Abstract
The objective of this study is to induce experimental diabetes mellitus by streptozotocin in normal adult Wistar rats via comparison of changes in body weight, consumption of food, volume of water, urine and levels of glucose, insulin and C-peptide in serum, between normal and diabetic rats. Intra-venous injection of 60 mg/kg dose of streptozotocin in 250–300 g (75–90 days) adult Wistar rats makes pancreas swell and causes degeneration in Langerhans islet β-cells and induces experimental diabetes mellitus in 2–4 days. For a microscopic study of degeneration of Langerhans islet β-cells of diabetic rats, biopsy from pancreas tissue of diabetic and normal rats, staining and comparison between them, were done. In this process, after collagenase digestion of pancreas, islets were isolated, dissociated and identified by dithizone method and then with enzymatic procedure by DNase and trypsin, the islet cells changed into single cells and β-cells were identified by immune fluorescence method and then assayed by flow-cytometer. Donor tissue in each step of work was prepared from 38 adult male Wistar rats weighted 250–300 g (75–90 days). Transplantation was performed in rats after 2–4 weeks of diabetes induction. In this study, the levels of insulin, C-peptide and glucose in diabetic rats reached to normal range as compared to un-diabetic rats in 20 days after transplantation of islet cells. Transplantation was performed under the cortex of testis as immunoisolated place for islet cells transplantation.
Keywords: Streptozotocin, Diabetes induction, Islet cells, Purification, Flow cytometry, Immunoisolated-transplantation
Introduction
Diabetes is a chronic disease that is relatively common throughout the world. In recent decades, various epidemiological studies have been carried out on prevalence of diabetes mellitus in Iran, according to which the population of diabetics was estimated to exceed 1.5 million. In the year 2004, according to the World Health Organization reports, more than 150 million people throughout the world suffered from diabetes [1, 5] while the mankind has been unable to solve this problem. The only simple, inexpensive, easy and available way is to refine the Langerhans islets and to graft them under subcutaneous cortex of testis. Inducing experimental diabetes mellitus is indeed the first step in the plan for transplanting the pancreatic Langerhans islets under subcutaneous cortex of testis. Experimental diabetes mellitus has been induced in laboratory animals by several methods. The generally effective method is to take the pancreas out of the body. However, to induce a notable form of diabetes, at least 90–95 % of the pancreas has to be removed. Otherwise, the Langerhans islets in the remaining pancreas may undergo hypertrophy and secrete a sufficient amount of insulin for fulfilling the natural metabolic needs. The second method for creating diabetes in animals is injecting drugs such as alloxan or streptozotocin. These materials inflate and ultimately degenerate the Langerhans islets β-cells [6]. A less reliable method for creating diabetes is injection of the anterior hypophysis extract [7]. The final symptoms of insulin deficiency are clearly seen in rats afflicted with diabetes chemically by streptozotocin [8]. For the purpose of transplantation of Langerhans islets of healthy rats under the subcutaneous cortex of testis of diabetic rats, we had to induce experimental diabetes in order to study the effect of grafting the Langerhans islets in diabetic rats. Therefore, the study made us, first, to induce experimental diabetes mellitus in order to study the effect of transplantation of the Langerhans islets in diabetic rats so as to be able to study the clinical parameters before and after the pancreas islet cells transplantation. In rat islets, approximately 70–80 % of the endocrine cells are insulin-containing β-cells, 15–20 % is glucagon-containing α-cells or pancreatic polypeptide-containing (pp) cells, and the remaining 5–10 % is somatostatin-containing δ-cells [2, 15]. The heterogeneity of islet cell types, their complex organization and the likelihood of cell-to-cell communications have limited characterization of the properties and functions of individual endocrine cell types [16, 17]. Development of methods to separate islet cells into homogeneous population would permit examination of specific characteristic of the different islet endocrine cell types in the absence of influences of other cell-types [18, 19]. In this study, we demonstrate that analysis of light scattering from single islet cells, by using flow cytometry, can be used to sort out rat islet cells into subpopulations enriched in β, α and δ-cells [20, 21] for transplantation of either the entire pancreas or transplantation of pancreas components while the latter is carried out much more easily than the former. Transplantation of pancreas components can be in one of the following forms: (1) transplantation of dissociated Langerhans islets; (2) transplantation of mass of the Langerhans islets cells; (3) transplantation of embryonic tissues; (4) transplantation of neonatal tissues, which has been carried out successfully in different areas such as the liver, kidneys, spleen, testis cortex subcutaneously. However, transplantation of the Langerhans islets as a logical solution for treating these patients is still argued [3, 4, 33, 34]. Transplantation of the Langerhans islets cells is a new method for treating diabetes. Standardizing and optimizing separation and purification conditions of Langerhans islets cells is one of the most important phases of the transplantation. It is only after achieving and stabilizing this method that the researchers will be able to carry out studies for solving the transplantation problems. Factors such as the number of implanted cells, capacity of performance of the new medium and the size of cell groups are effective in the relative control of the metabolism after transplantation [34].
Materials and Methods
Streptozotocin or streptozocin or izostazin or zanosar (STZ) is a synthetic antineoplastic agent that is classifically an anti-tumor antibiotic and chemically is related to other nitrosourea used in cancer chemotherapy. Streptozotocin sterile powders are provided and prepared as a chemotherapy agent. Each vial of sterilized streptozotocin powder contains 1 g of streptozotocin active ingredient with the chemical name, 2-deoxy-2<{[methyl(nitroso)amino]carbonyl}amino>-β-d-glucopyranose and 200 mg citric acid. Streptozotocin was supplied by Pharmacia Company. Streptozotocin is available for intravenous use as a dry-frozen, pale yellow, sterilized product. Pure streptozotocin has alkaline pH. When it is dissolved inside the vial in distilled water as instructed, the pH in the solution inside the vial will be 3.5–4.5 because of the presence of citric acid. This material is prepared in 1-g vials and kept in cold store and refrigerator temperature (2-8 °C) away from light. Collagenase, crystalline trypsin, bovine pancreatic DNase, 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethan-sulfonic acid (HEPES), silicon and bovine serum albumin V were obtained from Merck. Percol is a commercial solution made up of silicon particles, coated with polyvinyl pyrrolidone which is used for sterilized cellular separation. Ethylene glycol-bis (β-amino ethyl ether)-N,N,N′,N′,-tetra acetic acid (EGTA), CMRL-1066 medium, anti-insulin antibody and fluorescein-labeled goat anti–guinea pig (second antibody) were obtained from Sigma-Aldrich Co.
Induction of Diabetes in Rats
Six adult Wistar rats weighting 250–300 g (75–90 days old) were used for inducing diabetes. The animals were injected by streptozotocin at the dose of 60 mg/kg of the body weight intravenously. Streptozotocin induces diabetes within 3 days by destroying the β-cells [9]. Diabetic animals and non-diabetic control group were kept in metabolic cages individually and separately and under feeding and metabolism control. Glucose in the blood of diabetic rats exceeded that of the non-diabetic control ones. Food consumption was measured in terms of (g), water consumption and urine volume were measured in terms of (ml) on a daily basis while every 2–4 weeks in 80 days the levels of C-peptide, insulin and glucose in blood serum were also measured, so that chemical diabetes was verified in rats injected with streptozotocin [10].
Measurement of Glucose, Insulin and C-Peptide in Rats’ Serum
Normal and diabetic rats were anesthetized with ether (2 min. contact with ether does not affect blood glucose, insulin or C-peptide concentrations). One ml of blood was taken from rats in order to measure glucose, insulin and C-peptide [11]. Blood was taken from the heart. The samples were collected in sterilized tubes and kept at 4 °C and, after separating the clot, the serum was separated by centrifuging. Blood glucose was measured by the glucose-oxidase method and insulin and C-peptide by radio-immunoassay method. This phase of the work was carried out once every 2–4 weeks for 80 days in diabetic and control counterparts [12].
Pancreatic Biopsy of Normal and Diabetic Rats
For the study and comparison of pancreas Langerhans islet β-cells in diabetic rats inducted by streptozotocin and normal rats, pancreatic biopsy of normal and diabetic rats was done and samples were fixed in 10 % formalin, stained by Hematoxylin and Eosin and photographed by Leitz microscope with 4,000 times enlargement (Figs 1, 2). The comparison of these pictures shows that the tissue of pancreatic Langerhans and the β-cells of diabetic rats have been degenerated irreversibly. [13].
Fig. 1.
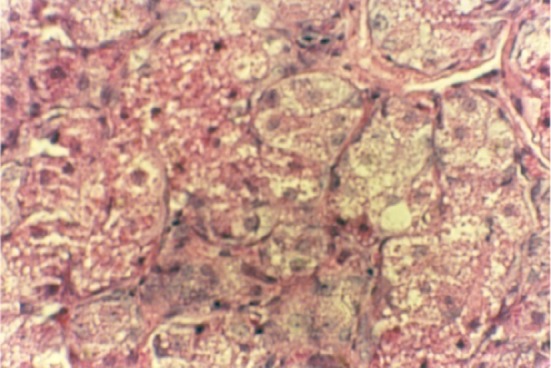
Pancreatic biopsy of normal rats
Fig. 2.
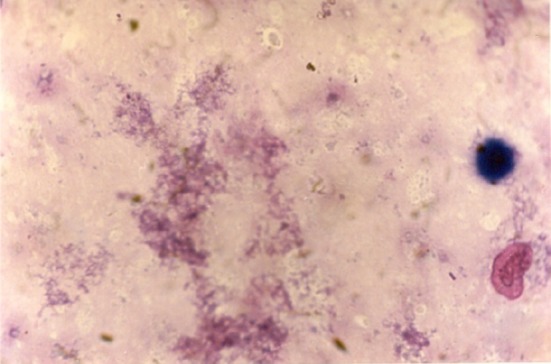
Pancreatic biopsy of diabetic rats that confirms the destruction of islets cells due to the effect of streptozotocin
Media Preparation
All media were filter sterilized through a 0.22 mm filter. Other reagents used were sterilized by autoclave or sterile media and glassware were employed. Glass wares used for collecting Langerhans islets cells were siliconized. They were coated with poly-l-lysine and incubated for 30 min with a sterile 10 mg/ml silicon solution followed by washing with distilled water. Separation of the Langerhans islets and the cellular purification were carried out in EHPES-buffered Earle’s-Hepes medium (EH) with following composition: 124 mM NaCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.4 mM KCl, 1.0 mM NaH2PO4, 14.3 mM NaHCO3, 2.8 mM Glucose, and 10 mM HEPES. The medium was supplemented with 2.5 % (w/v) bovine serum albumin, equilibrated with 5 % CO2 and adjusted to pH 7.30 at room temperature. The islets were isolated from male adult Wistar rats’ pancreas (250–300 g body weight) through collagenase digestion by the method of Lacy and Kostianovskt [21] which was modified to increase the yield.
Animals Used
The donor tissues were taken from 38 male adult Wistar rats weighting 250–300 g (75–90 days old). The Islet cells were purified from pancreas of 38 rats and transplanted to 19 rats and other 19 rats were taken as control. Transplantation was carried out in rats that were afflicted with diabetes for 2–4 weeks by intravenous injection of 60 mg/kg dose of streptozotocin. Streptozotocin induces diabetes within 3 days by destroying the β-cells. Transplanted animals and diabetic and non-diabetic control groups were kept individually and separately in metabolic cages and were controlled in terms of feeding and metabolism. The amount of food (g), water (ml) and urine (ml) were measured daily and every 2–4 weeks, the levels of C-peptide, insulin and glucose in blood serum were measured to confirm the chemical diabetes in rats which were injected with streptozotocin. The insulin level was minimized in these rats and signs of recovery were observed in rats receiving the transplantation [35].
Separation of Langerhans Islet Cells
For identifying the pancreas, 2 h before dissection, 32 male Wistar rats (200–250 g) were injected intraperitoneally with pilocarpine (0.2 ml from 0.2 % solution) [2, 23, 24]. To carry out dissection, first the animals were anesthetized in appropriate desiccators. Then, by opening the rat’s abdomen and closing the pancreatic canal, pancreas was distended by injection of 10 ml Earle’s-Hepes containing 1.5 mg/ml collagenase. The gland was removed, cleaned from lymph nodes and fat tissue and minced. After 15 s sedimentation, the supernatant fluid was discarded and the tissue suspension diluted with an equal volume of Earle’s-Hepes containing 4 mg/ml of collagenase. The tissue was digested at 37 °C for 10 mi. under continuous shaking (300 strokes/min) and then dispersed by gentle pipetting at room temperature for 3 min. The digest was filtered through a 500-mm nylon screen and the filtrate was washed by three successive centrifugation and re-suspension procedures in Earle’s-Hepes. The filter residue was re-suspended in EH without collagenase and further dispersed at 37 °C for 4 min in a shaking incubator (300 strokes/min). The second digest was finally washed in Earle’s-Hepes buffer. The washed filtrate and residue fractions were examined under a dissecting microscope and clean islets were aspirated from the preparation (Fig. 3) Using this procedure, 7,000–13,000 islets were routinely isolated from 32 rat pancreas within 2 h after beginning of the dissection [17].
Fig. 3.
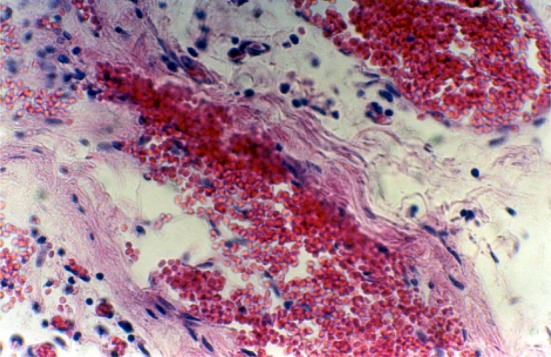
The existing transplanted Langerhans islet cells inside the testis subcutaneous of the diabetic rat which has been treated via transplantation of Langerhans islet cells, observed after taking a tissue from the testis and stabilizing in 10 % formaldehyde solution
Purification of the Langerhans Islets Cells
The isolated islets were washed by three sedimentation procedure in calcium-free EH and then re-suspended in calcium-free EH containing 1 mM EGTA, (5 ml/1,000 islets). This suspension was first maintained for 8 min at room temperature, continuously aspirated through a siliconized Pasteur pipette (9-inch), and then supplemented with trypsin (final concentration of 25 mg/ml) and DNase (final concentration of 2 mg/ml) at 30 °C. The degree of enzymatic dissociation was regularly checked under phase contrast microscope and stopped when 60–70 % of the cells occurred as single units. The islet cell suspension was then immediately diluted with 40 ml ice-cold calcium-free EH and filtered through a 63-mm nylon screen to remove large cell clumps and undigested material. An isotonic percoll solution with density of 1.040 g/ml was layered underneath the filtrate in order to remove debris and dead cells during a subsequent centrifugation at 300×g for 6 min [23, 32]. The percoll pellet was collected, suspended in 50 ml CMRL-1066 medium containing 2 mM glutamine and 0.2 % BSA and incubated for 20 min at 37 °C under 7.5 % CO2. At the end of this incubation, the cells were re-suspended and centrifuged at 100×g for 1 min. The pellet, which contained mostly small cell clump which had not been completely dissociated, was resubmitted to gentle pipetting in 10 ml calcium-free EH containing EGTA. This mechanical dispersion was done for 8 min, after which the preparation was filtered through a percoll layer (300×g, 5 min). Meanwhile, the initial supernatant, containing most single β-cells, were centrifuged at 300×g for 6 min. The pellets collected after the two latter centrifugations were combined, washed once in Earle’s-Hepes, and re-suspended in Earle’s-Hepes. The final cell suspension contained 7.5 × 10−107 cells obtained from 32 rat pancreases. This method should be carried out appropriately, carefully and rapidly so that the islets cells are less damaged [2, 17, 24].
Purity Determination of Islet Cells by Flow Cytometry
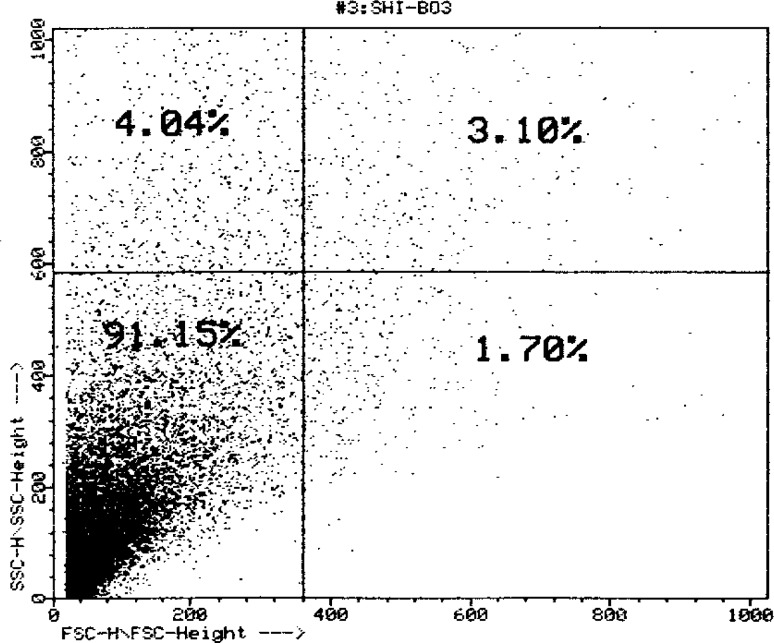
The freshly dissociated islet cells were submitted to auto fluorescence-activated cell sorting using a (FACC IV, Becton–Dickinson, Sunnyvale, Ca) equipped with two argon lasers (Argon 164-06 and UV-argon 171-17, Spectra-physics, Mountain view, Ca) [25], illuminating the cells at 488 nm, so that the emission at 510–550 nm could be taken as a parameter for their flavin adenine dinucleotide (FAD) content [26]. At 2.8 mM glucose, single β-cell displayed a threefold higher FAD fluorescence than single non β-cell. While its light scatter activity was also 50 % larger than non-β-cell. Selection of appropriate windows allowed the simultaneous isolation of single beta and single non-beta islet cells [27]. The Langerhans islet cells purified with the collagenase method were prepared with 91 % of β-cells in the cellular suspension (Figs. 4, 5).
Fig. 4.
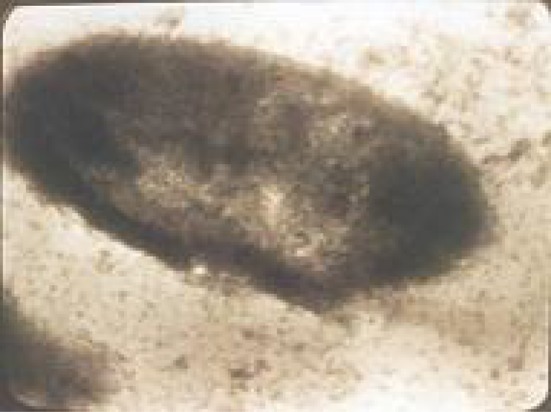
The electronic micrograph of purified β-cell of Langerhans islets at the cell suspension stained by osmium tetraoxide. The cell suspension made by using 32 pancreas contained 7.5 × 10−107 cells
Fig. 5.
Superficial distribution curve of Langerhans islets cells suspension obtained from flow cytometry, in the flow cytogram of a homogenous bulk of cells with purity of 91 % which belongs to the cells with fewer granularities among the langerhans islets cells, i.e. β-cells
Islet and β-Cell Identification
Islets were specifically stained by dithizone. 10 mg dithizone was dissolved in absolute ethyl alcohol and then 50 ml concentrated NH4OH was added and supplemented with 12 ml Hank’s solution (45 mM Na2HPO4, 2.5 mM citric acid, 0.1 % Triton X-100]. Before using, the solution was diluted with Hank’s solution (pH 7.8) by 1–100 and passed through a 0.22 μm filter membrane. Islet suspension was mixed with dithizone and placed 10 min and identified by light microscope [28].
Identification of β-Cells by Immunofluorescence Methods
β-cells were fixed by using Bouin’s solution [71.4 % picric acid solution (1.2 % w/v), 23.8 % formalin, and 4.8 % glacial acetic acid]. After 24 h, cells were rinsed three times with phosphate buffer saline solution (PBS), dehydrated and permeabilized with graded concentrations of ethanol, and incubated for 2 h at room temperature with an anti-insulin antibody diluted 1:1,000 in PBS. After rinsing, slides were incubated for 1 h at room temperature with fluoresce labeled goat anti-guinea pig second antibody (1:400). After rinsing in PBS, slides were covered with 0.02 % p-phenylenediamine in PBS–glycerol (1:2, V/V) and screened by fluorescence microscopy [29].
Flow Cytometry
Our aim in flow cytometry is to obtain information on the homogeneity of the β-cells and the percentage of homogeneity of these cells in cellular suspension obtained at the end of purification of the Langerhans islets cells so as to determine the percentage of β-cells in the suspension for transplantation. In view of the considerable difference in the size of types of Langerhans islets cells, a sample cellular suspension solution can be injected into the flow cytometry system and the appropriate graph can be prepared, which indicates the types of cells and their percentage in the suspension [21, 22].
Transplantation of Langerhans Islets β-Cells
In brief, the purification of Langerhans islets β-cells was carried out as follows: The Langerhans islets suspension was first kept in room temperature for 8 min and then aspired by a 9 inch length siliconized Pasteur pipette. Then, trypsin with final concentration of 25 μg/ml and DNase with final concentration of 1.5 μg/ml were added to it. The degree of enzymatic differentiation and dissociation were regularly examined with phase-contrast microscope and, when 50–60 % of the cells converted into single units, the work was stopped. This condition often occurs after 10 min. The suspension of the Langerhans islets cells was diluted immediately with 40 ml of EH buffer and the whole collection was put in ice bath and filtered by passing through a 63 μm nylon sheet. Thus, the undigested materials and the big cell masses were eliminated. The resulting product, which contains single cells, was centrifuged for 6 min in 300 g. The sediment was further changed into suspension and centrifuged. In this stage, the cellular sediment was suspended in isotonic Percoll solution with density of 1.040 g/ml, and was put in ice bath for 10 min so that the cellular suspension was layered and thus, the dead and destroyed cells and cell pieces obtained in consecutive centrifuging were eliminated. Finally, in the cellular suspension layer, the healthy cells were dissolved in the physiological serum. The purified cells of the Langerhans islets were transplanted to diabetic rats stimulated with STZ in a group of diabetic samples inside cortex of the testis subcutaneously and, in another group, to the peritoneal space. Transplantation of the cellular suspension in the physiological serum was carried out by using needle no. 20 in specified areas in each injected rat [35].
Biopsy and Histology
Two months after transplantation, the transplanted areas were vivisected in order to identify the Langerhans islets cells grown in the transplant receptor. For this purpose, the testes of the recipient rats were removed, stabilized in 10 % formalin buffer and given to the Electronic Microscope Department for light microscopic examination. After framing in paraffin, thin 3-μ tissue cuts were created. Staining was carried out by hematoxylin and eosin stain in order to recognize the transplanted islets (Fig. 6 with Leitz microscope).
Fig. 6.
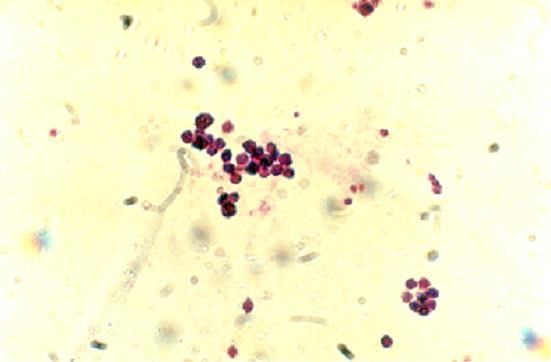
Existing cells of Langerhans islets at the cell suspension colored by the Gimsa and photographed by Litz microscope (×1,000)
Results
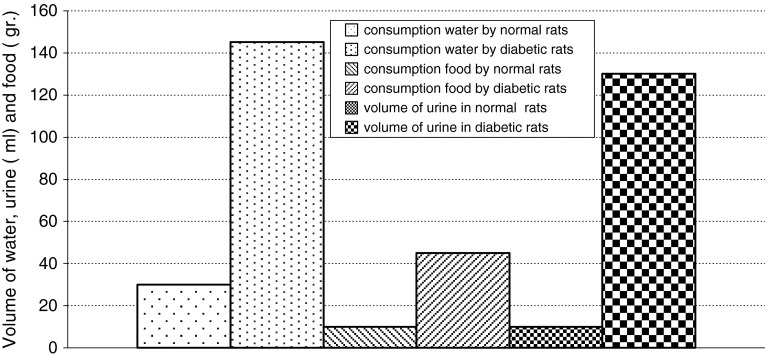
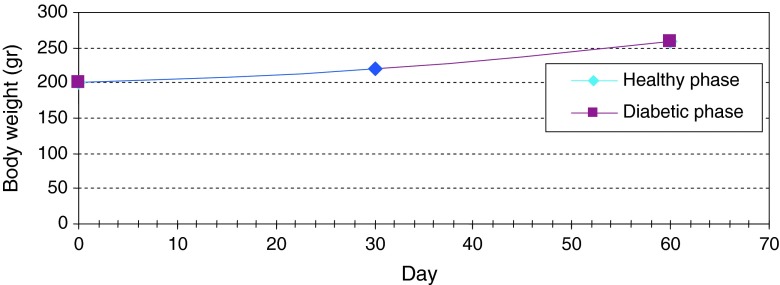
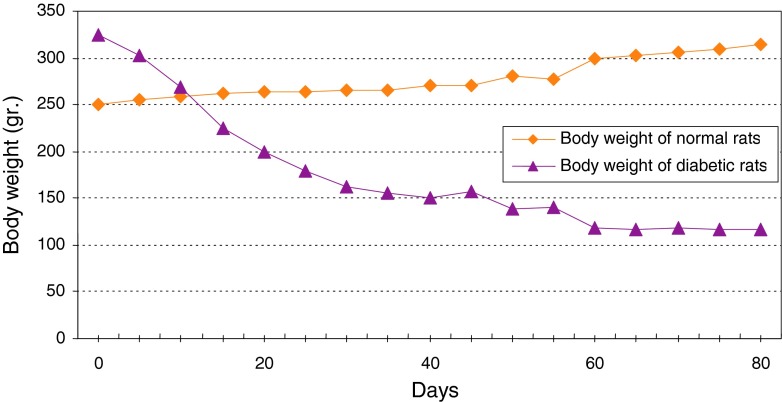
Normal levels of glucose, insulin and C-peptide in healthy adult rats were measured as 135 ± 5 mg/dl, 2 ± 0.2 mIU/l and 0.056 ± 0.003 ng/ml, respectively. Daily consumption of water and food in healthy adult rats were measured as 30 ± 5 ml and 10 ± 2 g, respectively. Daily urine volume in healthy adult rats was measured as 10 ± 1 ml. But in diabetic rats the levels of glucose, insulin and C-peptide were measured as 500 ± 20 mg/dl, 1.5 ± 0.2 mIU/l and 0.052 ± 0.002 ng/ml, respectively and daily consumption of water and food were measured as 145 ± 5 ml and 45 ± 4 g. Daily urine volume in diabetic rats was measured as 130 ± 5 ml (Table 1; Fig 7). Changes in average of body weight in 8 adult and non-adult diabetic rats varied. Since the non-adult diabetic rats were in the growing age, diabetic loss of weight was not seen in them and they even showed a slight weight gain (Fig. 8). In adult rats, however, diabetes was accompanied by loss of weight (Fig. 9). In each group, there were individual changing trends with respect to the amount of glucose, insulin, C-peptide and adult rats’ weight. Using replicated measurements, the data including a diabetic and a normal group underwent analysis of variance by SPSS 12 up to 80th day. According to the One-way ANOVA, a significant effect on diabetic and normal rats occurred, with rat blood glucose of SEM = 61.08, F = 1304.4, d.f = 1.8 and P < 0.001 (Fig. 10); rat blood insulin of SEM = 0.11, F = 19.3, d.f = 1.8 and P < 0.002 (Fig. 11); rat blood C-peptide of SEM = 0.002, F = 34.3, d.f = 1.8 and P < 0.001 (Fig. 12) and adult rats weight of SEM = 12.09, F = 56.3, d.f = 1.38 and P < 0.001 (Fig. 9) which showed the success of induction of diabetes by streptozotocin in rats. In addition, the changes in healthy and diabetic rats were apparently distinctive because, in addition to thinness of diabetic rats, the tails of the healthy rats were pink and they had a white velvet coat. Due to induction of diabetes, the tail became dark in color and stained and their coat turned from white velvet into pink or gray behind the head and in the lower part of the body. If the environment of the rats is kept clean, there will be a change of color from white to pink. Otherwise, the change will be from white to gray [13]. Pancreatic biopsy of normal and diabetic rats confirmed that the islet cells were destroyed due to the effect of streptozotocin in diabetic rats.
Table 1.
Data of number, weight, age, amount of streptozotocin, glucose, insulin, C-peptide of blood, consumed water, food and volume of urine in normal and diabetic rats
| State of rats | Streptozotocin and PBS injection | Blood glucose (mg/dl) | Blood insulin (mIU/ml) | Blood C-peptide (ng/ml) | Consumed water (ml) | Consumed food (g) | Volume of urine (ml) |
|---|---|---|---|---|---|---|---|
| Normal (n = 6) | 60 mg/kg (PBS) | 135 ± 5 | 2 ± 0.2 | 0.056 ± 0.003 | 30 ± 5 | 10 ± 2 | 10 ± 1 |
| Diabetic (n = 6) | 60 mg/kg (STZ) | 500 ± 20 | 1.5 ± 0.2 | 0.52 ± 0.002 | 145 ± 5 | 45 ± 5 | 130 ± 5 |
Fig. 7.
The mean of water, food consumption and volume of urine in normal and diabetic rats
Fig. 8.
Continuous changes in average of body weight in non-adult rats in two healthy and diabetic phase
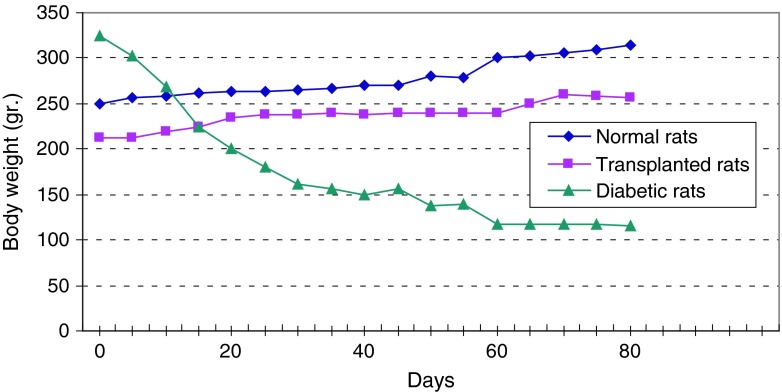
Fig. 9.
Comparison of the curves relating to the average of body weight in two groups of normal and diabetic rats. This graph reveals loss of weight and thinness in diabetic adult rats
Fig. 10.
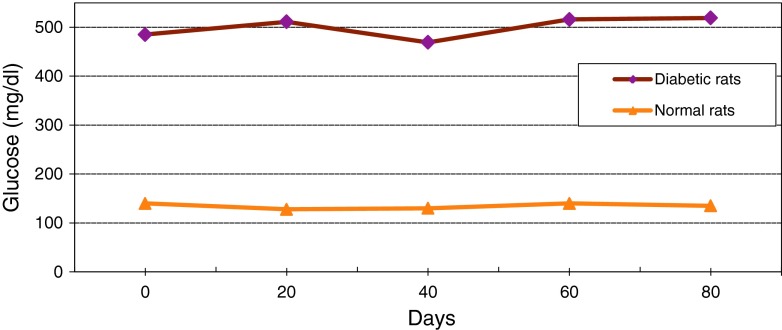
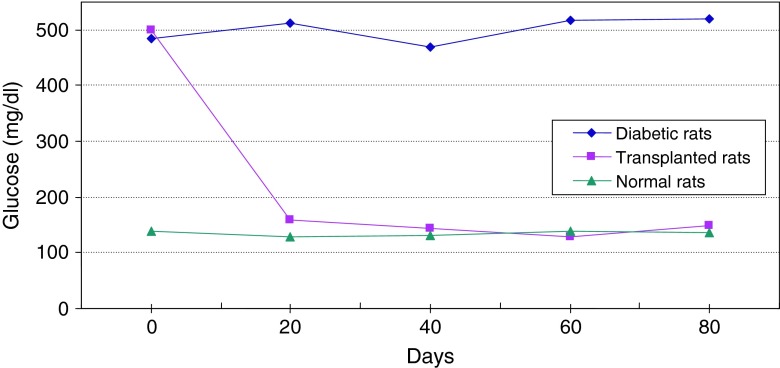
Changes of average level of glucose in serum of diabetic and normal rats during 80 days
Fig. 11.
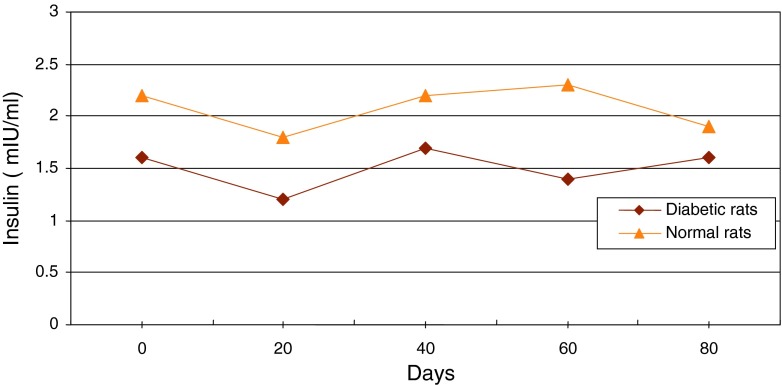
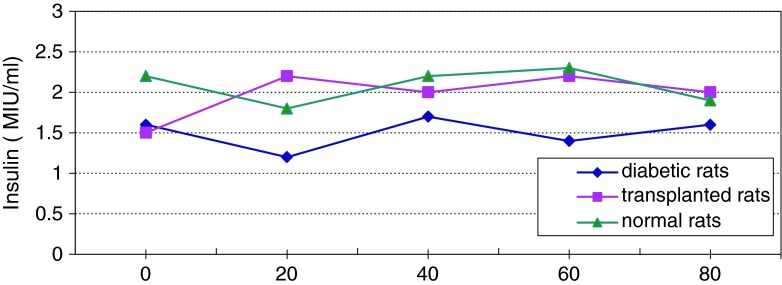
Changes of average level of insulin in serum of diabetic and normal rats during 80 days
Fig. 12.
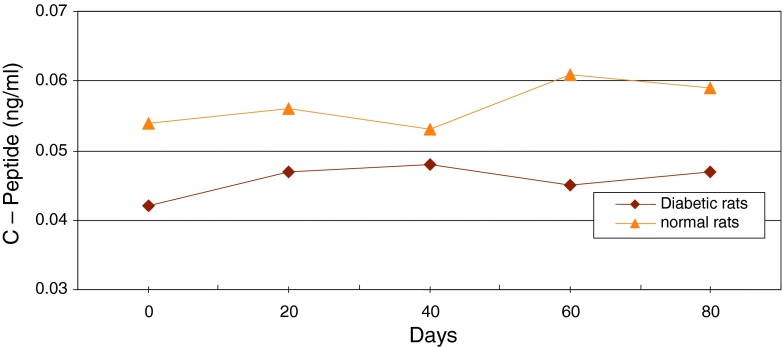
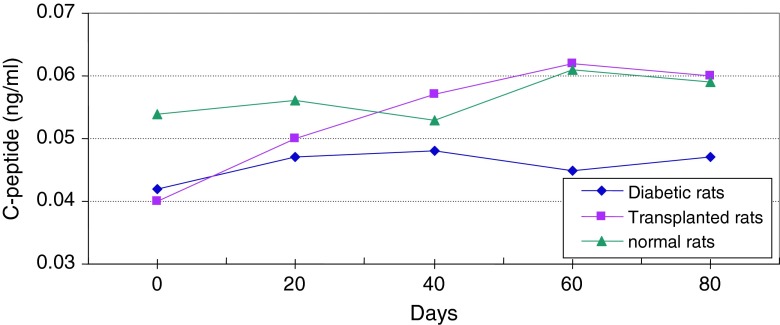
Changes of average level of C-peptide in serum of diabetic and normal rats during 80 days
Streptozotocin prevents DNA synthesis in mammalian and bacterial cells. In bacterial cells, it renders special reaction with cytosine groups, resulting in degeneration and destruction of DNA. The biochemical mechanism results in mammalian cell death. Streptozotocin prevents cellular reproduction with a much smaller dose than the dose needed for inhibiting the substrate connection to the DNA or inhibiting many of the enzymes involved in DNA synthesis [13]. Although streptozotocin prevents entry of cells into mitosis but no special phase of the cellular cycle is especially sensitive to its mortal effects. Streptozotocin, which is used in intravenously form by rapid injection or constant short diffusion, stimulates the tissues. Metabolically, a slight deviation of the glucose-bearing pain from the normal limit has been seen in patients treated with a certain dose of streptozotocin, which is generally reversible. However, the insulin shock, which is one of its other effects, is irreversible [14]. In this study, the clinical manifestations and also the amount of glucose, insulin and C-peptide after using a 60 mg/kg dose of streptozotocin, ensured induction of diabetes in rats. Hyperglycemia, hypoinsulinemia, polyphagia, polyuria and polydipsia accompanied by weight loss were seen in adult rats within 3 days of streptozotocin treatment and, within 1 week–10 days, the amounts of the relevant factors were almost stable, which indicates irreversible destruction of Langerhans islets cells. Moreover, researchers around the world have used streptozotocin to create experimental diabetes because it is a simple, inexpensive and available method [12–14]. Our results are similar with those of Elias [8], Ikebukuro [6]. We found their results are similar to ours with no significant difference between them. Flow cytometry is a technique by which the physicochemical specifications of the cells or any biological component are recorded individually when they pass against laser beam. The individuality and solution nature of the cells are important in flow cytometry. The sample must be a solution from the outset or be made into a solution with enzymatic methods, in which each tissue is prepared with special method of its own. Measurement of parameters such as size, form, DNA content, surface cell receptors, enzymatic activity, membrane permeability and calcium pump are possible with this method. In view of the considerable difference in the sizes of types of Langerhans islets cells, a sample cellular suspension solution can be injected into the flow cytometry system, model (FACC IV, Bacton Dickinson, Sunnyvale, Ca) and obtain the appropriate graph, which indicates the types of cells and their percentage in the suspension. The Langerhans islets cells purified with the collagenase method were prepared with 91 % of β-cells in the cellular suspension [28] (Fig. 4). The suspension of insulin-secreting β-cells was supplied by the pancreas of healthy rats with the collagenase-digestion method. Supply of pure Langerhans islets cells of rats requires combination of mechanical action (cutting the pancreas into pieces) and simultaneous use of the enzymes (trypsin and DNase). This method depends on conditions of dissociation of pancreatic cells from other tissues. Addition of DNase reduces the dissociated, slicked pancreatic cells and trypsin prevents formlessness of the dissociated cells. Moving to another container eliminates the smaller pieces and increases the concentration of larger particles of the islets. Filtration and incubation separate the purified cells from the slicked cells. The four quadrant of the cytogram shows that on the surface distribution curve, the Langerhans islets cells suspension is homogenous with the purity of 91 % that belongs to the cells with fewer granularities among the Langerhans islets cells i.e. β-cells (Fig. 4). There are many reports indicating the purification of islet cells from pancreas but none has shown the single pure β-cell as we have done [15, 18, 22, 25]. In addition, identification of two cell populations of the islets that were different in terms of size conformed to the small-sized pp cells, δ-cells and α-cells and the bigger-sized β-cells for identifying the insulin-secreting cells in the cellular suspension [31]. Pancreatic islets were obtained from adult rats using the collagenase digestion optimized and modified method of Lacy and Kostianovsky [21]. Isolated islets were dissociated in calcium-free medium containing trypsin and DNase. The islet cell suspension was cleared from debris and dead cells via centrifugation through a percoll layer of density 1.040 g/ml. After pre-incubating the cells for 10 min in ice bath, they were centrifuged at 800 g for 5 min and re-suspended in physiological serum. The cells were analyzed and separated in a fluorescence-activated cell sorter (FACS CA.) and obtained appropriate graph, which indicates the types of cells and their percentage in the suspension. Single cell analysis of β cell in Fig. 5 quadrant 3 on the x-axis sideward scatter and on the y-axis forward scatter is plotted. In quadrant 3, the total population is scattered as “Peacock tail” that most of the β cells are depolarized in quadrant 3, and 4.04 % cells are classified as live in quadrant 1 and 3.10 % cells classified as live in quadrant 2 and 1.70 % cells are classified as live in quadrant 4.
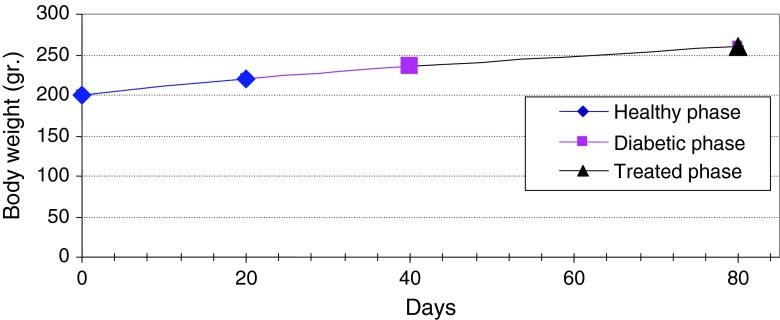
Figure 13 shows continuous changes in average of body weight in 8 non-adult rats in three healthy, diabetic and treated phases and a slight increase in the weight of non-adult rats in the three healthy, diabetic and treated phases. In adult rats, however, diabetes is accompanied by loss of weight. Two to four weeks after diabetes induction and observing its effects, transplantation of purified Langerhans islets cells was carried out by collagenase method with 91 % β-cells in the cellular suspension for treatment of diabetes. Figure 14) shows a comparison of the curves relating to the average changes in body weight in the three groups of healthy, diabetic and transplanted rats. This diagram reveals the loss of weight and thinness due to streptozotocin used for diabetes induction in adult rats and elimination of these effects after transplantation of pancreatic Langerhans islets cells. By analyzing of variance (ANOVA) with SPSS 12, the standard error mean (SEM) is found to be equal to 8.19, F = 40.87, d.f = 2.57, P < 0.001, which well indicates the weight loss in diabetic adult rats. By carrying out this operation, signs of recovery were gradually observed in the rats. So that the levels of glucose, insulin and C-peptide in transplanted rats were 145 ± 11.2 mg/dl, 1.98 ± 0.25 mIU/l and 0.042 ± 0.008 ng/ml, respectively. Figure 15 shows the average of the changes in the level of glucose in blood serum of 19 diabetic rats treated by transplantation of Langerhans islets cells and normal ones during 80 days in which the ANOVA on transplanted and diabetic rats shows the SEM is equal to 48.1, F = 903.18, d.f = 2.11, P < 0.001. Figure 16 shows the average of the changes in the level of insulin in blood serum of 19 diabetic rats treated by transplantation of Langerhans islets cells and normal ones during 80 days, in which the ANOVA on transplanted and diabetic rats shows the SEM is equal to 0.088, F = 8.53, d.f = 2.12, P < 0.005. Figure 17 shows the average of the changes in the level of C-peptide in blood serum of 19 diabetic rats treated by transplantation of Langerhans islets cells and normal ones during 80 days, in which the ANOVA on transplanted and diabetic rats shows the SEM is equal to 0.002, F = 4.85, d.f = 2.12, P < 0.029. Consequently, data analysis of glucose, insulin and C-peptide show the high significant difference between transplanted and diabetic rats serum and confirm the success of the transplantation project. Moreover, the daily consumption of water and food reached the relatively normal limit of 40 ± 5 cc and 30 ± 5 g, respectively and daily urine in treated rats was measured as 35 ± 5 cc.
Fig. 13.
Continuous changes in average of body weight in non-adult rats in three healthy, diabetic and treated phases
Fig. 14.
Comparison of the curves relating to the average changes in body weight in three phases of healthy, diabetic and transplanted rats. These curves reveal loss of weight and thinness due to streptozotocin used for diabetes induction in adult rats and elimination of these effects after transplantation of pancreatic Langerhans islets cells
Fig. 15.
Average changes in the level of glucose in blood serum of diabetic rats treated by transplantation of Langerhans islets cells and normal ones during 80 days
Fig. 16.
Average changes in the level of insulin in serum of diabetic rats treated by transplantation of Langerhans islets cells and normal ones during 80 days
Fig. 17.
Average changes in the level of C-peptide in serum of diabetic rats treated by transplantation of Langerhans islets cells and normal ones during 80 days
Discussion
With the transplantation of the obtained cellular suspension, it was expected that, due to secretion of insulin by transplanted β-cells, the level of blood serum glucose would fall to the normal, healthy limit and the amount of insulin and C-peptide of the blood would increase and the clinical manifestations of the disease, such as polyuria, polyphagia and polydipsia would be eliminated. All these were clearly seen immediately and completely, the day after transplantation [34]. The considerable point in this research is that inbred rats were not used as receptor or donor of the transplantation. In spite of this, however, no sign of rejection of the transplantation was observed [36]. To explain this, one must say that the phenomenon of immuno-isolation due to the effect of transplantation of Langerhans islets cells, in an immunity-quarantined space, prevented access of the immunity cells to the external transplanted cells and rejection of the transplantation [37]. Transplantation of Langerhans islets cells is generally used for treating a type of diabetes that results from the autoimmune destruction of β-cells of the islets. Therefore, as expected, this autoimmune process also continues with respect to the transplanted β-cells. In this research, by carrying out transplantation of Langerhans islets cells in parts of the body with a special immuno-isolated position, the risk of destruction of the transplanted β-cells by the autoimmune process in the transplantation receptor was completely eliminated [30]. The technique of transplantation of the Langerhans islets cells inside a capsule in the absence of immunological inhibitors to support the transplanted tissue against the host immunity system is a new way of success in this path [38]. In this process, the islets can be encircled in a semi-permeable membrane that allows food and oxygen to reach the Langerhans islets and the insulin to be released into the blood flow while, at the same time, it creates a mechanical barrier separating the potentially destructive immunity cells and the antibodies from the islets cells and thus preventing the rejection of the transplantation [39]. Statistical data relating to F, d.f, and P values of food, water, urine, body weight and SEM in the entire test groups, compared to the findings of Gray et al., and Pipellers et al. [17, 18] show the higher success of the transplantation of the Langerhans islets in rats as achieved in their work. The Langerhans islets constitute 1–2 % of the weight of the pancreas. Their diameter is 0.2–0.5 mm. The number of the separable Langerhans islets is 360,000 islets. Each islet has thousands of cells. In the infusion method of the islets through the portal vein into the liver and reproduction of islets and returning them back-up, approximately 9,000 Langerhans islets per kilogram of body weight of the rat are needed. In other words, for a rat weighting 250 g, approximately 2,500 islets are needed. In this method, other than the further need to islets, there is a major problem of the HLA compatibility complex of the main tissue in the receptor and donor of the islets, which we are studying further. This method is inexpensive and available to everyone. Figure 3 successfully shows the transplanted Langerhans islets in an immunity-quarantined space which prevents access of the immunity cells to the external transplanted cells and prevents rejection of the transplantation. The rat is cured 100 % as a result of secretion of the transplanted Langerhans islets cells. In this method, approximately 5,000 islets are needed for each kg of rat body weight. This method is important because of its simplicity and accessibility. Actually, by purifying the pancreas of an adult normal rat a successful transplantation to a diabetic rat can be done, while no change is observed in the tissue inside the testis subcutaneous of the normal and diabetic rats (Figs. 18, 19). We are still studying intra-peritoneal injection method.
Fig. 18.
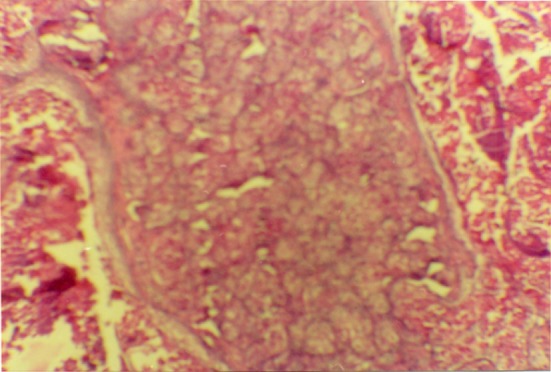
The cells of the tissue under the testis cortex subcutaneous of the normal rat
Fig. 19.
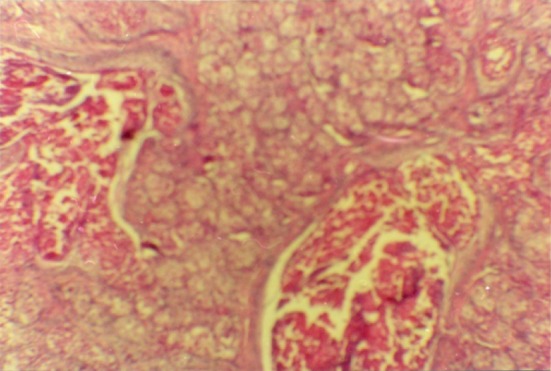
The cells of the tissue under the testis cortex subcutaneous of the diabetic rat
References
- 1.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi SH, Farhangi A, Allah Verdi A, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22:60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarzadeh A, Norouzian D, Farhangi A, Mehrabi MR, Jamshidi SH, Zare D, et al. Isolation and purification of rat islet cells by flow cytometry. Indian J Clin Biochem. 2008;23:56–61. doi: 10.1007/s12291-008-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarzadeh A, Noruzian D, Jamshidi SH, Farhangi A, Mehrabi MR, Lame Rad, et al. Treatment of streptozotocin induced diabetes mellitus in male rats by immunoisolated transplantation of purified Langerhans islet cells. Indian J Clin Biochem. 2007;22:57–61. [DOI] [PMC free article] [PubMed]
- 4.Akbarzadeh A, Noruzian D, Jamshidi SH, Farhangi A, Mehrabi MR, Lame Rad, et al. Treatment of streptozotocin induced diabetes mellitus in male rats by immunoisolated transplantation of purified Langerhans islet cells. Asian J Biochem. 2007;2:31–41. doi: 10.3923/ajb.2007.31.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization; http://www.who.int/medacenter/factsheets/fs/138/en/Page1-3.
- 6.Ikebukuro K, Adachi Y, Yamada Y, Fujimoto S, Seino Y, Oyaizu H. Treatment of streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone marrow cells via portal vein in rats. Transplantation. 2002;73(4):512–518. doi: 10.1097/00007890-200202270-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rastellini C, Shapiro R, Corry R, Fung JJ, Starzl TE, Rao AS. An attempt to reverse diabetes by delayed islet cell transplantation in humans. Transplant Proc. 1997;29:2238–2239. doi: 10.1016/S0041-1345(97)00313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias D, Prigozin H, Polak N, Rapoport M, Lohse AW, Cohen IR. Autoimmune diabetes induced by the β-cell toxin STZ. Diabetes. 1994;43:992–998. doi: 10.2337/diab.43.8.992. [DOI] [PubMed] [Google Scholar]
- 9.Karunanayake EH, Hearse DJ, Mellows G. The metabolic fate and elimination of streptozocin. Biochem Soc Trans. 1975;3:410–414. doi: 10.1042/bst0030410. [DOI] [PubMed] [Google Scholar]
- 10.Bhuyan BK, Kuentzel SL, Gray LG, Wallach D, Neil GL. Tissue distribution of streptozotocin (NSC 85998) Cancer Chemother Rep. 1974;58:157–165. [PubMed] [Google Scholar]
- 11.Levi JA, Wiernik PH, Diggs CH. Combination chemotherapy of advanced previously treated Hodgkin’s disease with streptozocin, CCNU, adriamycin and bleomycin. Med Pediatr Oncol. 1977;3:33–40. doi: 10.1002/mpo.2950030106. [DOI] [PubMed] [Google Scholar]
- 12.Thulesen J, Qrskov C, Holst JJ, Poulsen SS. Short term insulin treatment prevents the diabetogenic action of streptozotocin in rats. Endocrinology. 1997;138(1):62–68. doi: 10.1210/endo.138.1.4827. [DOI] [PubMed] [Google Scholar]
- 13.Holemans K, Bree RV, Verhaeghe J, Meurrens K, Assche AV. Maternal semi starvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr. 1997;127:1371–1376. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes mellitus-wikipedia, the free encyclopedia, http://encyclopedia.onlinereference.info/index.php Diabetes#Statistics.
- 15.Pipeleers DG, Pipeleers Marichal MA. A method for the purification of single A, B and D cells and for the isolation of coupled cells from isolated rat islets. Diabetologia. 1981;20:654–663. doi: 10.1007/BF00257436. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovitch A, Russel T. Preparation of rat islet B-cell enriched fractions by light-scatter flow cytometry. Diabetes. 1981;31:939–943. doi: 10.2337/diacare.31.11.939. [DOI] [PubMed] [Google Scholar]
- 17.Gray DWR, Morris PJ. Developments in isolated pancreatic islet transplantation. Transplantation. 1987;43(3):321–331. doi: 10.1097/00007890-198703000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Pipeleers DG, Pipeleers Marichal M, Hannaert JC, Berghmans M, Veld PAL, Rozing J, et al. Transplantation of purified islet cells in diabetic rats. Diabetes. 1991;40:908–919. doi: 10.2337/diab.40.7.908. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen DA, Lernmark A, Berelowitz M, Bloom GD, Steiner DF. Sorting of pancreatic islet cell subpopulations by light scattering using a fluorescence-activated cell sorter. Diabetes. 1982;31:229–306. doi: 10.2337/diab.31.4.299. [DOI] [PubMed] [Google Scholar]
- 20.The diabetes control and complications trial research group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–985. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 21.Lacy PE, Kostianovsky M, Louis S. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Pipeleers DG, Veld PA, Winkel MVD, Maes E, Schuit FC, GeptsW. A new in vitro model for the study of pancreatic A and B cells. Endocr Soc. 1985;117:806–16. [DOI] [PubMed]
- 23.Kuo WN, Hodgins DS, Kou JF. Adenylate cyclase in islets of Langerhans: isolation of islets and regulation of adenylate cyclase activity by various hormones and agents. J Biol Chem. 1973;248:2705–2715. [PubMed] [Google Scholar]
- 24.Olack BJ, Swanson CJ, Howard TK, Mohanakumar T. Improved method for the isolation and purification of human islets of Langerhans using Liberase enzyme blend. Hum Immunol. 1999;60:1303–1309. doi: 10.1016/S0198-8859(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 25.Winkel MVD, Maes E, Pipeleers D. Islet cell analysis and purification by light scatter and auto fluorescence. Biochem Biophys Res Commun. 1982;170:525–532. doi: 10.1016/0006-291X(82)91523-6. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch A, Russell T, Shienvold F, Noel J, Files N, Patel Y, Ingram M. Preparation of rat islet B-cell-enriched fractions by light-scatter flow cytometry. Diabetes. 1982;31:939–943. doi: 10.2337/diacare.31.11.939. [DOI] [PubMed] [Google Scholar]
- 27.Winkel MVD, Pipeleers D. Auto fluorescence activated cell sorting of pancreatic islet cells: purification of insulin-containing β-cells according to glucose-induced changes in cellular redox state. Biochem Biophys Res Commun. 1983;114(2):835–842. doi: 10.1016/0006-291X(83)90857-4. [DOI] [PubMed] [Google Scholar]
- 28.Lukowiak B, Vandewalle B, Riachy R, Kerr-Conte J, Gmyr V, Belaich S, et al. Identification and Purification of functional human-β cells by a new specific zinc-fluorescent probe. J Histochem Cytochem. 2001;49(4):519–27. [DOI] [PubMed]
- 29.Asada N, Shibuya I, Iwanaga T, Niwa K, Kanno T. Identification of β-cells in intact isolated islets of Langerhans by their characteristic cytoplasmic Ca2+ concentration dynamics and immunocytochemical staining. Diabetes. 1998;47:751–757. doi: 10.2337/diabetes.47.5.751. [DOI] [PubMed] [Google Scholar]
- 30.Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes. 2003;52:1716–1722. doi: 10.2337/diabetes.52.7.1716. [DOI] [PubMed] [Google Scholar]
- 31.Alexander von Mach M, Schlosser J, Weiland M, Feilen PJ. Cryopreservation of islets of Langerhans: optimization of protocols using rat pancreatic tissue. EXCLI J. 2003;2:6–21.
- 32.Titus T, Badet L, Gray DWR. Islet cell transplantation for insulin-dependent diabetes mellitus: perspectives from the present and prospects for the future. Expert Rev Mol Med. 2000;6:1–27. doi: 10.1017/S1462399400001861. [DOI] [PubMed] [Google Scholar]
- 33.Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Boyle PJ. Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients. Transplantation. 1991;51(1):76–85. doi: 10.1097/00007890-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Garcia–Ocana A, et al. Using β-cell growth factors to enhance human pancreatic islet transplantation. J Clin Endocrinol Metab. 2004;86(3):984–8. [DOI] [PubMed]
- 35.Montaña E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest. 1993;3:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keymeulen B, et al. Length of metabolic normalization after rat islet cell transplantation depends on endocrine cell composition of graft and on donor age. Diabetologia. 1997;40:1152–1158. doi: 10.1007/s001250050800. [DOI] [PubMed] [Google Scholar]
- 37.Winkel MVD, Maes E, Pipeleers D. Islet cell analysis and purification by light scatter and auto fluorescence. Biochem Biophys Res Commun. 1982;107(2):525–532. doi: 10.1016/0006-291X(82)91523-6. [DOI] [PubMed] [Google Scholar]
- 38.Titus T, Badet L, Gray DWR. Islet cell transplantation for insulin-dependent diabetes mellitus: perspectives form the present and prospects for the future. Expert Rev Mol Med. 2000;2(6):1–28. [DOI] [PubMed]
- 39.Sutherland DER, et al. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25:487–496. doi: 10.1007/s002680020342. [DOI] [PubMed] [Google Scholar]