Abstract
Normalizing neural responses by the sum of population activity allows the nervous system to adjust its sensitivity according to task demands, facilitating intensity-invariant information processing. In this issue of Neuron, two studies suggest that parvalbumin-positive interneurons in the olfactory bulb play a role in this process.
The brain's sensory systems transform information from the environment into the spiking activity of neurons. As the intensity of a stimulus increases, neurons tuned to that stimulus typically produce more action potentials, eventually reaching an asymptotic firing rate. This input-response function often takes a sigmoidal shape, responding minimally to low intensities, rising with a certain slope, and finally saturating. Neurons are most sensitive to changes in stimulus intensity at the center of the sigmoid. Interestingly, response functions are not fixed: the threshold, slope and saturation level of a sigmoid can be dynamically adjusted depending on the nature of sensory inputs. These changes in response functions are called “gain control” and are thought to be essential for efficient processing of behaviorally-relevant sensory information.
Although gain control has long played a prominent role in theories of olfaction, the neural mechanisms underlying this process have remained elusive. Recently, however, several studies have taken advantage of advanced molecular, genetic, optical, and electrophysiological techniques to reveal how gain control might be implemented at the circuit level (Olsen and Wilson, 2008; Olsen et al., 2010; Papadopoulou et al., 2011; Zhu et al., 2013). In a tour de force, two new studies in this issue of Neuron (Kato et al., 2013; Miyamichi et al., 2013) reveal one population of neurons—parvalbumin-positive GABAergic neurons (PVNs)—to be a key regulator of gain control in the olfactory system. These results support an emerging picture of a flexible and efficient olfactory system, which uses different circuit elements to implement different forms of gain control depending on the task at hand.
In this Preview, we begin by introducing the various types of gain control, referring to examples from the visual system, where gain control has been best studied. We then highlight the contribution of the two new studies to this growing field. Finally, we discuss other studies of gain control in olfaction, pointing out important differences between these studies and discussing future directions.
Suppose that in a given sensory environment, a neuron's firing takes the following form:
In this equation, R is the firing rate of a neuron, I is the input, and f is the input-response function, typically sigmoidal. Gain control can affect this curve in at least three ways (Figure 1): a shift along the x-axis (known as input gain control), a shift along the y-axis (known as output gain control), or a change in slope, without shifting along either axis (known as dynamic range compression).
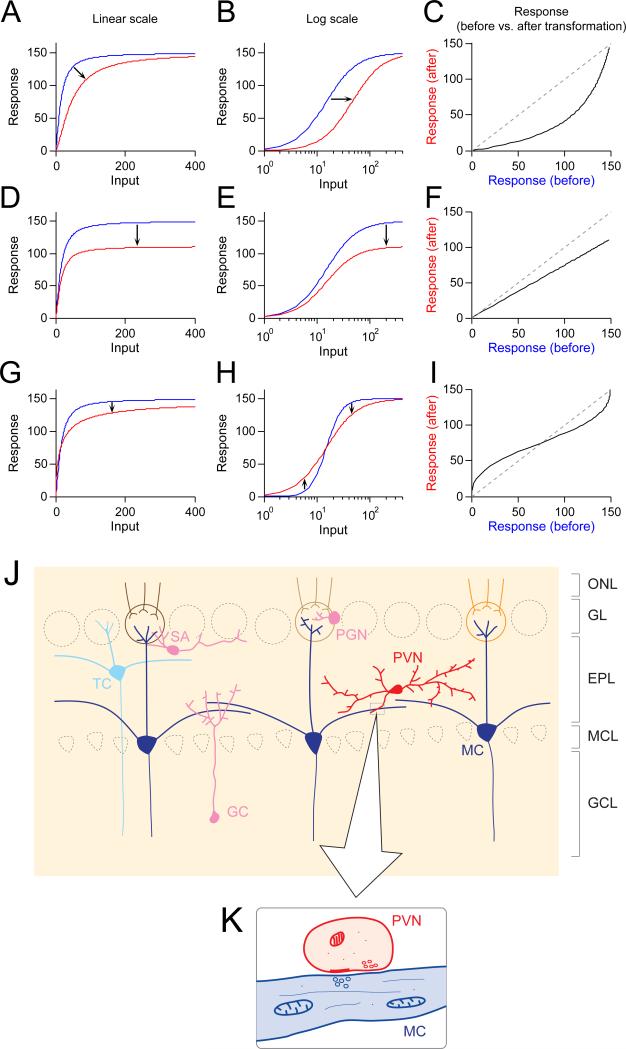
Figure 1. Different forms of gain control and the olfactory bulb.
(A-C) Input gain control. (D-F) Output gain control. (G-H) Dynamic range compression. See text for mathematical and conceptual descriptions of these forms of gain control. Input-response functions are modified after Olsen et al. (2010). (J) Major neuron types in the olfactory bulb. GC, granule cell. MC, mitral cell. SA, short axon cell. TC, tufted cell. PGC, periglomerular cell. PVN, parvalbumin-expressing interneuron. ONL, olfactory nerve layer. GL, glomerular layer. EPL, external plexiform layer. MCL, mitral cell layer. GCL, granule cell layer. (K) Dendrodendritic synapse between PVN and MC dendrites.
Input gain control scales the input (I) by some factor, α(Figure 1A).
In this case, the input has to be α times greater to cause the same level of response. This can be visually understood as a rightward shift of the input-response function on a logarithmic scale (Figure 1B). One important characteristic of input gain control is that it suppresses responses to weak inputs more than it suppresses responses to strong inputs, while keeping the saturation level constant (Figure 1C). Furthermore, if the response function is Gaussian rather than sigmoidal (e.g., in a tuning curve, where neuronal responses peak at a certain stimulus level and then show reduced firing), this transformation will change the shape of the response. In particular, any α> would cause a narrowing of the tuning curve.
Alternatively, in response gain control, responses to all input strengths, including the saturation level, are scaled by the same factor, α (Figure 1D-E). This effect is also referred to as multiplicative gain control:
Response gain control can be seen as a linear transformation of the output (Figure 1F). Importantly, it does not change the shape of the tuning curve; instead, it maintains neural sensitivity to different stimuli, just scaling it up or down.
Finally, dynamic range compression changes the slope of the response curve. For example, if f(I) is a sigmoid defined as
then dynamic range compression might cause the following change:
Such an effect allows a neuron to change its sensitivity to inputs without changing its response threshold or saturation level (Figure 1G-I).
When the scaling factor (α) reflects the summed activity of a pool of neurons, gain control is called “divisive normalization” (Carandini and Heeger, 2012). This can occur with any of the types of gain control discussed above. For example, neurons in the retina undergo input gain control according to the ambient light level. In contrast, in visual cortex, adding irrelevant stimuli outside the receptive field of a neuron causes output gain control.
The mathematical formulations above can explain gain control at an algorithmic level, yet how neural circuits actually implement gain control remains hotly debated (Carandini and Heeger, 2012).
To clarify how a specific neuronal population (often an inhibitory population) controls the gain of another population, the following two questions can be helpful: First, what drives the neurons? Do they receive a diverse set of inputs that can approximate total sensory input, or are they driven by a more specific set of inputs? How does their activity change depending on the animal's state (e.g., attention or arousal)? These questions can be addressed by monitoring the neurons’ activity patterns or by studying anatomical connectivity. Second, what is the impact of these neurons on postsynaptic neurons? How do they change the tuning curves or input-response functions of postsynaptic neurons? These questions can be addressed by either inhibiting or activating these neurons in an intact neural circuit. The two studies in this issue of Neuron address both of these questions.
In the olfactory bulb (OB, Figure 1J), the two major classes of interneurons are the periglomerular cells (PGCs) and the granule cells (GCs). These neurons regulate the activity of mitral and tufted cells (MTCs) through reciprocal connections via dendrodendritic synapses. In addition to PGCs and GCs, there are several minor classes of interneurons with distinct morphologies and molecular markers. These include short axon cells and PVNs. PVNs are located mostly in the external plexiform layer (EPL) in the OB. PVNs in EPL have multiple dendrites but typically lack apparent axons. They interact with MTCs through dendrodendritic synapses: thus, PVN dendrites are both postsynaptic (receiving glutamate released from MTCs) and presynaptic (releasing GABA to inhibit MTCs, Figure 1K).
To study the local connectivity of PVNs and GCs, Miyamichi and colleagues first modified the rabies virus-based transsynaptic circuit tracing system (Watabe-Uchida et al., 2012; Wickersham et al., 2007) to reduce non-specific labeling in injection sites. This improved rabies virus system allowed for the analysis of local connectivity. The authors showed that PVNs connect to widely distributed MTCs (<200-300 μm in distance). In stark contrast, GCs connect only to neighboring MTCs (<50-100 μm in distance). In agreement with these findings, Kato and colleagues performed paired recordings in acute OB slices and showed that PVNs form reciprocal connections with the majority of nearby MTCs whereas GCs have a low probability of connectivity with MTCs.
Next, the two groups examined the odor tuning properties of PVNs, GCs, and MTCs using optically-targeted loose patch recordings or optical imaging of genetically-targeted calcium sensors. Both experiments showed that PVNs are more broadly tuned to odors than GCs and MTCs. Indeed, PVNs were often activated by most of the odors tested (e.g. ~70% of PVNs responded to at least 4 out of 5 odors tested), while both GCs and MTCs were activated by fewer odors. Together, these results indicate that PVNs densely sample the activity of local MTCs distributed within several hundreds of micrometers.
So far, we know that PVNs are in a position to provide divisive normalization. But what is the functional impact of PVN activity on MTCs? To address this question, Kato et al. (2013) used pharmacogenetics to examine how PVN inactivation affects MTC odor tuning. After inactivation, the odor responses of MTCs were elevated in a largely multiplicative manner, with a small or negligible offset. In other words, PVNs cause output gain control. Taken together with the first set of results, it appears that PVNs sum activity from MTCs distributed in a local area (~ several hundreds of micrometers) to divisively normalize MTC odor responses. This result is consistent with recent studies of primary visual cortex, which showed that parvalbumin-expressing GABAergic neurons there are capable of output gain control on pyramidal neurons (Atallah et al., 2012; Wilson et al., 2012).
How does this role for PVNs differ from previous studies of gain control in olfaction? To address this question, it is important to distinguish forms of gain control as well as the spatial extent of sampling. Olsen and colleagues beautifully demonstrated that local interneurons (LNs) in Drosophila antennal lobes (ALs, the counterpart of vertebrate OB) perform divisive normalization via input gain control (Olsen and Wilson, 2008; Olsen et al., 2010) (Figure 1A-C). These LNs receive inputs from most or all glomeruli in the AL, in contrast to the relatively local connectivity of PVNs. Input gain control may decorrelate highly correlated ORN population input and facilitate concentration-invariant odor recognition. Similarly, in the locust, Papadopoulou et al. (2011) showed that a single “giant GABAergic neuron” samples activity from a broad swath of the mushroom body (MB, a counterpart of the piriform cortex) and performs divisive normalization of MB activity to maintain sparse representations. This normalization appears closest to output gain control.
Finally, in zebrafish, Zhu et al. (2013) showed that short axon cells in the OB exert the third form of gain control: dynamic range compression (Figure 1G-I). Short axon cells have dual functions in this process: at low levels of stimulation, electrical synapses from short axon cells boost MTC activation, while at increased levels of stimulation, short axon cells release GABA to suppress MTCs. Thus, short axon cells boost weak input and suppress strong input, changing the slope of the response function.
Compared to these previous examples, PVNs are unique in having relatively limited spatial pooling (although larger than GCs) while producing output gain control. As mentioned above, Drosophila LNs sample a nearly complete set of glomeruli; in contrast, the spatial extent to which PVNs pool MTC activity is far from complete. One limitation of Kato et al. (2013) and Miyamichi et al. (2013) is that the odors used to characterize odor tuning properties were chosen based on their ability to activate the dorsal surface of the OB, where recordings were made. This raises the possibility that a broader set of odors would reduce the probability of activating a given PVN. In other words, PVNs may not be as broadly tuned as Drosophila LNs. What are the consequences of relatively limited pooling of MTC populations by PVNs? It is important to note that the mammalian OB displays a coarse chemotopy (Soucy et al., 2009; Uchida et al., 2000), although whether this chemotopy holds at finer scales remains debated (Soucy et al., 2009). Glomeruli that respond to a given chemical class (e.g. fatty acids or tiglates) tend to cluster in specific “domains” (areas of several hundred millimeters) in the OB. Considering this coarse chemotopy, populations of PVNs located in slightly different locations in the OB are likely to pool the activity of slightly different sets of MTCs. This means that MTC responses are normalized by slightly different “denominators” depending on their locations. In fact, MTCs connected to the same glomerulus (sister MTCs) are scattered over 200-300 μm, and sister MTCs located farther from each other have more distinct odor tuning (Kikuta et al., 2013). Such an effect could be explained by local pooling from PVNs. It is important to note that analyses based on data pooled across neurons might miss finer, odor- and neuron-specific modification of odor tuning. Future studies should examine how PVNs shape odor tuning properties of individual MTCs using a larger panel of odor stimuli.
What could be the role of output gain modulation in the OB? In the visual system, output gain control has been observed in the attentional modulation of neuronal responses (e.g., Williford and Maunsell, 2006). These modulations may be important to amplify responses to behaviorally-relevant stimuli, without changing stimulus selectivity. It is important to note that interneurons in the OB receive inputs from the olfactory cortex and neuromodulatory systems, raising the possibility that gain control can itself be shaped by these inputs. Furthermore, considering the coarse chemotopy in OB and the spatial scale of PVN inputs, these gain modulations might be loosely domain- or chemical-specific. Future studies should examine whether the activity of PVNs is modulated by animal state, such as attention or arousal.
Gain control is a fundamental property of the brain, allowing for efficient coding of information in different sensory environments and behavioral states. The studies of Kato et al. (2013) and Miyamichi et al. (2013) bring us one step closer to understanding how gain control is implemented by neural circuits.
References
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Gillet SN, Peters AJ, Isaacson JS, Komiyama T. Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron. 2013 doi: 10.1016/j.neuron.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Fletcher ML, Homma R, Yamasoba T, Nagayama S. Odorant response properties of individual neurons in an olfactory glomerular module. Neuron. 2013;77:1122–1135. doi: 10.1016/j.neuron.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Shlomai-Fuchs Y, Shu M, Weissbourd BC, Luo L. Dissecting local circuits: Parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron. 2013 doi: 10.1016/j.neuron.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou M, Cassenaer S, Nowotny T, Laurent G. Normalization for sparse encoding of odors by a wide-field interneuron. Science. 2011;332:721–725. doi: 10.1126/science.1201835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat. Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford T, Maunsell JHR. Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Frank T, Friedrich RW. Equalization of odor representations by a network of electrically coupled inhibitory interneurons. Nat. Neurosci. 2013;16:1678–1686. doi: 10.1038/nn.3528. [DOI] [PubMed] [Google Scholar]