Abstract
This study is aimed to investigate the nanoliposomal artemisinin preparation, and its implementation on breast cancer cells. Side effects have been one of the common challenges of drug usage, as well as cancer treatment. In order to reduce such effects, nanotechnology has been a great help. Nanoliposomes are provided through reverse phase evaporation. In this method, certain proportions of phosphatidylcholine, cholesterol and artemisinin were mixed together. Besides, the obtained formulation was pegylated by using polyethylene glycol 2000 in order to increase its stability and solubility. The mean diameter of non-pegylated and pegylated liposomal artemisinin was determined by Zeta sizer system. The percent of drug released from liposome was performed by dialysis. The encapsulation efficiency of both formulations was estimated by spectrophotometry method. As a result, encapsulation and drug release of nanoliposomal formulation were more than the pegylation of the same formulation. In addition, this study indicated that cytotoxicity effect of pegylated nanoliposomal artemisinin was more, in comparison with nanoliposomal artemisinin.
Keywords: Artemisinin, Nanoliposome, Breast cancer, Drug delivery, Cytotoxicity
Introduction
Nanotechnology has recently revolutionized cancer diagnose and treatment [1]. While breast cancer is a fatal cause for wide death among women [2], by using nanotechnology, some nanoparticles are designed to safely target the cells and afterward, release their drug at the right place of the cancerous region [3]. Injected nano-scale carriers have selectively been studied as drug carrier agents, one kind of which are considered to be liposomes [4].
Liposomes are spherical phospholipid vesicles [5]. Which cause stability and improvement of pharmacokinetics properties of surrounded drugs, and declines their side effects [6]. Liposomes are biodegradable and essentially non-toxic vehicles, which can encapsulate both hydrophilic and hydrophobic molecules, and are used as drug carriers in drug delivery systems.
Artemisinin is used as an anti cancer drug, which is actually a terpene lactone taken from sweet warm wood leaves (Artemisia annua) [7]. Artemisinin molecule contains an endoperoxide bridge which releases carbon free radical after chemical reaction with Fe(II), which leads to molecular damage and consequently cellular death [7, 8]. Nevertheless, artemisinin carries some side effects such as: blood disorders and a serious allergic reaction [9, 10].
In this case study, the efficiency of nanoliposomal and pegylated nanoliposomal artemisinin are investigated in breast cancer cell lines and the result were compare with standard drug.
Materials and Methods
Materials
Artemisinin, phosphatidylcholine, cholesterol, polyethylene glycol 2000 (PEG 2000), and MTT solution (0.5 mg/ml) were supplied by Sigma Co. RPMI 1640 tissue culture was also purchased from Invitrogen Co. Ethanol and Isopropanol were provided from Merck company. MCF-7 cell line was purchased from Pasteur Institute of Iran.
Preparation of Nanoliposomal Drug and Pegylation
Cholesterol and phosphatidylcholine (ratio of 1 to 13.5) were dissolved in some certain amount of 98 % ethanol (400 rpm, room temperature) to produce a yellow, clear suspension. Afterwards, 1 mg of artemisinin was added and mixed by magnetic stirrer (300 rpm, 15 min, room temperature). Ethanol was then evaporated by means of Heidolph Co. Rotary evaporator and the reproduced gelose was dissolved in 12 ml physiological serum. PEG 2000 was also added to the mixture in order to produce pegylated nanoliposomal artemisinin formulation. Then, both formulations were sonicated for 5 min (Bandelin Sonorex Digitec, 60 Hz).
Size Measurement of Nanoliposomes
One mg of each formulation was dissolved in 10 ml of physiological serum and after the measuring of their absorption in 630 nm, the mean diameter of the nanoliposomes were determined by Zeta sizer (Malvern Instruments Ltd).
Encapsulation Efficiency
In order to determine the amount of entrapped drug, 1 mg of each formulation were centrifuged for 30 min at 4 °C and at 50,000 rpm. After that, optical density of the supernatant of each formulation were determined at 195 nm by means of spectrophotometer (UV 1601PC, Shimadzu Co.). Encapsulation efficiency was calculated by using Eq. (1) [11].
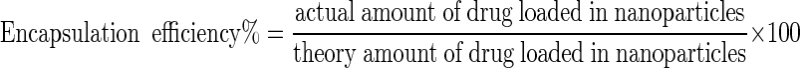 |
1 |
The density of the released drug in supernatant was determined by using standard curve.
In vitro Release Study
In order to study the release pattern of the nanoliposomal and pegylated nanoliposomal formulations, 1 mg of either artemisinin formulation was put in dialyze bag and while floating in 25 ml of PBS buffer (pH = 7.4), was placed on magnetic stirrer (300 rpm,48 h., room temperature). The amount of released drug in PBS buffer was determined after certain time periods through spectrophotometry method at 195 nm. The percentage of released drug was gained by means of drug standard curve.
Cytotoxicity Assay
After MCF-7 cell line culture, 100 μl of the suspension containing 10,000 cells was put in the wells of a 96-well plate and incubated by CO2, 5 % at 37°C. 24 h later, the supernatant of the cells was collected and different densities of nanoliposomal artemisinin product plus its control and also pegylated nanoliposomal artemisinin and their controls, as well as standard artemisinin drug were added to the cells; then it was incubated on the mentioned conditions for the following 24 h. Then the supernatant was decanted and 100 μl of the MTT solution (0.5 mg/ml) was added and after 3 h of incubation the purple color (due to formazane formation) was obtained by dissolving 100 μl of isopropanol in living cells. Absorption at 570 nm was measured (Power Eave XS spectrophotometer) and IC50 was calculated by using pharm Software.
Statistical Analysis
The results are expressed as mean ± standard deviation (SD, n = 3). The data were statistically analyzed by one-way analysis of variance using IBM Statistics SPSS software version 19, and significant difference was set at p < 0.05.
Results
Size Measurement of Nanoliposomes
Mean diameter of nanoliposomal artemisinin molecules was 500 nm and that of pegylated nanoliposomal artemisinin molecules was 455 nm, which was measured by means of Zeta sizer.
Encapsulation Efficiency
Encapsulation efficiency of both formulations was calculated through spectrophotometry method; which was 96.02 % ± 4.2 for nanoliposomal artemisinin formulation and 91.62 % ± 3.5 for pegylated nanoliposomal artemisinin (Fig. 1).
Fig. 1.
Encapsulation efficiency for both nanoliposomal and pegylated nanoliposomal formulations
In vitro Release Study
The amount of drug release in PBS buffer for both formulations was obtained for 2-, 4-, 6-, 24- and 48-hour periods by applying artemisinin standard curve. This was 8.21 % for nanoliposomal formulation and 5.17 % for pegylated nanoliposomal formulation (Fig. 2).
Fig. 2.
Artemisinin release pattern for both nanoliposomal and pegylated nanoliposomal formulations
Cytotoxicity Assay
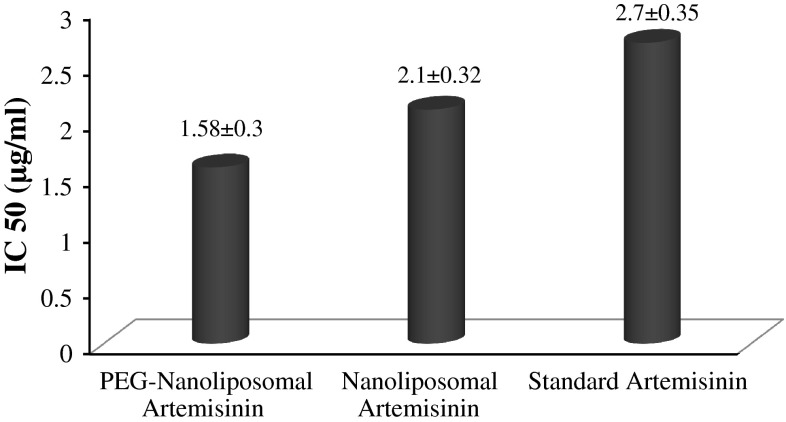
Drug toxicity with different concentration was studied through MTT technique. Figure 3 shows the obtained results of IC50 for nanoliposomal, pegylated nanoliposomal and standard drug by means of Pharm Software.
Fig. 3.
IC50 (μg/ml) of nanoliposomal, pegylated nanoliposomal and standard drug
Discussion
There was a great attempt to overcome breast cancer in the recent decades. In order to achieve such goal, artemisinin has been introduced as one of the most effective anti cancer drugs. Respective studies have proved that daily consumption of artemisinin can be positively affective on breast cancer prevention, as well as its extension [8, 12, 13]. This drug can affect the extension of cancerous cells through different mechanism one of which can kill precancerous cells, while another can directly kill cancerous ones [8]. In addition, this drug can be a proper candidate for chemotherapy due to its anti-angiogenic, anti-inflammatory, anti- metastasis effects, in addition to its preventing effect on cell development [14].
In this study, cytotoxicity effect of two formulations of nanoliposomal artemisinin and pegylated nanoliposomal artemisinin on MCF-7 line cell for breast cancer was investigated.
Mean diameter measurement of either formulation reveals that the diameter of nanoliposomal formulation is more than that of pegylated nanoliposomal one, which is resulted because of high infiltration susceptibility of PEG that penetrates into liposomal layers and compresses them together. Besides, these results verify the mean diameter of liposomal formulation to be in nano scale sizes [15].
Artemisinin encapsulation study results indicated that drug entrapment efficiency in nanoliposomal formulation is more than that of pegylated nanoliposomal one. Due to smaller liposomal molecules compared with pegylated liposomal ones, it is completely natural to see polyethylene chains would decrease the internal volume of liposomes after penetration [16]. At the same time, the results indicate that drug release of pegylated nanoliposomal formulation is slower; which is obvious to explain; since PEG surrounds nanoliposome, it takes longer time for the drug to exit liposome and PEG.
Cytotoxicity effect showed that the killing effect of pegylated nanoliposomal formulation is more than nanoliposomal one. PEG enhances drug solubility, as well as its more contact with the target cells, which is completely desirable.
Contributor Information
Seyed Ebrahim Alavi, Phone: +98-21-66968856, FAX: +98-21-66465132, Email: s.ebrahimalavi@gmail.com.
Azim Akbarzadeh, Email: azimakbarzadeh1326@gmail.com.
References
- 1.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 2.Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–1032. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed AR, Weston N, Coombes AG, Fitzgerald M, Perrie Y. Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int J Pharm. 2004;285:23–34. doi: 10.1016/j.ijpharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:591325. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lia T, Ho R. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90:667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 7.Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Lai H, Singh NP. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–48. doi: 10.1016/j.canlet.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi E, Gilvary G, White NJ, Nosten F. Severe allergic reactions to oral artesunate: a report of two cases. Trans R Soc Trop Med Hyg. 2001;95:182–183. doi: 10.1016/S0035-9203(01)90157-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27:4025–4033. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick J, Mercer AE, Park BK, Cosstick R, O’Neill PM. Synthesis and biological evaluation of extraordinarily potent C-10 carba artemisinin dimers against P. falciparum malaria parasites and HL-60 cancer cells. Bioorg Med Chem. 2009;17:1325–1338. doi: 10.1016/j.bmc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Sun B, Pan S, Jiang H, Sun X. Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs. 2009;20:131–140. doi: 10.1097/CAD.0b013e3283212ade. [DOI] [PubMed] [Google Scholar]
- 14.Lai HC, Singh NP, Sasaki T. Development of artemisinin compounds for cancer treatment. Investig New Drugs. 2013;31:230–246. doi: 10.1007/s10637-012-9873-z. [DOI] [PubMed] [Google Scholar]
- 15.Ning Z, Cheung CS, Fu J, Liu MA, Schnell MA. Experimental study of environmental tobacco smoke particles under actual indoor environment. Sci Total Environ. 2006;367:822–830. doi: 10.1016/j.scitotenv.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Yatuv R, Robinson M, Dayan-Tarshish I, Baru M. The use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophilia. Int J Nanomed. 2010;5:581–591. doi: 10.2147/ijn.s8603. [DOI] [PMC free article] [PubMed] [Google Scholar]