases/slides/margin convergence. |
| 3. Perform footprint preparation. |
| 4. Tie anterior and posterior sutures. |
| 5. Measure tear in coronal and sagittal dimensions. |
| 6. Place untied medial-row sutures on suture guide. |
| 7. Trim graft in semicircular shape. |
| 8. Pass sutures through medial 3 to 5 mm edge of graft. |
| 9. Tie innermost mattress sutures with sliding-locking knot. |
| 10. Advance graft into 8.25-mm clear cannula with blunt trocar. |
| 11. Slide knot onto repair. |
| 12. Tie remaining sutures in load-sharing fashion. |
NOTE. Arthroscopic repair of rotator cuff tears using ECM graft requires the standard shoulder arthroscopy instruments plus an arthroscopic 8.25-mm notched cannula.
Complete arthroscopic bursectomy and subacromial spur removal with preservation of the coracoacromial arch are performed next. After the bursectomy, debridement of the nonviable edges of the rotator cuff is performed. Preparation of the footprint and greater tuberosity and evaluation of the rotator cuff in terms of size, tendons, tissue quality, and mobility are carried out. Soft-tissue release follows, and removal of scar tissue and interval slides is performed to allow adequate mobility anterolateral to the prepared greater tuberosity.
Suture anchors are placed at the articular margin, and by use of a combination of simple and mattress sutures, the medial row is passed through the cuff. The most anterior and posterior simple sutures are pulled as far laterally as possible, tied, and cut. Then, the innermost mattress sutures are left untied and are tagged in an orderly fashion and placed on the suture guide. The defect is measured in the sagittal and coronal planes using a calibrated probe (Fig 1).
Fig 1.
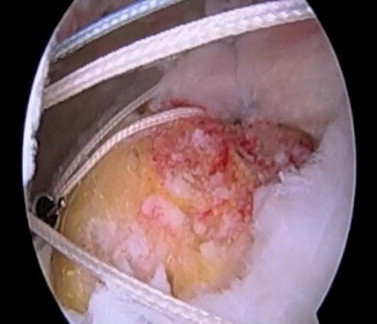
Arthroscopic evaluation of rotator cuff tear. The tear is measured in the coronal and sagittal dimensions, and untied medial-row sutures are placed on the suture guide.
In the subsequent stage, attention is directed toward the ECM graft. An appropriately sized graft is trimmed in a semicircular manner. The limbs of the sutures are passed through the medial 3- to 5-mm edge of the graft using a free needle according to the spacing of the mattress sutures and orientation of the defect. The innermost mattress sutures are tied using a sliding-locking knot. With the use of a blunt trocar, the folded graft is advanced into the clear cannula and through its bladder. The knot is advanced with the use of a knot pusher onto the cuff and tuberosity. The remaining sutures are tied in a similar load-sharing fashion (Fig 2). A limb of each tied suture is passed through a lateral-row anchor. A suture-bridge technique is used to secure the graft-incorporated repair and ensure adequate tension either through the lateral edge of the graft or 1 to 2 mm laterally to the edge of the graft. Then, excess graft material is trimmed with an arthroscopic biter and shaver (Fig 3).
Fig 2.
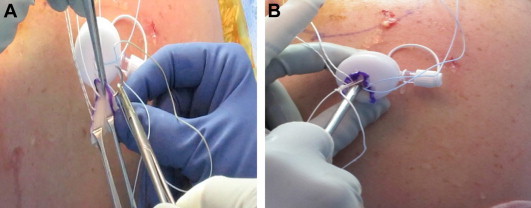
(A) Rotator cuff repair is performed with an ECM graft. The sutures are passed through the medial 3 to 5 mm edge of the graft, and the innermost mattress sutures are tied with a sliding-locking knot. (B) The ECM graft is advanced into an 8.25-mm clear cannula with a blunt trocar, and the surgeon slides the knot onto the repair; the remaining sutures are tied in a load-sharing fashion, adding the graft to the rotator cuff tear.
Fig 3.
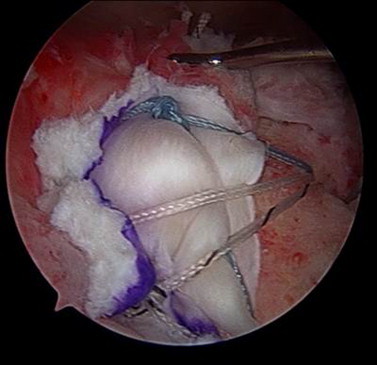
A suture-bridge technique is used to complete the repair. The final arthroscopic view of a complete massive rotator cuff repair using ECM graft augmentation is shown. The graft is incorporated using a medial-row load-sharing technique.
The wounds are closed in layers with No. 2 Vicryl (Ethicon) and No. 2-0 Prolene (Ethicon). A sterile dressing, abduction sling, and icepack are applied.
Discussion
Although surgical repair of large to massive rotator cuff tears has resulted in reliable pain relief and improved function,2,7 retear rates have remained relatively high despite advances in surgical techniques.2,3 The described technique is one of the most common and promising augmentation techniques using ECM scaffold graft. The advantages of using ECM scaffold grafts are that they are 3-dimensional and include structural and functional proteins, including collagen, elastin, growth factors, and proteoglycans.2-4
Repairs of large to massive rotator cuff tears have a relatively high rate of failure; therefore, improvement in repair strategies is needed. ECM scaffold grafts can decrease failure of the repair as a result of decreased gap formation at the repair site and improved load sharing. ECM scaffold grafts increase the supraspinatus thickness and decrease the retear rate. In addition, improved success rates are possible in patients who receive ECM augmentation, as shown by the revision rate and results of shoulder questionnaires. Our findings support the principle of rotator cuff repair with ECM, and it is necessary to conduct a randomized clinical trial to evaluate the effect of using the ECM technique in primary surgical treatment of large to massive rotator cuff tears.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
Supplementary Data
A 51-year-old man with a chronic, massive, retracted rotator cuff tear. The tear measured 5.6 cm (coronal) × 5.1 cm (sagittal) with grade 2 fatty infiltration and positive findings of atrophy. Arthroscopically, a rotator cuff repair was implemented using standard shoulder arthroscopy instruments plus an arthroscopic 8.25-mm notched cannula. The ECM graft was integrated using a medial-row load-sharing technique.
References
- 1.Ruiz-Moneo P., Molano-Muñoz J., Prieto E., Algora J. Plasma rich in growth factors in arthroscopic rotator cuff repair: A randomized, double-blind, controlled clinical trial. Arthroscopy. 2013;29:2–9. doi: 10.1016/j.arthro.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Derwin K.A., Badylak S.F., Steinmann S.P., Iannotti J.P. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467–476. doi: 10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Neyton L., Godeneche A., Nove-Josserand L. Arthroscopic suture-bridge repair for small to medium size supraspinatus tear: Healing rate and retear, pattern. Arthroscopy. 2013;29:10–17. doi: 10.1016/j.arthro.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Badylak S.F., Freytes D.O., Gilbert T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;1:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Dopirak R., Bond J.L., Snyder S.J. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Int J Shoulder Surg. 2007;1:7–15. [Google Scholar]
- 6.Barber F.A., Burns J.P., Deutsch A. A prospective randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Badhe S.P., Lawrence T.M., Smith F.D., Lunn P.G. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17:35S–39S. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A 51-year-old man with a chronic, massive, retracted rotator cuff tear. The tear measured 5.6 cm (coronal) × 5.1 cm (sagittal) with grade 2 fatty infiltration and positive findings of atrophy. Arthroscopically, a rotator cuff repair was implemented using standard shoulder arthroscopy instruments plus an arthroscopic 8.25-mm notched cannula. The ECM graft was integrated using a medial-row load-sharing technique.


