Abstract
Hydrogen breath tests are widely used to explore pathophysiology of functional gastrointestinal (GI) disorders. Small intestinal bacterial overgrowth (SIBO) and carbohydrate malabsorption are disorders detected by these tests that have been proposed to be of great importance for symptoms of GI diseases. Glucose hydrogen breath test is more acceptable for diagnosis of SIBO whereas lactose and fructose hydrogen breath tests are used for detection of lactose and fructose maldigestion respectively. Lactulose hydrogen breath test is also used widely to measure the orocecal transit time for GI motility. These methods are noninvasive and inexpensive. Many patients with functional gut disorders are unaware of the relationship between diet and GI symptoms they present. In particular, patients with chronic symptoms may regard their condition as normal and may not be aware that their symptoms can be effectively managed following a proper diagnosis. Patients with symptoms of abdominal pain, bloating, flatulence and altered bowel movements (diarrhea and constipation), or with a medical diagnosis of irritable bowel syndrome or celiac disease, may have undiagnosed carbohydrate malabsorption or SIBO. Hydrogen breath tests are specific and sensitive diagnostic tests that can be used to either confirm or eliminate the possibility of carbohydrate malabsorption or SIBO in such patients. Breath tests, though valuable tools, are underutilized in evaluating dyspepsia and functional bloating and diarrhea as well as suspected malabsorption. However, because of their simplicity, reproducibility and safety of procedure they are now being substituted to more uncomfortable and expensive techniques that were traditionally used in gastroenterology.
Keywords: Hydrogen breath tests, Carbohydrate malabsorption, Small intestinal bacterial overgrowth, Orocecal transit time
History
The breath analysis for various diseases goes back to the time of Hippocrates, father of medicine. He asked his students to detect the diseases by the smell of breath of patients, like the smell of rotten apples indicated diabetic ketoacidosis and urine like smell indicated renal failure. Further with more achievements in science and technology, Lavoisier (father of chemistry) discovered CO2 gas in breath. Alcohol breath test, first initiated in the early 1950s, has presented a number of issues relative to scientific inquiry. Harger et al. [1] presented a device for sampling breath to determine blood alcohol concentration. Borkenstein and Smith [2] applied the use of breath alcohol concentration testing to forensic purposes. The scientification of medicine and gastroenterology began during the latter part of the nineteenth century when the discovery of bacterial causes of disease revealed the potential of research in the discovery of new knowledge. It was Linus Pauling’s milestone discovery of 250 unique substances present in exhaled breath that offered promising insight into breath testing [3].
In 1970s the concept of lactose intolerance (LI) was evaluated using Hydrogen breath test. Newcomer and his associates in 1975 studied lactose malabsorption by analyzing breath H2 and CO2 labelled lactose and blood sugar [4]. They showed the supremacy of H2 breath measurement for detection of lactose malabsorption. Moreover in 1978, Bond and Levitt [5] used breath H2 to conclude that some disaccharides remain unbroken and unabsorbed in small intestine due to incomplete digestion. They concluded this due to the fact that there was change in H2 concentration in expired air after ingested sugar reached colon undigested and intact. The most well-known application of breath test back then was to study of lactose malabsorption or intolerance. During those times, H2 breath test replaced blood test in which lack of blood glucose response to lactose indigestion lead to conclusion that lactose was not digested.
Basic Principle of Hydrogen Breath Test
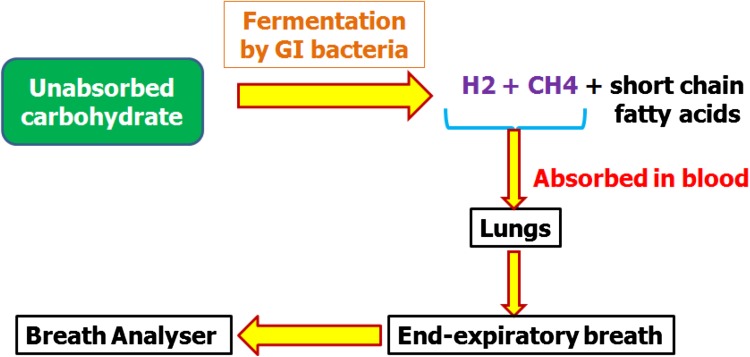
Bacteria in the bowel generally produce hydrogen gas on fermentation of carbohydrates. In bowel, bacteria can only do this when dietary carbohydrates are not absorbed in small intestine and stay as undigested material as it travels along the digestive tract into large intestine. Though some of the hydrogen gas produced by the bacteria is expelled as flatus or in making other molecules such as sulphides, acetate and short chain fatty acids but most of the gas is absorbed across the lining of the large intestine into blood stream. The gas is then transported to lungs via the blood stream and from blood it is exchanged into the airways of lungs and breathed out. The only source of hydrogen gas in the breath can be from bacterial fermentation in the bowel. The same applies to the gas called methane which is exhaled by some people and not all. The bacteria in their large bowel make methane from the hydrogen. The amount of hydrogen and methane gases breathed out from the lungs can be easily measured by taking a breath sample, and measuring by a breath-testing machine.
Uses of Hydrogen Breath Testing
Hydrogen breath testing is used in the diagnosis of three conditions.
First condition is for diagnosing bacterial overgrowth of the small bowel [small intestinal bacterial overgrowth (SIBO)], a condition in which larger-than-normal numbers of colonic bacteria are present in the small intestine.
Second condition is that in which dietary sugars are not digested normally i.e. carbohydrate malabsorption. The most common sugar that is poorly digested is lactose, the sugar in milk. Individuals who are unable to properly digest lactose are referred to as lactose intolerant. Testing also may be used to diagnose problems with the digestion of other sugars such as sucrose, fructose and sorbitol.
Third condition is that hydrogen breath testing can also be used for diagnosing rapid or slow passage of food through the small intestine.
All three of these conditions may cause abdominal pain, bloating, distention, flatulence and diarrhea.
Preparation for Hydrogen Breath Testing
Patients should not take antibiotics for 4 weeks before the test.
Patients are advised not to eat any slowly digested foods (such as beans, bran and high-fiber cereals) for 24 h prior to the test and should take low fibre diet.
They should not take any fiber supplements and laxatives 24 h before the test
Patients should fast for 12 h before the procedure.
Patients should not smoke and sleep 2 h before and during the test.
Types of Hydrogen Breath Tests
Glucose hydrogen breath test Under physiological conditions, glucose is readily absorbed in the small intestine [6]. However if there is bacterial overgrowth in the (upper) small intestine, bacterial fermentation of glucose and production of H2 can take place prior to the absorption of glucose. So, a breath hydrogen test showing a rise after consuming glucose will mean that there are too many bacteria in the small bowel (i.e. bacterial overgrowth is present).Generally, rise of H2 ≥10 ppm over baseline value in 2 consecutive readings is considered as SIBO.
Lactose hydrogen breath test This test determines if one has a problem digesting lactose products (i.e. milk, cheese, ice cream etc.). Symptoms of LI include diarrhea, gas, cramping, and bloating. LI generally occurs when lactose is not hydrolyzed to galactose and glucose due to deficiency or absence of enzyme lactase. Generally, rise of H2 ≥20 ppm over baseline value in 2 consecutive readings is considered as SIBO.
Fructose hydrogen breath test This test is done to find out if the patient has problem digesting fructose. Fructose is a naturally occurring sugar found in honey, vegetables, fruits and grains. It is a single molecule sugar which many people cannot effectively absorb. Fructose malabsorption is a digestive disorder in which proper absorption of fructose does not take place in the small intestine. This poorly absorbed fructose moves through to the large bowel, where it gets fermented by the flora present in the intestinal tract. The fermentation by bacteria produces gas and other gastrointestinal (GI) symptoms.
Sorbitol hydrogen breath test Sorbitol is a sugar alcohol (polyol) commonly used as a sweetener in sugar-free sweets and chewing gum, diet and diabetic foods, amongst other products. It is produced by the human body, occurs in fruit, beer and berries, is also contained in some medicines (e.g. mouth washes, cough syrups and laxatives) and cosmetics. Sorbitol has fewer calories and is less likely to cause dental caries than normal household table sugar, sucrose. Absorption in the small intestine occurs passively and is much slower than other sugars. This allows even moderate doses to be malabsorbed, reaching the colon for fermentation, especially in individuals with a rapid intestinal transit. A high proportion of healthy individuals develop abdominal bloating, gas, cramps and diarrhea at doses of 5 g and above.
Lactulose hydrogen breath test Lactulose is a disaccharide composed of galactose and fructose. As there is no naturally occurring lactulose enzyme in the body to hydrolyze this sugar in the small intestine, lactulose is not broken down in the small bowel and is transported intact to the colon where it is fermented by colonic bacteria. The products of its metabolism include hydrogen. The time interval between ingestion of lactulose and rise in breath hydrogen 20 ppm above basal is a measure of orocecal transit time (OCTT).
Carbohydrate Malabsorption and Hydrogen Breath Tests
Lactose Intolerance (LI)/Malabsorption
Lactose malabsorption is a common condition caused by reduced expression or activity of lactase in the small intestine. In such patients, LI is characterized by abdominal symptoms (e.g. nausea, bloating, and pain) after ingestion of dairy products. Lactase activity is highest at birth and declines after weaning. The age at which this decline starts and the proportion of the adult population with lactase levels are low enough to be considered having hypolactasia are both strongly related to ethnicity. Highest rates of lactose malabsorption are in Asian populations, Native Americans and African Americans (60–100 %) and lowest rates in people of northern European origin and the US white population (2–22 %) [7]. Newcomer et al. [4] found that measurement of breath hydrogen is sensitive and specific, and does not require ethanol or isotopes. It is noninvasive, and is not influenced by gastric emptying or metabolic factors. It was believed to be the most suitable test for population screening for lactase deficiency.
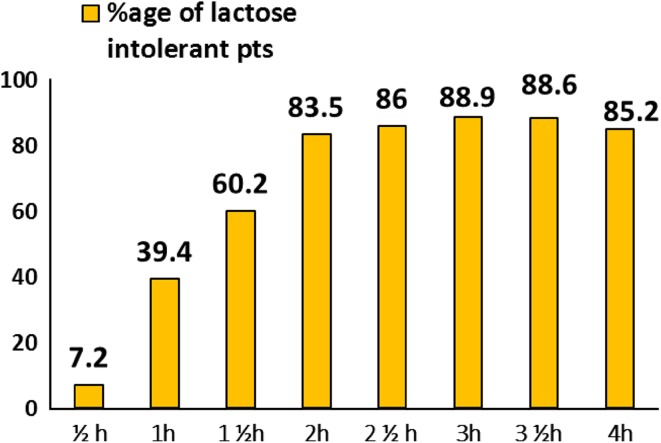
Initially, 50 g of lactose was used for the breath test [8], but this caused diarrhea and flatulence in patients during the test. Therefore a study was conducted in our laboratory [9] to modify dose of lactose for lactose hydrogen breath test. Patients positive with 50 g lactose were repeated with 25 and 12.5 g lactose in 1-week interval. 91.4 % patients were detected of LI with 25 g lactose and 42.8 % with 12.5 g lactose. Symptoms of diarrhea (63 %) and flatulence (97 %) decreased to 22.8 and 34.2 % respectively with 25 g lactose. Thus, 25 g lactose dose was set for detecting lactose deficiency. Similarly, a study by Qiao et al. [10] also observed that in Chinese population even with LI, can tolerate 25 g cow’s milk powder (6.25 g lactose). 20 g cow’s milk powder (5 g lactose, make 160 ml of milk solution) was lowest acceptable intake of healthy adults. Although original procedure of breath testing involved confinement of patients in continuous re-breathing system [11]. Modern methods involve interval sampling of H2 breath samples after carbohydrate load. No consensus on frequency and duration of collection of breath samples was present. Therefore, a study was planned in our laboratory to assess the number of breath samples required to detect LI by hydrogen breath test. Study was done in 375 suspected lactose intolerant patients. Out of which 236 patients (62.9 %) had abnormal Lactose hydrogen breath test. Results suggested that for lactose malabsorption, breath H2 samples should be taken at fasting and at least 2 or 3 h (Figs. 1, 2).
Fig. 1.
Principle of Hydrogen Breath Testing
Fig. 2.
Breath samples at fasting, 2 and 3 h can estimate Lactose Intolerance among subjects
Another study [12] also showed that 3-sample breath test has excellent sensitivity and specificity for lactase deficiency. In this study, 1049 lactose hydrogen breath test were performed. 337/1049 (32 %) showed a positive result. Two-sample tests had sensitivity and specificity of 52.5 and 100.0 % (0–60 min), 81.9 and 99.7 % (0–90 min), and 92.6 and 99.2 % (0–120 min), respectively. Three-sample tests had sensitivity and specificity of 83.4 and 99.7 % (0–60–90 min), 95.0 and 99.2 % (0–60–120 min), and 95.0 and 98.9 % (0–90–120 min), respectively.
Lactase activity with age has been reported in wide variety of population globally. Most of these studies in human have ignored to assess age stratified lactose maldigestion. A study by Rana et al. [13] showed that lactose maldigestion is not associated with age stratification among north Indians. Study in Chinese population however showed that the prevalence of LI among Chinese children was 12.2 % at age 3–5 years, 33.1 % at age 7–8 years, and 30.5 % at age 11–13 years, respectively [14].
LI has also been linked to various GI diseases. Frequency of LI was high and comparable among irritable bowel syndrome (IBS) patients (89/124; 72 %) and controls (32/53; 60 %) from northern India in a study by Gupta et al. [15]. Patients with IBS more often reported symptoms following lactose ingestion despite levels of breath hydrogen being similar to healthy subjects. The frequency of LI in patients with IBS–diarrhea was comparable to that in patients with other types of IBS. However controversial results [16] are available in which it was observed that patients with diarrheal type of IBS (44 %) have a higher incidence of LI as compared to spastic type (28 %) as well as patients with features of both types (28 %). Moreover, frequency and degree of lactose malabsorption was high in southern than in northern Indian healthy populations due to genetic differences in these populations [17].
Incidence of LI (41.7 %) in idiopathic ulcerative colitis (IUC) patients was found comparable with controls (40 %). However, incidence of LI (59.1 %) in active colitis patients was significantly higher (P < 0.05) than remission group or during quiescent disease (31.5 %) [18]. Similar finding was observed by Cabrera-Acosta et al. [19]. They found no significant difference in symptoms, extension or progression of chronic IUC between patients that could digest and those who could not digest lactose. Similarly, no significant difference in the prevalence of lactose malabsorption was found between patients with ulcerative colitis and controls by Ginard et al. [20].
Fructose Malabsorption
Incomplete fructose absorption is detected by breath testing for hydrogen production after consumption of a fructose-containing beverage or food. When accompanied by GI symptoms, this condition is increasingly referred to as dietary fructose intolerance [21]. In 1978, Andersson and Nygren [22] first reported cases of fructose malabsorption in which patients received a positive result using the breath hydrogen test. It has been suggested that fructose malabsorption or intolerance occurs more frequently in individuals with compromised gut function as compared with healthy individuals [23, 24]. Study by Rao et al. [25] revealed that healthy subjects completely absorbed a dose of 15 g fructose. About 10 % of subjects malabsorbed a dose of 25 g, but they were asymptomatic. Therefore, 25 g fructose can distinguish a normal from an abnormal capacity to absorb fructose. A positive breath test with this dose suggests an abnormally low capacity to absorb dietary fructose. Thus, 25 g of fructose is the appropriate dose for testing subjects with suspected dietary fructose malabsorption. Breath samples obtained at 30-min intervals, and for duration of 3 h, and analyzed for both hydrogen concentration can detect most individuals with dietary fructose malabsorption. In another study in Thai population it was observed that fructose malabsorption was found in 11 females with significant increase in breath-H2 level at 30, 60, 90 and 120 min. Only 1/11 females with increased breath H2 had GI symptoms. In all fructose malabsorbers, excess breath-H2 reverted to normal when fructose solution was mixed and administered with 25 g glucose [26]. It has been reported that prevalence of fructose intolerance (FI) in patients with functional GI disorders (FGID) range between 38 and 75 % [26]. In one study, 25 IBS patients underwent fructose breath test with 25 g fructose. Fructose H2BT was considered positive when concentrations of H2 were ≥20 ppm or rise >5 ppm in 3 consecutive samples was detected. According to Rome II criteria, 10 patients (40 %) had IBS-C, 9 (36 %) had IBS-D and 6 (24 %) had IBS-M .13 (52 %) of IBS patients had fructose intolerance while only 4 (16 %) of controls. They concluded that fructose intolerance may be responsible for GI symptoms in at least half of IBS patients especially in group of D-IBS [27].
Small Intestinal Bacterial Overgrowth and Hydrogen Breath Tests
Concept of SIBO has breathed new controversy into world of FGID. It is characterized by nutrient malabsorption associated with excessive number of bacteria in proximal small intestine. Over time, our understanding of SIBO has evolved with a growing knowledge of the gut microbiota and its bidirectional interaction with immune function, digestion, metabolism and brain-gut communication. It is defined as increased in microorganisms >104 CFU/ml of jejunum aspirate or more colonic type bacteria within small intestine. Pathology of this condition involves competition between bacteria and human host for ingested nutrients. In contrast to small bowel aspiration for quantitative culture, breath testing provides a more readily available, safe, inexpensive, and noninvasive alternative to jejunal aspiration culture for the diagnosis of SIBO. The glucose breath test (GBT) was introduced in 1976 in the assessment of SIBO [28]. In an individual with SIBO, the proximally displaced bacteria theoretically should lead to the fermentation of glucose and a resultant increase in breath hydrogen excretion. In the classic description of this test, a single peak in the hydrogen concentration after the ingestion of glucose is indicative of SIBO.
20 % of general population has IBS but cause is unknown. Symptoms of IBS generally resemble SIBO [29]. SIBO has been implicated in pathogenesis of IBS. The concept of a possible association of SIBO with IBS has some logic as several symptoms of these two conditions are similar, and that treatment of SIBO with non-absorbable antibiotics has shown to result in improvement of symptoms in patients with IBS [30].Various studies are present in literature to confirm the association of SIBO with IBS. One study was planned in our laboratory to evaluate prevalence of SIBO in patients with IBS as compared to healthy controls. In 225 IBS patients and 100 healthy controls SIBO was estimated by using non-invasive GBT. The study indicated that prevalence of SIBO in IBS patients was ~11.1 %, which was less than reported prevalence [31]. Similar study was planned in Pakistani patients to determine SIBO in 119 D-IBS patients and 115 controls with chronic nonspecific diarrhea (CNSD). SIBO was detected by lactose H2BT in 32/234 (14 %) cases out of which 22/119 (19 %) cases were with D-IBS and 10/115 (9 %) cases with CNSD. This study showed that significant number of D-IBS patients had SIBO [32]. Study by Sachdeva et al. [33] also showed that SIBO was more frequent in patients with IBS as compared to healthy controls. D-IBS subtype, female gender and history of bloating were possible predictors of SIBO in patients with IBS. Identification of one or more of these factors could aid in development of successful management plan in patients with IBS. Similar results were obtained by other studies [34, 35].
A proportion of patients with celiac disease have a poor response to a gluten-free diet, which may be due to SIBO. Treatment of SIBO in patients with poorly responsive celiac disease using antibiotics has been shown to be successful in alleviating symptoms [36–38]. This has been demonstrated primarily in small, observational studies, typically using breath testing.
SIBO has also been considered a predisposing factor of spontaneous bacterial peritonitis (SBP) in cirrhotic patients by bacterial translocation or hematogenous spread during spontaneous bacteremia. The prevalence of SIBO using GBT and its relationship with the severity of liver dysfunction and the presence of ascites was assessed in 45 cirrhotic patients and 28 healthy subjects. SIBO was documented in 16 (35.6 %) of the 45 cirrhotic patients and in 1 (3.6 %) of the 28 healthy controls. The prevalence of SIBO was significantly higher in patients with Child–Pugh class B or C (50 %) than in those with class A (19 %) and had no relationship with the presence or absence of ascites [39]. In another study [40], the prevalence of intestinal bacterial overgrowth was significantly higher in cirrhotics with ascites (37.1 %) than in those with no evidence of ascites (5.3 %) and among patients with Pugh–Child class C (48.3 %) than in patients with class A (13.1 %) or B (27 %). Twelve (17.1 %) of the 70 patients with ascites developed an episode of SBP. The prevalence of SBP was significantly higher in patients who had intestinal bacterial overgrowth (30.7 %) than in patients who did not (9.09 %). Similarly, Gupta et al. [41] observed that fifty-seven (55.9 %) patients with cirrhosis had minimal hepatic encephalopathy (MHE). Among these patients with MHE, 22 (38.6 %) had SIBO, while 4 (8.9 %) without MHE had SIBO.
Due to discrepancy about the use of glucose or lactulose breath test for detection of SIBO, a study was conducted in our laboratory to compare lactulose breath test with GBT to diagnose SIBO in IBS patients and controls [42]. SIBO was positive in 60/175 (34.3 %) patients by lactulose and in 11/175 (6.2 %) patients by GBT. In controls, LBT was positive for SIBO in 45/150 (30 %) patients and in 1/150 (0.66 %) patients by GBT. Positive LBT for SIBO was not significantly different in patients and controls; while using GBT, SIBO was significantly higher (P < 0.01) in patients as compared to controls. By using GBT as gold standard for SIBO, sensitivity, specificity, positive predictive value and negative predictive value of LBT in IBS patients was 63.6, 67.7, 11.7 and 96.6 % respectively. Thus, it was concluded that GBT is a far better test as compared to lactulose breath test to discriminate SIBO patients.
Orocecal Transit Time and Hydrogen Breath tests
OCTT can be defined as how long it takes for food to pass through intestines. It’s important because it influences how efficiently nutrients are absorbed from food and influences fermentation associated with healthy gut flora. Short transit time may be due to inflammation caused by intestinal infection, food allergies, or by absence of healthy intestinal flora. Long transit time is usually due to eating too much refined and processed foods, low thyroid, dehydration, lack of dietary fiber, serotonin deficiency and insufficient salt intake. Several factors affect the orocaecal transit time such as: physiological (age, sex, menstrual cycle), usage of stimulants (coffee, alcohol, cigarette smoking), as well as pathological conditions.
It is quite frequent to recognize celiac patients who show GI motor abnormalities in clinical practice. In fact, in 30–60 % of patients, physical examination and dyspeptic symptoms (epigastric discomfort, early satiety, and more rarely, hyporexia and vomiting) suggest a GI motility disorder [43, 44]. Small bowel motor abnormalities may be involved in this pathological condition. However, there is no study addressing small bowel transit in patients of celiac disease from Northern India [45]. Mouth-to-cecum transit time was studied in 80 celiac patients and 80 age and sex matched apparently healthy controls. OCTT in celiac patients was significantly delayed being 180 ± 10.6 min as compared to 105 ± 12.4 min in apparently healthy controls. This prolonged OCTT could be due to impaired small bowel function (deranged motility) in patients with celiac disease.
In another study also similar results of prolonged OCTT were obtained [46].They studied the mouth-to-cecum transit time of a caloric liquid meal in a homogeneous group of celiac patients presenting with clinical and biochemical evidence of malabsorption and complaining of diarrhea. At the time of the diagnosis, mouth-to-cecum transit time was significantly prolonged in celiacs with respect to controls (243 ± 10 vs 117 ± 6 min, P = 0.0001). After the gluten-free diet period, mouth-to-cecum transit time in celiacs was significantly reduced compared to pre-diet transit (134 ± 8 vs 243 ± 10 min, P = 0.0001) and did not show statistical difference when compared to that found in controls (P = 0.1).
Accidental/suicidal ingestion of corrosive substances is also very common in North India. Decreased gastric secretion and delayed gastric emptying in the chronic phase of corrosive injury has been documented at our center. So a study was planned in our laboratory to measure orocecal transit time in patients in the chronic phase of corrosive injury [47]. 30 patients (27 with acid ingestion and 3 with alkali ingestion) with corrosive injury to their GIT with its sequelae were enrolled. OCTT was significantly prolonged in study group (135.4 ± 15) as compared to control group (90.6 ± 10.4 min). OCTT was prolonged maximally in patients with lower-third oesophageal cicatrization. This may be result of autovagotomy due to vagal entrapment in cicatrization process involving lower third of oesophagus.
Study in microscopic colitis which also leads to chronic diarrhea however showed no significant difference in OCTT in patients with microscopic as compared to controls [48].
Diarrhea, constipation, flatulence, and abdominal pain are also common complaints among diabetic patients. Impaired intestinal motility is often followed by SIBO. Even if this problem has been attributed to autonomic neuropathy, the pathophysiological mechanisms responsible are not completely defined. Various studies have shown the relation of OCTT and SIBO in diabetes. One study showed that bacterial overgrowth may contribute to the delay of intestinal transit as confirmed by its significant improvement after eradication therapy [49]. Study by Rana et al. [50] also observed that OCTT in type 2 diabetes patients with SIBO was significantly delayed (P < 0.001) compared with type 2 diabetes patients without SIBO and they concluded that SIBO in diabetes patients may be due to delayed OCTT. In type 1 diabetes also there was a statistically significant increase in OCTT values in patients (79 ± 41 min) in comparison with controls (54 ± 17 min) (P = 0.01). Individual analysis showed that OCTT was above the upper limit (mean + 2 SD) in 30.8 % of patients [51].
Gallstone disease (GSD) is one of the most prevalent abdominal diseases. Heaton et al. [52] reported that one particular factor associated with GSs is the slow intestinal transit time. In their study, the normal weight women along with age-matched controls with healthy gallbladders underwent measurement of whole-gut transit time by radio-opaque markers. It was found by them that the mean transit time was significantly longer in women with GSs than in controls. Cholecystectomy is widely performed abdominal operation worldwide. Even after surgical procedure, there are about 15–20 % patients have persistence of vague dyspeptic symptoms. Change in small intestinal motility and SIBO may cause this problem. A study in our lab [53] showed that OCTT was significantly higher in gallstone patients as compared to controls which might be the reason of SIBO in gallstone patients. OCTT was further increased after cholecystectomy which may be the reason of post cholecystectomy symptoms in these patients.
Thus, measurement of OCTT in various diseases with GI complications is very important. This helps physician to treat the patient more effectively as OCTT influences the gut microbiota in intestine to a large extent.
Summary
There are numerous potential advantages for breath analysis as a clinical test. The method is non-invasive (the sample is relatively easy and painless to acquire), the sample is likely to be rich with information (a single test can scan for signatures of many abnormalities or markers of disease), it has the potential for low cost, and lends itself to easy administration. The field of breath testing has grown tremendously in recent years and with evolving technologies in sampling, sensor design, standardization, and analytical methods breath analysis has the potential to clinically benefit individuals on a global scale in the future. Moreover, this simple can diagnose SIBO, carbohydrate malabsorption and OCTT by breath analysis following ingestion of a substrate. It gives quick and accurate results and is of great help to physicians to treat patients accordingly.
References
- 1.Harger RN, Forney RB, Barnes HB. Estimation of the level of blood alcohol from the analysis of breath. In: First international conference on alcohol and traffic, Stockholm, Sweden; 1950. pp. 107–21.
- 2.Borkenstein RF, Smith H. The breathalyzer and its application. Med Sci Law. 1961;2:13–22. [Google Scholar]
- 3.Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas–liquid partition chromatography. Proc Natl Acad Sci USA. 1971;68:2374–2376. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newcomer AD, McGill DB, Thomas PJ, Hofmann AF. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med. 1975;293:1232–1236. doi: 10.1056/NEJM197512112932405. [DOI] [PubMed] [Google Scholar]
- 5.Bond JH, Levitt MD. Effect of dietary fiber on intestinal gas production and small bowel transit time in man. Am J Clin Nutr. 1978;31(10 Suppl):S169–S174. doi: 10.1093/ajcn/31.10.S169. [DOI] [PubMed] [Google Scholar]
- 6.Bond JH, Levitt MD. Use of breath hydrogen (H2) to quantitate small bowel transit time following partial gastrectomy. J Lab Clin Med. 1977;90:30–36. [PubMed] [Google Scholar]
- 7.Srinivasan R, Minocha A. When to suspect lactose intolerance; symptomatic, ethnic, and laboratory clues. Postgrad Med. 1998;104:109–123. doi: 10.3810/pgm.1998.09.577. [DOI] [PubMed] [Google Scholar]
- 8.Solomons NW, García-Ibañez R, Viteri FE. Hydrogen breath test of lactose absorption in adults: the application of physiological doses and whole cow’s milk sources. Am J Clin Nutr. 1980;33:545–554. doi: 10.1093/ajcn/33.3.545. [DOI] [PubMed] [Google Scholar]
- 9.Rana S, Bhasin DK, Gupta D, Mehta SK. Assessment of optimal dose of lactose for lactose hydrogen breath test in Indian adults. Indian J Gastroenterol. 1995;14:13–14. [PubMed] [Google Scholar]
- 10.Qiao R, Hung CY, Zeng G, Vonk RJ, Li L, Ye S. Study on the lowest acceptable intake of cow’s milk for healthy adults. Wei Sheng Yan Jiu. 2006;35:747–749. [PubMed] [Google Scholar]
- 11.Levitt MD, Engel RR. Intestinal gas. Adv Intern Med. 1975;20:151–165. [PubMed] [Google Scholar]
- 12.Oberacher M, Pohl D, Vavricka SR, Fried M, Tutuian R. Diagnosing lactase deficiency in three breaths. Eur J Clin Nutr. 2011;65:614–618. doi: 10.1038/ejcn.2010.287. [DOI] [PubMed] [Google Scholar]
- 13.Rana SV, Bhasin DK, Naik N, Subhiah M, Ravinder P. Lactose maldigestion in different age groups of north Indians. Trop Gastroenterol. 2004;25:18–20. [PubMed] [Google Scholar]
- 14.Yang Y, He M, Cui H, Bian L, Wang Z. The prevalence of lactase deficiency and lactose intolerance in Chinese children of different ages. Chin Med J (Engl). 2000;113:1129–1132. [PubMed] [Google Scholar]
- 15.Gupta D, Ghoshal UC, Misra A, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: a case–control study. J Gastroenterol Hepatol. 2007;22:2261–2265. doi: 10.1111/j.1440-1746.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- 16.Rana SV, Mandal AK, Kochhar R, Katyal R, Singh K. Lactose intolerance in different types of irritable bowel syndrome in north Indians. Trop Gastroenterol. 2001;22:202–204. [PubMed] [Google Scholar]
- 17.Babu J, Kumar S, Babu P, Prasad JH, Ghoshal UC. Frequency of lactose malabsorption among healthy southern and northern Indian populations by genetic analysis and lactose hydrogen breath and tolerance tests. Am J Clin Nutr. 2010;91:140–146. doi: 10.3945/ajcn.2009.27946. [DOI] [PubMed] [Google Scholar]
- 18.Kochhar R, Mehta SK, Goenka MK, Mukherjee JJ, Rana SV, Gupta D. Lactose intolerance in idiopathic ulcerative colitis in north Indians. Indian J Med Res. 1993;98:79–82. [PubMed] [Google Scholar]
- 19.Cabrera-Acosta GA, Milke-García MP, Ramírez-Iglesias MT, Uscanga L. Deficient lactose digestion and intolerance in a group of patients with chronic nonspecific ulcerative colitis: a controlled, double-blind, cross-over clinical trial. Rev Gastroenterol Mex. 2012;77:26–30. [PubMed] [Google Scholar]
- 20.Ginard D, Riera J, Bonet L, Barranco L, Reyes J, Escarda A, et al. Lactose malabsorption in ulcerative colitis. A case–control study. Gastroenterol Hepatol. 2003;26:469–474. doi: 10.1016/S0210-5705(03)70396-3. [DOI] [PubMed] [Google Scholar]
- 21.Skoog SM, Bharucha AE, Zinsmeister AR. Comparison of breath testing with fructose and high fructose corn syrups in health and IBS. Neurogastroenterol Motil. 2008;20:505–511. doi: 10.1111/j.1365-2982.2007.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson DE, Nygren A. Four cases of long-standing diarrhoea and colic pains cured by fructose-free diet—a pathogenetic discussion. Acta Med Scand. 1978;203:87–92. doi: 10.1111/j.0954-6820.1978.tb14836.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelis GF, Vermeeren MA, Jansen W. Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology. 1990;99:1016–1020. doi: 10.1016/0016-5085(90)90621-7. [DOI] [PubMed] [Google Scholar]
- 24.Symons P, Jones MP, Kellow JE. Symptom provocation in irritable bowel syndrome. Effects of differing doses of fructose–sorbitol. Scand J Gastroenterol. 1992;27:940–944. doi: 10.3109/00365529209000167. [DOI] [PubMed] [Google Scholar]
- 25.Rao SSC, Attaluri A, Anderson L, Stumbo P. The ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5:959–963. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Densupsoontorn N, Jirapinyo P, Thamonsiri N, Wongarn R. Fructose malabsorption in Thai adult. Asia Pac J Clin Nutr. 2007;16:209–212. [PubMed] [Google Scholar]
- 27.Reyes-Huerta JU, de la Cruz-Patiño E, Ramírez-Gutiérrez de Velasco A, Zamudio C, Remes-Troche JM. Fructose intolerance in patients with irritable bowel syndrome: a case–control study. Rev Gastroenterol Mex. 2010;75:405–411. [PubMed] [Google Scholar]
- 28.Metz G, Gassull MA, Drasar BS, Jenkins DJ, Blendis LM. Breath-hydrogen test for small-intestinal bacterial colonisation. Lancet. 1976;1(7961):668–669. doi: 10.1016/S0140-6736(76)92779-3. [DOI] [PubMed] [Google Scholar]
- 29.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 31.Rana SV, Sinha SK, Sikander A, Bhasin DK, Singh K. Study of small intestinal bacterial overgrowth in North Indian patients with irritable bowel syndrome: a case control study. Trop Gastroenterol. 2008;29:23–25. [PubMed] [Google Scholar]
- 32.Yakoob J, Abbas Z, Khan R, Hamid S, Awan S, Jafri W. Small intestinal bacterial overgrowth and lactose intolerance contribute to irritable bowel syndrome symptomatology in Pakistan. Saudi J Gastroenterol. 2011;17:371–375. doi: 10.4103/1319-3767.87176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26(Suppl 3):135–138. doi: 10.1111/j.1440-1746.2011.06654.x. [DOI] [PubMed] [Google Scholar]
- 34.Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40–46. doi: 10.5056/jnm.2010.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddymasu SC, Sostarich S, McCallum RW. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC Gastroenterol. 2010;10:23. doi: 10.1186/1471-230X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana SV, Sinha SK, Lal S, Sikander A, Singh K. Small intestinal bacterial overgrowth in North Indian patients with celiac disease. Trop Gastroenterol. 2007;28:159–161. [PubMed] [Google Scholar]
- 37.Ghoshal UC, Ghoshal U, Misra A, Choudhuri G. Partially responsive celiac disease resulting from small intestinal bacterial overgrowth and lactose intolerance. BMC Gastroenterol. 2004;4:10. doi: 10.1186/1471-230X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839–843. doi: 10.1111/j.1572-0241.2003.07379.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang CY, Chang CS, Chen GH. Small-intestinal bacterial overgrowth in patients with liver cirrhosis, diagnosed with glucose H2 or CH4 breath tests. Scand J Gastroenterol. 1998;33:867–871. doi: 10.1080/00365529850171549. [DOI] [PubMed] [Google Scholar]
- 40.Morencos FC, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995;40:1252–1256. doi: 10.1007/BF02065533. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Dhiman RK, Kumari S, Rana SV, Agarwal R, Duseja A, et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Rana SV, Sharma S, Kaur J, Sinha SK, Singh K. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion. 2012;85:243–247. doi: 10.1159/000336174. [DOI] [PubMed] [Google Scholar]
- 43.Usai P, Usai Satta P, Lai M, Corda MG, Piras E, Calcara C, et al. Autonomic dysfunction and upper digestive functional disorders in untreated adult coeliac disease. Eur J Clin Invest. 1997;27:1009–1015. doi: 10.1046/j.1365-2362.1997.2340781.x. [DOI] [PubMed] [Google Scholar]
- 44.Giorgetti GM, Tursi A, Brandimarte G, Rubino E, Gasbarrini G. Dysmotility-like dyspeptic symptoms in coeliac patients: role of gluten and Helicobacter pylori infection. Dig Liver Dis. 2000;32:73–74. doi: 10.1016/S1590-8658(00)80051-1. [DOI] [PubMed] [Google Scholar]
- 45.Rana SV, Sharma S, Sinha SK, Prasad KK, Bhasin DK, Singh K. Orocecal transit time in patients with celiac disease from North India: a case control study. Trop Gastroenterol. 2008;29:98–100. [PubMed] [Google Scholar]
- 46.Chiarioni G, Bassotti G, Germani U, Battaglia E, Brentegani MT, Morelli A, et al. Gluten-free diet normalizes mouth-to-cecum transit of a caloric meal in adult patients with celiac disease. Dig Dis Sci. 1997;42:2100–2105. doi: 10.1023/A:1018878703699. [DOI] [PubMed] [Google Scholar]
- 47.Rana SV, Kochhar R, Pal R, Nagi B, Singh K. Orocecal transit time in patients in the chronic phase of corrosive injury. Dig Dis Sci. 2008;53:1797–1800. doi: 10.1007/s10620-007-0096-7. [DOI] [PubMed] [Google Scholar]
- 48.Rana SV, Sinha SK, Prasad KK, Sharma SK, Kaur J, Rana SS, et al. W1838 Orocecal transit time and small intestinal bacterial overgrowth in patients with microscopic colitis. Gastroenterology. 2010;138:S-750. [Google Scholar]
- 49.Cuoco L, Montalto M, Jorizzo RA, Santarelli L, Arancio F, Cammarota G, et al. Eradication of small intestinal bacterial overgrowth and orocecal transit in diabetics. Hepatogastroenterology. 2002;49:1582–1586. [PubMed] [Google Scholar]
- 50.Rana S, Bhansali A, Bhadada S, Sharma S, Kaur J, Singh K. Orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetes patients from North India. Diabetes Technol Ther. 2011;13:1115–1120. doi: 10.1089/dia.2011.0078. [DOI] [PubMed] [Google Scholar]
- 51.Faria M, Pavin EJ, Parisi MC, Lorena SL, Brunetto SQ, Ramos CD, et al. Delayed small intestinal transit in patients with long-standing type 1 diabetes mellitus: investigation of the relationships with clinical features, gastric emptying, psychological distress, and nutritional parameters. Diabetes Technol Ther. 2013;15:32–38. doi: 10.1089/dia.2012.0158. [DOI] [PubMed] [Google Scholar]
- 52.Heaton KW, Emmett PM, Symes CL, Braddon FE. An explanation for gallstones in normal-weight women: slow intestinal transit. Lancet. 1993;341(8836):8–10. doi: 10.1016/0140-6736(93)92479-D. [DOI] [PubMed] [Google Scholar]
- 53.Rana SV, Kaur J, Gupta R, Gupta V, Rana SS, Bhasin DK. Su1190 Effect of cholecystectomy on orocecal transit time and small intestinal bacterial overgrowth in patients with gallstones. Gastroenterology. 2012;142(5)(Suppl 1):S-447.