ABSTRACT
OBJECTIVES
To identify patient demographics and characteristics associated with compliance to statin therapy after switching from branded to generic agents
DESIGN
Retrospective cohort study using electronic health records and pharmacy claims data from Sutter Health’s ambulatory-care medical network
PATIENTS
Managed-care beneficiaries, ≥ 18 years of age, who were switched from branded to generic statins between 1 January 2003 and 31 December 2012
MAIN MEASURES
Compliance was calculated as days of therapy dispensed divided by days from first to last generic prescription fill over 6 months, and was defined as a medication possession ratio ≥ 0.80. We used multivariable logistic regression to assess factors associated with compliance. Adjusted ORs and 95 % CI were generated.
KEY RESULTS
We identified 5,156 patients who were switched from branded to generic statins; 73 % of patients were compliant in the 6 months after switching. After statistical adjustment, higher compliance was associated with each 10-year increase in age (OR: 1.13; 95 % CI: 1.07, 1.19; p < 0.001), receipt of a generic statin equivalent in potency to the prior branded statin (OR: 1.41; 95 % CI: 1.16, 1.70; p < 0.001), and compliance with prior branded statin (OR: 4.68; 95 % CI: 4.07, 5.39; p < 0.001). Lower compliance was seen among Hispanic patients compared to non-Hispanic white patients (OR: 0.68; 95 % CI: 0.52, 0.91; p = 0.009). Also, a switch to a higher potency generic statin, regardless of prior dose/potency, was negatively associated with compliance after switching (OR: 0.87; 95 % CI: 0.80, 0.94; p = 0.001).
CONCLUSIONS
The majority of patients switched from branded to generic agents were compliant with therapy in the first 6 months after switching. The potential for non-compliance to generic statin therapy, particularly among younger or Hispanic patients or when dose/potency changes are made, should be considered prior to switching. For these patients, counseling or close monitoring may be required to optimize generic interchange.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2933-7) contains supplementary material, which is available to authorized users.
KEY WORDS: generics, switching, drug interchange, statins, medication compliance
INTRODUCTION
Prescriptions for lipid-modifying agents in the United States totaled $20.1 billion in 2011; statins, specifically, branded agents, accounted for the majority of this cost.1 With most statins available off-patent, the interchange of generic for branded agents has become common practice among healthcare systems and is encouraged by payers to reduce healthcare spending.
Many studies have shown that lower out-of-pocket costs for patients, as would be expected with the utilization of generic drugs, correlates with improved compliance to statin therapy.2–5 Nevertheless, some patients still perceive generics as less safe and efficacious and of lower quality than brand-name drugs.6–8 In particular, patient surveys have shown that African Americans, individuals with lower health literacy, and those with lower incomes hold negative views about generic drugs.8–10 Both African Americans and Hispanics are also more likely than non-Hispanic whites to report that generic drugs have more side effects than branded drugs.11 Furthermore, older patients are less likely to believe that generic drugs are as safe as branded drugs, and women are more likely than men to believe that generics are a better value.6 Such beliefs may hinder compliance to treatment after switching and lead to suboptimal clinical outcomes.
In practice, however, it is unknown in which patients generic statin interchange can be effective, as few studies have measured compliance after a switch from branded to generic agents. Accordingly, we sought to identify both demographic and clinical predictors of compliance in an ambulatory-care setting among patients who were switched from branded to generic statins.
METHODS
Study Design and Setting
This retrospective study was conducted with the electronic health records (EHR) and pharmacy claims data from Sutter Health, an ambulatory-care medical network in Northern California. This study was approved by the appropriate institutional review board. Unique patient identifiers were removed prior to analyses.
Subject Identification
Managed-care beneficiaries who initiated treatment with branded statin monotherapy and subsequently were switched to a generic statin were identified between 1 January 2003 and 31 December 2012 (observation period). Eligible patients had at least two consecutive statin monotherapy pharmacy claims for branded statins, followed by at least two consecutive statin monotherapy claims for generic statins during the study period. The first pharmacy claim for a branded statin was designated the start date and the first pharmacy claim for a generic statin was designated the switch date. Patients had to be ≥ 18 years of age at the start date and have EHR activity or any medication orders or pharmacy claims ≥ 12 months before the start date. Patients meeting inclusion criteria were administratively censored on 31 December 2012 or when a statin prescription was filled under a new health plan, indicating a change in insurance coverage. The date of the last statin pharmacy claim prior to censoring was designated the end date.
Patients were excluded if they had: claims or medication orders for statins in the 12 months prior to the start date; any claims or medication orders for fixed-dose combination or non-statin lipid-modifying agents during the pre-switch period; < 60 days of treatment with a branded statin; or were missing or had invalid statin doses. Lovastatin and pravastatin, which became available as $4 generics in late 2006, would not have been recorded in our database if purchased without insurance claims. For patients prescribed these agents, gaps in pharmacy claims might have been inaccurately interpreted as non-compliance to treatment. Thus, we also excluded patients with medication orders after 30 June 2006 for lovastatin or pravastatin, as generic agents or branded agents (without a dispense-as-written flag).
Data Sources and Collection
Patient demographics, including self-reported race/ethnicity, clinical characteristics, and International Classification of Disease 9th Revision, Clinical Modification (ICD-9 CM) diagnoses, were abstracted from the EHR. All baseline variables were captured as of the switch date. Charlson Comorbidity Index (CCI) score was calculated using ICD-9 CM codes.12,13 Patients with coronary heart disease (CHD) or CHD-risk equivalents were identified by ICD-9 CM and Current Procedural Terminology codes.14 We identified statin prescription fills and copayments in the pharmacy claims database. Branded and generic statins were differentiated by a generic indicator flag associated with each claim. Daily statin doses were calculated from prescribed medication strength (mg), quantity dispensed, and days supply. Total copayments and statin copayments were each estimated for a 30-day supply during follow-up.
Statin potency after switching was categorized as equipotent, less potent, or more potent, based on the established relative low-density lipoprotein cholesterol (LDL-C) lowering efficacy of statins (Supplemental Table; available online).14,15 Substitution type was categorized as generic (receipt of a generic statin biochemically identical to the prior branded statin) or therapeutic (receipt of a generic statin biochemically different from the prior branded statin). Compliance with statin therapy was measured as medication possession ratio (MPR), and was calculated as follows:
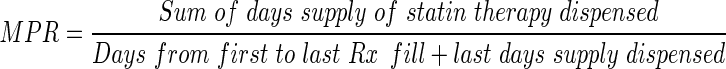 |
1 |
MPR was calculated separately during the pre-switch period and during the first 6 months after switching. If patients filled prescriptions early, overlapping days supplies were counted only once. Based on established convention, compliance was defined as an MPR ≥ 0.80.16
Statistical Analyses
Pairwise comparisons of baseline variables were performed using t-tests (parametric) or Wilcoxon rank-sum tests (non-parametric) for continuous variables and chi-square tests for categorical variables. We used multivariable logistic regression with a stepwise approach to assess predictors of compliance with statin therapy using baseline covariates (Table 1). Baseline variables that were statistically different between compliant and non-compliant patients (p < 0.20) were included in a multivariable logistic regression model. Variables that were statistically significant (p < 0.05) in the presence of other variables were retained in the final model. A post-hoc Pearson goodness-of-fit test was performed to assess differences between the data and the final model. We used logistic regression to calculate the adjusted percentage of patients compliant with generic statin therapy, with statistical adjustment for multivariable model covariates and stratification by race/ethnicity, and by compliance with pre-switch branded statin. Because multiple comparisons were made for this exploratory endpoint, a Bonferroni-corrected critical value of < 0.01 was used to reduce the chance of a type 1 statistical error. All analyses were performed in STATA 12.0 (StataCorp; College Station, TX).
Table 1.
Baseline Demographics and Characteristics
| All Switchers (N = 5,156) | Switchers Non-Compliant with Generic (N = 1,377) | Switchers Compliant with Generic (N = 3,779) | p Value* | |
|---|---|---|---|---|
| Mean age ± SD, years | 65.7 ± 13.7 | 63.2 ± 14.4 | 66.6 ± 13.3 | < 0.001 |
| Female, n (%) | 2,519 (48.9) | 646 (46.9) | 1,873 (49.6) | 0.092 |
| Race/ethnicity, n (%) | < 0.001 | |||
| NHW | 2,794 (54.4) | 645 (46.8) | 2,059 (54.5) | |
| African American | 205 (4.00) | 69 (5.01) | 137 (3.63) | |
| Hispanic | 284 (5.51) | 102 (7.41) | 182 (4.82) | |
| Asian | 154 (2.99) | 40 (2.90) | 11 (3.02) | |
| Other† | 591 (11.5) | 180 (13.1) | 411 (10.9) | |
| Unknown | 1,217 (23.6) | 341 (24.8) | 876 (23.2) | |
| Substitution type, n (%) | < 0.001 | |||
| Generic | 3,417 (66.3) | 842 (61.2) | 2,575 (68.1) | |
| Therapeutic | 1,739 (33.7) | 535 (38.8) | 1,204 (31.9) | |
| Statin potency level at switch, n (%) | 0.104 | |||
| 0 | 28 (0.54) | 2 (0.15) | 26 (0.69) | |
| 1 | 361 (7.00) | 92 (6.68) | 269 (7.12) | |
| 2 | 2,324 (45.1) | 615 (44.7) | 1,709 (45.2) | |
| 3 | 1,717 (33.3) | 464 (33.7) | 1,253 (33.2) | |
| 4 | 572 (11.1) | 153 (11.1) | 419 (11.1) | |
| 5 | 154 (2.99) | 51 (3.70) | 103 (2.73) | |
| Potency change at switch, n (%) | < 0.001 | |||
| Less potent | 761 (14.8) | 253 (18.4) | 508 (13.4) | |
| Equipotent | 4,056 (78.7) | 1,020 (74.1) | 3,036 (80.3) | |
| More potent | 339 (6.57) | 104 (7.55) | 235 (6.22) | |
| Diabetes, n (%) | 1,532 (29.7) | 405 (29.4) | 1,127 (29.8) | 0.775 |
| Hypertension, n (%) | 1,632 (31.6) | 383 (27.8) | 1,249 (33.0) | < 0.001 |
| Mean CCI score ± SD | 2.69 ± 1.99 | 2.43 ± 1.95 | 2.78 ± 1.99 | < 0.001 |
| Mean medication count at switch ± SD | 1.81 ± 1.21 | 1.70 ± 1.18 | 1.85 ± 1.22 | < 0.001 |
| High risk for CHD, n (%) | 2,423 (47.0) | 630 (45.8) | 1,793 (47.4) | 0.281 |
| Pre-switch statin compliance, n (%) | 3,378 (65.5) | 543 (39.4) | 2,835 (75.0) | < 0.001 |
| Statin supply at switch, n (%) | < 0.001 | |||
| 30 days | 3,604 (69.9) | 1,130 (82.1) | 2,474 (65.5) | |
| 90 days | 1,472 (28.6) | 226 (16.4) | 1,246 (33.0) | |
| Miscellaneous | 80 (1.55) | 21 (1.53) | 59 (1.56) | |
| Mean time on brand statin ± SD, days | 796.5 ± 715.4 | 766.0 ± 711.2 | 807.6 ± 716.7 | 0.006 |
| Mean time on generic statin ± SD, days | 155.7 ± 40.7 | 164.9 ± 29.8 | 152.2 ± 43.7 | < 0.001 |
| Mean change in total copayment ± SD, $ | −5.90 ± 30.7 | −5.26 ± 41.6 | −6.13 ± 25.6 | 0.368 |
| Mean change in statin copayment ± SD, $ | −18.9 ± 22.2 | −19.4 ± 21.2 | −18.8 ± 22.6 | 0.317 |
CCI Charlson Comorbidity Index; CHD coronary heart disease; IQL interquartile limit; NHW non-Hispanic White; SD standard deviation
*Pairwise differences between compliant and non-compliant groups assessed by t-tests or Wilcoxon rank-sum tests for parametric and non-parametric continuous variables, respectively, and by chi-square tests for categorical variables
† Pacific Islander, Asian Indian, Native Alaskan, or reported as multiple races or “other”
RESULTS
Baseline Demographics and Characteristics of Compliant and Non-Compliant Switchers
We identified 5,156 patients who were switched from branded to generic statins and met study eligibility criteria. Overall, patients were most frequently switched to generic simvastatin (57 %) or atorvastatin (35 %) (Table 2). Among patients receiving branded atorvastatin in the pre-switch period, 57% were switched to generic atorvastatin and 36% were switched to generic simvastatin; 97% of patients receiving branded simvastatin were switched to generic simvastatin. A small proportion of patients switched between lovastatin, fluvastatin, pravastatin, and rosuvastatin (< 10 %).
Table 2.
Frequency Distribution of Post-Switch Generic Statins by Prior Branded Statins (%)
| Post-Switch Generic Statin | ||||||
|---|---|---|---|---|---|---|
| Prior Branded Statin | Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Simvastatin | TOTAL |
| All prior, N = 5,156 | 35.1 | 0.12 | 7.03 | 0.95 | 56.8 | 100 |
| Atorvastatin N = 3,128 | 57.1 | 0 | 6.36 | 0.34 | 36.2 | 100 |
| Fluvastatin N = 141 | 0 | 4.20 | 27.0 | 2.10 | 66.7 | 100 |
| Lovastatin N = 3 | 0 | 0 | 100 | 0 | 0 | 100 |
| Pravastatin N = 117 | 0.80 | 0 | 49.6 | 27.4 | 22.2 | 100 |
| Rosuvastatin N = 126 | 18.2 | 0 | 11.1 | 2.50 | 68.2 | 100 |
| Simvastatin N = 1,641 | 0.06 | 0 | 3.14 | 0 | 96.8 | 100 |
The majority of patients (n = 3,779; 73 %) were compliant with generic statin therapy in the first 6 months after switching. Compliant patients were older than non-compliant patients (66.6 vs. 63.2 years, respectively), and were more frequently non-Hispanic White (NHW) (54 % vs. 47 %) (Table 1). Compliant versus non-compliant patients more frequently received generic substitutions (68 % vs. 61 %) and received equipotent dosing (80 % vs. 74 %) after switching. Compliant patients also more frequently had a diagnosis of hypertension (33 % vs. 28 %) and were taking more concomitant medications (mean: 1.85 vs. 1.70 medicines) than non-compliant patients. Approximately 75 % of patients who were compliant after switching were also compliant with their prior branded statin, compared with 39 % of patients who were non-compliant after switching. Compliant patients more often had a 90-day supply of generic statins dispensed at the time of switching (33 % vs. 16 %).
Multivariable Logistic Regression Model
After statistical adjustment for all covariates, each 10-year increase in age (odds ratio [OR]: 1.13; 95 % CI: 1.07, 1.19; p < 0.001) and receipt of a generic statin dose equivalent in potency to the prior branded statin (OR: 1.41; 95 % CI: 1.16, 1.70; P < 0.001) were significantly associated with greater odds of compliance after switching (Table 3). Compliance with prior branded statin (OR: 4.68; 95 % CI: 4.07, 5.39; p < 0.001) and prescription of a 90-day supply of statin therapy was also significantly associated with greater odds of compliance (OR: 2.10; 95 % CI: 1.76, 2.49; p < 0.001).
Table 3.
Multivariate Logistic Regression: Compliance 6 Months after Switching
| OR (95 % CI) | p value | |
|---|---|---|
| 10-year increase in age | 1.13 (1.07, 1.19) | < 0.001 |
| Race/ethnicity | ||
| NHW (ref) | 1.00 | |
| African American | 0.88 (0.63, 1.22) | 0.448 |
| Hispanic | 0.68 (0.52, 0.91) | 0.009 |
| Asian | 0.92 (0.61, 1.37) | 0.681 |
| Other† | 0.88 (0.70, 1.09) | 0.227 |
| Unknown | 0.96 (0.81, 1.14) | 0.643 |
| Unit increase in statin potency level at switch | 0.87 (0.80, 0.94) | 0.001 |
| Potency change at switch | ||
| Less potent (ref) | 1.00 | |
| Equipotent | 1.41 (1.16, 1.70) | < 0.001 |
| More potent | 1.15 (0.84, 1.56) | 0.373 |
| Brand statin compliance | 4.68 (4.07, 5.39) | < 0.001 |
| Statin supply at switch | ||
| 30 days (ref) | 1.00 | |
| 90 days | 2.10 (1.76, 2.49) | < 0.001 |
| Miscellaneous | 0.89 (0.52, 1.51) | 0.658 |
| 60-day increase in brand statin treatment | 1.01 (1.00, 1.02) | 0.001 |
| 60-day increase in generic statin treatment | 0.51 (0.42, 0.60) | < 0.001 |
| Pearson’s chi-square test, p = 0.310 | ||
NHW non-Hispanic white
† Pacific Islander, Asian Indian, Native Alaskan, or reported as multiple races or “other”
Hispanic versus NHW race/ethnicity (OR: 0.68; 95 % CI: 0.52, 0.91; p = 0.009) and an increase in statin potency (OR: 0.87; 95 % CI: 0.80, 0.94; p = 0.001) were associated with lower odds of compliance after switching (Table 3). Each 60-day increase in treatment with a generic statin was associated with a 49% reduced odds of compliance (OR: 0.51; 95 % CI: 0.42, 0.60; p < 0.001).
Compliance with Generic Statins by Pre-Switch Compliance
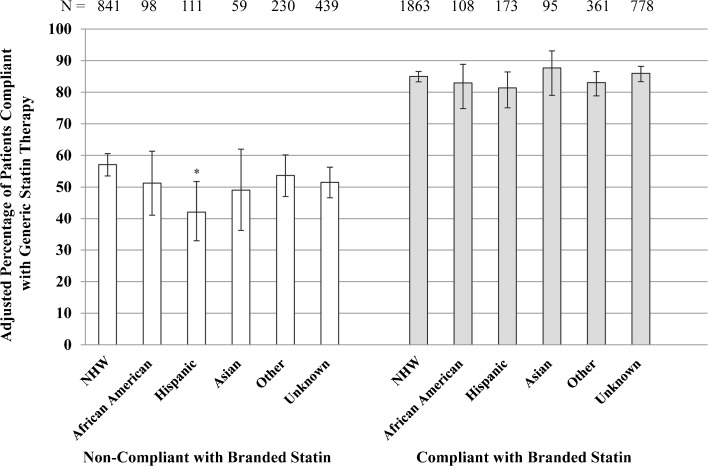
Across all race/ethnic groups, for patients who were compliant with pre-switch branded statins, more than 80 % were compliant with generic statins after switching (Fig. 1). For patients who were non-compliant with pre-switch branded statins, less than 60 % were compliant with generic statins after switching, and of these, a significantly smaller percentage of Hispanics than NHWs were compliant after switching (42 % vs. 57 %; p = 0.008).
Figure 1.
Adjusted percentage of patients compliant with generic statins by race/ethnicity and compliance with prior branded statin. Percentages adjusted by logistic regression for age, statin efficacy level at switch, potency change at switch, statin supply at switch, and duration of branded and generic statin treatment. Vertical error bars represent 95 % CIs. Compliance was defined as medication possession ratio ≥ 0.80. *Statistically significant versus non-Hispanic white (NHW) group at Bonferroni-adjusted critical value < 0.01 (p = 0.008).
DISCUSSION
In this retrospective analysis of managed-care beneficiaries, most patients (73 %) who were switched from branded to generic statins were compliant with treatment. Multivariable analysis revealed several demographic and clinical factors associated with compliance, including age, race/ethnicity, statin potency, dose changes at the time of switching, and prior compliance.
We are aware of only one other study, by Chapman and colleagues, that evaluated factors associated with switching from branded to generic statins.17 While their study used a large national cohort (N > 40,000) of commercially insured patients, the switch period evaluated was relatively short (July 2006 – December 2006) and focused on patients who were switched to generics at the time that simvastatin and pravastatin lost patent exclusivity. Furthermore, data on race/ethnicity or prescription copayments were not available in their analysis. To our knowledge, our study is the first to evaluate race/ethnic differences in compliance after switching, as well as the association between changes in cost sharing and compliance. Moreover, the switch period utilized in our study was intentionally broad (2003–2012), and was inclusive of patients who switched to all currently available generic statins, including atorvastatin, which became available in generic form in November 2011. Thus, our analysis may provide relatively better applicability to contemporary clinical practice.
In our study, older patients were more compliant with treatment after switching than younger patients. We show that each 10-year increase in age was associated with a 13% increased odds in compliance. These findings are similar to those reported by Chapman et al.17 Increased age has been previously associated with improved compliance with chronic-disease medication regimens, including antihypertensive18–20 and lipid-modifying drugs.21 Consistent with a health belief model of compliance,22 older patients may perceive their condition as more severe than younger patients and therefore may be more apt to take medications as prescribed.
We also showed that after switching from branded to generic statins, Hispanic patients had a 32% lower odds of compliance relative to NHW patients. However, stratified analysis revealed that this relationship held only among patients who were non-compliant in the pre-switch period. In a study by Mann et al. that investigated predictors of compliance to statin therapy for primary prevention, the authors found that Hispanic versus non-Hispanic ethnicity was significantly associated with poorer compliance (OR: 3.9; 95 % CI: 1.0, 6.3).21 Furthermore, another study showed that relative to NHWs, Hispanics (OR: 10.3; 95 % CI: 1.3, 79.4) and African Americans (OR: 10.2; 95 % CI: 1.4, 76.4) are more likely to report that generic drugs have more side effects than branded drugs.11 If such concerns are prevalent in these communities, this might drive lower rates of compliance. Despite studies showing that African Americans hold negative views about generic drugs,8–10 we did not find a significant difference in compliance between African American and NHWs after switching (OR: 0.84; 95 % CI: 0.60, 1.17; p = 0.297). We note that our sample of African Americans was relatively small, and therefore, the analyses were likely not powered to detect statistically significant differences in this population relative to NHWs.
Patients receiving higher potency therapy were less compliant after switching, with each increasing level of potency associated with a 13 % reduced odds of compliance. Higher potency therapy is associated with an increased rate of adverse effects, particularly myalgias, which may hinder compliance.23,24 We found that patients receiving therapy of lower potency than when on their branded statin were also significantly less compliant with therapy compared to patients receiving equipotent doses. Although these findings are seemingly contradictory, patients who experienced adverse events and required dose decreases at the time of switching may have been less inclined to continue on statin therapy, even at lower doses. Concerns about adverse effects have been previously reported by patients as a barrier to compliance.21,25,26
Notably, changes in patients’ out-of-pocket costs for all medications, and for statin therapy alone, were not significantly associated with compliance to treatment after switching. Numerous studies have shown that lower prescription copayments correlate with better compliance with statin therapy.2–5,27 However, to our knowledge, these studies were not conducted in populations switching from branded to generic agents. For example, Watanabe and colleagues reported that among new statin users, patients who did not pay out-of-pocket costs had a nearly 20 % increased odds of compliance (MPR ≥ 0.80) relative to patients with any out-of-pocket costs.5 Although patients saved an average of $20 per 30-day supply of statin therapy, we have previously shown that this cohort of switchers is as compliant as patients who remain on branded statins.28 Importantly, while patients who switched had marked reductions in statin costs, savings for all medication regimens were on average only $5 per month and may not have been large enough to influence compliance. Furthermore, although copayments decreased on average, as would be expected with conversion to generic agents, copayments for some patients actually increased. This may have been due to changes to cost-sharing policies in patients’ health insurance plans.
We found that the strongest predictor of compliance with generic statins after switching was compliance with prior branded statins. Patients who were compliant with branded statins had a 4.7-fold increase in odds of compliance with post-switch generic statins. These data are consistent with findings by Chapman and colleagues, who showed that compliance with pre-switch branded statins was significantly associated with a 5.5-fold increase in compliance with generic statins.17 Despite adjusting for compliance with prior branded statins in the multivariable model, the aforementioned covariates were still significantly associated with compliance after switching. These data indicate that prior compliance can predict much, but not all, of compliance after switching.
In our analysis, we also found that additional factors, such as duration of treatment and dispensed supply of statin therapy, were associated with compliance. Patients with longer durations of branded statin treatment had greater odds of compliance after switching to generic statins. These patients may have become accustomed to taking statins, and may also have had greater perceived self-efficacy than patients taking branded statins for shorter periods of time prior to switching.
Implications and Future Research
Conversion of patients from branded to generic drugs requires careful consideration of patients’ beliefs, attitudes, and experiences. Culturally competent strategies may be needed to improve compliance after switching to generic statins, particularly among Hispanic patients who were previously noncompliant with a branded statin. Such strategies may include providing medical/drug information in a patient’s native language. Indeed, culturally appropriate interventions have been previously used in Hispanic communities to modify health-related behaviors, including medication compliance.29,30 Patients who require dose changes at the time of generic interchange may need additional counseling at the time of switching, as well as close monitoring for compliance after switching. This counseling could be in the prescriber’s office or in the pharmacy.
Generic substitutions may be initiated by the prescriber, or as permitted by law in most states including California, the pharmacist dispensing the medication.31,32 Therapeutic substitutions, on the other hand, are often performed for efficacy or safety reasons, and require prior authorization or a prescription from the provider. Thus, interventions aimed at improving compliance after a generic interchange will necessitate tailored approaches based on the reasons for the switch and the point in care at which switching occurs (e.g., physician’s office or community pharmacy). This may require communication with the prescriber that a pharmacist-initiated switch has occurred. As interventions are being developed to improve compliance at this critical time, additional studies might explore how potency changes at the time of switching influence compliance. Furthermore, additional analyses are warranted to further understand how medication copayments influence compliance after generic interchange.
Limitations
Several limitations should be considered when interpreting our study’s findings. Due to the retrospective, observational design of this study, causal inferences based on statistical associations are limited. We cannot know the effect that missing pravastatin and lovastatin claims had on outcomes. However, we note that these agents were prescribed at very low rates even prior to the availability of $4 generics. Furthermore, patients were required to have at least four pharmacy claims to calculate pre-switch and post-switch MPR. Approximately 25 % of patients were excluded based on this criterion. While this might have resulted in the selection of more compliant patients, we do not believe that this would differentially affect predictors of compliance. Other factors that are likely associated with compliance after switching, such as health literacy and patient beliefs, are not available in an EHR database, and therefore could not be included in this analysis. We also did not have information on the cost-sharing structure or changes in cost sharing of patients’ health plans, or whether switching was performed for costs, safety, and/or efficacy. MPR is an approximation of compliance based on pharmacy claims, but does not account for medication consumed. Lastly, there may be limited generalizability of study findings to other than managed-care beneficiaries and to drugs in other medication classes.
Conclusions
The majority of patients switched from branded to generic statins were compliant with therapy in the first six months after switching. The potential for non-compliance to generic statin therapy, particularly among younger or Hispanic patients or when dose/potency changes are made, should be considered prior to switching. For these patients, improved management or monitoring may be required to optimize generic interchange.
Electronic supplementary material
(DOCX 15 kb)
Acknowledgements
Study Funding
This study was funded internally by Sutter Health. No external funding was received for the conduct of this study or the preparation of the manuscript.
Prior Presentation
None.
Conflicts of Interest
The clinical outcomes research group, of which Robert J. Romanelli was formerly a member, has previously received research funding from AstraZeneca and Daiichi Sankyo. Jodi B. Segal declares she does not have any conflicts of interest.
REFERENCES
- 1.IMS Institute for Health Informatics. The Use of Medicines in the United States: Review of 2011. Available at: http://www.environmentalhealthnews.org/ehs/news/2013/pdf-links/IHII_Medicines_in_U.S._Report_2011-1.pdf. Accessed May 26 2014.
- 2.Gibson TB, Mark TL, Axelsen K, Baser O, Rublee DA, McGuigan KA. Impact of statin copayments on adherence and medical care utilization and expenditures. Am J Manag Care. Dec 2006;12 Spec no.:SP11-19. [PubMed]
- 3.Gibson TB, Mark TL, McGuigan KA, Axelsen K, Wang S. The effects of prescription drug copayments on statin adherence. Am J Manag Care. 2006;12(9):509–517. [PubMed] [Google Scholar]
- 4.Schneeweiss S, Patrick AR, Maclure M, Dormuth CR, Glynn RJ. Adherence to statin therapy under drug cost sharing in patients with and without acute myocardial infarction: a population-based natural experiment. Circulation. 2007;115(16):2128–2135. doi: 10.1161/CIRCULATIONAHA.106.665992. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe JH, Kazerooni R, Bounthavong M. Association of copayment with likelihood and level of adherence in new users of statins: a retrospective cohort study. J Manag Care Pharm. 2014;20(1):43–50. doi: 10.18553/jmcp.2014.20.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients’ perceptions of generic medications. Health Aff (Millwood) 2009;28(2):546–556. doi: 10.1377/hlthaff.28.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrank WH, Joseph GJ, Choudhry NK, et al. Physicians’ perceptions of relevant prescription drug costs: do costs to the individual patient or to the population matter most? Am J Manag Care. 2006;12(9):545–551. [PubMed] [Google Scholar]
- 8.Iosifescu A, Halm EA, McGinn T, Siu AL, Federman AD. Beliefs about generic drugs among elderly adults in hospital-based primary care practices. Patient Educ Couns. 2008;73(2):377–383. doi: 10.1016/j.pec.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sewell K, Andreae S, Luke E, Safford MM. Perceptions of and barriers to use of generic medications in a rural African American population, Alabama, 2011. Prev Chronic Dis. 2012;9:E142. doi: 10.5888/pcd9.120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piette JD, Heisler M, Harand A, Juip M. Beliefs about prescription medications among patients with diabetes: variation across racial groups and influences on cost-related medication underuse. J Health Care Poor Underserved. 2010;21(1):349–361. doi: 10.1353/hpu.0.0247. [DOI] [PubMed] [Google Scholar]
- 11.Omojasola A, Hernandez M, Sansgiry S, Jones L. Perception of generic prescription drugs and utilization of generic drug discount programs. Ethnicity & disease. 2012;22(4):479–485. [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 14.Tunceli K, Sajjan SG, Ramey DR, et al. Switching from high-efficacy lipid-lowering therapies to simvastatin and low-density lipoprotein cholesterol goal attainment in coronary heart disease/coronary heart disease-equivalent patients. J Clin Lipidol. 2010;4(6):491–500. doi: 10.1016/j.jacl.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration. FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. http://www.fda.gov/drugs/drugsafety/ucm256581.htm#sa. Accessed May 26, 2014.
- 16.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapman RH, Benner JS, Girase P, et al. Generic and therapeutic statin switches and disruptions in therapy. Curr Med Res Opin. 2009;25(5):1247–1260. doi: 10.1185/03007990902876271. [DOI] [PubMed] [Google Scholar]
- 18.Zeng F, Patel BV, Andrews L, Frech-Tamas F, Rudolph AE. Adherence and persistence of single-pill ARB/CCB combination therapy compared to multiple-pill ARB/CCB regimens. Curr Med Res Opin. 2010;26(12):2877–2887. doi: 10.1185/03007995.2010.534129. [DOI] [PubMed] [Google Scholar]
- 19.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. The American journal of medicine. 2007;120(1):26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Ishisaka DY, Jukes T, Romanelli RJ, Wong KS, Schiro TA. Disparities in adherence to and persistence with antihypertensive regimens: an exploratory analysis from a community-based provider network. J Am Soc Hypertens. 2012;6(3):201–209. doi: 10.1016/j.jash.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44(9):1410–1421. doi: 10.1345/aph.1P150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health-behavior change in primary care. Am J Prev Med. 1999;17(4):275–284. doi: 10.1016/S0749-3797(99)00094-X. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PloS one. 2012;7(8):e42866. doi: 10.1371/journal.pone.0042866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408–416. doi: 10.1016/j.amjmed.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Fung V, Sinclair F, Wang H, Dailey D, Hsu J, Shaber R. Patients’ perspectives on nonadherence to statin therapy: a focus-group study. The Permanente Journal. 2010;14(1):4–10. doi: 10.7812/tpp/09-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casula M, Tragni E, Catapano AL. Adherence to lipid-lowering treatment: the patient perspective. Patient Preference and Adherence. 2012;6:805–814. doi: 10.2147/PPA.S29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz JS, O’Donnell JC, McDonough KL, Sasane R, Meyer J. Determinants of compliance with statin therapy and low-density lipoprotein cholesterol goal attainment in a managed care population. Am J Manag Care. 2005;11(5):306–312. [PubMed] [Google Scholar]
- 28.Romanelli RJ, Jukes T, Segal JB. Compliance after switching from branded to generic statins. Pharmacoepidemiology and drug safety. May 9 2014. [DOI] [PubMed]
- 29.Bender MS, Nader PR, Kennedy C, Gahagan S. A culturally appropriate intervention to improve health behaviors in Hispanic mother-child dyads. Child Obes. 2013;9(2):157–163. doi: 10.1089/chi.2012.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Stable EJ, Salazar R. Issues in achieving compliance with antihypertensive treatment in the Latino population. Clinical Cornerstone. 2004;6(3):49–61. doi: 10.1016/S1098-3597(04)80064-4. [DOI] [PubMed] [Google Scholar]
- 31.Christensen TP, Kirking DM, Ascione FJ, Welage LS, Gaither CA. Drug product selection: legal issues. J Am Pharmaceut Assoc. 2001;41(6):868–874. doi: 10.1016/s1086-5802(16)31328-6. [DOI] [PubMed] [Google Scholar]
- 32.Vivian JC. Generic-Substitution Laws. US Pharm. 2008;33(6):30–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)