Synopsis
Nutritional insufficiencies of omega-3 highly unsaturated fatty acids (HUFAs) may have adverse effects on brain development and neurodevelopmental outcomes. A recent meta-analysis of ten randomized controlled trials of omega-3 HUFAs reported a small to modest effect size for the efficacy of omega-3 for treating symptoms of ADHD in youth. Several controlled trials of omega-3 HUFAs combined with micronutrients (vitamins, minerals) show sizeable reductions in aggressive, antisocial, and violent behavior in youth and in young adult prisoners. Meta-analyses report efficacy for depressive symptoms in adults, and preliminary findings suggest anti-suicidal properties in adults, but studies in youth are insufficient to draw any conclusions regarding mood. Dietary adjustments to increase omega-3 and reduce omega-6 HUFA consumption are sensible recommendations for youth and adults based on general health considerations, while the evidence base for omega-3 HUFAs as potential psychiatric treatments develops.
Keywords: Omega-3 fatty acids, eicosapentaenoic acid, docosahexaenoic acid, arachidonic acid, child neurodevelopment, ADHD, conduct disorder, learning disorders
Introduction
Anyone who has observed children knows that their behavior changes dramatically when they are hungry. However, an important consideration is that children today may be consuming adequate or excessive calories, but their brains nonetheless can be starved of vital nutrients critical for optimal brain function, thus increasing risk for behavioral disorders and adverse developmental trajectories. Among these vital nutrients are iodine, folate, B vitamins, iron, zinc, micronutrients and omega-3 essential fatty acids. Dietary Guidelines for Americans, 2010 [1] address both specific nutrients and patterns of health eating to optimize physical health outcomes. Similarly, this article considers both specific nutrients and multiple interactive nutrients to optimize mental health outcomes.
The primary focus of the article is the effects of deficits in the dietary intake of omega-3 highly unsaturated fatty acids (HUFAs); the associated potential increase in risk for attention deficit hyperactivity disorder (ADHD) and similar behavioral disorders, and the hypothesis that omega-3 HUFAs has some treatment efficacy. Deficits of omega-3 HUFAs in depressive and aggressive disorders are also especially relevant to children; however, the main body of observational data and treatment studies has been conducted in adults. The proposition that nutritional insufficiencies in early development may have residual behavioral and cognitive deficits merits critical consideration.
The first part of the article will introduce the reader to nutritional requirements for optimal brain development and the impacts of nutritional inadequacy during pregnancy on adverse long-term developmental outcomes. Basic science issues related to neurological function and essential fatty acid metabolism underlying these findings will be addressed in the context of the dramatic differences between current dietary patterns and those during hominid evolution. Finally observational and treatment studies will be assessed for the plausibility of the efficacy of nutritional treatments for psychiatric disorders in children and adolescents.
Essential Nutrients and Risk of Deficiencies
The peak vulnerability to harm from nutritional deficiencies occurs during pregnancy, when the central nervous system is first developing. The quality of the maternal diet is particularly dependent on the intake of micronutrients (such as vitamins A and B, choline, and folate, trace elements (such as iodine, iron, zinc, and copper), and HUFAs, especially docosahexaenoic acid (DHA) and the omega-6 HUFA arachidonic acid (AA). These nutrients are especially critical during the fetal and early postnatal stage, when most areas of the brain are undergoing their most rapid development.
It is well-established that nutritional deficiencies (and excesses) may affect the infant brain and alter subsequent development and behavior permanently [2–4]. For example, the link between iodine deficiency and mental retardation is widely documented. In developing countries, about 38 million children are born at risk of iodine-associated mental retardation every year [5]. Deficiencies in iodine and iron (i.e., anemia) during infancy have been linked to a range of suboptimal developmental outcomes, including:
abnormal neuronal development [6]
disruptions in regulatory processes, such as the sleep-wake cycle [7]
sub-optimal performance in global measurements of cognition, motor skills, and social-emotional behavior [8–12]
mental retardation and cognitive deficits associated with reductions in learning capacity and productivity [6]
In animal models of nutritional deficiencies, a similar pattern of cognitive, motor, and behavioral changes is observed , along with alterations in dopaminergic function and lower dopamine levels in the cerebrospinal fluid compared to controls [13]. Iron deficiency also affects other neurotransmitters and other neuronal processes, including metabolism in hippocampus and striatum, myelination, dendritogenesis, and both gene and protein profiles [14–16]. HUFAs, including the omega-3 DHA, are similarly proposed to play a critical role during sensitive periods of neurodevelopment during early childhood and also in the regulation of cognitive function throughout the life span [17, 18]. While the beneficial effects of EPA and DHA for cardiovascular diseases and stroke are well established, their potential for preventing mild cognitive dysfunction and reducing the risk for Alzheimer's disease require further evaluation in large long-term clinical trials [19].
The Lipid Substance of the Brain
About 50 to 60% of the dry weight of an adult brain is comprised of lipid, and at least 35% of the lipid content is made up of highly unsaturated fatty acids (HUFAs). Given the high brain content of HUFAs, it is remarkable that these fatty acids are dietary essential: HUFAs cannot be synthesized de novo but must be either ingested directly from dietary sources or metabolized from essential polyunsaturated fatty acid (PUFA) precursors [20–22]. These fatty acids are highly specialized, with very specific metabolic functions and unique biophysical properties.
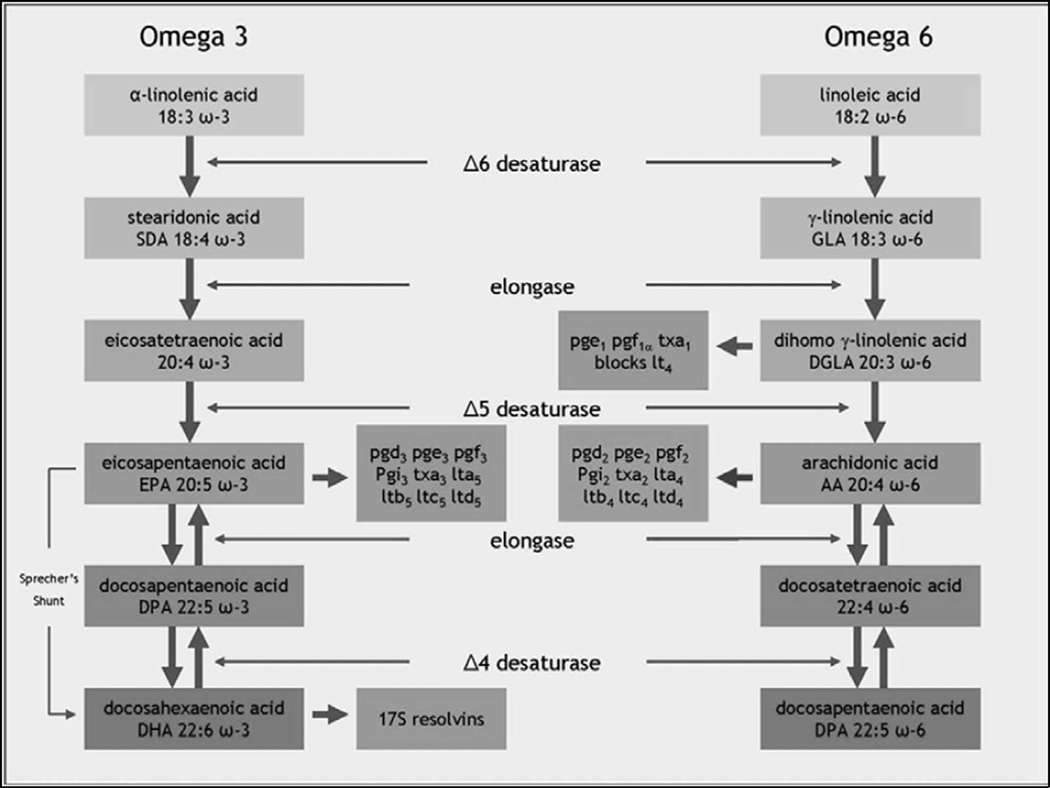
The biosynthetic pathways and metabolic interactions among the omega-3 and omega-6 series of fatty acids are complex. The parent compounds for the large number of HUFAs are two PUFAs: α-linolenic acid (ALA) is the precursor for the omega-3 fatty acids, and linoleic acid (LA) is the precursor for the omega-6 fatty acids [23]. These two precursor nutrients are the only fatty acids that are definitely essential, in the sense that the human body has no way to synthesize them, and they must be ingested in the diet. Until the 1950s, α-linolenic acid and linoleic acid were collectively known as Vitamin F.
Linoleic acid (LA), the omega-6 precursor, is the most abundant PUFA in the Western diet. In addition to its role in the brain, the omega-6 series are vital for mammalian reproduction [24]. Linoleic acid is primarily sourced from practically every commercially manufactured foods in the market place; in particular, it is sourced from soybean oil (the most frequently consumed oil), corn oil, and sunflower oil [25]. Typical dietary intake of omega-6 PUFAs in Western diets are excessive and thought to be in the region of 12 to 17 grams daily [26]. Linoleic acid is a metabolic precursor to γ-linolenic acid (gamma-linolenic acid, GLA) and arachidonic acid [26], having been converted by an elongase and two desaturase enzymes. Arachidonic acid is particularly abundant in the lipids of inner cell membranes, is important in the vasculature [24], and plays a crucial role in the production of eicosanoids. Although arachidonic acid can be synthesized from linoleic acid, the main dietary sources of arachidonic acid are red meat and dairy products including eggs [27].
The omega-3 precursor α-linolenic acid is metabolized into EPA and DHA, which are considered the two major omega-3 fatty acids. α-linolenic acid is readily available in vegetable sources (especially green leafy vegetables, plants, vegetable oils, nuts and seeds such as flax and canola). The richest direct source of EPA and DHA is marine fish (such as mackerel, salmon, herring and sardine) and seafood [23]. These two omega-3 fatty acids, EPA and DHA, are associated with many important functions related to neural activity, such as cell membrane fluidity, neurotransmission, ion channels, enzyme regulation, gene expression, and myelination [28, 29]. DHA alone makes up approximately 30% of the phosphoglycerides in the gray matter of the brain [30, 31] and is essential for optimal neuronal functioning [32]. Within brain tissues, DHA preferentially accumulates in growth cones, astrocytes, synaptosomes, myelin, and microsomal and mitochondrial membranes [33, 34]. Omega-3 fatty acids mediate a variety of key neurotransmitter functions, including serotonergic responsivity, signal transduction, and phospholipid turnover [21, 35, 36].
The eicosanoids, which can be derived from either omega-3 or omega-6 fatty acids, are a variety of compounds that are involved in the regulation of blood flow (vasodilatory prostacyclin), halting of blood flow in the case of injury (anti-thrombotic thromboxanes), the resolution of inflammation (anti-inflammatory prostaglandins), and tissue homeostasis [24, 26]. Diets which are depleted in omega-3 result in reductions of DHA in the brain and a simultaneous increase in the turnover of arachidonic acid to eicosanoids [37]. These effects can be reversed by adding omega-3 to the diet.
Eicosanoids have varying crucial yet complex functions in the brain and control over numerous bodily systems. A growing body of evidence suggests that inadequate omega-3 fatty acid levels during critical stages of neurogenesis may alter parameters of cell signalling, including within neurotransmitter systems, resulting in impairments in behavior, learning and cognition [38–41]. Eicosanoids have been shown to be involved in long-term potentiation, synaptic plasticity, spatial learning, and sleep induction; they also reduce neuroinflammation and have neuroprotective properties [36].
Excess Linoleic Acid and the Dietary Balance of Omega-3 and Omega-6 HUFAs
Both omega-3 and omega-6 fatty PUFA precursors are metabolized to their respective HUFAs by common enzyme pathways, which are influenced by many factors including diet, oxidative stress, alcohol, smoking, age and genetic factors [42–46]. These common pathways can be overloaded, leading to a bottleneck in the metabolism of both omega-3 and omega-6 HUFAs [47, 48]. The dietary balance of the ratio of omega-6 to omega-3 PUFAs therefore has important metabolic implications. For instance, excessive intake of the omega-6 linoleic acid, which is abundant in modern diets rich in vegetable oils, may inhibit the synthesis of the omega-3 α-linolenic acid to EPA and DHA, and thereby reduce the availability of EPA and DHA. An excessive dietary intake of the pro-inflammatory omega-6 HUFAs may reduce the synthesis and functioning of anti-inflammatory omega-3 compounds, leading to a tilt toward inflammatory processes such as cardiovascular disease, metabolic disorders, immunological conditions, and cancer [49, 50]. Similarly, in the brain, an excessive intake of omega-6 or an insufficient intake of omega-3 can potentially increase the risk of depression, speculatively by altering serotonergic and catecholaminergic neurotransmission [51–54].
The imbalance of omega-3 and 6 fatty acids present in modern diets today is a focal point of much scientific debate. Recent calculations estimate that omega-6 to omega-3 ratios in dietary intake have risen from about 1:1 to 2:1 to approximately 20:1 [55]. It has been suggested that these ratio increases are predominantly a result of the increased consumption of linoleic-rich soybean oil during the last century [25]. In a randomized clinical trial in which the intake of linoleic acid was selectively increased (n = 221) from approximately 6 to 15 % of dietary energy, increased mortality was observed from both cardiovascular disease (Hazard ratio 1.70, 95% confidence interval 1.03–2.80, p = 0.04) compared to controls (n =237) and coronary heart disease (Hazard ratio 1.74, 95% confidence interval 1.04 to 2.92, p = 0.04), which are findings consistent with other linoleic-selective trials [56] (see the Sydney Diet Heart Study updated meta-analysis for more information). Therefore, reducing omega-6 linoleic acid intake to ensure a good dietary balance of omega-3 and 6 fatty acids may be a key for optimal health outcomes.
Impact of Inadequate DHA Intake on Brain Development in Animals
A large body of research has confirmed the essential role of DHA in the development and function of the brain. The negative impact of inadequate DHA during critical periods of brain development has been well studied in animals and, to a lesser extent, in humans. Maternal nutritional deficiencies during neurogenesis and angiogenesis have long been associated with behavioral impairments in both animal models [57–59] and in humans [40, 60, 61]. It appears that HUFA insufficiency during lactation can lead to some irreversible changes [62], presumably due to impaired connectivity.
In animal studies, prenatal and postnatal DHA insufficiency has been associated with a variety of structural changes, such delayed neuronal migration, disrupted dendritic arborization, abnormal neuronal development in the hippocampus [52], and abnormalities in timed apoptosis. Neurochemical studies have shown alterations of several neurotransmission systems, including the dopaminergic and serotonergic systems [63]. The resulting altered or impaired connectivity may result in permanent disturbances [64]. Subsequent functional deficits include cognitive impairments, such as memory and learning [57] as well as deficits in emotional regulation and behavior, such as depression, anxiety, and aggression in animal models [65, 66]. Repletion of both omega-3 and omega-6 fatty acids into the diet during lactation in animals restores the brain fatty acid composition and some parameters of neurotransmitter function [62], but only partially.
In humans, DHA insufficiency in utero has been hypothesized to be linked to impaired magnocellular neurite growth associated with dyslexia [67]. Some studies have also reported findings of abnormal omega-3 HUFA levels in the erythrocytes of children and young adults with ADHD [68–75]. In addition, a growing body of clinical research has reported improvements in symptoms of ADHD [76–78], depression [79], learning difficulties and/or dyslexia [80] following supplementation with omega-3/6 fatty acids relative to placebo.
There is very little research however investigating the potential effects of omega-3 intervention in healthy control children. One functional magnetic resonance (fMRI) imaging study reported changes in cortical attention networks in healthy boys following DHA supplementation [81]. A recent randomized placebo-controlled study reported that DHA supplementation improved both reading and behavior in healthy but underperforming school children [82], although another clinical trial reported little or no effect of HUFA intervention compared to placebo on the cognitive ability and behavior of school children [83]. A more detailed review of the randomized placebo-controlled clinical trials in this area is provided later in the article.
Omega-3 interventions are more likely to demonstrate benefits among children with omega-3 deficiencies than among healthy children with omega-3 sufficiency. The collective findings from both animal and human studies has led researchers to postulate that deficits of omega-3 HUFAs during critical periods of brain development may increase the risk for neurodevelopmental disorders, such as ADHD and possibly predispose toward the later appearance of depressive and aggressive behaviors [54, 84, 85].
A Dietary Evolution: Unfortunate Consequences of Agricultural Developments
Crawford and others have argued that the fossil evidence indicates that the lacustrine (lake and shore) and marine food chains were being extensively exploited during the period when cerebral expansion took place, suggesting that the transition from the archaic to modern humans took place at the land/water interface. At the land/water interface in regions of hominid evolution, diets consumed from wild foods were lower in saturated fats [range 11–12 in percentage energy (en%)], higher in omega-3 HUFAs [2.26–17.0 grams per day (g/d)], lower in the omega-6 linoleic acid (range 2.3–3.6 en%), and a lower α-linolenic acid /linoleic acid (ALA/LA) ratio (range 1.12 to 1.64 g/d), indicating a lower omega-3/omega-6 ratio than contemporary diets [55]. The paleolithic diet is a modern dietary regimen which seeks to mimic the presumed diets of pre-agricultural hunter-gathers. Arguably, compared to modern Western diets consumed today, the paleolithic diets of our ancestors provided more DHA, which is a known key omega-3 constituent of the brain (and visual photoreceptor signalling systems). The shore-based theory has provided considerable evidence for our ancestors settling along the river-banks in Africa with access to fish, clams, frogs and seafood as their stable diet. Dr. Stephen Cunnane from the University of Sherbrooke and Dr. Kathy Stewart from the Canadian Museum of Nature in Ottawa have extensively studied fossil material excavated from numerous Homo habilis sites in eastern Africa which have revealed a bevy of chewed fish bones, particularly catfish. 172,173 Their theory is that a rich and secure shore-based diet fuelled and provided the essential nutrients to make our brains what they are today. Crawford and colleagues have also proposed that the availability of DHA was crucial to permit evolution of the human brain.[86, 87] DHA is both conserved and irreplaceable in neuronal signalling, and it is involved in the expression of several hundred genes [88], highlighting its unique and dominant place in brain evolution and biology in general.
The most dramatic and unfortunate changes from our paleolithic heritage of whole and unrefined foods are direct consequences of the Agricultural Revolution. Between the 17th and the end of the 19th century, and then continuing with a second wave after the World War II, new farming and technological changes brought the advent of mass food production, which has resulted in the problematic “modern refined diet” consumed today [49, 89–91]. Contemporary Western diets also have low quantities of key micronutrients (minerals, vitamins and trace elements), amino acids, antioxidants, fibre, and helpful phytochemicals, and are overloaded with sodium and refined sugars and grain products that carry a high glycemic load [89].
Even seemingly healthy parts of the modern Western diet have progressively lost their nutrient value. In the 1970s, poultry and eggs were the major land-based sources of protein and omega-3 fatty acids, especially DHA, and poultry was considered a healthy lean alternative to fatty red meat [92]. However, a laboratory analysis of modern supermarket chickens revealed that their energy from fat now actually exceeds energy from protein, and that their omega-6/omega-3 ratio is 9:1 rather than the recommended 2:1 ratio [93]. The loss of omega-3 HUFAs from chicken may result from feeding farm animals with soy-based products that are relatively deficient in omega-3 HUFAs, and the use of severely cramped bird cages that prevent exercise and reduce mitochondria-rich muscle mass [93]. This shift toward excessive omega-6 and insufficient omega-3 HUFAs in the human diet is argued to have adverse health consequences [94].
Despite its critical biological role, essential fatty acids cannot be synthesized or stored by the body for very long periods of time, and therefore must be obtained in the diet, and so these modern dietary changes have significant biological consequences. The recent rise in obesity [95] and diabetes (Word Health Organization 2002) in both children and adults is likely due to both the sedentary lifestyle and the excessive consumption of energy-dense refined foods rich in salt, sugar, and fats. “Type B malnutrition” is now recognized as a new type of malnutrition directly resulting multiple micronutrient depletion and very likely deriving from the globalization of the Western food systems [24]. Several scientific and governmental bodies have made dietary recommendations, including the Dietary Guidelines for Americans (2010), to increase intake of fish and seafood during pregnancy to prevent suboptimal brain development in utero and residual problems in cognitive and visual development [1]. In view of the prediction by the World Health Organization of a 50% increase in child mental ill-health by 2020, the promotion of optimal nutrient requirements for the developing brain warrants examination as a means to reduce the risk of potential developmental and functional consequences.
Inadequate HUFA Intake during Pregnancy on Human Developmental Outcome
Fish and seafood are the richest sources of omega-3 fats in the human diet, but also contain multiple nutrients that are beneficial to optimal brain development. Two major studies of inadequate seafood intake during pregnancy are offered here as examples to provide an overview of the potential impact of intrauterine nutrient inadequacies on behavioral and cognitive deficits later in childhood.
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a longitudinal study of health care outcomes with pregnant mothers and their children conducted at Bristol University in the U.K. In 1991, over 14,000 mothers were enrolled during pregnancy, and the developmental and health trajectory of their children has been charted ever since [96]. Among the numerous nutritional factors examined, the effects of the 2004 Food Drug Administration (FDA) advisory to limit seafood intake during pregnancy was directly evaluated in order to determine whether eating seafood (i.e., exposure to trace methyl mercury) or avoiding seafood (risk of nutritional deficiencies) was associated with greater harm [97]. One purpose of the advisory was to protect against impaired verbal development. However, detrimental effects on verbal development were found among children whose mothers consumed less than 12 ounces (oz) of seafood per week (Odd Ratio= 1.48 for greater risk of low verbal IQ). Low maternal seafood intake during pregnancy was also associated with suboptimal outcomes for fine motor skills, communication, pro-social behavior, and social development scores [96]. A net effects analysis by the World Health Organization [98] and the FDA [99], found that the nutritional benefits of fish far exceed the toxicological effects of metal mercury. These findings may result in an update of the 2004 FDA advisory on EPA [97].
Another longitudinal study conducted in Australia, known as The Raine Study [100] followed 2,868 live births to age 14 and then assessed the adolescents for dietary patterns and ADHD diagnosis. Data were available for 1,799 adolescents, including 115 with a diagnosis of ADHD. The two main dietary patterns were assessed and categorized as “Healthy” and “Western.” The “Western” dietary pattern was correlated with higher intakes of total and saturated fat, salt, and refined sugars, and was inversely correlated with intake of folate, fiber and omega-3 fatty acids[100]. In contrast, the “Healthy” dietary pattern was positively correlated with fiber, folate, and omega-3 fatty acid intake and inversely correlated with the amount of refined sugars and the total fat/saturated fat ratio [100]. The results showed that an increased likelihood of an ADHD diagnosis was significantly associated with the Western dietary pattern, after adjustment for potential confounding variables from pregnancy to adolescence. The ADHD diagnosis was not associated with the Healthy dietary pattern. Clearly, firm conclusions cannot be drawn regarding the causal nature of dietary patterns on likelihood of ADHD because of the cross-sectional nature of the study design. The observations may also be bidirectional; that is, the diagnosed ADHD may be either indicative of poorer food choices or the Western diet may have promoted the expression of attention deficits. This study does however highlight the necessity for a closer inspection of the role of dietary patterns in ADHD [100].
Effect of HUFA Supplementation in Healthy Children
Early dietary intake of HUFAs, specifically during pregnancy and breastfeeding, have been associated with subsequent improvements in an array of functions, including visual acuity at age 12 months [101, 102], problem-solving ability in infants [103], and alterations in cortical attention networks in school children [81].
For example, the effects of DHA supplementation on attention networks in the brain have been examined in 38 healthy boys (ages 8 to 10 years) using fMRI [81]. Participants were randomly allocated to receive DHA (n = 12, dose: 400 mg or n = 14, dose: 1200 mg) or placebo (n = 12), although five children were lost to follow up, withdrawal or non-compliance. Their cortical brain activity was recorded at baseline and 8 weeks later. The results found that DHA erythrocyte membrane composition increased by 47 to 70% at 8 weeks in comparison to the placebo group [81]. Both DHA dose groups had increased activation of the dorsolateral prefrontal cortex and greater reductions in activation in the occipital and cerebral cortex during a sustained attention task compared to controls. Decreases in cerebellar activation were larger in the 1200 mg group compared to the 400 mg group [81]. DHA erythrocyte levels were positively associated with dorsolateral prefrontal cortex activation and negatively associated with reaction time (RT), which improved (that is, became faster) as brain function increased This was the first study to demonstrate that dietary intake of DHA changed cortical attention networks in healthy boys.
Osendarp and colleagues [104] assessed the effects of randomization to a fortified drink containing either (a) a mix of micronutrient intervention (zinc, iron, vitamins A, B6, B12, and C, folate) alone, (b) DHA 88 mg and/or EPA 22 mg daily alone, (c) the micronutrient intervention plus DHA 88 mg and/or EPA 22 mg daily, or (d) placebo. Two groups of 6- to 10-year old school children were enrolled and classified as well-nourished (a group in Adelaide Australia, n=396) or marginally nourished (a group in Jakarta, Indonesia n=384 [104]. A total of 120 children completed the study. The micronutrient treatment resulted in significant increases in verbal learning and memory in the Australian group (estimated effect size: 0.23; 95% CI: 0.01 to 0.46) and similar effect was observed among Indonesian girls (estimated effect size: 0.32; 95% CI: −0.01 to 0.64). No effects were found on tests measuring general intelligence or attention. No effects of DHA+EPA on the cognitive tests were observed [104]. Overall, the authors concluded that micronutrient intervention can have beneficial effects on cognitive performance, even in well-nourished children. The failure of EPA and DHA to produce significant effects on cognitive function, especially in marginally nourished school children, may be accounted for by the very low combined dose of EPA and DHA in this study (DHA 88 mg and EPA 22 mg daily), compared to daily doses in the region of 1000 mg used in other supplementation trials.
Together, these two early reports on HUFA supplementation in healthy children provide preliminary evidence that DHA might result in increased activation of the dorsolateral prefrontal cortex, thus improving the functioning of cortical attentional networks, and improved reaction times. Micronutrients, including vitamins and minerals, may improve verbal memory and learning, but might not improve attentional functions, in healthy children.
HUFA Abnormalities in ADHD
In children and adults with ADHD, abnormalities in fatty acid blood profiles have been reported [68, 72–75, 105], but it is unclear whether the observed irregularities in omega-3 and omega-6 HUFAs are due to low dietary intakes of HUFAs in ADHD and/or an abnormality in HUFA metabolism.
For example, Stevens et al. [70] reported 6- to 12-year old boys with ADHD both arachidonic acid and DHA levels were significantly lower than matched controls (n = 43) in addition approximately 40% of (n = 53) had excessive thirst and skin problems, which are classic signs of fatty acid deficiency from the animal literature. Combining the ADHD and controls and then classifying them into “low” or “high” omega-3 and omega-6 groups, the researchers found that lower omega-6 concentrations were associated with several signs of fatty acid deficiency, such as excessive thirst, skin problems, frequent urination, rough and dry skin/hair, frequent colds and antibiotic use. The low omega-3 group were also found to have learning and behavioral difficulties, such as hyperactive-impulsive behavior, anxiety, temper tantrums and conduct disorder symptoms [106].
Another study by Antalis et al [68] compared 35 young adult males with ADHD to healthy controls, and found that the ADHD group had a higher total omega-6/omega-3 ratio (i.e., the sum of total omega-6 over the total of omega-3) and a 36% higher ratio of arachidonic acid/EPA in erythrocytes compared to the controls. Erythrocyte levels of arachidonic acid were also approximately 10% greater in the ADHD group compared to the control group. DHA levels were 53% lower in plasma and 36% lower in erythrocytes in the ADHD group compared to controls. An identical pattern was also observed for total omega-3 ratio. Plasma α-linolenic acid levels were greater in ADHD, but all α-linolenic acid metabolites were lower. Correlational analysis was conducted to assess the strength of the relationships between behavior and omega-3 fatty acid levels. The percentage of DHA in the phospholipid fraction of blood correlated with the ADHD symptoms (inattention r = −0.47, impulsivity/hyperactivity r = −0.45, and DSM-IV total ADHD scores r = −0.047). Similar trends were observed for total omega-3 fatty acid levels in both plasma phospholipids and erythrocytes. These findings suggest that lower omega-3 levels were associated with greater severity of ADHD-like symptoms.
Colter et al. [75] also assessed 11 adolescents with ADHD and 12 healthy controls for fatty acid levels and severity of behavioral symptoms . The ADHD group self-reported more checklist symptoms of fatty acid deficiency. Both total fat and saturated fat intake were positively associated with scores of oppositional, problematic and hyperactive behaviors. Adolescents with ADHD had lower blood concentrations of DHA and total omega-3 (α-linolenic acid, DHA, EPA and docosapentaenoic acid) levels and higher levels of the omega-6 linoleic acid. DHA levels were negatively correlated and total omega-6 levels were significantly positively correlated with inattention, behavioral problems, oppositional behavior, restlessness, and total DSM-IV symptoms on Conners’ parent scores. The diet records did not reveal any differences in total omega-3 fatty acid consumption, potentially suggesting that the lower DHA levels in ADHD may be due to higher oxidative metabolism rather than dietary intake [75]. Confounding variables in this study were group differences in vitamin and mineral supplementation (50% of the ADHD group, 25% of the controls) and in gender, which is relevant as males and females can differ in their metabolism of PUFAs [107].
Researchers in Taiwan have examined 58 children (aged 4–12 years) with a clinical diagnosis of ADHD compared to 52 controls. They found no differences in dietary patterns of the children with ADHD, except for higher intake of iron and vitamin C. The ADHD children were found to have lower linoleic acid , arachidonic acid and DHA fatty acid levels in red blood cells (and higher iron levels in their blood) compared to controls [105].
Across these studies on HUFA abnormalities in ADHD, ADHD and ADHD behaviors in youth appear to be characterized by physical symptoms characteristic of essential fatty acid deficiency, low levels of DHA and other omega-3 HUFAs, and a high levels of omega-6 HUFAs. Patients with low DHA levels were associated with more severe symptoms of inattention and hyperactivity/impulsivity, the hallmark symptoms of ADHD, and showed more learning problems and conduct disorder symptoms. Findings on arachidonic acid and omega-6 levels were more mixed, but there was a suggestion that omega-6/omega-3 ratio may be a marker for more ADHD symptoms. However, generalized essential fatty acid deficiencies are unlikely, as these subjects were replete with omega-6 linoleic acid. These studies raise the question of whether omega-3 fatty acid supplementation, even in the face of adequate omega-6 fatty acid levels, might ameliorate symptoms of ADHD and some associated physical signs of essential fatty acid insufficiency.
Most supplementation studies have utilized mixtures of omega-3 HUFAs and omega-6, γ-linolenic acid, making it difficult to isolate the potential effects of individual fatty acids. Some of the early research in children with ADHD focused on supplementation with DHA alone, and showed little or no therapeutic effect [108, 109]. Other clinical trials which have investigated supplementation with evening primrose oil or formulas rich in omega-6 in children with ADHD have also reported little or no effect on ADHD [110–112]. Very few trials employed comparable experimental designs, supplements, doses or duration of supplementation, and most studies were not adequately powered, so their non-significant findings and wide variations in findings are often difficult to interpret.
Differential Effects of EPA and DHA in the Brain
Little is known about the biological role of EPA in the brain, especially regarding its impact on cognition and mood. There are some preliminary research studies reporting differential effects of EPA and DHA in relation to cognitive and emotional responses [113, 114].
Resting State EEG
Sumich and colleagues [113] reported differential associations between DHA and EPA erythrocyte levels and EEG components in children and adolescents with ADHD: DHA levels were associated with more fast activity (alpha activity during eyes open and beta during eyes closed resting states), and EPA levels were associated with more slow activity (theta during both eyes open and eyes closed resting states. No associations were found with omega-6 HUFA levels. Alpha activity was found to be positively associated with performance for language fluency involving semantic memory, while theta activity was negatively associated with verbal memory performance [113].
Emotion-Elicited Event-Related Potentials
Gow and colleagues [114] have reported the relationship between erythrocyte HUFA levels and event-related potential (ERP) responses to the presentation of happy, sad and fearful faces during an emotional processing task in a small sample of adolescent boys with ADHD. The authors created an ERP cognitive bias paradigm based on 2 premises (1) Beck’s theory of depression which postulated that individuals with depression gravitate towards negative schema and stimuli and (2) evidence which suggests that children with ADHD have difficulty correctly identifying the emotional expression of others especially in relation to fear and anger [115]. The authors therefore hypothesized that EPA (due to its association with regulating and improving mood) would be positively associated with a bias in the overt P300 response towards facial expressions of happiness relative to fear or sadness. The happy-fear cognitive bias was calculated by subtracting the midline frontal P300 amplitudes to fearful faces from those of happy faces (P300H–F). A similar calculation was made for a happy–sad bias (P300H–S). The findings showed there was a significant positive association between EPA levels and a cognitive bias in orientation to overt expressions of happiness, relative to both sad and fearful faces, as indexed by midline frontal P3 amplitude. In contrast, DHA levels were associated with the right temporal N170 response to fear.
Memory-Related Event-Related Potentials In Healthy Fish-Eating Children
To the best of our knowledge, only one study has investigated HUFA status and event-related potentials in healthy children. Boucher et al [116] examined the prenatal omega-3 fatty acid intake of the mothers of 154 children in the fish-eating Inuit community, and compared the cord plasma concentrations to the child’s subsequent memory functioning evaluated at a mean age of 11.3 years old. This prospective longitudinal study employed neurophysiologic data (event-related potentials during a continuous visual recognition task) and neurobehavioral measures of memory (Wechsler Intelligence Scales for Children, 4th edition, and the California Learning Test Children’s version). Children with higher prenatal concentrations of DHA (as measured in cord plasma) displayed, at age 11, a shorter N4 ERP latency deflection and larger late positive component (LPC), which is a positive-going event-related potential component derived from EEG recordings and thought to be associated with recognition memory processes. Elevated DHA measures were related with enhanced N4 amplitudes, and positive associations were also observed between cord DHA and neurobehavioral performance on memory tasks.
Collectively, these three EEG studies provide preliminary evidence to suggest that EPA and DHA may be implicated in different functional roles related to features of cognitive and affect processing in ADHD.
Milte et al [117]. (2011) examined 75 children (ages 7–12 years) with symptoms of ADHD with or without learning difficulties in the context of a larger study. This study reports data only from the baseline time-point (after controlling for covariates). Pearson correlational analysis examined associations between literacy (word reading, spelling and vocabulary) and behavior (using the Conners’ Parent Rating Scales), and found that higher DHA (omega-3) predicted better word reading (p < 0.001), while higher omega-6 predicted poorer levels of reading, vocabulary, spelling and attention [117]. Higher levels of total omega-6 and arachidonic acid levels at baseline also predicted lower anxiety/shyness after 4 months. In a similar fashion, both increased levels of EPA and total omega-3 were associated with decreased anxiety/shyness. However, a key limitation of this study is that the correlation analyses were employed with no statistical correction for multiple testing, so it may be that these were spurious findings. The findings are provocative enough to warrant replication employing correction for multiple testing.
Individual HUFA Supplementation Trials in Youth with ADHD or ADHD-Type Symptoms
Several dietary supplementation trials with omega-3 and omega-6 fatty acids have reported some improvement in ADHD symptoms, typically using either the Parent or Teacher-rated Conners’ scales and/or the Clinical Global Impression (CGI) scales as primary outcomes [76, 78, 118–120].
Omega 3/6 in Relation to Learning, Behavior, and Motor Skills
The Oxford-Durham study (2005) was arguably the landmark study linking omega-3 or omega-6 supplementation to improvements in behavior and concentration in underachieving mainstream school children [76]. Although not primarily concerned with ADHD, this examination of ADHD-like symptoms in children was a pivotal study in this field. This placebo-controlled double-blind randomized clinical trial (RCT) examined 117 school children, ages 5 to12 years old, with untreated DSM-IV developmental coordination disorder (also known as dyspraxia, or informally as clumsiness). Although none of the children recruited had previously received a clinical diagnosis of ADHD, cognitive and behavioral ADHD-like symptoms are frequently associated with developmental coordination disorder, and 31% of the sample at baseline scored 2 standard deviations above the mean on the DSM-IV total score on the Conners’ Teacher Rating Scale (CTRS-L, which assessed each of 59 items of child behavior on a 4-point scale). The participants were treated with either fish oil (n = 60) or placebo (n = 57) for 3 months. The daily dose of 6 capsules provided a combination of omega-3 fatty acids (EPA 558 mg and DHA 174 mg) and omega-6 fatty acid γ-linoleic acid 60 mg, and vitamin E 9.6 mg (in the natural form, α-tocopherol). Placebo treatment consisted of olive oil capsules, which were carefully matched to the active treatment in both appearance and flavor. After 3 months, the placebo group was crossed over to the active supplement, and the active group continued with active treatment, for another 3 months [76]. Outcome variables included learning, literacy (including word reading and spelling), motor skills, and teacher ratings of behavior linked to ADHD.
Fish oil supplementation for 3 months did not improve motor skills in these children with developmental coordination disorder, but fish oil was found to produce significant improvements in reading, spelling and ADHD-like symptoms. The mean achievement scores at baseline for reading and spelling were 1 year below chronological age in both fish oil and placebo groups. Compared to controls, the fish oil group showed significant gains in reading age (9.5 ± 13.9 months vs. 3.3 ± 6.7 months for placebo, z = 2.87, p < .004), gains in spelling age (6.0 ± 11.4 months vs. 1.2 ± 5.0 months for placebo, z = 3.36, p < .001), and improved behavior (CTRS-L scores decreased from 75 ± 27 to 58 ± 28, p < .05 over three months of treatment. When the placebo group was crossed over to the active treatment, they showed similar improvements, while the active group continued to improve up to the end of the 6 month trial. Improvements for those children continuing on active treatment between 3 to 6 months were characterized by a gain in reading age of 13.5 ± 11.9 months and a gain in spelling age of 6.2 ± 6.8 months. Those children in the active treatment group demonstrated improvements above their chronological age (reading age gain 10.9 ± 11.8 months; spelling age gain: 5.3 ± 6.9 months). The placebo-to-active crossover group demonstrated behavioral improvements in teacher ratings (CTRS-L global scales) at 6 months, comparable to those of the active group after 3 months.
The findings from the Oxford-Durham trial suggest that omega-3/6 supplementation may help children with ADHD-like behavioral symptoms and educational difficulties associated with developmental coordination disorder.
Randomized Double-Blind Placebo-Controlled Clinical Trials in School Children
Other trials have attempted to replicate the findings of the Oxford-Durham study in either children with symptoms of ADHD or children with actual clinical diagnoses of ADHD, with less success. For example, in the well-known Adelaide trial, Sinn and Bryan (2007) conducted a randomized placebo-controlled double-blind study with 7- to 12-year olds who did not have a clinical diagnosis of ADHD but were entered into the study if they scored 2 standard deviations above the mean on Conners’ Abbreviated ADHD Index [78]. These psychostimulant-free children were randomly allocated into one of three groups: (1) an active group (n = 41) who were given the same fish oil supplements used in the Oxford-Durham trial but with an additional multivitamin/mineral supplement containing daily recommended amounts, (2) a fish oil only group (n = 36), and (3) a placebo group (n = 27). There were no significant differences between any of the treatment groups on Conners’ Teacher Rating Scale scores at either 15 or 30 weeks, but several differences were observed on the Conners Parents’ Rating Scale (CPRS). Parent ratings at 15 weeks showed mostly moderate effect sizes in the two HUFA groups compared to placebo in 9 out of 14 symptom subscales, including DSM-IV inattentive subscale (effect size: 0.61), Conners ADHD Index (ES: 0.59), CPRS cognitive problems and inattention (ES: 0.52), DSM-IV total (ES: 0.49), Conners Global Index restless/impulsive (ES: 0.45), Oppositional (ES: 0.43), Conners Global total (ES: 0.39), DSM-IV hyperactive/impulsive subscale (ES: 0.20), and CPRS hyperactivity (ES: 0.17). The effects on hyperactivity/impulsivity were statistically significant despite small effect sizes, especially in comparison to the larger effect sizes on cognitive measures. When the placebo group crossed over to the active fish oil plus micronutrient treatment at week 15, parent ratings showed additional significant improvements between weeks 15 and 30 (on 9 of 14 subscales), but once again no improvement was observed by the teachers. There were no significant differences in children treated with or without the supplemental micronutrients on parent or teacher ratings, suggesting that the therapeutic effects on ADHD were due to the fish oil, and that the vitamin-mineral supplementation did not add to the fish oil effect. However, the doses of micronutrients were quite low (at or below Recommended Daily Allowances), which may have accounted for the negative findings. The authors interpreted the parent ratings as more reliable than the teacher ratings for a number of reasons, including: (1) there was a high teacher turnover, (2) classrooms being taught by different teachers on different days, (3) long vacations and service leaves, and (4) children moving to different schools during their enrollment in the study.
During the Adelaide trial, Sinn et al [77] also conducted neuropsychological assessments of cognition in the same sample of children. In the HUFA-treated groups, significant improvements were observed in the ability to switch and control attention (as measured by the Creature Counting Task) at 15 weeks compared with placebo (effect size 0.43), and a similar change was seen in the placebo group after switching to active treatment between 15 and 30 weeks (effect size 0.93, p <0.001). The observed improvements in cognitive performance were found to mediate the parent-rated improvements in hyperactivity/impulsivity and attention scores. However, no significant improvements were noted in any other cognitive test in a large assessment battery.
In short, the Oxford-Durham study found that fish oil did not improve motor skills in children with developmental coordination disorder, but did improve reading, spelling, and ADHD-like symptoms such as inattention, hyperactivity, and oppositional behavior. The Adelaide trial, which was the replication by Sinn and colleagues, in children with symptoms of ADHD confirmed that fish oil improved both inattention and hyperactivity/impulsivity symptoms, but neuropsychological testing showed only very limited areas of cognitive improvement.
Johnson et al [120] conducted a randomized placebo-controlled clinical trial with 75 youths (8–18 years old, 64 boys) with ADHD (35 with the combined type and 40 with the inattentive type) to evaluate the efficacy of omega-3/6 fish oil supplementation in reducing core symptoms of ADHD and to establish subtypes of responders based on a detailed evaluation of symptoms and comorbid problems. The study used the same formula and dose of fish oil as well as the one-way crossover (the placebo group was crossed over at 3 months into the active group for another 3 months) that was used in the Oxford-Durham and the Adelaide studies. There were 37 randomized to the active group and 38 in the placebo group. The investigators carried out a medical examination including psychiatrists’ assessment of diagnosis and comorbidity using DSM-IV criteria. The two primary outcome measures were the investigator-rated ADHD Rating Scale IV-Parent version (which has been validated as a clinician-rated parent interview to allow evaluation of symptom severity across different settings as well as clinicians’ overall experience of the patients) and the Clinical Global Impression (CGI) severity scales [120]. Overall, the findings of this study were negative, with a lack of statistically significant differences in either of the outcome measures between active and placebo groups at 3 or 6 months. In a post hoc analysis of the diagnostic subtypes, a higher number of responders were found in the inattentive subtype group (p = .03) compared to the ADHD combined type group. The inattentive group was more likely to have a comorbid developmental disorder (e.g., reading and writing disorder, learning disability or autistic traits). None of the responders had comorbid conduct disorder, oppositional defiant disorder, depression or anxiety [120]. It is unclear why the subgroup of children with ADHD-Inattentive Type benefited from the omega-3/6 intervention, but a similar pattern was observed in the Adelaide trial, warranting closer attention to this potential finding of improved attention in future clinical trials.
Omega 3/6 Relation to Dyslexia
Richardson and Puri [119] conducted an earlier 12-week randomized double-blind placebo-controlled study of 41 previously undiagnosed children (aged 8–12) with high ADHD symptom ratings (according to DSM-IV criteria) and specific learning difficulties, including dyslexia [119]. The active treatment group (n = 22) received omega-3 (EPA 186 mg, DHA 480 mg) and omega-6 (γ-linolenic acid 96 mg, cis-linoleic acid 864 mg, arachidonic acid 42 mg) HUFAs, vitamin E 60 IU, and thyme oil 8 mg; and the placebo group (n = 19) received olive oil. At the end of 12 weeks, the treatment group had significantly lower scores than the placebo group on inattention (effect size 0.61) and a global behavioral scale (effect size 0.58), but not on other measures. However, given the large number of measures, very few positive findings, lack of adequate power, and a questionable control (the olive oil placebo contained bioactive properties), it is unclear whether any firm conclusions can be drawn from this study.
Omega 3/6 Studies Relating to Attention and Behavior
Stevens et al [121] conducted a randomized double-blind placebo-controlled study with omega-3 or omega-6 HUFA supplementation in 50 children (ages 6–13 years old) with either a clinical or suspected diagnosis of ADHD. The parents completed a fatty acid deficiency questionnaire, and children who were rated as having 4 or more symptoms of fatty acid deficiency were recruited for the study. The fatty acid deficiency questionnaire included thirst, frequent urination, dry hair, dandruff, dry skin, brittle nails and follicular keratosis, which parents rated on a scale of 1–4; Stevens and colleagues had previously used this questionnaire and found significantly higher scores (p=0.0009) in an ADHD compared to a control group [70]. The children with both ADHD and fatty acid deficiency symptoms were randomized to receive either an active treatment (DHA 80 mg, EPA 80 mg, arachidonic acid 40 mg, γ-linolenic acid 96 mg, and Vitamin E 24 mg daily) or placebo (olive oil 6.4 mg) for 4 months. HUFA supplementation resulted in a 50% increase in plasma and erythrocyte levels of EPA, DHA and vitamin E. HUFA treatment was significantly better than placebo on only 2 out of 16 measures: parent-rated conduct problems (-42.7 vs. −9.9%, n = 47, p = .05) and teacher-rated attention (-14.8 vs. +3.4%, n = 47, p = 0.03). There were significant correlations between higher EPA levels and reduced parent-rated disruptive behavior scores (r = 0.38, n = 31, p = < 0.05), and similarly, correlations between higher EPA or DHA levels with reduced teacher-rated disruptive behavior (r = 0.49, n = 24, p = < 0.05). Oppositional behavior scores also showed significant improvements. Although these findings were not strong, this study suggests that HUFA supplementation may improve both attention and behavior in children with ADHD or ADHD-like symptoms.
Pharmacological Intervention and Omega-3 HUFAs for ADHD
There are few studies comparing omega-3 with standard pharmacological treatment or exploring the plausibility of HUFAs as an adjunct therapy for ADHD. Perera et al [122] recruited 98 children (ages 6–12 years) whose parents reported that methylphenidate produced no improvement in either behavior or learning and randomly assigned them to receive either an omega-3/6 combination (fish oil 296 mg, evening primrose oil 181 mg) (n = 48) or placebo (n = 46) containing sunflower oil in an identical capsule. The children continued their methylphenidate treatment (0.7 to 1.0 mg/kg/d) and home/school-based behavioral interventions throughout the 6-month study period. Both treatment groups also took unspecified micronutrients to guard against any other potential deficiencies. HUFA treatment improved parent ratings of both ADHD behavior symptoms (aggressiveness, fighting, restlessness, inattention, easy anger, impulsiveness waiting for turn, cooperation) and learning (completing work, academic performance) more than placebo in 5 out of 11 measurements at 3 months and in all 11 measurements at 6 months. However, HUFA treatment did not improve distractibility scores. The largest significant improvements in behavior were reported for aggressiveness (effect size 0.98) and restlessness (effect size 1.11).
Harding et al [123] (2003) recruited 20 children (ages 7–12 years) with a clinical diagnosis of ADHD and non-randomly allocated them (by parental choice) to methylphenidate (n = 10) or a dietary supplementation (n = 10) consisting of essential fatty acids, vitamins, minerals, amino acids, phospholipids, probiotics and phytonutrients for a period of 4 weeks. Mean daily doses of methylphenidate were not reported, and parent ratings were collected but not reported. A nonstandard continuous performance test was employed. No differences were found between methylphenidate and the dietary supplementation, and the authors inferred that the dietary supplementation and methylphenidate were equivalent in effectiveness. However, because there was no placebo group, it is possible that the nonstandard continuous performance testing was unable to measure any clinical change, even by the methylphenidate. This small open-label study does not permit any inferences about effectiveness.
Meta-analyses and Reviews of Clinical Efficacy of Omega-3 HUFAs in ADHD
To examine the clinical efficacy of omega-3 and omega-6 interventions in child ADHD, Bloch and Qawasmi [124] conducted a recent meta-analysis of 10 well-designed trials involving 699 children. To represent effects sizes, the standard mean difference (SMD) was chosen as the summary statistic for meta-analysis and calculated by pooling the standardized mean improvement of each study using RevMan 5 (The Cochrane Collaboration). The exact methodology was applied for the secondary analysis to test the effect of omega-3 HUFAs on symptoms of inattention and hyperactivity/ impulsivity independently.
This meta-analysis found that omega-3 supplementation had a small but statistically and clinically significant effect in the treatment of ADHD (effect size SMD = 0.31, 95% CI 0.16 to 0.47, z = 4.04, p = < .001). The meta-analysis demonstrated also similar effect sizes of omega-3 supplementation in the treatment of both the inattention (SMD = 0.29, 95% CI 0.07 to 0.50, z = 2.63, p = < .009) and hyperactivity/impulsivity symptom clusters (SMD = 0.23, 95% CI 0.07 to 0.40, z = 2.78, p = < .005). When the different omega-3 fatty acids were considered separately, higher doses of EPA (which collectively ranged from 80 to 750 mg daily) compared to α-linolenic acid and DHA were significantly but modestly correlated with omega-3 efficacy in the treatment of ADHD (β = 0.36, 95% CI 0.01 to 0.72, t = 2.30, p = .04, R2 = 0.37) [124]. Doses of other omega-3 HUFAs such as DHA and α-linolenic acid were not significantly related to efficacy (DHA: β = 0.24, 95% CI 0.54 to 1.02, t = 0.70, p = .50; α-linolenic acid: β = −1.71, 95% CI −4.62 to 1.19, t = −1.33, p = .22). The effect size of 0.2 to 0.3 for omega-3 fatty acid supplementation was modest in comparison to standard pharmacological interventions for ADHD: The effect size for psychostimulants is about 0.8 (where 0.2 to 0.3 is small, 0.3 to 0.5 is medium, and 0.6 to 0.8 or more is a large effect size).
Bloch and Qawasmi raised a critical point regarding adequate power and sample size [124]. They noted that, in order to have sufficient power (β = 80%, 2-tailed α = 0.05) to detect a significant benefit (effect size of 0.31), clinical trials of omega-3 intervention compared to a placebo would need a sample size of approximately 330 children. Therefore, the actual sample sizes in the clinical trials to date are considerably under-powered and likely to account for the inconsistent findings in the literature to date. Taking into consideration the relatively mild side effect profile, the authors concluded that it may be reasonable to use omega-3 fatty supplementation to augment traditional pharmacologic interventions or to treat youths whose families decline other psychopharmacologic options, despite the evidence of only moderate efficacy [124].
In summary, these clinical studies have been criticized by reviewers, such as the Cochrane Collaboration, as both inconsistent and fragmentary [125]. The present article has reviewed several randomized placebo-controlled double-blind trials and open-label or pilot studies on omega-3 supplementation in child literature. We recognize the wide variation in design and methodological issues, as well as a range of formulations, doses, and durations of supplementation: All of these factors have implications for interpretation and replication. Some improvements in behavior, concentration, and literacy have been reported following supplementation with HUFA in various populations, including underachieving mainstream school children with developmental coordination disorder and associated ADHD-like symptoms [76], community samples of children with ADHD symptoms[77, 78] and youth with verified clinical diagnoses of ADHD [122]. The neurophysiological and imaging research on HUFAs in children is extremely limited, [79, 106–108] with only two studies on ADHD. Some evidence suggests abnormal erythrocyte fatty acids levels of children and young adults with ADHD [68, 71–75]. To the best of our knowledge, there are no published HUFA supplementation trials in adults with ADHD. Despite several reports of HUFA benefits for symptoms of ADHD in children, 5 trials have found little or no effect [108, 110–112, 126]. Bloch and Qawasmi’s recent meta-analysis [124] (2011), which found some efficacy of EPA in improving ADHD symptoms in children, highlighted the very valid point that available studies have small sample sizes and lack the statistical power to demonstrate a substantive effect size.
Future recommendations include carefully designed clinical trials with longer supplementation periods (i.e., 6 months or more) and adequate sample sizes conducted in both children and adults with ADHD. Sensitive measures, such as event-related potentials combined with fMRI (due to their temporal and spatial precision, respectively) may be better suited to capture changes in cortical function.
HUFA’s and Youth with Aggressive or Conduct-Disordered Related Behaviors
In addition to the putative role of HUFAs in ADHD, there is accumulating evidence that omega-3 HUFA and other micronutrient (vitamins and minerals) deficits may also be linked to antisocial and aggressive behaviors [127–131]. Given the comorbidity of conduct disorder in ADHD, coupled with the over-representation of ADHD in incarcerated adults [132] the use of HUFAs for treating aggressive and delinquent behaviors deserves serious examination.
In a recent clinical study, Gow et al [133] recruited a sample of 29 young male adolescents (ages 12–16 years) with ADHD and found a significant association between low EPA levels and high scores of callous-unemotional traits (r = −0.597, p = 0.009), as assessed by the Inventory of Callous-Unemotional (CU) Traits [134, 135]. The ADHD group also showed a correlation between high callous-unemotional scores and oppositional behaviors (Conners’ Parent Rating Scale subscale, r = 0.464, p = 0.011) and with low total omega-3 levels (r = −0.498, p = 0.027). Though not quite statistically significant, the ADHD group also showed trends towards a correlation between low DHA levels and high callous-unemotional scores (r = −0.436, p = 0.054) as well as a trend toward an association of low total omega-3 levels and antisocial behaviors (measured by the Antisocial Process Screening Device). This study suggests a link between low levels of EPA and high callous-unemotional behavior related to conduct disorder in male children with ADHD. Callous-unemotional personality traits are known to represent a distinct developmental vulnerability to persistent antisocial behavior [136, 137]. Further research is needed to investigate whether early intervention with EPA may benefit youths with callous-unemotional traits and help prevent the subsequent emergence of conduct disorder.
HUFAs and Young Adult Prisoners
Gesch et al [127] conducted a randomized double-blind placebo-controlled trial on the effects of combined supplementation of HUFAs and micronutrients in a population of 231 young adult prisoners (ages 18 and over) treated for a minimum of two weeks (mean duration 142 days). The nutrient supplementation consisted of linoleic acid 1260 mg, γ-linolenic acid 160 mg, EPA 80 mg, DHA 44 mg daily, and a broad spectrum of vitamins and minerals at recommended daily levels. The results revealed that nutrient supplementation (N=172) at routine low daily doses produced a marked reduction (35 to 37% vs. 7 to 10% in the placebo group) in antisocial behavior and violent offenses for the active group in comparison to baseline. This landmark study demonstrated that a nutrient supplement combining HUFAs and micronutrients can have a pronounced effect on the problem behaviors in a prison population of young adults (18 years old and up). This study is instructional in showing that nutritional supplementation can affect major antisocial and aggressive behaviors, but it does not isolate the role of HUFAs in producing this benefit. The findings of Gesch et al. [127] were later replicated and confirmed in a randomized controlled trial in the Netherlands of 221 young adult prisoners (mean age 21.0, range 18–25 years) who received comparable nutritional supplementation (N=115) over a period of 1–3 months [138]. This study produced similar findings of a nearly 30% reduction in major behavioral and conduct incidents [138].
The efficacy of nutritional supplementation, especially the combination of HUFAs with vitamins and minerals at routine doses, in producing relatively rapid reductions in major misconduct has now been shown in these two large randomized double-blind placebo-controlled studies in young adult prisoners. Future studies of HUFA and micronutrient supplementation in prison populations could assess for comorbid ADHD and mood disorders. This may determine whether HUFAs produce a direct effect on conduct and aggression or whether the observed HUFA effects are mediated by improvements in ADHD or mood. These findings highlight the critical importance of nutrition and the potentially powerful impact that simple dietary interventions involving HUFAs (and micronutrients) can have in improving antisocial and major misbehavior in young adult prison populations. However, more clinical research is needed in youths with ADHD and/or comorbid conduct disorder to establish whether HUFAs with or without micronutrients may be a safe and effective intervention in youth at risk for the development of more serious delinquent, violent and criminal behaviors.
Omega-3 HUFA Supplementation Trials in Youth and Adults with Depression
Several lines of evidence in adults support the notion that low omega-3 HUFA deficits are associated with depression and that omega-3 HUFA supplementation can treat depression [79, 139, 140]. Other ecological studies by our research group on negative affect in adult populations are informative, but do not establish causation because of their correlational nature [141–143]. For example, a cross-national ecological study reported a highly significant association between low seafood consumption and low maternal milk DHA composition with a 50-fold greater risk for postpartum depression (r = −0.81, p = 0.0001) [143]. More broadly, in comparing countries with the lowest and highest consumption of seafood, low omega-3 HUFA intake is associated with a 65-fold higher lifetime risk for depression (r = −0.84, p = 0.0001) [144], a 30-fold higher risk for bipolar disorder (r = −0.80, p = 0.0003) [145], and a 10-fold higher risk of death from homicide (r = −0.63, p = 0.0006) [146].
Dietary Intake in Youth with Depressive Symptoms
Only one study provides comparable data in youth. A cross-sectional study evaluated dietary intake data from food frequency questionnaires among 3,067 boys and 3,450 girls (ages 12 to 15) in relation to their depression rating scores using the Center for Epidemiologic Studies Depression Scale (CESD) [147]. Boys with depressive symptoms had a lower mean value of EPA, DHA, vitamin B6, and folate. It is uncertain why this was not observed in girls, but possibilities include a greater heterogeneity of contributing causes to girls’ depressive symptoms or a more efficient endogenous conversion of α-linolenic acid to EPA among females compared to males [148]. For girls, higher intake of B vitamins was positively associated with EPA and DHA intake. Fewer depressive symptoms also appeared associated with higher dietary intakes of riboflavin (in girls), folate, pyridoxine (vitamin B6), and (in girls) riboflavin (but not with vitamin B12). In girls, but not in boys, higher riboflavin intakes were associated with fewer depressive symptoms (OR [95% CI], 0.85 [0.67, 108]; p for trend = .03). In boys and girls, higher folate and pyridoxine (vitamin B6) intakes were associated with lower risk of depressive symptoms. No clear association was seen with vitamin B12 intake [147]. These findings suggest that lower EPA and DHA levels are associated with depressive symptoms in boys but not girls, and that combined vitamins and mineral status may be important than HUFAs to prevent depressive symptoms in youth. In another study, an apparent association between HUFAs and depression scores in an adolescent population disappeared after adjusting for lifestyle confounders [149].
Omega-3 in Children with Depression
The only randomized double-blind placebo-controlled monotherapy study thus far to investigate omega-3 HUFAs in childhood depression was conducted by Nemets et al [79]. These researchers examined omega-3 HUFAs (EPA 400 mg and DHA 200 mg daily) in 20 children (ages 6 to 12 years) who completed at least a 1 month trial and were treated for a total of 16 weeks. The findings reported strong statistically significant effects of omega-3 fatty acids. By week 8, omega-3 HUFA treatment was superior to placebo (olive oil) on several depression scales: Greater than 50% reductions in Childhood Depression Rating Scale (CDRS) scores were observed in 7 of 10 children treated with omega-3 HUFAs and in none of the 10 placebo-treated children (p= 0.003, Fisher’s exact test); and remission (CDRS score of less than 29 at the study endpoint) was observed in 4 of the omega-3 HUFA-treated children (p > 0.05). Further research in children and adolescents is needed with larger sample sizes to pursue this promising lead.
Omega-3 in Adults with Depression
Studies in adult depression have examined the use of omega-3 HUFAs as monotherapy or adjunctive therapy [150–152]. In addition, several meta-analyses and review articles have evaluated the efficacy of omega-3 HUFAs in reducing depressive symptoms [152–158]. The overall conclusion is that, much in like the ADHD literature, significant heterogeneity is reported in the omega-3 HUFA formulation, depression severity, experimental design, and study populations in most of these studies [153]. In these studies, only preparations containing more than 50% EPA appear to be consistently effective [155, 158, 159]. Another source of heterogeneity in several meta-analyses may be the inclusion of negative trials of subjects without clinically significant depression; the severity of depressive symptoms at study entry is a major determinant of the ability to detect efficacy of any antidepressant treatment [160]. Meta-analyses of omega-3 HUFA trials that distinguish clinical and non-clinical population populations report antidepressant effects among those subjects who have significant depressive symptoms [157]. Given the heterogeneity in design and dose formulation, it is difficult to determine if omega-3 fatty acids are effective as monotherapies alone or only as adjunctive therapies in treating major depression.
Ultimately, omega-3 HUFA monotherapy may be attractive for child and adolescent populations in view of their general health benefits, low side effect profile, and reports of efficacy in treatment-resistant depression, but caution should be exercised as data are insufficient to support this recommendation for treating major depression in youth.
Omega-3 and Suicide
Several studies in adults suggest that omega-3 HUFAs might have some clinical value in reducing suicidal thinking and attempts [161, 162]. Sublette and colleagues [163, 164] demonstrated that low DHA plasma levels strongly predicted future suicide risk and were associated with both hyperfunction of the limbic forebrain and hypofunction of the parietal and temporal cortex. In a case control study of 1,600 active duty US military, low DHA status was a significant risk factor for suicide death [162]. All US military personnel in this study had low omega-3 HUFA levels in comparison to North American, Australian, Mediterranean, Chinese and other Asian civilian populations. These US military personnel had a significantly higher odds of a suicide attempt (OR 4.8, 95% CI 1.7 to 14.3, p < .0003) compared to the highest quartile [165] of the Chinese. In view of these promising data in adults, a $10 million trial on the efficacy of omega-3 HUFAs (4 grams daily) for the prevention of significant suicidal behaviors among United States Military Veterans is currently being conducted at the Medical College of South Carolina; it is being conducted in collaboration with National Institute on Alcohol Abuse and Alcoholism (NIAAA) and funded by the Defense Medical Research Program at the United States Department of Defense. To the best of our knowledge, no trials of omega-3 fatty acids and suicide attempt have yet been conducted in youth.
Omega-3 HUFA Prevention of Psychosis in High-Risk Youth
Among the most interesting new developments is the potential for omega-3 HUFA treatment is to prevent adolescents at high risk for psychosis from transitioning to schizophrenia. In a study of 81 adolescents at ultra-high risk for psychotic disorder [166], participants were randomized to omega-3 PUFA (1.2 grams daily) or placebo for a 12-week active intervention period, followed by a 40-week monitoring period. At the end of the 12-month study, transition to a psychotic disorder occurred in only 2 of 41 individuals (4.9%) in the omega-3 group, but in 11 of 40 (27.5%) in the placebo group (p = 0.007). For example, the reduction in transition to psychosis was reduced by 22.6% in that 12-month period (95% confidence interval 4.8 to 40.4). The omega-3 HUFA intervention significantly reduced positive symptoms (p = .01), negative symptoms (p = .02), general symptoms (p = .01), and improved functioning (p = .002) in comparison to the placebo group. Changes in intracellular phospholipase A2 (PLA2s) activity (key enzymes in phospholipid metabolism which release fatty acids from the second carbon group of glycerol) and erythrocyte membrane fatty acids comparing the omega-3 HUFAs vs. placebo groups were investigated as potential mechanism of action. The levels of membrane omega-3 and omega-6 PUFAs and in PLA2 (from pre- to post-treatment) were significantly related to functional improvement, as indicated by increased Global Assessment of Functioning scores between baseline and the study endpoint at 12 weeks. Supplementation with omega-3 PUFA also resulted in a significant decrease in inPLA2 activity. This study is the first to demonstrate that a 12-week intervention trial with omega-3 fatty acids significantly reduced the transition rate from the prodromal stage to psychosis, and furthermore resulted in significant functional and symptomatic improvements during the entire follow-up period at 12 months [166]. Replication of these findings is currently being attempted in a multinational multisite trial
Clinical Recommendations in relation to HUFAs
Clinicians can currently be guided by the 2006 treatment recommendations of a subcommittee of the Committee on Research on Psychiatric Treatments of the American Psychiatric Association that adults with major depression and other psychiatric illnesses should minimally consume 1 gram daily of omega-3 HUFAs or consume seafood 2 to 3 times per week, if only because these patients are at high risk for cardiovascular and other medical illnesses [152]. In more recent 2010 report of the Task Force on Complementary and Alternative Medicine of the American Psychiatric Association [122], certain alternative treatments for adults with major depressive disorder showed promising results, such as omega-3 fatty acids, hypericum (St. John’s Wort), S-adenosyl-L-methionine (SAMe), folate, acupuncture, mindfulness psychotherapies, light therapy and exercise. In relation to omega-3 fatty acids, this review recommended more research is necessary to clarify whether EPA is more effective than EPA plus DHA, optimum doses, and the value of fish intake versus capsules. They also highlighted the need for preventive studies, and the evaluation of omega-3 as a treatment intervention in recurrent major depressive disorder both as an adjunct and monotherapy. The American Psychiatric Association report also highlighted concerns about confounding variables, such as smoking (which can inhibit absorption of omega-3s into red blood cells) and methods to control for total omega-3 intake obtained from the diet in research trials [122].
Cautionary Findings on Omega-3 Supplementation
Not all research is clear-cut, of course, and negative findings can be as important and insightful as positive outcomes. There are a number of studies which have investigated HUFAs in behavior, ADHD symptoms, school attendance, and physical aggression in youth and which have reported little or no effect of omega-3 supplementation [108, 110–112, 126].
Hirayama et al [126] conducted a randomized double-blind placebo-controlled study in 40 children (aged 6–12 years) with ADHD who were placed on a specific diet (fermented soybean milk, steamed bread and bread rolls) that was fortified with either fish oil (n = 20, containing DHA 514 mg daily) or placebo (n = 20, olive oil 1,300 mg daily). At baseline and 2 months later, measurements were obtained of DSM-IV ADHD symptoms (hyperactivity, impulsivity, inattention), aggression (reported by teachers and parents), visual perception, visual and auditory short-term memory, visual-motor integration, a continuous performance task measuring sustained attention, and impatience. After two months of treatment, the DHA treatment group did not improve on any ratings of ADHD-related symptoms compared to the olive oil group. In fact, the DHA group showed significantly less improvement on commission errors on the continuous performance test (a marker of impulsivity) and on visual short-term memory. These negative results highlight several methodological issues:
The selection of fatty acid supplement: Although the dose of DHA was moderate, previous studies using DHA-rich oils have also been found ineffective at improving cognitive functioning and ADHD-type symptoms. It seems that DHA alone is not effective in ameliorating ADHD symptoms. The dose of EPA (100 mg daily) in the fish oil group was extremely low and unlikely to have any noticeable effect.
Choice of placebo: Olive oil is bioactive, with known anti-inflammatory and antioxidant effects, so its use as a control treatment is questionable. There was no indication of the amount of DHA or EPA that was received by the placebo-treated group in their fortified diet or olive oil.
Bioavailability of the fatty acids: Arguably, the fortification process may have lowered the bioavailability of the fatty acids, whereas a direct source of HUFAs would be more potent.
Short treatment duration: Without knowing the pre-supplementation blood fatty acids levels, it is difficult to know whether a longer trial than 2 months was needed to correct a nutrient deficiency.
There is such wide variation of EPA and DHA that researchers would be advised to measure it in finger prick blood tests in order to allocate a more appropriate dose.
Itomura et al [167] conducted a randomized double-blind placebo-controlled trial to test whether fish oil could reduce aggression in a normative population of 166 school children (ages 9 to 12 years). Similar to Hirayama’s study, the participants were randomized to receive fortified foods with fish oil (bread, sausage and spaghetti containing DHA 514 mg plus EPA 120 mg daily) or placebo (n = 83) for 3 months. A self-rated questionnaire did not identify an effect of fish oil on physical aggression (although physical aggression ratings increased in girls in the control sample). When aggressive tendencies were assessed by psychological testing, fish oil had the unexpected effect of appearing to increase aggressive responses, while the controls remained unchanged. When DSM-IV ADHD behavioral impulsivity symptoms were assessed by parents, fish oil appeared to reduce impulsivity in girls (relative to controls), but not in boys. The findings paint a somewhat mixed and confusing picture, and they suggest a variety of methodological problems, especially in the assessment instruments, in addition to the questions raised by the Hirayama study. Furthermore, in this study, the authors mention that a control sausage contained EPA 40 mg and DHA 80 mg, but clearly control foods should not have contained any of the active omega-3 intervention at all. Otherwise, this means that the placebo was not a true placebo, a problem that was not adequately discussed by the authors. Furthermore, both groups received the omega-6 linoleic acid (514 to 1,114 mg daily). The addition of linoleic acid in the fish oil group is a major confound, because omega-3 and omega-6 compete for incorporation into the red blood cells, and a higher amount of one would suppress the absorption of the other. In this case, omega-6 would have likely been the dominant fatty acid in the diet.
Both of these studies are extremely problematic from a methodological point of view, so it is hard to know how seriously to accept their somewhat contra-intuitive and inconsistent findings, but they are mentioned here mainly for completeness.
A final study by Hamazaki et al. [168] examined the effects of DHA-rich fish oil on behavior and school attendance in a randomized double-blind placebo-controlled trial in school children (aged 9–12) in Indonesia, who received either omega-3 supplementation (DHA 650 mg and EPA 100 mg, n = 116) or placebo (soybean oil capsules, n = 117). Omega-3 supplementation did not appear to improve measures of aggression or impulsivity relative to placebo treatments. Interestingly, though, school absenteeism appeared reduced in the children treated with DHA-rich fish oil relative to the control group (OR 0.40, 95% CI = 0.23–0.71, p = 0.002). Although the effects of nutritional supplementation on endemic diseases might conceivably have been a mediating factor, this particular finding warrants further exploration because school absenteeism is often linked with depression and anxiety in Western populations.
Collectively, these studies suggest that DHA-rich formulas show little or no effect in improving aggressive behaviors, ADHD symptoms, or cognitive performance, although these studies are particularly problematic from a methodological point of view. The fortified food studies appeared the least promising, and it is probably preferable to use more directly quantifiable sources of omega-3 fatty acids. Also, it may be speculated that a higher EPA-to-DHA ratio in needed for treating school children, consistent with the findings of a recent meta-analysis that suggested that higher EPA is associated with better clinical efficacy in improving symptoms of ADHD-type behavior [124]. More research is needed to answer these basic questions about dose and formulation in child populations.
Safety and Adverse Effects
Omega-3 supplementation is considered to be a safe intervention by the United States Food Drug Administration (FDA), which currently advises that dietary dosage of up to 3 grams daily of omega-3 fatty acids from marine sources are “Generally Recognized as Safe” (GRAS). The European Food Safety Authority has set a Safe Upper Limit of 5 grams daily for total omega-3 fatty acids, including DHA, EPA and docosapentaenoic acid. These standards are set for adults, with no separate designations for children or adolescents.
Currently, there is no established procedural system for reporting adverse events of food supplements, so systematically collected information is not available on a national scale. Several omega-3 products have been approved by the FDA for marketing in the United States as medications rather than as food supplements and the manufacturing companies have reported mostly minor but some severe side effects. In addition, there is some information about adverse effects from clinical research trials, some of which use relatively higher omega-3 dosing regimens. Given the lack of systematic data, the monitoring of adverse effects in research and clinical settings should receive higher prioritization. Nonetheless, some general observations on adverse effects can be made (see Table 1).
Table 1.
Potential Adverse Effects of Omega-3 HUFA Supplementation
| Common (1 to 10%) | Dyspepsia, nausea, diarrhea, increased bleeding |
| Uncommon (0.1 to 1%) | Gastrointestinal symptoms, upper abdominal pain, allergic hypersensitivity, dizziness, headache, increased low-density lipoprotein (LDL) levels, increased alanine aminotransferase (ALT) levels, mania, depression |
| Rare (1/1,000 to 1/10,000) | Hyperglycemia, headache, gastrointestinal pain, hepatotoxicity, acne, pruritic rash, ventricular tachycardia |
| Very rare (Less than 1/10,000) | Hypotension, urticaria, nasal dryness, gastrointestinal hemorrhage, hemorrhagic stroke, increased blood lactate dehydrogenase (LDH), hemolytic anemia |
Frequency figures are estimates for the general population, and certain individuals may be especially vulnerable based on their medical status, concurrent medication usage, and other individual factors.
Omega-3 fatty acids can increase the incidence of excessive bleeding, including gastrointestinal bleeding and even hemorrhagic stroke. For patients who are anticipating surgery, it is advisable to make certain that surgeons are aware when their patients are consuming HUFAs, because many surgeons will want to discontinue their use 3 to 7 days prior surgery, depending on the type of surgery.
Gastrointestinal effects of omega-3 fatty acids are common, and may include loose stools, diarrhea, nausea, vomiting, reduced appetite; some of these adverse effects may be mitigated by administration with meals or more gradual dose elevation. Omega-3 HUFAs may have hepato-protective properties, and omega-3s have been used to treat non-alcoholic fatty liver disease (NAFLD); but there have been reports of increased liver transaminases (mainly ALT).
Short-term memory loss and headache have been reported, although increasing the EPA-to-DHA ratio has been reported to be a possible treatment for headache [169].
Chronic treatment with HUFAs can cause vitamin E deficiency; so many clinicians provide advice about concurrent supplementation with Vitamin E, especially at higher HUFA doses. Also, many commercial HUFA products include Vitamin E as a preservative to reduce oxidation.
Many patients object to the fishy odor of some products, a result of the vulnerability of fatty acids to oxidation, which is especially problematic when weak manufacturing processes are used. High-quality preparations are also recommended to reduce the risks of exposure to potential contaminants, including mercury, dioxins, and polychlorinated biphenyls (PCBs), although many products are now manufactured in accordance with World Health Organization standards, which call for methods to reduce contaminants.
It should be emphasized that most data on the adverse effects of omega-3 HUFAs are derived from observations on adults, and the side effect profile might be somewhat different in children and adolescents.
Conclusions
Western diets can lead to imbalances in HUFA metabolism and alter molecular substrates in the brain. Omega-3 insufficiency and omega-6 excess can have particularly important effects on brain development, structure, and functioning. Malnutrition and inadequate nutrient intakes are well-established risk factors for both impaired cognitive development and adverse behavioral outcomes.
The case for nutritional intervention to reduce the burden of ADHD, aggressive and delinquent behaviors, and possibly major depressive disorder in youth is promising, but the research data at present are mainly suggestive. In adults, omega-3 HUFA supplementation is an inexpensive and safe adjunctive treatment for reducing symptom severity in mood disorders [150, 170] and depression [151]. The role of omega-3 in adults with ADHD has not yet been investigated, but current efforts are being undertaken by our research team in the Section of Nutritional Neurosciences at the National Institutes of Health.
In applying the early intervention research to clinical practice, it is notable that omega-3 HUFAs are “Generally Recognized as Safe” (GRAS) by the Food and Drug Administration in doses up to 3 grams daily for adults, with no added specifications concerning children. This designation does not indicate that higher levels are unsafe, but rather that most cardiovascular and other health benefits are likely to have been achieved by that level of intake.
The American Psychiatric Association has recommended at least one gram daily of omega-3 fatty acids, based on likely benefits for patients with certain psychiatric conditions as well as its more general medical benefits, but no specific APA recommendations have been offered for youth [152]. International guidelines recommend the consumption of a minimum of dose of 200 mg of DHA daily for pregnant and lactating women [171] to support the developing nervous system, but we are not aware of similar recommendations for children and adolescents. The Dietary Guidelines for Americans 2010 recommend consumption of seafood 2 to 3 times per week for children [1]. For children who refuse to eat fish, dietary supplements, fortified foods, or smoothie drinks may be more feasible sources to ensure daily recommended allowances.
In selecting a particular formulation of omega-3 fatty acids, clinicians should be aware that there are marked differences in quality among manufacturers. Total concentrations and ratios of omega-3 HUFAs vary widely across commercially available products (see Table 2). In old, spoiled, or poorly manufactured products, oxidized omega fatty acid HUFAs smell strongly of adverse fish odors. Fresh preparations have a clean smell akin to fresh seafood, unless masked by other natural flavors such as lemon, lime or strawberry, and are more desirable to consume. Naturally flavored emulsions or capsules may also be beneficial to children or adults with olfactory neurosensory issues. In addition, honey placed on broiled salmon (or any fish) may increase the likelihood of consumption by children.
Table 2.
Contents and Ratios of Omega-3 HUFAs Vary Widely Across Commercially Available Products
| Total oils in capsules/serving |
Total Omega-3 HUFA ( mg) |
EPA (mg) |
DHA (mg) |
% of EPA in Total Omega-3 HUFA |
Number of capsules needed to deliver HUFA at 2 grams daily |
Daily Cost |
|---|---|---|---|---|---|---|
| Unconcentrated fish oil (1,000 mg capsules) | 300 | 120 | 180 | 40% | 7 caps | $ |
| Molecularly distilled (1,000 mg capsules) | 500 | 300 | 200 | 60% | 4 caps | $$ |
| Highly purified (1,100 mg capsules) | 1,000 | 600 | 400 | 60% | 2 caps | $$$ |
| Algal oils (625 mg capsules) | 500 | 180 | 320 | 36% | 4 caps | $$$ |
| Algal oils (500 mg capsules) | 200 | nil | 200 | ~100% | 10 caps | $$$ |
| Liquid oil (highly concentrated) (1 teaspoon = 5 ml) | 2510 | 1450 | 1060 | 58% | 3/4 tsp | $$ |
| Emulsion (highly concentrated) (15 ml serving) | 1,500 | 910 | 590 | 60% | 20 ml (1.25 tablespoons) | $$$ |
| Sardines, canned in oil (3.75 oz) | 921 | 435 | 486 | 47% | 2 cans | $ |
| Salmon, Atlantic, farmed, broiled (3 oz) | 1824 | 586 | 1,238 | 32% | 3.3 oz | $$ |
| White tuna, canned (3 oz) | 733 | 198 | 535 | 27% | 8 oz | $ |
| Omega-3 eggs (one egg, 50 gm) | 130 | 5 | 125 | 4% | 15 eggs | $ |
Due to the small number of well-powered trials in adults and youth, more research is needed to better determine the optimal ratios and doses of EPA and DHA. The majority of studies indicate that symptom improvement in adults with depression and youth with ADHD symptoms appears to be more likely using preparations containing higher levels of EPA than DHA. The mechanisms of action of this tentative clinical finding are not well understood.
Supplementation with omega-3 fatty acids has proven to be safe, easy to use, and relatively inexpensive and, despite open questions regarding mechanisms of action in different psychiatric disorders, these treatments have a clear biological rationale. Preparations which are EPA-rich (as opposed to DHA-rich) appear to be linked to improvement in symptoms of both affect and cognition. However, more well-powered studies are needed in youths with ADHD, conduct disorder, and mood disorders before clinical treatment recommendations can be made (See Tables 3 and 4).
Table 3.
Evidence-Based Treatment Evaluations Derived from the Medical Literature (Quality of Evidence / Strength of Recommendations for Each Treatment and Indication)
| EPA Alone | DHA Alone | Fish Oil | |
|---|---|---|---|
| ADHD | Good/Recommend | Insufficient data | Fair/Recommend |
| Depression | Fair/Recommend | Poor/Insufficient data | Fair/Recommend |
| Aggression/Defiance | Fair/Insufficient data | Insufficient data | Fair/Recommend |
| Cognitive Parameters | Fair/Insufficient data | Insufficient data | Good/Recommend |
| Dyspraxia | Insufficient data | Insufficient data | Good/Recommend |
| Basis for ADHD in Youth | Multiple RCTs | Multiple RCTs | Meta-analyses |
Levels of evidence based on US Preventive Services Task Force: http://www.uspreventiveservicestaskforce.org
Table 4.
Expert Clinical Opinion regarding HUFA Treatment of ADHD (Quality of Evidence / Strength of Recommendations for Each Treatment and Indication)
| Treatment | ADHD | Opinion |
|---|---|---|
| EPA >60% | Good/Recommend | Effects are consistent, but not as large as stimulant medications |
| DHA >60% | Insufficient data | Case reports for decreased anxiety and disruptive behaviors are encouraging |
| Fish oil | Fair/Recommend | Effects may be larger for aggression and mood than for inattention |
Levels of evidence based on US Preventive Services Task Force: http://www.uspreventiveservicestaskforce.org
Figure 1.
Biochemical Pathways for the Omega-3 and Omega-6 Fatty Acids
Box 1: Explanation of Modified United States Preventive Services Task Force Grading System Used in Tables 3 and 4.
Tables 3 and 4 use a modified form of the United States Preventive Services Task Force (USPSTF) system
Each treatment is assigned a grade for “Quality of Evidence” and a grade for “Strength of Recommendations.”
The Quality of Evidence grade is a qualitative ranking of the strength of the published evidence in the medical literature regarding a treatment:
| • Good | Consistent benefit in well-conducted studies in different populations. |
| • Fair | Data shows positive effects, but weak, limited, or indirect evidence. |
| • Poor | Can’t show benefit due to data weakness. |
The Strength of Recommendations grade provides a qualitative ranking of the clinical recommendations that can be drawn from findings of the studies:
| • Insufficient Data | |
| • Recommend Against | Fair evidence of ineffectiveness or harm. |
| • Neutral | Fair evidence for, but appears risky. |
| • Recommend | Fair evidence of benefit and of safety. |
| • Recommend Strongly | Good evidence of benefit and safety. |
Adapted from U.S. Preventive Services Task Force Grade Definitions. May 2008.
http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. Accessed Feb 26 2014; with permission.
Key Points.
Omega-3 highly unsaturated fatty acids (HUFAs) are critical for both structure and function of the brain
The omega-3 HUFA docosahexaenoic acid (DHA) and the omega-6 HUFA arachidonic acid (AA) are especially critical for the development of the central nervous system.
Omega-3 and omega-6 fatty acids have distinct roles and require a balance of omega-3/6 for optimal physical and mental health. An excessive intake of one fatty acid may inhibit the conversion of the other kind.
EPA-rich formulas are linked to improvements in mood and ADHD symptoms.
The APA Task Force on Complementary and Alternative Medicine recommended that the dietary intake of omega-3 for patients with poor impulse control, mood or psychotic disorders should include EPA + DHA at a daily dose of about one gram.
Lower concentrations of omega-3 fatty acids have been present in both plasma and red blood cells of children and young adults with ADHD in comparison to healthy controls.
Supplementation with omega-3 HUFAs in clinical trials have found some improvement in learning capacity and behavior in youths who are academically underachieving, have ADHD-like symptoms, and/or have severe misconduct.
The relationship between nutritional deficiencies, in particular of omega-3 fats, and symptoms of mental ill-health, warrants closer examination by clinicians and mental health practitioners.
Acknowledgments
Funding Sources: Intramural Research Program, National Institute of Alcohol Abuse and Alcoholism (NIAAA) and Barlean’s Organic Oils, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
References
- 1.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- 2.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. The American journal of clinical nutrition. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 3.Rees GA, Doyle W, Srivastava A, Brooke ZM, Crawford MA, Costeloe KL. The nutrient intakes of mothers of low birth weight babies - a comparison of ethnic groups in East London, UK. Matern Child Nutr. 2005;1:91–99. doi: 10.1111/j.1740-8709.2005.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle W, Rees G. Maternal malnutrition in the UK and low birthweight. Nutr Health. 2001;15:213–218. doi: 10.1177/026010600101500410. [DOI] [PubMed] [Google Scholar]
- 5.UNICEF. Sustainable Elimination of Iodine Deficiency. New York: UNICEF; 2008. Available only in free downloadable format at: www.unicef.org/publications/index_44271.html. [Google Scholar]
- 6.The Lancet: The Iodine deficiency - Way to go yet [editorial] The Lancet. 2008;372:88. doi: 10.1016/S0140-6736(08)61009-0. [DOI] [PubMed] [Google Scholar]
- 7.Peirano PD, Algarin CR, Chamorro R, Reyes S, Garrido MI, Duran S, Lozoff B. Sleep and neurofunctions throughout child development: lasting effects of early iron deficiency. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 1):S8–S15. doi: 10.1097/MPG.0b013e31819773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–666S. doi: 10.1093/jn/131.2.649S. discussion 666S-668S. [DOI] [PubMed] [Google Scholar]
- 9.McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. The American journal of clinical nutrition. 2007;85:931–945. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- 10.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:S560–S571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 11.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public health nutrition. 2005;8:117–132. doi: 10.1079/phn2004677. [DOI] [PubMed] [Google Scholar]
- 12.Venturi S, Donati FM, Venturi A, Venturi M. Environmental iodine deficiency: A challenge to the evolution of terrestrial life? Thyroid. 2000;10:727–729. doi: 10.1089/10507250050137851. [DOI] [PubMed] [Google Scholar]
- 13.Coe CL, Lubach GR, Bianco L, Beard JL. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Dev Psychobiol. 2009;51:301–309. doi: 10.1002/dev.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36:1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 17.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- 18.Karr JE, Alexander JE, Winningham RG. Omega-3 polyunsaturated fatty acids and cognition throughout the lifespan: A review. Nutritional Neuroscience. 2011;14:216–225. doi: 10.1179/1476830511Y.0000000012. [DOI] [PubMed] [Google Scholar]
- 19.Siegel G, Ermilov E. Omega-3 fatty acids: benefits for cardio-cerebro-vascular diseases. Atherosclerosis. 2012;225:291–295. doi: 10.1016/j.atherosclerosis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain research. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 21.Haag M. Essential fatty acids and the brain. Can J Psychiatry. 2003;48:195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 22.Elmadfa I, Kornsteiner M. Fats and fatty acid requirements for adults. Ann Nutr Metab. 2009;55:56–75. doi: 10.1159/000228996. [DOI] [PubMed] [Google Scholar]
- 23.Brenna JT, Salem N, Jr., Sinclair AJ, Cunnane SC. [alpha]-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans, Prostaglandins. Leukotrienes and Essential Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Crawford MA, Bazinet RP, Sinclair AJ. Fat intake and CNS functioning: ageing and disease. Ann Nutr Metab. 2009;55:202–228. doi: 10.1159/000229003. [DOI] [PubMed] [Google Scholar]
- 25.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM, Fat deposition. fatty acid composition and meat quality: A review. Meat Science. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain research. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 29.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 30.Brenna JT, Diau G-Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition, Prostaglandins. Leukotrienes and Essential Fatty Acids. 2007;77:247–250. doi: 10.1016/j.plefa.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diau G-Y, Hsieh A, Sarkadi-Nagy E, Wijendran V, Nathanielsz P, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Medicine. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell DC, Gawrisch K, Litman BJ, Salem N., Jr. Why is docosahexaenoic acid essential for nervous system function? Biochem Soc Trans. 1998;26:365–370. doi: 10.1042/bst0260365. [DOI] [PubMed] [Google Scholar]
- 33.Bourre JM, Bonneil M, Chaudiere J, Clement M, Dumont O, Durand G, Lafont H, Nalbone G, Pascal G, Piciotti M. Structural and functional importance of dietary polyunsaturated fatty acids in the nervous system. Adv Exp Med Biol. 1992;318:211–229. doi: 10.1007/978-1-4615-3426-6_18. [DOI] [PubMed] [Google Scholar]
- 34.Jones CR, Arai T, Rapoport SI. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochem Res. 1997;22:663–670. doi: 10.1023/a:1027341707837. [DOI] [PubMed] [Google Scholar]
- 35.Condray R, Yao JK, Steinhauer SR, van Kammen DP, Reddy RD, Morrow LA. Semantic memory in schizophrenia: association with cell membrane essential fatty acids. Schizophr Res. 2008;106:13–28. doi: 10.1016/j.schres.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions. J Neurosci Res. 1999;56:565–570. doi: 10.1002/(SICI)1097-4547(19990615)56:6<565::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 37.Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pacific journal of clinical nutrition. 2008;17(Suppl 1):220–228. [PubMed] [Google Scholar]
- 38.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–449. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- 39.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 40.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins, leukotrienes, and essential fatty acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Mostofsky DI, Yehuda S, Salem N. Fatty Acids: Physiological and Behavioral Functions. New Jersey: Humana Press; 2001. [Google Scholar]
- 42.Lands B. A critique of paradoxes in current advice on dietary lipids. Prog Lipid Res. 2008;47:77–106. doi: 10.1016/j.plipres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Horrobin DF. Essential fatty acids, prostaglandins, and alcoholism: an overview. Alcohol Clin Exp Res. 1987;11:2–9. doi: 10.1111/j.1530-0277.1987.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 44.Marangoni F, Colombo C, De Angelis L, Gambaro V, Agostoni C, Giovannini M, Galli C. Cigarette smoke negatively and dose-dependently affects the biosynthetic pathway of the n-3 polyunsaturated fatty acid series in human mammary epithelial cells. Lipids. 2004;39:633–637. doi: 10.1007/s11745-004-1276-5. [DOI] [PubMed] [Google Scholar]
- 45.Agostoni C, Riva E, Giovannini M, Pinto F, Colombo C, Rise P, Galli C, Marangoni F. Maternal smoking habits are associated with differences in infants' long-chain polyunsaturated fatty acids in whole blood: a case-control study. Arch Dis Child. 2008;93:414–418. doi: 10.1136/adc.2007.129817. [DOI] [PubMed] [Google Scholar]
- 46.Pawlosky RJ, Salem N., Jr. Perspectives on alcohol consumption: liver polyunsaturated fatty acids and essential fatty acid metabolism. Alcohol. 2004;34:27–33. doi: 10.1016/j.alcohol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 48.Brookes KJ, Chen W, Xu X, Taylor E, Asherson P. Association of Fatty Acid Desaturase Genes with Attention-Deficit/Hyperactivity Disorder. Biological psychiatry. 2006;60:1053–1061. doi: 10.1016/j.biopsych.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 50.Hibbeln JR, Nieminen LR, Lands WE. Increasing homicide rates and linoleic acid consumption among five Western countries, 1961–2000. Lipids. 2004;39:1207–1213. doi: 10.1007/s11745-004-1349-5. [DOI] [PubMed] [Google Scholar]
- 51.Chalon S. The role of fatty acids in the treatment of ADHD. Neuropharmacology. 2009;57:636–639. doi: 10.1016/j.neuropharm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Yavin E, Himovichi E, Eilam R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162:1011–1022. doi: 10.1016/j.neuroscience.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 53.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins, leukotrienes, and essential fatty acids. 2010;83:185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Kuipers RS, Luxwolda MF, Dijck-Brouwer DA, Eaton SB, Crawford MA, Cordain L, Muskiet FA. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. The British journal of nutrition. 2010;104:1666–1687. doi: 10.1017/S0007114510002679. [DOI] [PubMed] [Google Scholar]
- 56.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. Bmj. 2013;346:e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salem N, Jr., Moriguchi T, Greiner RS, McBride K, Ahmad A, Catalan JN, Slotnick B. Alterations in brain function after loss of docosahexaenoate due to dietary restriction of n-3 fatty acids. Journal of molecular neuroscience : MN. 2001;16:299–307. doi: 10.1385/JMN:16:2-3:299. discussion 317–221. [DOI] [PubMed] [Google Scholar]
- 58.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Calatayud S, Redondo C, Martin E, Ruiz JI, Garcia-Fuentes M, Sanjurjo P. Brain docosahexaenoic acid status and learning in young rats submitted to dietary long-chain polyunsaturated fatty acid deficiency and supplementation limited to lactation. Pediatr Res. 2005;57:719–723. doi: 10.1203/01.PDR.0000156506.03057.AD. [DOI] [PubMed] [Google Scholar]
- 60.Al MD, van Houwelingen AC, Hornstra G. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. The American journal of clinical nutrition. 2000;71:285S–291S. doi: 10.1093/ajcn/71.1.285s. [DOI] [PubMed] [Google Scholar]
- 61.Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aid S, Poumes-Ballihaut C, Champeil-Potokar G, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev. 2004;44:509–538. doi: 10.1051/rnd:2004063. [DOI] [PubMed] [Google Scholar]
- 62.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins, leukotrienes, and essential fatty acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284:25–28. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 64.Hibbeln JR, Ferguson TA, Blasbalg TL. Omega-3 fatty acid deficiencies in neurodevelopment, aggression and autonomic dysregulation: opportunities for intervention. Int Rev Psychiatry. 2006;18:107–118. doi: 10.1080/09540260600582967. [DOI] [PubMed] [Google Scholar]
- 65.Fedorova I, Salem JN. Omega-3 fatty acids and rodent behavior. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Mathieu G, Denis S, Lavialle M, Vancassel S. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;78:391–401. doi: 10.1016/j.plefa.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 68.Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75:299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Ward PE. Potential diagnostic aids for abnormal fatty acid metabolism in a range of neurodevelopmental disorders. Prostaglandins, leukotrienes, and essential fatty acids. 2000;63:65–68. doi: 10.1054/plef.2000.0193. [DOI] [PubMed] [Google Scholar]
- 70.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, Burgess JR. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. The American journal of clinical nutrition. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 71.Chen J-R, Hsu S-F, Hsu C-D, Hwang L-H, Yang S-C. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. The Journal of Nutritional Biochemistry. 2004;15:467–472. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell EA, Aman MG, Turbott SH, Manku M. Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatr (Phila) 1987;26:406–411. doi: 10.1177/000992288702600805. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell EA, Lewis S, Cutler DR. Essential fatty acids and maladjusted behaviour in children. Prostaglandins Leukot Med. 1983;12:281–287. doi: 10.1016/0262-1746(83)90006-9. [DOI] [PubMed] [Google Scholar]
- 74.Germano M, Meleleo D, Montorfano G, Adorni L, Negroni M, Berra B, Rizzo AM. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (ADHD) Nutr Neurosci. 2007;10:1–9. doi: 10.1080/10284150601153801. [DOI] [PubMed] [Google Scholar]
- 75.Colter AL, Cutler C, Meckling K. Fatty acid status and behavioural symptoms of Attention Deficit Hyperactivity Disorder in adolescents: A case-control study. Nutrition Journal. 2008;7:8. doi: 10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson AJ, Montgomery P, The Oxford-Durham, study: a randomized. controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115:1360–1366. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- 77.Sinn N, Bryan J, Wilson C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: A randomised controlled trial. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;78:311–326. doi: 10.1016/j.plefa.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. 2007;28:82–91. doi: 10.1097/01.DBP.0000267558.88457.a5. [DOI] [PubMed] [Google Scholar]
- 79.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 80.Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:233–239. doi: 10.1016/s0278-5846(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 81.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. The American journal of clinical nutrition. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic Acid for Reading, Cognition and Behavior in Children Aged 7–9 Years: A Randomized, Controlled Trial (The DOLAB Study) PloS one. 2012;7:e43909. doi: 10.1371/journal.pone.0043909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA, A double-blind. placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Research in Developmental Disabilities. 2010;31:718–730. doi: 10.1016/j.ridd.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 84.McNamara RK. The emerging role of omega-3 fatty acids in psychiatry. Prostaglandins, leukotrienes, and essential fatty acids. 2006;75:223–225. doi: 10.1016/j.plefa.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 85.McNamara RK, Able J, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. J Psychiatr Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crawford MA, Hassam AG, Williams G. Essential fatty acids and fetal brain growth. Lancet. 1976;1:452–453. doi: 10.1016/s0140-6736(76)91476-8. [DOI] [PubMed] [Google Scholar]
- 87.Fiennes RN, Sinclair AJ, Crawford MA. Essential fatty acid studies in primates linolenic acid requirements of capuchins. J Med Primatol. 1973;2:155–169. doi: 10.1159/000460319. [DOI] [PubMed] [Google Scholar]
- 88.Crawford MA, Leigh Broadhurst C, Guest M, Nagar A, Wang Y, Ghebremeskel K, Schmidt WF. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins, leukotrienes and essential fatty acids. 2013;88:5–13. doi: 10.1016/j.plefa.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. The American journal of clinical nutrition. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 90.Eaton SB, Eaton SB, 3rd, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. World Rev Nutr Diet. 1998;83:12–23. doi: 10.1159/000059672. [DOI] [PubMed] [Google Scholar]
- 91.Eaton SB, Eaton SB, 3rd, Konner MJ. Paleolithic nutrition revisited: a twelve-year retrospective on its nature and implications. European journal of clinical nutrition. 1997;51:207–216. doi: 10.1038/sj.ejcn.1600389. [DOI] [PubMed] [Google Scholar]
- 92.Crawford M, Crawford S. Stein and Day. New York: 1972. What we eat today. [Google Scholar]
- 93.Wang Y, Lehane C, Ghebremeskel K, Crawford MA. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public health nutrition. 2010;13:400–408. doi: 10.1017/S1368980009991157. [DOI] [PubMed] [Google Scholar]
- 94.Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, Kristiansen K, Froyland L, Hibbeln JR. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity. 2012;20:1984–1994. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 97.US Food and Drug Administration and the Environmental Protection Agency. What You Need to Know About Mercury in Fish and Shellfish: EPA and FDA Advice For Women Who Might Become Pregnant. Women Who are Pregnant, Nursing Mothers, and Young Children. 2004 Mar; EPA-823-R-04-00. [Google Scholar]
- 98.Moriguchi T, Greiner RS, Salem N., Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. Journal of neurochemistry. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 99.FDA. Federal Register - 74 FR 3615. 2009. Draft Risk and Benefit Assessment Report and Draft Summary of Published Research Report of Quantitative Risk and Benefit Assessment of Commercial Fish Consumption, Focusing on Fetal Neurodevelopmental Effects (Measured by Verbal Development in Children) and on Coronary Heart Disease and Stroke in the General Population, and Summary of Published Research on the Beneficial Effects of Fish Consumption and Omega-3 Fatty Acids for Certain Neurodevelopmental and Cardiovascular Endpoints. in: Notice of Availability January 21 (Ed.), 2009. [Google Scholar]
- 100.Freeman MP, Mischoulon D, Tedeschini E, Goodness T, Cohen LS, Fava M, Papakostas GI. Complementary and alternative medicine for major depressive disorder: a meta-analysis of patient characteristics, placebo-response rates, and treatment outcomes relative to standard antidepressants. The Journal of clinical psychiatry. 2010;71:682–688. doi: 10.4088/JCP.10r05976blu. [DOI] [PubMed] [Google Scholar]
- 101.SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105:1292–1298. doi: 10.1542/peds.105.6.1292. [DOI] [PubMed] [Google Scholar]
- 102.Birch EE, Carlson SE, Hoffman DR, Fitzgerald-Gustafson KM, Fu VL, Drover JR, Castaneda YS, Minns L, Wheaton DK, Mundy D, Marunycz J, Diersen-Schade DA. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. The American journal of clinical nutrition. 2010;91:848–859. doi: 10.3945/ajcn.2009.28557. [DOI] [PubMed] [Google Scholar]
- 103.Forsyth JS, Willatts P, DiModogno MK, Varma S, Colvin M. Do long-chain polyunsaturated fatty acids influence infant cognitive behaviour? Biochem Soc Trans. 1998;26:252–257. doi: 10.1042/bst0260252. [DOI] [PubMed] [Google Scholar]
- 104.Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, Karyadi SJ, van Klinken BJ, van der Knaap HC, Lukito W, Mikarsa W, Transler C, Wilson C. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. The American journal of clinical nutrition. 2007;86:1082–1093. doi: 10.1093/ajcn/86.4.1082. [DOI] [PubMed] [Google Scholar]
- 105.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem. 2004;15:467–472. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 106.Stevens LJ, Zentall SS, Abate ML, Kuczek T, Burges JR. Omega-3 fatty acids in boys with behavior, learning, and health problems. Physiology & Behavior. 1996;59:915–920. doi: 10.1016/0031-9384(95)02207-4. [DOI] [PubMed] [Google Scholar]
- 107.Pawlosky R, Hibbeln J, Lin Y, Salem N., Jr. n-3 fatty acid metabolism in women. Br J Nutr. 2003;90:993–994. doi: 10.1079/bjn2003985. discussion 994–995. [DOI] [PubMed] [Google Scholar]
- 108.Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139:189–196. doi: 10.1067/mpd.2001.116050. [DOI] [PubMed] [Google Scholar]
- 109.Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit//hyperactivity disorder [mdash] a placebo-controlled double-blind study. European journal of clinical nutrition. 58:467–473. doi: 10.1038/sj.ejcn.1601830. (0000) [DOI] [PubMed] [Google Scholar]
- 110.Arnold LE, Kleykamp D, Votolato NA, Taylor WA, Kontras SB, Tobin K. Gamma-linolenic acid for attention-deficit hyperactivity disorder: placebo-controlled comparison to D-amphetamine. Biological psychiatry. 1989;25:222–228. doi: 10.1016/0006-3223(89)90167-4. [DOI] [PubMed] [Google Scholar]
- 111.Belanger SA, Vanasse M, Spahis S, Sylvestre MP, Lippe S, L'Heureux F, Ghadirian P, Vanasse CM, Levy E. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr Child Health. 2009;14:89–98. doi: 10.1093/pch/14.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raz R, Carasso RL, Yehuda S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: a double-blind placebo-controlled study. J Child Adolesc Psychopharmacol. 2009;19:167–177. doi: 10.1089/cap.2008.070. [DOI] [PubMed] [Google Scholar]
- 113.Sumich A, Matsudaira T, Gow RV, Ibrahimovic A, Ghebremeskel K, Crawford M, Taylor E. Resting state electroencephalographic correlates with red cell long-chain fatty acids, memory performance and age in adolescent boys with attention deficit hyperactivity disorder. Neuropharmacology. 2009;57:708–714. doi: 10.1016/j.neuropharm.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 114.Gow RV, Matsudaira T, Taylor E, Rubia K, Crawford M, Ghebremeskel K, Ibrahimovic A, Vallee-Tourangeau F, Williams LM, Sumich A. Total red blood cell concentrations of omega-3 fatty acids are associated with emotion-elicited neural activity in adolescent boys with attention-deficit hyperactivity disorder. Prostaglandins, leukotrienes, and essential fatty acids. 2009;80:151–156. doi: 10.1016/j.plefa.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 115.Singh SD, Ellis CR, Winton AS, Singh NN, Leung JP, Oswald DP. Recognition of facial expressions of emotion by children with attention-deficit hyperactivity disorder. Behav Modif. 1998;22:128–142. doi: 10.1177/01454455980222002. [DOI] [PubMed] [Google Scholar]
- 116.Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. The American journal of clinical nutrition. 2011;93:1025–1037. doi: 10.3945/ajcn.110.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milte CM, Sinn N, Buckley JD, Coates AM, Young RM, Howe PR. Polyunsaturated fatty acids, cognition and literacy in children with ADHD with and without learning difficulties. J Child Health Care. 2011 doi: 10.1177/1367493511403953. [DOI] [PubMed] [Google Scholar]
- 118.Richardson AJ. Clinical trials of fatty acid treatment in ADHD, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;70:383–390. doi: 10.1016/j.plefa.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 119.Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:233–239. doi: 10.1016/s0278-5846(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 120.Johnson M, Ostlund S, Fransson G, Kadesjo B, Gillberg C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Atten Disord. 2009;12:394–401. doi: 10.1177/1087054708316261. [DOI] [PubMed] [Google Scholar]
- 121.Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, Zentall SS, Arnold LE, Burgess JR. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids. 2003;38:1007–1021. doi: 10.1007/s11745-006-1155-0. [DOI] [PubMed] [Google Scholar]
- 122.Freeman MP, Fava M, Lake J, Trivedi MH, Wisner KL, Mischoulon D. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association Task Force report. The Journal of clinical psychiatry. 2010;71:669–681. doi: 10.4088/JCP.10cs05959blu. [DOI] [PubMed] [Google Scholar]
- 123.Harding KL, Judah RD, Gant C. Outcome-based comparison of Ritalin versus food-supplement treated children with AD/HD. Altern Med Rev. 2003;8:319–330. [PubMed] [Google Scholar]
- 124.Bloch MH, Qawasmi A. Omega-3 Fatty Acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50:991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swan GE, Carmelli D, Rosenman RH. Psychological characteristics in twins discordant for smoking behavior: a matched-twin-pair analysis. Addictive behaviors. 1988;13:51–60. doi: 10.1016/0306-4603(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 126.Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder - a placebo-controlled double-blind study. European journal of clinical nutrition. 2004;58:467–473. doi: 10.1038/sj.ejcn.1601830. [DOI] [PubMed] [Google Scholar]
- 127.Gesch CB, Hammond SM, Hampson SE, Eves A, Crowder MJ. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners. Randomised, placebo-controlled trial. Br J Psychiatry. 2002;181:22–28. doi: 10.1192/bjp.181.1.22. [DOI] [PubMed] [Google Scholar]
- 128.Corrigan F, Gray R, Strathdee A, Skinner R, Van Rhijn A, Horrobin D. Fatty acid analysis of blood from violent offenders. Journal of Forensic Psychiatry & Psychology. 1994;5:83–92. [Google Scholar]
- 129.Schoenthaler SJ. Diet and criminal behavior: A criminological evaluation of the Arlington, Virginia proceedings. Personality and Individual Differences. 1991;12:339–340. [Google Scholar]
- 130.Schoenthaler SJ. The Alabama diet-behavior program: An evaluation at the Coosa Valley regional detention center. Personality and Individual Differences. 1991;12:336–336. [Google Scholar]
- 131.Schoenthaler SJ, Bier ID. The effect of vitamin-mineral supplementation on juvenile delinquency among American schoolchildren: a randomized, double-blind placebo-controlled trial. J Altern Complement Med. 2000;6:7–17. doi: 10.1089/acm.2000.6.7. [DOI] [PubMed] [Google Scholar]
- 132.Weller EB, Weller RA, Rooney MT, Fristad MA. Children’s Interview for Psychiatric Syndromes (ChIPS) Arlington, VA: American Psychiatric Press, Inc; 1999. [DOI] [PubMed] [Google Scholar]
- 133.Gow RV, Vallee-Tourangeau F, Crawford MA, Taylor E, Ghebremeskel K, Bueno AA, Hibbeln JR, Sumich A, Rubia K. Omega-3 fatty acids are inversely related to callous and unemotional traits in adolescent boys with attention deficit hyperactivity disorder, Prostaglandins. kLeukotrienes and Essential Fatty Acids. 2013 doi: 10.1016/j.plefa.2013.03.009. in press. [DOI] [PubMed] [Google Scholar]
- 134.Frick PJ, Stickle TR, Dandreaux DM, Farrell JM, Kimonis ER. Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. Journal of Abnormal Child Psychology. 2005;33:471–487. doi: 10.1007/s10648-005-5728-9. [DOI] [PubMed] [Google Scholar]
- 135.Frick PJ, White SF. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 136.Viding E. Annotation: understanding the development of psychopathy. J Child Psychol Psychiatry. 2004;45:1329–1337. doi: 10.1111/j.1469-7610.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 137.Viding E, Jones AP, Frick PJ, Moffitt TE, Plomin R. Heritability of antisocial behaviour at 9: do callous-unemotional traits matter? Dev Sci. 2008;11:17–22. doi: 10.1111/j.1467-7687.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 138.Zaalberg A, Nijman H, Bulten E, Stroosma L, van der Staak C. Effects of nutritional supplements on aggression, rule-breaking, and psychopathology among young adult prisoners. Aggressive Behavior. 2010;36:117–126. doi: 10.1002/ab.20335. [DOI] [PubMed] [Google Scholar]
- 139.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 140.Peet M, Murphy B, Shay J, Horrobin D. Depletion of Omega-3 Fatty Acid Levels in Red Blood Cell Membranes of Depressive Patients. Biological psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 141.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res. 2007;152:1–10. doi: 10.1016/j.psychres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 142.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosomatic medicine. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 143.Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. Journal of affective disorders. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 144.Hibbeln JR. Fish consumption and major depression. The Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 145.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. American Journal of Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 146.Hibbeln JR. Seafood consumption and homicide mortality. A cross-national ecological analysis. World Rev Nutr Diet. 2001;88:41–46. doi: 10.1159/000059747. [DOI] [PubMed] [Google Scholar]
- 147.McNamara RK, editor. Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. Hauppauge (New York): Nova Science Pub Incorporated; 2013. [Google Scholar]
- 148.Pawlosky R, Hibbeln J, Lin Y, Salem N., Jr. n-3 fatty acid metabolism in women. British Journal of Nutrition. 2003;90:993–994. doi: 10.1079/bjn2003985. discussion 994–995. [DOI] [PubMed] [Google Scholar]
- 149.Ginsberg Y, Lindefors N. Methylphenidate treatment of adult male prison inmates with attention-deficit hyperactivity disorder: randomised double-blind placebo-controlled trial with open-label extension. The British Journal of Psychiatry. 2012;200:68–73. doi: 10.1192/bjp.bp.111.092940. [DOI] [PubMed] [Google Scholar]
- 150.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 151.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 152.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr., Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. The Journal of clinical psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 153.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. The American journal of clinical nutrition. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 154.Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS. Efficacy of omega-3 fatty acids in mood disorders - a systematic review and metaanalysis. Psychopharmacology bulletin. 2009;42:39–54. [PubMed] [Google Scholar]
- 155.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. Journal of the American College of Nutrition. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 156.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Molecular psychiatry. 2012;17:1144–1149. doi: 10.1038/mp.2012.25. discussion 1163–1147. [DOI] [PubMed] [Google Scholar]
- 157.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. The Journal of clinical psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, Su KP. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Molecular psychiatry. 2012;17:1161–1163. doi: 10.1038/mp.2012.111. author reply 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Molecular psychiatry. 2012;17:1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS medicine. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hibbeln JR. Depression, suicide and deficiencies of omega-3 essential fatty acids in modern diets. World Rev Nutr Diet. 2009;99:17–30. doi: 10.1159/000192992. [DOI] [PubMed] [Google Scholar]
- 162.Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. The Journal of clinical psychiatry. 2011;72:1585–1590. doi: 10.4088/JCP.11m06879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 164.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 165.Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biological psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 166.Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 167.Itomura M, Hamazaki K, Sawazaki S, Kobayashi M, Terasawa K, Watanabe S, Hamazaki T. The effect of fish oil on physical aggression in schoolchildren — a randomized, double-blind, placebo-controlled trial. The Journal of Nutritional Biochemistry. 2005;16:163–171. doi: 10.1016/j.jnutbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 168.Hamazaki K, Syafruddin D, Tunru IS, Azwir MF, Asih PB, Sawazaki S, Hamazaki T. The effects of docosahexaenoic acid-rich fish oil on behavior, school attendance rate and malaria infection in school children--a double-blind, randomized, placebo-controlled trial in Lampung, Indonesia. Asia Pacific journal of clinical nutrition. 2008;17:258–263. [PubMed] [Google Scholar]
- 169.Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, Hibbeln JR, Loewke J, Smith S, Coble R, Suchindran C, Gaylord SA. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Freeman MP. Omega-3 fatty acids and perinatal depression: a review of the literature and recommendations for future research. Prostaglandins, leukotrienes, and essential fatty acids. 2006;75:291–297. doi: 10.1016/j.plefa.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 171.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. The New England journal of medicine. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Plourde M, Kathy Stewart K, Crawford MA. Docosahexaenoic acid and shore-based diets in hominin encephalization: A rebuttal. American Journal of Human Biology. 2007;19:578–581. doi: 10.1002/ajhb.20673. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Stewart KM. Human Brain Evolution: The Influence of Freshwater and Marine Food Resources. Hoboken NJ: Wiley-Blackwell; 2010. [Google Scholar]