Summary
Epidermal stem cells have been in clinical application as a source of culture-generated grafts. Although applications for such cells are increasing due to aging populations and the greater incidence of diabetes, current keratinocyte grafting technology is limited by immunological barriers and the time needed for culture amplification. We studied the feasibility of using human fetal skin cells for allogeneic transplantation and showed that fetal keratinocytes have faster expansion times, longer telomeres, lower immunogenicity indicators, and greater clonogenicity with more stem cell indicators than adult keratinocytes. The fetal cells did not induce proliferation of T cells in coculture and were able to suppress the proliferation of stimulated T cells. Nevertheless, fetal keratinocytes could stratify normally in vitro. Experimental transplantation of fetal keratinocytes in vivo seeded on an engineered plasma scaffold yielded a well-stratified epidermal architecture and showed stable skin regeneration. These results support the possibility of using fetal skin cells for cell-based therapeutic grafting.
Highlights
-
•
Properties of fetal and adult keratinocytes are compared in tissue culture and grafts
-
•
Fetal skin cells can be engrafted and show stable human-to-mouse skin regeneration
-
•
Fetal keratinocytes are stem cell rich and need no differentiation before grafting
-
•
Fetal keratinocytes are able to suppress proliferation of stimulated T cells in vitro
Lane, Chan, and colleagues demonstrate that fetal human keratinocytes have good potential to become a cell source for therapeutic skin grafting. Cultured second-trimester skin keratinocytes show higher progenitor/stem cell properties, greater clonogenic potential, higher proliferation, and lower immunogenicity characteristics than adult keratinocytes. They also exhibit adult-type differentiation in vitro and can regenerate a viable epidermis after grafting in a SCID model.
Introduction
The grafting of cultured keratinocytes to promote regeneration represents one of the oldest clinical examples of stem cell therapy (Green, 2008). The skin constitutes an essential barrier between the living tissues of the body and the external environment, and skin tissues have evolved to maintain that barrier: water is retained and noxious substances and invasive organisms are excluded, and new skin normally can be regenerated rapidly in the event of a break in this barrier. However, large interruptions in the skin are life threatening: burns can result in deep, extensive wounds that are slow to close without medical intervention. The gold-standard treatment for large wounds is autologous split-skin grafts, but this is not possible for extensive full- or partial-thickness burns covering over 50% of the body surface area. In addition to acute skin injuries, chronic wounds are now a growing medical challenge as nonhealing wounds become more common in aging populations of the developed world, and increase further with rising rates of diabetes and resulting circulatory deficiencies. Large wounds are usually grafted with cadaveric skin (if available) to form a temporary barrier until the allogeneic cells are immunologically rejected. Alternatively, cultured epithelial autografts can be used for covering such wounds. The patient’s own epidermal cells are isolated, expanded in the laboratory, and used to replace the damaged skin (Green et al., 1979; Compton et al., 1989) without any tissue rejection. The major disadvantage of this approach is that it takes at least 3 weeks to grow enough cells for successful grafting, due to the low number of keratinocyte stem cells recovered from skin biopsies.
Much work has also been directed toward developing bioengineered skin substitutes using cultured cells (keratinocytes and/or fibroblasts) with a suitable matrix (Pham et al., 2007), but the difficulty of achieving permanent wound coverage for patients with large or intransigent wounds persists (Turk et al., 2014; Kamel et al., 2013). Bioengineered products have been hampered by immune rejection, vascularization problems, difficulty of handling, and failure to integrate due to scarring and fibrosis. Furthermore, no currently available bioengineered skin replacement can fully replace the anatomical and functional properties of the native skin, and appendage development is absent in the healed area of full-thickness culture-grafted wounds.
Thus, alternative sources of cells for engineering skin substitutes are urgently required to address this area of clinical need. One possibility is to use fetal skin as a potential cell source for tissue-engineered skin. Several types of fetal cells have been shown to have higher proliferative capacities and to be less immunogenic than their adult counterparts, suggesting potential allogeneic applications (Guillot et al., 2007; Davies et al., 2009; Montjovent et al., 2009; Götherström et al., 2004; Zhang et al., 2012). Lying between embryonic and adult cells in the developmental continuum, fetal cells offer several advantages as cell sources for therapeutic applications. Fetal cells are likely to harbor fewer of the mutations that accumulate over the lifetime of an organism, and may also possess greater proliferative potential and plasticity than adult stem cells. Although all stem cells are self-renewing and multipotent by definition, it is believed that stem cells from younger donors should have greater potential (Van Zant and Liang, 2003; Roobrouck et al., 2008). In addition, fetal cells may possess immunomodulatory properties associated with the fetal/maternal interface (Gaunt and Ramin, 2001; Kanellopoulos-Langevin et al., 2003). The use of early or midtrimester fetal tissue for skin tissue engineering was first suggested by Hohlfeld et al. (2005), who developed dermal-mimetic constructs using fetal dermal fibroblasts. Although their technique was reported to promote healing of severe burns, engraftment was only temporary and did not provide permanent cover.
Here, we demonstrate that second-trimester fetal keratinocytes can be isolated and expanded in a robust and stable manner under conditions in which they maintain genetic stability and high proliferative potential. We also show that fetal keratinocytes are capable of differentiating in organotypical cultures and can fully differentiate upon grafting. Together with the fact that these cells show low expression of major histocompatibility complex (MHC) proteins, these findings suggest that these cells have significant potential as an allogeneic source of skin cells for life-saving culture-generated grafts.
Results
Histological Differences between Adult and Fetal Skin
To understand the developmental state in situ of the fetal skin from which cells were being cultured, we analyzed fetal dorsal trunk skin histologically at various second-trimester gestational ages (13–22 weeks gestation) and compared it with adult skin. We analyzed keratin expression during development by immunofluorescence using a panel of well-characterized monospecific monoclonal antibodies to keratins. Expression of keratin 14 (K14, a marker for basal keratinocytes [Fuchs and Green, 1980]) and K15 (which is enriched in stable basal cells [Porter et al., 2000] and some epidermal stem cell niches [Lyle et al., 1998]) was similar in fetal and adult skin (Figure 1A; Figure S1B available online). In contrast, expression of K18, K17, and K19 was seen in the basal layer of fetal epidermis, but not in adult interfollicular epidermal keratinocytes. In adult skin, K18, K17, and K19 are associated with appendages, stress responses, and stem cell compartments (Lane et al., 1991; Michel et al., 1996). Results from further staining with other markers are summarized in Table S1 (see also Figures S1–S3).
Figure 1.
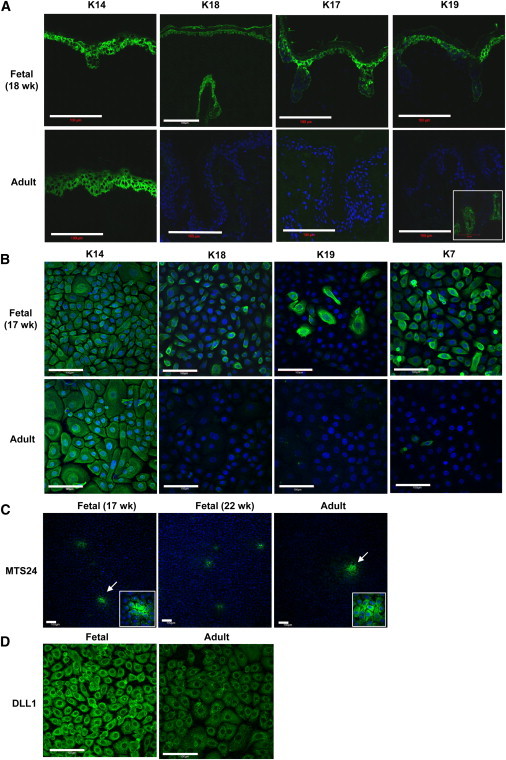
Characterization of Fetal Skin
(A) Immunofluorescence staining of keratins in fetal (18 weeks) and adult epidermis. K18, K17, and K19 were present in fetal epidermis, but not in adult epidermis (except for adult hair follicles, which show expression of K19 [inset]). Scale bar, 100 μm. See also Figures S1–S3 for a full range of images.
(B) Expression of K14, K18, and K19 in cultured fetal keratinocytes isolated from dorsal skin (17 weeks) at passage 4 and adult keratinocytes grown to 90% confluence. Fetal keratinocytes show higher expression of K18 and K19, consistent with the expression in in vivo tissue sections.
(C) Expression of MTS24 in fetal (17 and 22 weeks) and adult keratinocytes.
(D) Expression of Delta-like 1 (DLL1) in fetal and adult keratinocytes. Images in the micrographs were taken with the same exposure time. Scale bar, 100 μm. See also Figure S4 for further results for cultured cells.
Culture and Characterization of Human Fetal Keratinocytes
We developed a robust method for culturing fetal keratinocytes from skin at 15–22 weeks gestation. Samples from <15 weeks gestation were very small and cells isolated before 18 weeks were poorly adherent, so it was necessary to coat the culture flasks with 0.1% gelatin to achieve adequate cell attachment. Fibroblast contamination was not significant because fibroblasts were easily removed from first-passage cultures by 5 min incubation in 0.02% EDTA; no fibroblasts were observed at subsequent passages (Figure S4). In serum-free culture conditions, fetal keratinocytes exhibited a typical cobblestone epithelial pattern of growth, but were noticeably smaller than their adult counterparts (diameter: fetal = 16.7 ± 0.1 μm, adult = 20.8 ± 0.6 μm, p < 0.01; volume: fetal = 2.4 ± 0.03 pL, adult = 4.7 ± 0.4 pL, p < 0.01; n = 3 adult and 3 fetal samples; Figures S4F–S4G).
K14 and K7 were uniformly expressed in fetal keratinocyte cultures, whereas K18 and K19 were positive in 94.4% ± 4.0% and 14.6% ± 4.8% of cells in the cultures, respectively (n = 4), revealing a heterogeneous population of keratinocytes (Figure 1B). In contrast, adult keratinocytes did not express either K18 or K19, and only a minority of cells (6.5% ± 6.7%) expressed K7.
When we tested the cultures for expression of keratinocyte stem/progenitor markers, we observed expression of MTS24 as previously reported (Nijhof et al., 2006; Depreter et al., 2008) in clusters, with more clusters found in fetal cultures (17 and 22 weeks gestation) than in adult cultures (n = 2; Figure 1C). Delta-like-1 (DLL1) (Tan et al., 2013; Lowell et al., 2000) was expressed in both fetal and adult keratinocytes, although the staining was subjectively observed to be much stronger in fetal cells than in adult cells under the same culture conditions (Figure 1D). Other stem cell-associated markers (MCSP, NFATc1, and Thy-1) were also tested, and gave staining in all cells in the culture, with no significant differences between adult and fetal keratinocytes (data not shown).
To establish the stability of the fetal cells, the karyotype of fetal cultures at passage 3 to passage 7 was examined and observed to be normal (i.e., 46 XX or 46 XY), showing no gross karyotypic abnormalities as determined by G-banding (Figure 2A). Fetal keratinocytes also showed reproducibly high recovery rates (82% ± 9%) after 3 years of storage in liquid nitrogen following cryopreservation via a gradual freezing method in a routine lab setting (Figure 2B).
Figure 2.
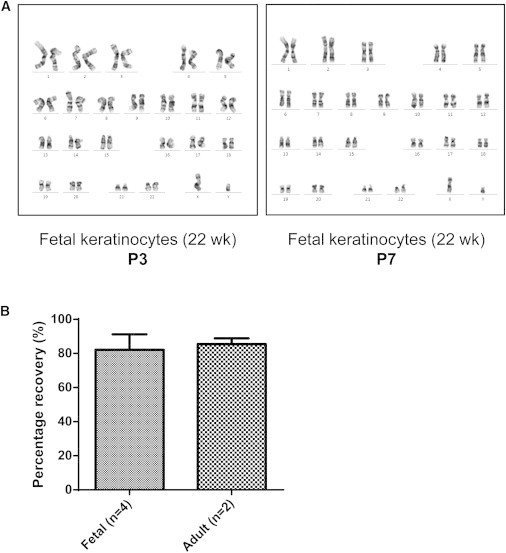
Karyotyping Analysis and Recovery of Cryopreserved Fetal Keratinocytes
(A) Fetal keratinocytes maintain a normal karyotype after serial passaging. Karyotype analysis by G-banding is represented here by 22-week fetal keratinocytes. The chromosome complement remained normal as far as P7.
(B) Fetal keratinocytes cryopreserved in low-serum-containing medium (70% serum-free medium/20% FBS/10% DMSO) show reproducibly high recovery after thawing. Percentage recovery is defined as the percentage of frozen cells that remained viable after thawing. Fetal keratinocytes (15, 16, 17, and 22 weeks) at P2 were recovered 2–3.5 years after cryopreservation. Recovery is comparable to that observed for adult keratinocytes recovered after 1.5–2 years. Data are represented as mean ± SD of n biological replicates.
Fetal Keratinocytes Have Higher Proliferation Potential than Adult Keratinocytes
Two independent lines of evidence indicate that fetal keratinocytes have higher proliferation potential than adult keratinocytes. First, in tissue sections, immunohistochemical staining with the nuclear cell proliferation marker Ki67 showed the highest proportion of Ki67-positive cells in the youngest samples tested, with a decreasing trend in the proliferation index (PI) with increasing sample age (Figure 3A). Second, in parallel, cultured fetal keratinocytes reached a higher cumulative population doubling (pd) before senescence than adult cells (20 pd [fetal] versus 12 pd [adult] by 40 days of culture: Figure 3B). Two adult and four fetal skin samples were assayed with five technical replicates each. Typical pd times for fetal keratinocytes (14–22 weeks) were 30.3 ± 7.5 hr compared with 49.3 ± 8.4 hr for adult keratinocytes (p < 0.0001; Figures 3C and S5). Telomere lengths were longer in fetal keratinocytes (14 and 19 weeks gestation) than in adult keratinocytes, and shortened by 7.6% over two passages in fetal cultures and 8.9% in adult cultures (Figure 3D).
Figure 3.
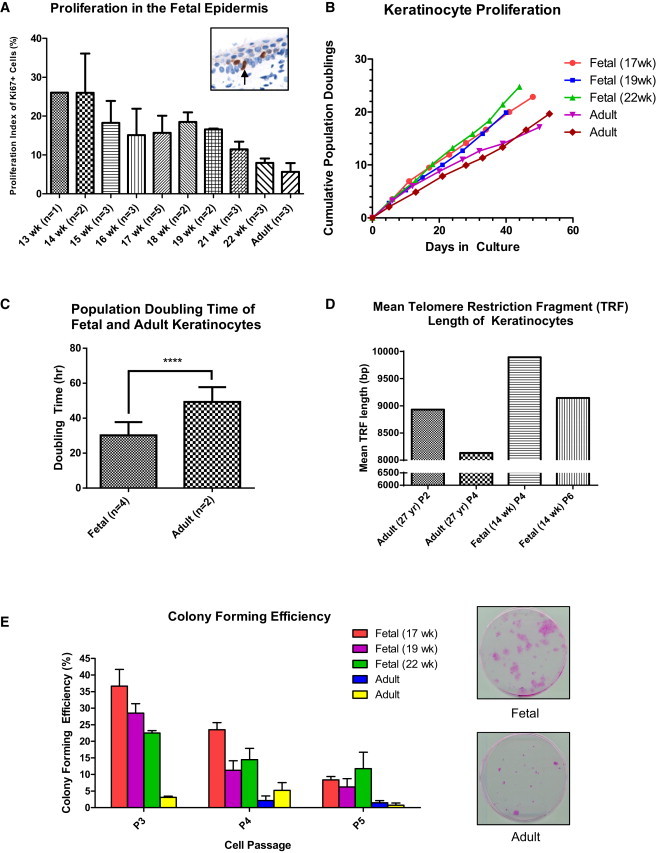
Fetal Keratinocytes Have a Higher Proliferation Potential than Adult Keratinocytes
(A) Proliferation index (PI) in fetal and adult epidermis. The PI was defined as the number of Ki67-positive cells divided by the total number of cells at the basal layer × 100%. Data are represented as mean ± SEM of n biological replicates.
(B) Comparison of the proliferation rates of fetal keratinocytes (17, 19, and 22 weeks) and adult keratinocytes. Data were generated by counting the number of cells after each passage, subcultured at 70%–80% confluence.
(C) Population doubling (pd) times are derived from each exponential phase of the growth curves monitored by a real-time cell analyzer (see Figure S5). Fetal (14, 16, 19, and 22 weeks) and adult keratinocytes were plated in 96-well plates at 2,500 cells per well. Cell growth was monitored over a period of 1 week. Data are represented as mean ± SD of n biological replicates. ∗∗∗∗p < 0.0001.
(D) Mean telomere restriction fragment (TRF) length of fetal (14 weeks) and adult keratinocytes. DNA (2 μg) prepared from keratinocytes was digested with Hinf I and Rsa I, and then separated on a 0.9% agarose gel by gel electrophoresis. It was then transferred to nylon, probed with a Dig-labeled telomere probe (TTAGGG), and detected via chemiluminescence. The average TRF length was determined by comparing the location of the TRF on the blot relative to a molecular weight standard. Fetal keratinocytes have longer telomeres than adult keratinocytes. In both adult and fetal keratinocytes, telomeres shorten with increasing passage number.
(E) Comparison of colony-forming activity between cultured fetal (17, 19, and 22 weeks) and adult keratinocytes. The colony-forming efficiency (CFE) was defined as the percentage of colonies formed over the number of cells seeded. A colony was defined as a cluster of >1 mm2. After 14 days, culture was arrested and the colonies were stained with Rhodamine B. Fetal keratinocytes form more colonies than adult keratinocytes. Data are represented as mean ± SD of three technical replicates.
We further evaluated the self-renewal capacity of these keratinocytes by performing colony-forming efficiency (CFE) assays (Barrandon and Green, 1985). Fetal keratinocytes had a 9.8-fold higher CFE than adult keratinocytes at low passage (30.3% versus 3.1%, p < 0.001). Although the CFE for both fetal and adult keratinocytes was reduced with increasing passages, the fetal keratinocytes maintained a superior clonogenic ability compared with their adult counterparts (Figure 3E). High clonogenic potential is widely regarded as a characteristic of stem or progenitor cells.
Fetal Keratinocytes Express Lower MHC Antigen Levels than Adult Keratinocytes
Both MHC I and MHC II antigens were weakly detected in fetal skin, with some positive cells in the dermis and hair germs in the epidermis, but most of the epidermis was negative for MHC I (Figure 4A). Expression of MHC I increased with increasing gestational age and was expressed ubiquitously in adult skin. Expression of MHC II in fetal skin was scattered, with sporadic positive cells in the dermis and epidermis, possibly due to the presence of Langerhans cells and other antigen-presenting cells that express MHC II. MHC II expression was higher in adult skin than in fetal skin, but was similarly scattered.
Figure 4.
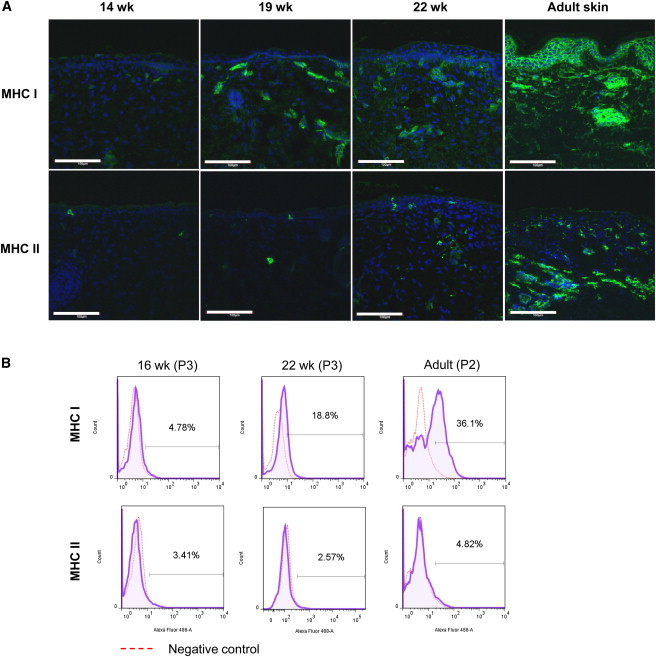
Expression of MHC Markers on Fetal and Adult Skin
(A) Fetal skin was observed to express lower levels of MHC molecules than adult skin. Scale bar, 100 μm.
(B) Flow-cytometric analysis for MHC I and MHC II was performed on cultured fetal (16 and 22 weeks) and adult keratinocytes. Purple tracings represent the stained population, whereas red tracings set the background reference with an isotype-matched antibody. Positive staining (%) was defined by a gate that included 3% of the background population.
MHC I expression in fetal keratinocytes was low, with 5% expression at 16 weeks, but increased to 19% by 22 weeks. More than a third of adult keratinocytes were positive for MHC I. MHC II was similarly expressed in <5% of both fetal and adult keratinocytes (Figure 4B).
Fetal Keratinocytes and Fetal Fibroblasts Are Able to Suppress T Cell Proliferation
As T cell activation is one of the early, key events that may initiate allograft rejection, we asked whether fetal keratinocytes and fetal fibroblasts can activate T cells in coculture conditions. Neither adult nor fetal cells (keratinocytes and fibroblasts) induced T cell proliferation (Figures 5A and 6A). When adult keratinocytes were added into a CD3/28 bead-induced T cell proliferation assay, they were found to enhance T cell proliferation. In contrast, high doses of fetal keratinocytes were able to suppress T cell proliferation in the same assay, suggesting that these cells have some innate ability to modulate the immune function of T cells in a dose-dependent manner (Figures 5B and 5C). The immunosuppressive ability was more prominent in fetal than adult fibroblasts, with the suppression increasing in a dose-dependent manner (Figure 6B).
Figure 5.
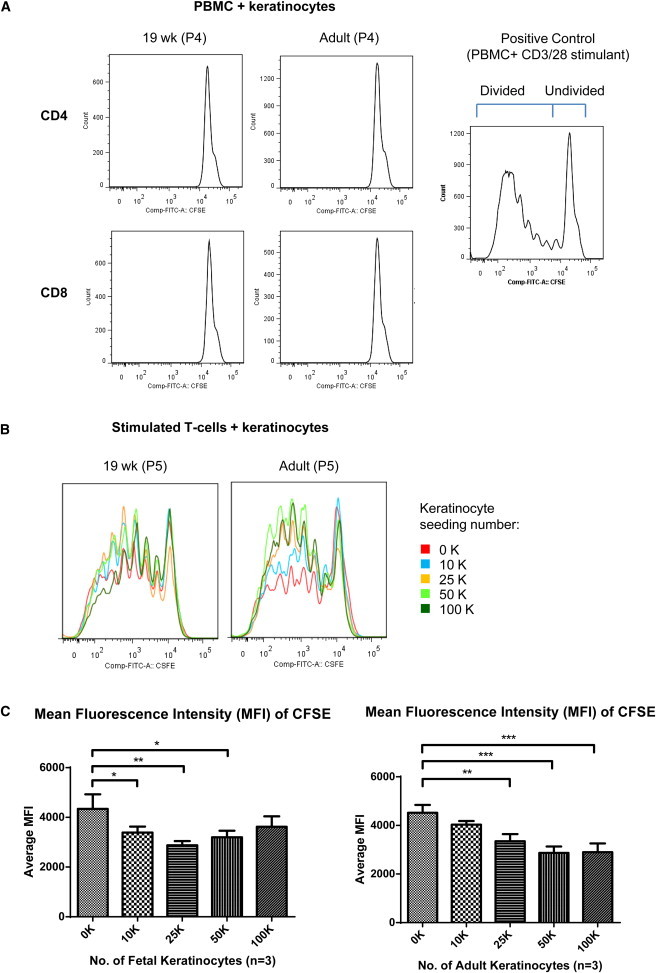
Fetal Keratinocytes Suppress T Cell Proliferation at High Numbers
Proliferation of T cells after 8 days of incubation with cultured keratinocytes.
(A) PBMCs labeled with CFSE were cocultivated with keratinocytes for 8 days. Proliferating T cells (divided) were CFSEdim and resting T cells (undivided) remained CFSEbright. Adult and fetal keratinocytes (19 weeks) did not cause T cells to proliferate.
(B) Proliferation of stimulated T cells after 8 days of incubation with cultured fetal (19 weeks) and adult keratinocytes. CD4 T cells were stained with CFSE, stimulated with CD3/28 beads, and cocultured with keratinocytes for 8 days before flow-cytometry analysis.
(C) Mean fluorescence intensity (MFI) of CFSE. Lower MFI values indicate more proliferation of cells due to the dilution of CFSE as the cells divide. Left: MFI of CFSE when cocultured with fetal keratinocytes (17, 19, and 22 weeks). Right: MFI of CFSE when cocultured with adult keratinocytes. Data are represented as mean ± SD of three biological replicates and using Dunnett’s post hoc analysis. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Figure 6.
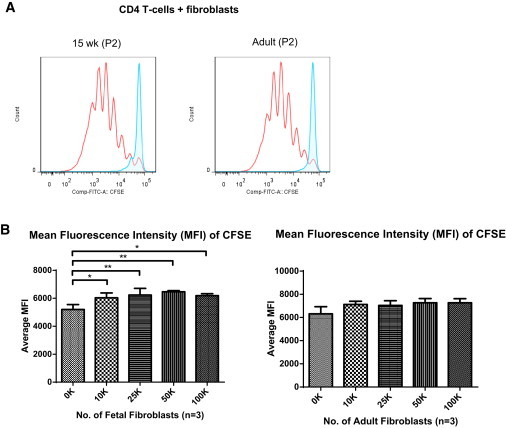
Fetal Fibroblasts Suppress CD3/28 Bead-Induced T Cell Proliferation
(A) Proliferation of T cells after 8 days of incubation with cultured fetal (15 weeks) and adult fibroblasts. CD4 T cells were labeled with CFSE and cocultured with fetal and adult fibroblasts. Blue tracings represent unstimulated T cells (undivided) that remained CFSEbright. Red tracings represent proliferating T cells (divided) stimulated with CD3/28 beads that were CFSEdim in the presence of 1 × 105 fibroblasts.
(B) MFI of CFSE. Lower MFI values indicate more proliferation of cells due to the dilution of CFSE as the cells divide. Left: MFI of CFSE when cocultured with fetal fibroblasts (15, 17, and 19 weeks). Right: MFI of CFSE when cocultured with adult fibroblasts. In all experiments, three adult and three fetal samples were used. Data are represented as mean ± SD of three biological replicates and using Dunnett’s post hoc analysis. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Cultured Human Fetal Keratinocytes and Fetal Fibroblasts Can Be Successfully Engrafted with Stable Human-to-Mouse Skin Regeneration
When tested in an organotypic culture system, fetal keratinocytes were able to generate a multilayered epithelial structure with suprabasal expression of K10, which is typical of normal epidermal differentiation (Figures 7A and S6). Therefore, we grafted fetal keratinocyte-based skin mimetic constructs onto SCID mice, using a previously described method (Llames et al., 2004) with some minor modifications (see Experimental Procedures), to further challenge their ability to differentiate. Successful grafting was confirmed at 8 weeks posttransplantation. The grafts showed histological structure similar to mature human skin, with five to seven epidermal layers and a cornified layer, as well as good seamless integration between graft and host tissues (Figures 7B, 7C, and S7). Staining with human-specific antibodies to nucleus LP4N (Figure 7D; Jeppe-Jensen et al., 1993), K10 (Figure 7E; Leigh et al., 1993), involucrin (Figure 7F; Llames et al., 2004), and vimentin (Figure 7G; Bohn et al., 1992) demonstrated the persistence of human cells in the full thickness of the graft. alpha-Smooth muscle actin (α-SMA) was expressed in the dermis of the regenerated skin at 7 days and 14 days postgrafting, confirming the expected presence of myofibroblasts (Figures 7H and 7I) associated with a wound-healing state. By 8 weeks postgrafting, the myofibroblasts had disappeared, reflecting the fully healed state of the skin graft, and α-SMA staining was limited to blood vessels in the regenerated skin (Figure 7J). Fetal fibroblasts were also able to successfully integrate into the grafts when adult keratinocytes were used (Figures 7K–7M).
Figure 7.
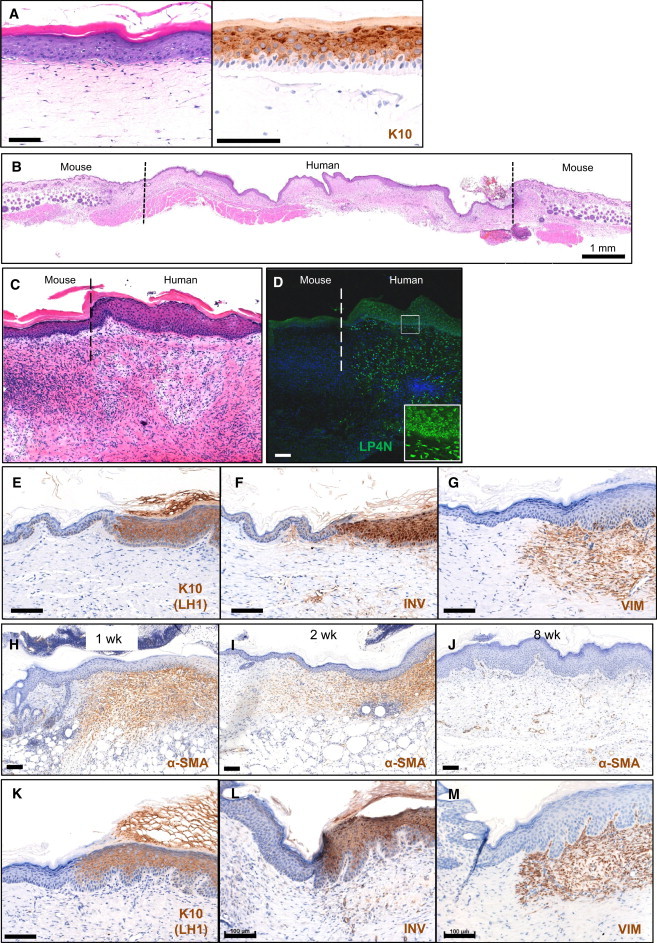
Fetal Keratinocytes Can Stratify in In Vitro Skin Equivalent Models and Can Be Engrafted Successfully with Stable Human-to-Mouse Skin Regeneration
(A) Hematoxylin and eosin staining of skin equivalents of cultured keratinocytes. Fetal and adult keratinocytes were seeded on a fibroblast-embedded collagen gel and cultured in a submerged condition before initiating differentiation of the epidermal layer at the air-liquid interface for 14 days. Expression of K10 in suprabasal layers demonstrates that fetal keratinocytes can stratify in vitro. Scale bar, 100 μm. See also Figure S6.
(B) Histological appearance of culture-generated human fetal skin on the back of a SCID mouse 3 weeks postgrafting.
(C and D) Immunofluorescence staining with the antibody LP4N, which is specific for human cells. The nuclei of human epidermis were stained and the nuclei of mouse epidermis were unstained.
(E–G) Human-specific keratin 10 (K10), involucrin (INV), and vimentin (VIM) immunostaining at the junction of regenerated human skin. Antibody staining shows normal stratification in grafted skin 8 weeks postgrafting.
(H–J) Immunostaining of α-SMA at the junction of regenerated human skin. α-SMA was expressed in the dermis of the regenerated skin at 1 week postgrafting, indicating the presence of myofibroblasts. At 8 weeks postgrafting, α-SMA marked the presence of blood vessels in the regenerated skin, but myofibroblasts had disappeared by that time.
(K–M) Human-specific K10, INV, and VIM immunostaining at the junction of regenerated human skin grafted with fetal fibroblasts and adult keratinocytes. Scale bar, 100 μm.
See also Figure S7.
Discussion
We report here the isolation and characterization of fetal keratinocytes derived from second-trimester fetuses. Fetal keratinocytes were found to be more proliferative and clonogenic, and to have longer telomeres and lower expression of MHC proteins than adult keratinocytes. We also show that fetal keratinocytes are capable of differentiating in organotypical cultures and can be successfully grafted in a well-described mouse model. These findings suggest that fetal skin cells have significant potential as an allogeneic source of cells for life-saving culture-generated grafts. The work presented here has implications for a next generation of cost-effective, user-friendly, bioengineered skin constructs based on nonanimal products. The possibility of “off-the-shelf” availability is important, especially in cases where treatment must be carried out early and at very short notice, such as massive burn wounds.
Human fetal dorsal skin, from which the fetal keratinocytes were cultured for this study, was analyzed histologically to correlate morphologic changes of the skin to biochemical changes in structural proteins during development. K18, which along with K8 is typical of simple epithelia and early embryonic stages (Moll et al., 1982), and K19, which is expressed in adult mixed epithelial regions and possibly is a stem cell niche indicator (Stasiak et al., 1989), were still expressed in the basal epidermal layer and periderm of fetal skin up until 22 weeks. K17, which is typically expressed by “activated” keratinocytes, was present in fetal epidermis but reduced with increasing age. If fetal cells are ultimately to be used for clinical applications, quality-control measures will be needed to ensure that the cells being propagated retain their defined state. Thus, it is significant that the fetal phenotype persists in tissue culture, as shown by the retention of fetal keratin expression in culture. Fetal keratinocyte cultures can therefore be distinguished from their adult counterparts by expression of K18 and K19.
We have shown that fetal keratinocytes can be stably and successfully cultured in vitro while maintaining their normal phenotype and karyotype. No slowing of growth was observed until cells were beyond 20 pds, about twice as many cell divisions as observed for similar adult cells. This significantly higher proliferative potential suggests that fetal cells can provide a long-lived (and thus more economical and more accessible to a greater number of patients) cell source for tissue-regeneration applications. This will facilitate exhaustive characterization of a single fetal keratinocyte bank prior to clinical use. By using the isolation and culture techniques described here, one can induce a 4 cm2 sample of fetal skin to generate sufficient cells to expand to an area of 16 m2 within 1 week of recovering live cells from a frozen cell bank (Figure S5B). Here, we cryopreserved cells using a progressive freezing method and achieved a recovery of >80% even after 3 years of liquid nitrogen storage, showing that these cells are robust in tissue culture. We have achieved this efficiency using serum-free culture without mouse-derived fibroblast feeder cells; therefore, the process should be easily and quickly adapted to meet GMP culture requirements. With this yield and efficiency, additional steps to enrich for stem cells may be unnecessary—anything that reduces handling will increase cell viability and thus further increase cell yield.
In spite of the developmental immaturity of the starting material, second-trimester fetal keratinocytes are clearly capable of achieving fundamentally normal adult-type differentiation in vitro, as they can form a stratified epidermal structure in an organotypical culture system that expresses major structural proteins of adult epidermis. Proof of principle was established in a preclinical human-to-mouse model using immune-deficient mice (Del Rio et al., 2002) optimized for grafting cultured human fetal keratinocytes and fibroblasts. The successful engraftment and stable skin regeneration achieved using cultured fetal skin cells show that these cells can generate mature, differentiated epidermis in vivo.
The biggest obstacle to skin grafting using anything other than the patient’s own cells is immune rejection. The data presented here reveal low MHC I expression and no MHC II expression in the fetal skin cells. The fetal cells were also shown to elicit no proliferative response in naive T cells. Coculture with fetal keratinocytes or fetal fibroblasts even led to suppression of T cell proliferation. This may be due to production of factors with immunosuppressive activity (Kehrl et al., 1986; Lúdvíksson et al., 2000; Taylor et al., 2006) or other mechanisms that operate in the state of mutual immune tolerance that exists between the fetus and mother during pregnancy (Munn et al., 1998; Meisel et al., 2004; Hunt et al., 2005). The effect of fetal skin cells on regulatory T cells (Treg), which are capable of modulating tolerance in the immune response (Sakaguchi et al., 2001), may also play a role. In a previous study, fetal liver mesenchymal stem cells were shown to exhibit various levels of inhibitory immune effects (Götherström et al., 2004). In a related study, Zuliani et al. (2013) recently reported evidence of suppression of peripheral blood mononuclear cell (PBMC) proliferation in a sample of fetal keratinocytes, although they made no comparison with adult cultures. These authors suggested a role for indoleamine 2,3 dioxygenase (IDO) in the immunosuppressive effects. The in vitro data presented here suggest that fetal keratinocytes may have an “immunological advantage” that could be of significant benefit in future clinical applications of these cells.
Although the present study focuses predominantly on keratinocytes, it has been known for many years that fibroblasts play an important role in wound healing and in remodeling the extracellular matrix. Thus, an ideal bioengineered graft will always need to incorporate fibroblasts as well as keratinocytes. The combination of keratinocytes and fibroblasts with a keratinocyte/fibroblast ratio of 1:9 in a spray device was shown clinically to be very effective in promoting wound closure (Goedkoop et al., 2010; Kirsner et al., 2012). However, the use of such growth-arrested cells still requires time to stimulate wound coverage, whereas keratinocytes and fibroblasts grown in a fibrin scaffold can be grafted instantly. Here, we have shown that fetal cells in such a combination grow well for at least 8 weeks in a human-to-mouse skin graft, suggesting that they are a viable option for covering open wounds.
The improved method of preparing fibrin gels directly from whole plasma will be useful for constructing bioengineered skin equivalents to provide a more cost-effective and clinically suitable product. Fetal cells have also been shown to adapt well to various biocompatible materials with high survival rates (Montjovent et al., 2005; De Buys Roessingh et al., 2006), favoring their use in tissue engineering. De Buys Roessingh et al. (2006) reported that fetal fibroblasts are also resistant to various environmental stresses and low oxygen conditions, suggesting that they are likely to survive when grafted into hostile wound environments.
There is much discussion about the potential of induced pluripotent stem cells (iPSCs) as an autologous cell source to generate large numbers of tissue cells for therapeutic applications, including the generation of keratinocytes (Guenou et al., 2009). When compared with the handling methods required for iPSCs and embryonic and mesenchymal stem cells, the isolation and cell-culture procedures used for fetal skin cells are technically less demanding. Expansion and maintenance of iPSCs in an undifferentiated state and during subsequent reprogramming require the addition of many specific growth factors, presenting a financial obstacle against upscaling of stem cell cultures for clinical applications. Unlike stem cells, fetal keratinocytes are already programmed for efficient, full epidermal differentiation, and also have high expansion potential and low immunogenicity.
Although it is speculative at this point, another possible benefit of using immature cells such as those described here for grafting is that it may be possible to reinitiate appendage formation from fetal cells. The absence of appendages such as hair follicles and sweat glands is one of the most difficult consequences of large-scale grafting. Holbrook et al. (1993) and Holbrook and Minami (1991) reported that fetal skin tissue from a critical window of time (9–12 weeks gestation) could initiate follicle morphogenesis in vitro. In addition, primary cultures of mouse fetal and neonatal skin cells containing both epidermal and dermal cells will reform skin, complete with hair follicles, if transplanted into subcutaneous sites in the mouse (Yuspa et al., 1970; Worst et al., 1982). The factors that control sweat-gland development are even less understood, but recent publications have begun to address the nature of sweat-gland stem cells (Lu et al., 2012) and the interaction between progenitor cells and extracellular matrix in generating sebaceous glands (Horsley et al., 2006).
Human-to-mouse culture grafts using cultured fetal cells as described here will be useful for studies on wound healing and diseases. Wound healing in adults usually results in scarring, which can cause functional restrictions in movement as well as negative physical and psychological effects on the patient. Formation of hypertrophic scars and keloids is also a burden and is difficult to treat medically. The developing fetus has a remarkable ability to heal skin wounds by regenerating normal epidermis and dermis with restoration of skin architecture, strength, and function in the absence of any scar formation (Bullard et al., 2003).
Although there is strong clinical support for developing cellular therapies, and the use of such therapies is already reaching clinical translation (Götherström et al., 2014), ethical issues associated with the collection and use of fetal tissue for research and therapy still remain. Concerned political and religious groups have lobbied against funding for research using fetal tissues that have been obtained from clinically indicated termination of pregnancies, restricting progress in the field. Donation of fetal skin is considered as an organ donation by law in Singapore and most other countries, but this process is highly regulated under strict guidelines and human tissue transplantation laws, including ethics committee approval of the procedure.
The future of fetal cell therapy is likely to be beset with numerous ethical issues, not the least of which is the reluctance of some patients to receive grafts from fetal cells. This issue will likely be less acute in emergency life-saving situations such as extensive burns, which would probably be the first context in which fetal grafts would be tested, but will have to be dealt with sensitively. All human tissue is precious. As we begin to understand much more about the mechanisms behind skin regeneration, a new approach to grafting is timely, and this may well be the most significant phase in the evolution of skin reconstruction.
Experimental Procedures
Skin Biopsies
Human fetal tissues were obtained from National University Hospital, Singapore, with approval from the local institutional review board (IRB). Women undergoing clinically indicated termination of pregnancy gave written informed consent for the use of fetal tissue for this research. Fetal skin samples were collected from the back after completion of termination, with gestational ages ranging between 13 and 23 weeks of amenorrhoea (n = 60 samples). Human adult skin samples were obtained from discarded surgical material from healthy donors with their informed consent and with approval from the local IRB. Skin samples were fixed in 10% neutral buffered formalin or snap-frozen in liquid nitrogen for subsequent processing for histochemistry.
Histological and Immunostaining Analysis
Formalin-fixed skin samples were processed for paraffin wax embedding. Endogenous peroxidase activity in sections was quenched by incubation with 1% H2O2 for 30 min and antigen access was recovered by heating in citrate buffer pH 6. Sections were incubated with primary antibodies for 90 min at room temperature (Table S2) after blocking in 10% goat serum. Sections were washed in water and then incubated in secondary antibody using the EnVision system (Dako) for 30 min at room temperature. Peroxidase activity was detected with diaminobenzidine tetrahydrochloride (DAB) substrate (Dako). Sections were counterstained with hematoxylin.
For immunofluorescence, frozen sections or cells grown on ibiTreat chambers (Ibidi) were fixed in methanol/acetone (1:1) at –20°C for 10 min and blocked in 10% goat serum as above. Sections or cells were incubated with primary antibodies for 60 min (Table S2), followed by incubation with secondary antibody coupled to Alexa Fluor 488 (Molecular Probes; Life Technologies) and counterstained with DAPI.
Isolation and Culture of Epidermal Keratinocytes and Dermal Fibroblasts
Fetal skin biopsies were washed in 4× antibiotic/antimycotic solution (Sigma-Aldrich), cut into small pieces, and soaked in 0.125% trypsin at 4°C overnight. Specimens were dissociated into single-cell suspensions and filtered using a cell strainer (BD Biosciences). Cells were cultured in DermaLife K serum-free keratinocyte culture media (Lifeline Cell Technology) to establish keratinocyte cultures. For fetal skin at <18 weeks gestation, a 0.1% gelatin solution (StemCell Technologies) was used to coat culture flasks to improve cell attachment for keratinocytes. Fibroblast cultures were established using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% serum. For keratinocyte cultures, contaminating fibroblasts were removed by treatment with 0.02% EDTA (Sigma-Aldrich) for 5 min (Figure S4).
To isolate adult keratinocytes, adult skin was soaked in Dispase (Roche Applied Science) at 4°C overnight, followed by separation of the epidermis from the dermis. The epidermis was incubated with 0.125% trypsin for 20 min and the cell suspension was centrifuged and seeded on tissue culture flasks. Adult fibroblasts were isolated by collecting cells growing out from minced tissue organoids in culture.
Organotypic Culture
Adult fibroblasts embedded in rat-tail collagen I (BD Biosciences) were prepared and placed into a hanging-cell culture insert (1 μm pore size; Merck Millipore). Fetal keratinocytes (1 × 105 cells) were seeded on top of collagen gels and cultured in a submerged state for 7 days and then at the air-liquid interface for 14 days. Keratinocytes in the submerged state were maintained with serum-free medium, and fibroblasts in collagen gels were maintained with growth medium consisting of a mixture of three parts DMEM to one part Ham’s nutrient mixture F12 medium supplemented with 10% fetal bovine serum (FBS), 5 μg/ml insulin, 0.4 μg/ml hydrocortisone, 5 μg/ml transferrin, 1.8 × 10−4 M adenine, 10 ng/ml epidermal growth factor (EGF), and 2 × 10−11 M triiodothyronine. At the air-liquid interface, EGF and serum were removed from the medium. Samples were fixed in 10% neutral buffered formalin and embedded in paraffin for histology (Figure S6).
T Cell Proliferation Assay
T cell proliferation was evaluated by flow cytometry after PBMCs labeled with carboxyfluorescein succinimidyl ester (CFSE) had been incubated for 8 days with cultured keratinocytes or fibroblasts. The cell-surface dye CFSE is retained on the cells but gradually dilutes as cells divide and share the remaining dye between the daughter cells, resulting in lower fluorescence intensity in a proliferating cell population. As a positive control, beads coated with anti-CD3 and anti-CD28 were used to stimulate T cells to divide in vitro. For the immunomodulatory assay, isolated CD4+ T cells were stained with CFSE prior to stimulation with CD3/28 beads, and 200,000 cells were cocultured with keratinocytes (P5) or fibroblasts (P2) with different seeding densities. Lymphocyte cell division was assessed after 8 days.
Plasma-Based Skin-Equivalent Transplants
A dermal-equivalent construct was generated from pig blood plasma (Equitech-Bio) using methods modified and optimized from a published protocol (Llames et al., 2004, 2006). Fibroblasts were resuspended at a concentration of 1 × 106 cells/ml before preparation of the fibrin gel. Then, 3 ml of mixed plasma, fibroblasts, and thrombin (6 NIH U/ml) was added into each culture insert (BD Biosciences). The mixture was incubated at 37°C for 2 hr to allow thrombin-cleaved plasma fibrinogen to form a fibrin gel before addition of medium. The following day, 5 × 105 keratinocytes in 200 μl suspension were seeded on top of the gel and then cultured in a submerged state until the keratinocytes reached confluence.
Human-to-mouse grafting was performed as described previously (Del Rio et al., 2002) using SCID mice, with minor modifications. A 3 cm × 3 cm area of mouse skin, comparable in size to the graft, was surgically excised and devitalized by freezing (by immersion in liquid nitrogen for 2 min) and heating (in an 80°C water bath for 2 min) cycles for three times. The devitalized skin flap was fixed in place with a continuous running stitch around half of the flap to form a pocket. The gel with fibroblasts and confluent keratinocytes was carefully lifted and placed inside the pocket, which was then closed by a continuous suture. No further bandaging was required. The mouse was placed in a clean and warm environment to recover. At scheduled time points of the study, mice were euthanized and skin tissue was collected for histology. All procedures were approved by the local IACUC, and animals were cared for in accordance with institutional guidelines.
Statistical Analysis
Data are presented as the mean and SD or SEM where appropriate. Data analysis was performed by one-way ANOVA followed by post hoc Dunnett’s test for multiple comparisons of at least three groups or by unpaired Student’s t test for two groups using Prism 5.0 (Graphpad Software); p values < 0.05 were considered statistically significant.
Acknowledgments
We thank John Lim and the IMB Microscopy Unit for technical support. We also thank M. del Rio and F. Larcher (CIEMAT, Madrid) for instruction in the early days of learning techniques for human-to-mouse grafting, and T.C. Lim for providing normal skin fragments. This work was supported by the Biomedical Research Council of Singapore through the Institute of Medical Biology, A∗STAR. K.K.B.T. was funded by a Graduate Scholarship from the NUS Graduate School for Integrative Sciences and Engineering, Singapore, and J.K.Y.C. received salary support from the National Medical Research Council (NMRC/CSA/043/2012).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Contributor Information
Jerry K.Y. Chan, Email: jerrychan@nus.edu.sg.
E. Birgitte Lane, Email: birgit.lane@imb.a-star.edu.sg.
Supplemental Information
References
- Barrandon Y., Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc. Natl. Acad. Sci. USA. 1985;82:5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn W., Wiegers W., Beuttenmüller M., Traub P. Species-specific recognition patterns of monoclonal antibodies directed against vimentin. Exp. Cell Res. 1992;201:1–7. doi: 10.1016/0014-4827(92)90341-5. [DOI] [PubMed] [Google Scholar]
- Bullard K.M., Longaker M.T., Lorenz H.P. Fetal wound healing: current biology. World J. Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- Compton C.C., Gill J.M., Bradford D.A., Regauer S., Gallico G.G., O’Connor N.E. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab. Invest. 1989;60:600–612. [PubMed] [Google Scholar]
- Davies S.B., Chui J., Madigan M.C., Provis J.M., Wakefield D., Di Girolamo N. Stem cell activity in the developing human cornea. Stem Cells. 2009;27:2781–2792. doi: 10.1002/stem.209. [DOI] [PubMed] [Google Scholar]
- De Buys Roessingh A.S., Hohlfeld J., Scaletta C., Hirt-Burri N., Gerber S., Hohlfeld P., Gebbers J.O., Applegate L.A. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplant. 2006;15:823–834. doi: 10.3727/000000006783981459. [DOI] [PubMed] [Google Scholar]
- Del Rio M., Larcher F., Serrano F., Meana A., Muñoz M., Garcia M., Muñoz E., Martin C., Bernad A., Jorcano J.L. A preclinical model for the analysis of genetically modified human skin in vivo. Hum. Gene Ther. 2002;13:959–968. doi: 10.1089/10430340252939069. [DOI] [PubMed] [Google Scholar]
- Depreter M.G.L., Blair N.F., Gaskell T.L., Nowell C.S., Davern K., Pagliocca A., Stenhouse F.H., Farley A.M., Fraser A., Vrana J. Identification of Plet-1 as a specific marker of early thymic epithelial progenitor cells. Proc. Natl. Acad. Sci. USA. 2008;105:961–966. doi: 10.1073/pnas.0711170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Gaunt G., Ramin K. Immunological tolerance of the human fetus. Am. J. Perinatol. 2001;18:299–312. doi: 10.1055/s-2001-17861. [DOI] [PubMed] [Google Scholar]
- Goedkoop R., Juliet R., You P.H., Daroczy J., de Roos K.P., Lijnen R., Rolland E., Hunziker T. Wound stimulation by growth-arrested human keratinocytes and fibroblasts: HP802-247, a new-generation allogeneic tissue engineering product. Dermatology (Basel) 2010;220:114–120. doi: 10.1159/000277380. [DOI] [PubMed] [Google Scholar]
- Götherström C., Ringdén O., Tammik C., Zetterberg E., Westgren M., Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am. J. Obstet. Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Götherström C., Westgren M., Shaw S.W., Aström E., Biswas A., Byers P.H., Mattar C.N., Graham G.E., Taslimi J., Ewald U. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl. Med. 2014;3:255–264. doi: 10.5966/sctm.2013-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. The birth of therapy with cultured cells. BioEssays. 2008;30:897–903. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenou H., Nissan X., Larcher F., Feteira J., Lemaitre G., Saidani M., Del Rio M., Barrault C.C., Bernard F.X., Peschanski M. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet. 2009;374:1745–1753. doi: 10.1016/S0140-6736(09)61496-3. [DOI] [PubMed] [Google Scholar]
- Guillot P.V., Gotherstrom C., Chan J., Kurata H., Fisk N.M. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- Hohlfeld J., de Buys Roessingh A., Hirt-Burri N., Chaubert P., Gerber S., Scaletta C., Hohlfeld P., Applegate L.A. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366:840–842. doi: 10.1016/S0140-6736(05)67107-3. [DOI] [PubMed] [Google Scholar]
- Holbrook K.A., Minami S.I. Hair follicle embryogenesis in the human. Characterization of events in vivo and in vitro. Ann. N Y Acad. Sci. 1991;642:167–196. [PubMed] [Google Scholar]
- Holbrook K.A., Smith L.T., Kaplan E.D., Minami S.A., Hebert G.P., Underwood R.A. Expression of morphogens during human follicle development in vivo and a model for studying follicle morphogenesis in vitro. J. Invest. Dermatol. 1993;101(1, Suppl):39S–49S. doi: 10.1111/1523-1747.ep12362616. [DOI] [PubMed] [Google Scholar]
- Horsley V., O’Carroll D., Tooze R., Ohinata Y., Saitou M., Obukhanych T., Nussenzweig M., Tarakhovsky A., Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J.S., Petroff M.G., McIntire R.H., Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- Jeppe-Jensen D., Clausen H., Leigh I.M., Lane E.B., Dabelsteen E. Three monoclonal antibodies differentiate human from murine epidermis. Epithelial Cell Biol. 1993;2:100–106. [PubMed] [Google Scholar]
- Kamel R.A., Ong J.F., Eriksson E., Junker J.P., Caterson E.J. Tissue engineering of skin. J. Am. Coll. Surg. 2013;217:533–555. doi: 10.1016/j.jamcollsurg.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos-Langevin C., Caucheteux S.M., Verbeke P., Ojcius D.M. Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod. Biol. Endocrinol. 2003;1:121. doi: 10.1186/1477-7827-1-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J.H., Wakefield L.M., Roberts A.B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M.B., Fauci A.S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsner R.S., Marston W.A., Snyder R.J., Lee T.D., Cargill D.I., Slade H.B. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2012;380:977–985. doi: 10.1016/S0140-6736(12)60644-8. [DOI] [PubMed] [Google Scholar]
- Lane E.B., Wilson C.A., Hughes B.R., Leigh I.M. Stem cells in hair follicles. Cytoskeletal studies. Ann. N Y Acad. Sci. 1991;642:197–213. doi: 10.1111/j.1749-6632.1991.tb24388.x. [DOI] [PubMed] [Google Scholar]
- Leigh I.M., Purkis P.E., Whitehead P., Lane E.B. Monospecific monoclonal antibodies to keratin 1 carboxy terminal (synthetic peptide) and to keratin 10 as markers of epidermal differentiation. Br. J. Dermatol. 1993;129:110–119. doi: 10.1111/j.1365-2133.1993.tb03511.x. [DOI] [PubMed] [Google Scholar]
- Llames S.G., Del Rio M., Larcher F., García E., García M., Escamez M.J., Jorcano J.L., Holguín P., Meana A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation. 2004;77:350–355. doi: 10.1097/01.TP.0000112381.80964.85. [DOI] [PubMed] [Google Scholar]
- Llames S., García E., García V., del Río M., Larcher F., Jorcano J.L., López E., Holguín P., Miralles F., Otero J., Meana A. Clinical results of an autologous engineered skin. Cell Tissue Bank. 2006;7:47–53. doi: 10.1007/s10561-004-7253-4. [DOI] [PubMed] [Google Scholar]
- Lowell S., Jones P., Le Roux I., Dunne J., Watt F.M. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Lu C.P., Polak L., Rocha A.S., Pasolli H.A., Chen S.C., Sharma N., Blanpain C., Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lúdvíksson B.R., Seegers D., Resnick A.S., Strober W. The effect of TGF-beta1 on immune responses of naïve versus memory CD4+ Th1/Th2 T cells. Eur. J. Immunol. 2000;30:2101–2111. doi: 10.1002/1521-4141(200007)30:7<2101::AID-IMMU2101>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Lyle S., Christofidou-Solomidou M., Liu Y., Elder D.E., Albelda S., Cotsarelis G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci. 1998;111:3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Michel M., Török N., Godbout M.J., Lussier M., Gaudreau P., Royal A., Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J. Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W.W., Schiller D.L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Montjovent M.O., Mathieu L., Hinz B., Applegate L.L., Bourban P.E., Zambelli P.Y., Månson J.A., Pioletti D.P. Biocompatibility of bioresorbable poly(L-lactic acid) composite scaffolds obtained by supercritical gas foaming with human fetal bone cells. Tissue Eng. 2005;11:1640–1649. doi: 10.1089/ten.2005.11.1640. [DOI] [PubMed] [Google Scholar]
- Montjovent M.O., Bocelli-Tyndall C., Scaletta C., Scherberich A., Mark S., Martin I., Applegate L.A., Pioletti D.P. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng. Part A. 2009;15:1523–1532. doi: 10.1089/ten.tea.2008.0222. [DOI] [PubMed] [Google Scholar]
- Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Nijhof J.G.W., Braun K.M., Giangreco A., van Pelt C., Kawamoto H., Boyd R.L., Willemze R., Mullenders L.H.F., Watt F.M., de Gruijl F.R., van Ewijk W. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- Pham C., Greenwood J., Cleland H., Woodruff P., Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33:946–957. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Porter R.M., Lunny D.P., Ogden P.H., Morley S.M., McLean W.H., Evans A., Harrison D.L., Rugg E.L., Lane E.B. K15 expression implies lateral differentiation within stratified epithelial basal cells. Lab. Invest. 2000;80:1701–1710. doi: 10.1038/labinvest.3780180. [DOI] [PubMed] [Google Scholar]
- Roobrouck V.D., Ulloa-Montoya F., Verfaillie C.M. Self-renewal and differentiation capacity of young and aged stem cells. Exp. Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Shimizu J., Yamazaki S., Sakihama T., Itoh M., Kuniyasu Y., Nomura T., Toda M., Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- Stasiak P.C., Purkis P.E., Leigh I.M., Lane E.B. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J. Invest. Dermatol. 1989;92:707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- Tan D.W.M., Jensen K.B., Trotter M.W.B., Connelly J.T., Broad S., Watt F.M. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development. 2013;140:1433–1444. doi: 10.1242/dev.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Verhagen J., Blaser K., Akdis M., Akdis C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E., Karagulle E., Turan H., Oguz H., Abali E.S., Ozcay N., Moray G., Haberal M. Successful skin homografting from an identical twin in a severely burned patient. J. Burn Care Res. 2014;35:e177–e179. doi: 10.1097/BCR.0b013e3182957572. [DOI] [PubMed] [Google Scholar]
- Van Zant G., Liang Y. The role of stem cells in aging. Exp. Hematol. 2003;31:659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Worst P.K., Mackenzie I.C., Fusenig N.E. Reformation of organized epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res. 1982;225:65–77. doi: 10.1007/BF00216219. [DOI] [PubMed] [Google Scholar]
- Yuspa S.H., Morgan D.L., Walker R.J., Bates R.R. The growth of fetal mouse skin in cell culture and transplantation to F1 mice. J. Invest. Dermatol. 1970;55:379–389. doi: 10.1111/1523-1747.ep12260498. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Teoh S.H., Hui J.H., Fisk N.M., Choolani M., Chan J.K. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33:2656–2672. doi: 10.1016/j.biomaterials.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Zuliani T., Saiagh S., Knol A.C., Esbelin J., Dréno B. Fetal fibroblasts and keratinocytes with immunosuppressive properties for allogeneic cell-based wound therapy. PLoS ONE. 2013;8:e70408. doi: 10.1371/journal.pone.0070408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


