Abstract
To determine the normal range of Hemoglobin and cutoff values in healthy adults of Southern India, blood samples were analyzed for parameters of RBC and iron metabolism in 177 male and 203 female medical students. The data were compared with the American white population (NHANES III) and the WHO criteria for detection of anemia. The mean values for hemoglobin and hematocrit in male students differed minimally from American white males. However, values for parameters of iron metabolism were lower except total iron binding capacity (TIBC) which was higher. In female students, hemoglobin, hematocrit and parameters of iron metabolism were lower than American white females, except TIBC which was higher. Lower 5th percentile cutoff point (Mean − 1.645 SD) in males and females were 13.5 and 10 g/dl respectively. In conclusion, South Indian adult males have Hb values similar to American male adults, but South Indian females have considerably lower Hb levels than American females, raising the questions about appropriateness of WHO or US criteria for detection of anemia in Indian females.
Keywords: Hemoglobin, Anemia, South Indian, Serum iron
Introduction
For the purpose of international surveys, in 1959 the World Health Organization (WHO) proposed iron deficiency anemia to exist in males with hemoglobin (Hb) <14 and non-pregnant women <12 g/dl (Table 1) [1]. This was based on a large body of hematological data derived from studies of apparently normal persons throughout the world and personal observation of the group members. As further studies became available, in 1968 WHO revised cutoff values for diagnosing anemia as Hb <13 for adult males and <12 g/dl for non-pregnant females (Table 1) [2]. In 1997, the Institute of Medicine (IOM), USA, after examining data from the third National Health and Nutrition Examination Survey (NHANES III, 1988–1994), suggested age specific hemoglobin cutoff values for diagnosing iron deficiency anemia [3]. According to this study, Hb cutoff values for the age group of 20–49 years was <13.7 in males and <12 g/dl in females. Unlike the arbitrary value proposed previously, the hemoglobin cutoff values were calculated as the mean of the reference group minus 1.645 SD which corresponds to the 5th percentile values (Table 1). NHANES III data also revealed that when compared to whites, black females and males have lower hemoglobin levels 0.9 and 0.6 g/dl respectively (Table 1) [4].
Table 1.
Normal and cutoff values for hemoglobin
| Year | Study | Database | Mean values ± SD (g/dl) | Cutoff (g/dl) | Derivation method for cutoff value | ||
|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | ||||
| 1959 | WHO [1] | Available studies | – | – | 14 | 12 | Arbitrary |
| 1968 | WHO [2] | Modified based on interim studies | – | – | 13 | 12 | Arbitrary |
| 1997 | Institute of Medicine [3] | NHANES III (Age 20–49 years) | 15.3 + 0.97 | 13.5 + 0.91 | 13.7 | 12 | Mean − 1.645 SD |
| 2005 | NHANES III (Age 20–29 years) [4] | Whites | 15.49 + 0.88 | 13.3 + 1.0 | – | – | – |
| Blacks | 14.85 + 0.9 | 12.42 + 1.1 | – | – | – | ||
| 1995–1996 | NIN criteria, Vietnam [5] | National samplea | 14.7 + 1.1 | 12.8 + 1.0 | 12.5 | 11 | – |
| 2005 | Hinduja Hospital, India [6] | Subjects from health checkup program | 14.35 + 1.08 | 12.7 + 0.85 | 12.3 | 11 | Mean − 2 SD |
| 12.6 | 11.3 | Mean − 1.645 SDb | |||||
| 2010 | Hyderabad, India | Medical students | 15.6 + 1.3 | 12.6 + 1.6 | 13 | 9.4 | Mean − 2 SD |
| 13.5 | 10 | Mean − 1.645 SD | |||||
aSince mean Hb levels were 1 g/dl less than the Caucasian population, lower limits were proposed as 1 g/dl less than the Caucasians values
bValues were calculated by the authors from the data
In 1995, the UNICEF supported Vietnam National Nutrition Anemia and Intestinal Helminth Survey, by National Institute of Nutrition (NIN), suggested that healthy Vietnamese population had mean hemoglobin 1 g/dl lower than the value for the Caucasian population. This prompted development of Vietnam specific anemia criteria for estimation of iron deficiency anemia [5]. According to the Vietnam-NIN criteria, the Hb cutoff values were set 1 g/dl lower than the criteria for Caucasian population (Hb <12.5 g/dl in males; Hb <11 g/dl in non-pregnant females) (Table 1). Prevalence of anemia in the Vietnam population is markedly lower based on NIN criteria when compared to the WHO criteria for males and non-pregnant females (10.1 vs. 28.3 % and 19 vs. 43 % respectively).
There is a dearth of studies with respect to establishing reference values for India. Ashavaid et al., based upon the healthy subjects from heath checkup program at Hinduja hospital, Mumbai, western India, after excluding the subjects with known patho-physiological conditions, found hemoglobin range (95 % confidence interval) for apparently normal individuals to be 12.3–16.4 for males and 11.0–14.4 g/dl for females [6]. Thus the lower limit of hemoglobin for non-pregnant adult Indian females was 1 g/dl less than the WHO criteria and 1 g/dl less than IOM criteria for both males and females (Table 1). Similar studies are not found from other parts of India. Given the geographic and ethnic variations in hemoglobin levels, use of a single cutoff value for diagnosing anemia may not be applicable for different subpopulations. According to the National Family Health Survey-3 (NFHS-3; 2005–06) 55 % of females and 24 % of males in India are reported to be anemic, based on the WHO criteria, with higher prevalence in Andhra Pradesh (63 % females and 23 % males) [7]. However, with use of the WHO criteria we are inclined to overestimate prevalence of anemia as suggested by western Indian and Vietnam (NIN) studies. Hence, the need to undertake special studies to validate the WHO criteria and establish the cutoff points for detection of anemia in different subgroups of Indian population is all the more important for assessing the extent of variation in the prevalence of anemia in different regions of India. The present study is an attempt to fill the knowledge gap in this immense health problem.
Methodology
A cross-sectional study of the medical students was undertaken at the MediCiti Institute of Medical Sciences (MIMS), Medchal, in Andhra Pradesh, India. The subjects, who are studying at MIMS, were recruited using convenience sampling. Most students belonged to the middle or upper socioeconomic class. Participation in the study was voluntary. The sample size was calculated using the formula (Standard deviation × critical factor/acceptable error)2; based on the previous studies we used the standard deviation of 1, critical values as 1.96 which corresponds to 95 % confidence level and an acceptable error of 0.2 g/dl. The sample size calculated to be 96. We planned to recruit double the number, but succeeded in recruiting 177 males and 203 females. After explaining the study protocol, informed consent was obtained and a questionnaire was administered. The students were asked to exclude themselves from the study if they have any of the following patho-physiological states: pregnancy, dehydration, chronic smoker (≥10 pack year), acute or chronic infection or illness (e.g. Malaria), abnormal bleeding (e.g. excessive menstrual bleeding, gastrointestinal bleeds or any other bleeding disorder), radiotherapy, hematopoietic drugs, history of recent blood transfusion. Height and weight measurements were taken for all eligible subjects. The study was approved by the Ethics Committee of MIMS and all the subjects gave their informed consent prior to participating in the study.
Two blood samples, each of 4 ml were collected from the superficial vein in the cubital fossa, between 0900 and 1300 h. The first sample was collected in an EDTA coated vacutainer for measurement of hemoglobin (Hb), hematocrit (Hct), red blood cell (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC). These vacutainers were stored in an ice pack and were tested within 2 h of the sample collection. The second sample was collected in a plain vacutainer for measurement of serum iron (SI), total iron binding capacity (TIBC), serum ferritin (SF) and serum creatinine. Blood in the plain vacutainer was allowed to clot at room temperature (27 °C) and then centrifuged at 2,000 rpm for 10 min to separate serum from the whole blood. After testing for serum creatinine, serum iron and TIBC the remaining serum was pipetted and stored at −20 °C for serum ferritin estimation which was performed once a week.
The hematological tests were done using a Lablife H3D automated hematological analyzer-Diagnova, India. Quality check was performed daily as per the instrument protocol, before the samples were tested. Hemoglobin was measured using a method similar to cyanmethemoglobin method, RBC’s were measured using impedance method and MCV was estimated from RBC histogram. MCH (pg), MCHC (g/dl) and Hct (%) were calculated: [MCH (pg) = Hb (g/dl)/RBC (million/μl/mm3) × 10; MCHC (g/dl) = Hb (g/dl)/Hct (%) × 100; Hct (%) = RBC (million/μl/mm3) × MCV (fl)/10].
Serum Iron, serum TIBC and serum creatinine tests were done on Dimension® Xpanplus; Dade Behring, India. Serum Iron was measured using IRN method, which is an adaptation of direct iron assays developed by Smith et al. using the chromophore Ferene®. Serum TIBC was measured by proprietary IBCT method (Siemens Healthcare Diagnostics Inc. Newark, DE 19714, USA). Transferrin saturation (TS) was calculated using the following formula: Transferrin saturation (%) = (SI/TIBC) × 100. Serum creatinine was measured using the CREA method. Serum ferritin was measured using a two-site sandwich immunoassay using direct chemiluminometric technology, on AVIDA Centaur® CP Immunoassay system; Bayer Health Care, India.
Statistical analysis was done using STATA version 9.2 [8]. The independent t test was used to compare sex-specific hematological values and parameters of iron metabolism between the MIMS population and Non-Hispanic whites of age range 20–29 years from the US National Health and Nutrition Examination Survey (NHANES III) data [4].
Results
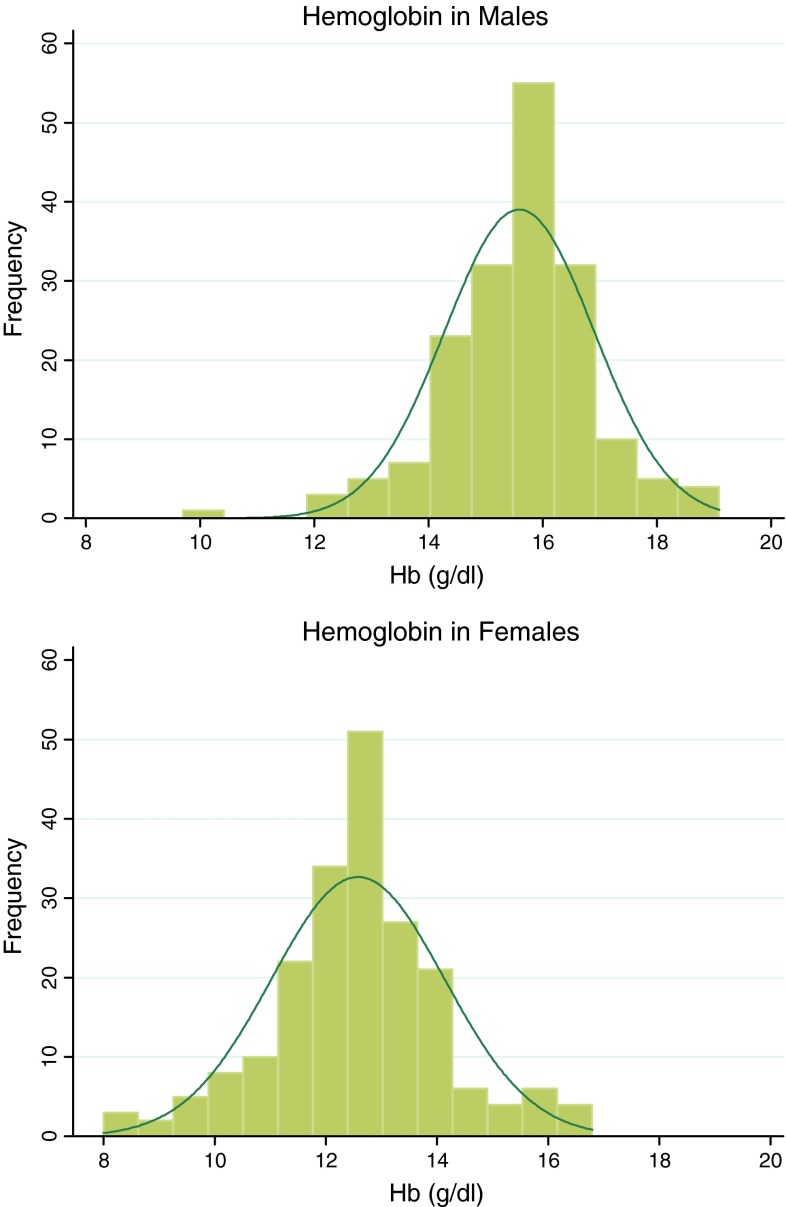
The age of the subjects ranged between 18 and 29 years with mean age of males being 22.3 ± 2.6 years and females 20.4 ± 2.0 years (Table 2). On an average, males were about 2 years older, taller (171 vs. 157 cm) and heavier (64.4 vs. 53.7 kg) when compared to females. However, there was no significant difference in BMI (21.9 vs. 21.7 kg/m2). Fifty-seven percent subjects of either sex had normal BMI and approximately 30 % of either sex were either overweight or obese (Table 3). Serum creatinine values were lower for females (0.7 ± 0.2 mg/dl) than for males (0.9 ± 0.1 mg/dl). The frequency distribution of Hb by sex is shown in Fig. 1. When compared to males, the curve for females had a lower peak and wider base. The mean, median and mode Hb values were 15.6, 15.7 and 15.5 g/dl for males, and 12.6, 12.6 and 11.9 g/dl for females, respectively.
Table 2.
Anthropometric values of the males and females
| Characteristic | Males (n = 177) | Females (n = 203) | p | ||
|---|---|---|---|---|---|
| Mean | 1 SD | Mean | 1 SD | ||
| Age (years) | 22.3 | 2.6 | 20.4 | 2.0 | <0.0001 |
| Height (cm) | 171 | 7.4 | 157 | 6.0 | <0.0001 |
| Weight (kg) | 64.4 | 12.3 | 53.7 | 8.6 | <0.0001 |
| BMI (kg/m2) | 21.9 | 3.5 | 21.7 | 3.2 | =0.56 |
Table 3.
Prevalence of BMI stratified by gender
| BMI (kg/m2) | Males (n = 177) | Females (n = 203) |
|---|---|---|
| Under weight (<18.5) | 20 (11.3 %) | 29 (14.3 %) |
| Normal weight (≥ 8.5 < 23) | 100 (56.5 %) | 115 (56.7 %) |
| Over weight (≥ 23 < 25) | 22 (12.4 %) | 30 (14.8 %) |
| Obese (≥25) | 35 (19.8 %) | 29 (14.3 %) |
Fig. 1.
Hemoglobin distribution; compared to males, hemoglobin distribution curve in females is shifted to left with a lower peak and broader base
The mean Hb (Table 4) of Indian males (15.6 ± 1.3 g/dl) was similar to white males (15.5 ± 0.9 g/dl). RBC and MCH were also similar. Hematocrit (Hct), MCV, and MCHC were significantly different but absolute differences were negligible. The lower 95 % cutoff value (Mean − 1.645 SD) of hemoglobin for males was calculated to be 13.5 g/dl (Table 1).
Table 4.
Comparison of parameters of RBC and iron metabolism: hyderabad (India) versus. American whites (NHANES III)
| Parameters | MIMS | NHANES III (Non-Hispanic whites) | ||
|---|---|---|---|---|
| Males (n = 177) | Females (n = 203) | Males (n = 381–384) | Females (n = 477–480) | |
| RBC (/mm3) | 5.17 ± 0.4a,c | 4.4 ± 0.4d | 5.11 ± 0.35f | 4.38 ± 0.35 |
| Hb (g/dl) | 15.6 ± 1.3a,c | 12.6 ± 1.6d,e | 15.5 ± 0.9f | 13.3 ± 1 |
| Hct (%) | 44.3 ± 3.2a,b,c | 36.4 ± 4d,e | 45 ± 2f | 39 ± 3 |
| MCV (fl) | 86 ± 6.1a,b,c | 83 ± 7.7d,e | 89.1 ± 3.6f | 89.9 ± 4 |
| MCH (pg) | 30.3 ± 2.6a | 28.7 ± 3.1d,e | 30.4 ± 1.4 | 30.4 ± 1.5 |
| MCHC (g/dl) | 35.2 ± 1.2a,b,c | 34.5 ± 1.7d,e | 34 ± 0.8f | 33.8 ± 0.8 |
| Serum ferritin (ng/ml) | 68.6 ± 47.6a,b,c | 21.9 ± 22.3d,e | 130.2 ± 77.9f | 45.1 ± 35.8 |
| Serum iron (μg/dl) | 96 ± 40a,b | 59.7 ± 34d,e | 108.6 ± 42.7f | 97.1 ± 46.5 |
| Transferrin saturation (%) | 26.7 ± 12.3a,b | 15.5 ± 10.1d,e | 31.5 ± 13.3f | 25.7 ± 12.5 |
| TIBC (μg/dl) | 371 ± 56a,b,c | 411 ± 64d,e | 351.3 ± 49.3f | 383.8 ± 64.8 |
| Serum creatinine (mg/dl) | 0.9 ± 0.1a,b,c | 0.7 ± 0.2d,e | 1.14 ± 0.08f | 0.93 ± 0.08 |
P < 0.03
aIndian males versus Indian females
bIndian males versus American males
cIndian males versus American females
dIndian females versus American males
eIndian females versus American females
fAmerican males versus American females
Indian females (Table 4) had lower values for all parameters of RBC when compared to Indian males. However, when compared to American white females, the RBC count was same but mean Hb was 0.7 g/dl lower. Consequently, MCH was lower in Indian females (28.7 vs. 30.4 pg). MCV was lower by 7 points and, therefore, Hct was also lower by 3 points. Although Hb and Hct were both lower, MCHC was higher, but absolute differences were negligible. The Cutoff value (Mean − 1.645 SD) for considering anemia in Indian females was calculated to be 10 g/dl (Table 1).
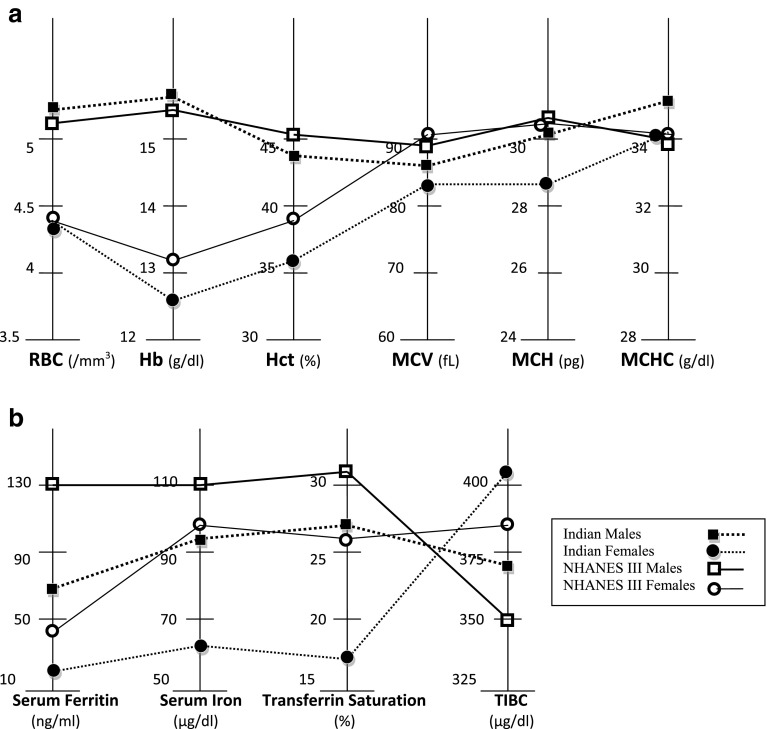
Parameters of iron metabolism, serum ferritin, serum iron, transferrin saturation, were significantly lower and TIBC was significantly higher in Indians than in American whites. Values of serum iron and transferrin saturation in Indian males were as low as American females (Fig. 2). Indian males and females had lower serum creatinine than American white males (0.9 vs. 1.14 mg/dl) and females (0.7 vs 0.93 mg/dl) (Table 4).
Fig. 2.
Comparison between South Indian’s and American whites (NHANES III, USA). a Parameters of red blood cell, b parameters of iron metabolism
Indian females when compared to Indian males and American white females had significantly lower values for all parameters of iron metabolism except TIBC which was significantly elevated (Fig. 2). Serum creatinine was also lower in Indian females when compared to American white females (0.7 vs. 0.93 mg/dl) (Table 4).
Discussion
Although selection of the medical students was opportunistic, it also provided young and well educated subjects belonging to middle or upper socioeconomic class as evidenced by prevalence of overweight and or obesity in one-third of the subjects. Selection minimized the effects of nutritional deficiency which is common to low income group countries and degenerating diseases of advancing age on Hb and other related parameters of interest.
In our male subjects (18–29 years), the mean hemoglobin value of 15.6 was similar (15.5 g/dl) to American white males (20–29 years), and just marginally higher than the IOM value (15.3 g/dl) in the NHANES III population which included older individuals (20–49 years) representing all ethnic groups. The mean Hb in males in the current study was about 1 g/dl higher than the value for the Southeast Asian Nation, Vietnam (14.7 g/dl) and 1.3 g/dl higher than the value obtained by Western Indian study (14.3 g/dl). The difference may not necessarily represent true geographical variation but may be related to differences in the sample selection. The Western Indian study was based upon healthy subjects of all ages selected from health checkup program of the Hospital. Since Hb is stable for adults between age 20–59 years for males and 20–49 years for females but deviates from the adults on either end of the spectrum [12], our values derived from subjects aged 18–29 years are valid for adults. The lower mean values in men obtained from Western Indian study may be partly due to inclusion of those age groups who tend to have lower Hb than adults—younger than 20 and older than 49 years [4].
The statistical definition for lower limit of normal hemoglobin varied from study to study. The cutoff value of 13 g/dl (males) for lower limit of hemoglobin set by WHO was rather arbitrary and without statistical basis., while the 13.7 g/dl by IOM was derived as mean − 1.645 SD, and 12.3 g/dl by the Western Indian study was derived as mean − 2 SD. The corresponding cutoff values arrived at in the current study for males were 13 g/dl (Mean − 2 SD) and 13.5 g/dl (Mean − 1.645 SD). We prefer the later definition considered by the IOM, which represents 5th percentile. Our value of 13.5 g/dl closely approximates the value of 13.7 g/dl recommended by the IOM for American male adults but 0.5 g/dl higher than the WHO criteria for adult males. Prevalence of anemia in male medical students of the study is 5 % by our definition, but as per the WHO criteria it calculates to be 4 and 5.6 % by the IOM criteria. Thus, estimates of anemia rates based on the WHO criteria minimally underestimate and the IOM criteria overestimate prevalence of anemia in male medical students. Therefore, the prevalence of anemia in Indian males, of 24 % nationally and 23 % in the state of Andhra Pradesh, as estimated by NFHS-3 study using the WHO criteria, is indirectly validated by our study.
It is intriguing that in spite of Hb values comparable to American whites, serum values for parameters of iron metabolism were lower in Indian males. Differences in laboratory techniques may contribute to this difference. But we are also intrigued by lower creatinine in Indian males compared to American white males. Lower creatinine levels may indicate lower intake of protein and iron or low muscle mass, which is known to adversely affect Hb levels, particularly in the elderly [9].
Indian females had the same number of RBC as American white females, but the RBC were smaller in size with a corresponding decrease in MCH and lower hemoglobin. Thus Indian females RBC’s were microcytic but normochromic. The mean hemoglobin of Indian females, when compared to American white females was 0.7 and 0.9 g/dl lower than the NHANES III and IOM values respectively. For our female subjects, mean Hb was very close to values obtained from Vietnamese and Western Indian females. The cutoff value of 10 g/dl derived from our study is 2 g/dl lower than the WHO and IOM criteria and approximately 1 g/dl less than the values suggested by Vietnam and Western Indian studies. As per the statistical definition of cutoff value, the prevalence of anemia in female medical students is 5 %, but using the WHO and IOM criteria it calculates to be 33.5 %, which suggests 28.5 % (33.5–5 %) overestimation using the latter criteria. Our data thus suggests that, the NFHS-3 might have overestimated prevalence of anemia in Indian females by 28.5 %. The true prevalence may be only 26.5 % (55−28.5 %) nationally and 34.5 % (63–28.5 %) statewide. The true prevalence of anemia 26.5 % thus calculated nationally in females closely approximates to prevalence in males (24 %) and the gross differences between the two sexes disappear. These markedly lower estimates of prevalence of anemia in females are similar to what was observed in Vietnam. Since treatment for Hb between 8–12 g/dl, anemia by WHO criteria, improves Hb but not symptoms in non-pregnant women, an unduly high cutoff value inappropriate for a community may exaggerate the prevalence of anemia in a community without any obvious benefit [10].
It is worth speculating why Indian women have a lower Hb level and iron parameters. It has been a conventional wisdom to dismiss lower Hb values in females compared to males due to menstrual loss. There has never been any evidence for this presumption. Close examination of NHANES III age specific data for parameters of iron metabolism and RBC suggests that Hb remains relatively constant in women throughout the life, but lower than men after puberty, irrespective of menstrual phase. Neither menstruation decreases Hb nor menopause increases it. On the other hand puberty induces increased muscle mass in men with concomitant increases in parameters of iron and Hb. Therefore it is possible that low muscle mass, as evidenced by lower serum creatinine levels than american white females, may at least partially explain the low iron and hemoglobin levels observed in the female medical students [11].
Although the sample size is taken from a single medical institute situated in one corner of Hyderabad, students come from all directions of the state. Many parents of the students are immigrants from other districts of Andhra Pradesh with a population of 84.5 million. Thus students of the study represent microcosm of the ‘well to do’ population of Andhra Pradesh. However, similar studies should be conducted in different regions of the state and across India to increase our knowledge of Hb distributions in the Indian population and subpopulations.
In conclusion, male subjects from a medical college in state of Andhra Pradesh, India, have Hb values similar to American adult males, but non-pregnant females from the same medical college have considerably lower Hb levels than American non-pregnant females, raising the question about the appropriateness of the WHO or US criteria for detection of anemia in adult non-pregnant female in India. Further research needs to be conducted from various regions of the state and India to investigate relevance of these observations to various subpopulations of India or to generalize them to the state or the country.
Acknowledgments
Research reported in this publication was conducted by scholars in the Fogarty International Center of the National Institutes of Health training program under the Award Number D43 TW 009078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank all the research laboratory staff at MediCiti Institute of Medical Sciences and mentoring of Clareann H. Bunker, Ph.D, Raj Warrier, MD, C. Venkata S. Ram, MD, Balasubramanian K, Ph.D, Sathyanarayana P, MD, Saurabh Malhotra, MD, MPH. This research work was internally supported by the MediCiti Institute of Medical Sciences and SHARE India.
Conflict of interest
The authors declare that they do not have any conflict of interest.
References
- 1.WHO Tech Rep Ser. 1959;182:4. [PubMed]
- 2.Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed]
- 3.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of Iron Deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 4.Hematological and Iron-Related Analytes—Reference data for persons aged 1 year and over: United States, 1988–94, vital & health statistics, series 11, number 247, March 2005. [PubMed]
- 5.Yip R. Final report of the 1995 Viet Nam National Nutrition Anemia and Intestinal Helminth Survey: a recommended plan of action for the control of iron deficiency for Viet Nam. Jakarta, United Nations Children’s Fund [Indonesia], 1996.
- 6.Ashavaid TF, Todur SP, Dherai AJ. Establishment of reference intervals in Indian population. Indian J Clin Biochem. 2005;20:110–118. doi: 10.1007/BF02867409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005–06: India: Volume I. Mumbai: IIPS. 2007.
- 8.Stata 9.2, Stata Corp LP, 4905 Lakeway Drive, College Station, Texas 77845, USA.
- 9.Frisoli A, Jr, Chaves PH, Pinheiro MM, Szejnfeld VL. The effect of nandrolone decanoate on bone mineral density, muscle mass, and hemoglobin levels in elderly women with osteoporosis: a double-blind, randomized, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2005;60(5):648–653. doi: 10.1093/gerona/60.5.648. [DOI] [PubMed] [Google Scholar]
- 10.Elwood PC, Wood MM. Effect of oral iron therapy on the symptoms of anaemia. Br J Prev Soc Med. 1966;20:172–175. doi: 10.1136/jech.20.4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guralnik JM, Ershler WB, Schrier SL, Picozzi VJ. Anemia in the elderly: a public health crisis in hematology. Hematol Am Soc Hematol Educ Program. 2005:528–32. [DOI] [PubMed]
- 12.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]