Abstract
Effects of Cu2+, Ni2+ or Cu2+ + Ni2+ on lipid peroxide and glutathione (GSH) levels in U937 cells were investigated. Cells were treated with 0, 5,10, and 20 µM of Cu2+ and/or Ni2+ and H2O2 (0.01 mM) and incubated for 24 hours at 37°C. Lipid peroxides were measured by the thiobarbituric acid assay (TBA). GSH intracellular levels were assayed by the GSH assay kit from EMD/Calbiochem (San Diego, California, USA). Cu2+ or Ni2+ significantly (P < 0.01) increased lipid peroxides in a dose-dependent manner, compared to controls. The effect was more pronounced for Cu2+, compared to the Ni2+-treated samples. Cu2+ + Ni2+ increased lipid peroxides in a significant (P < 0.001), dose-dependent manner, compared to Cu2+ or Ni2+ alone (i.e., ratio of 2.5:1-fold for combined versus single treatments, respectively). Cu2+ or Ni2+ significantly decreased GSH levels in U937 cells, with the effect being pronounced for Cu2+. Cu2+ + Ni2+ metal ions significantly (P < 0.001) depleted cells of GSH in a dose-dependent manner. Ethylene diamine tetraacetic acid (EDTA) at 50 or 100 µM moderately reduced the Cu2+- or Ni2+-induced effects on GSH levels. Interestingly, GSH levels generally decreased to half (except for the combined metal dose of 20 µM at 100 µM EDTA) of its level at the highest metal concentration tested for both the single or combined treatments. In conclusion, multiple exposures of cells to metal ions may be lethal to cells, compared to their single treatments.
Keywords: Copper, glutathione, lipid peroxides, nickel, thiobarbituric acid assay, U937 cells
Introduction
Glutathione (GSH) is an important neuronal antioxidant and is critical for the detoxification of H2O2 and prevention and repair of peroxidative damage to lipids, proteins, and nucleic acids (Bains and Shaw, 1997). It is a tripeptide of glutamine, cysteine, and glycine, synthesized by glutathione synthase. The glutamine is bound through the gamma-carboxyl group. GSH exists in a reduced (GSH) and oxidized (GSSG) form, but the reduced form is, by far, the predominant species in healthy cells (Wataha et al., 2000) (500:1). The cell regenerates GSH from GSSG using reduced nicotinamide adenine dinucleotide phosphate and the enzyme, glutathione reductase. It also has important roles in maintaining the intra- and extracellular redox environments and modulating intracellular transport of copper (Cu2+) into metalloproteins immediately after uptake, preventing toxicity from unbound intracellular redox active Cu2+ (White and Cappai, 2003). When the ratio of GSH to GSSG drops, it serves as one important indicator of oxidative stress in cells (Lakritz et al., 1997). For example, GSH depletion can activate neuronal 12-lipoxygenase (12-Lox), which, in turn, generates increased intracellular peroxide levels through catabolism of arachidonic acid (Li et al., 1997). The subsequent oxidative damage from H2O2 and hydroxyl radicals (OH) may play an important role in neuronal dysfunction and/ or death in neurodegenerative diseases. Thus, GSH plays a vital role in combating oxidative stress in cells.
Studies by Li et al. (1993) have indicated thatincubation of nickel (Ni2+) with cultured 3T3 cells resulted in a dose-dependent decrease in cytoskeletal protein sulfhydryls as well as cellular GSH content. Further, aggregation of microtubules was found to occur in the above cells in the presence of Ni2+, which was believed to be the result of sulfhydryl oxidation with the formation of disulfide bonds between individual microtubular polymers. These studies support the role of oxidative mechanisms in the cytotoxicity of Ni2+. Studies by White and Cappai (2003) indicated that the neurotoxic effects of Cu2+ in GSH-depleted neurons involved the generation of Cu+ and subsequent free-radical–mediated oxidative stress.
Although many of the studies on the effects of Ni2+ and Cu2+ on GSH levels have involved the use of single dose-dependent treatments (i.e., for each of the above metals), their combined dose-dependent effects on the modulation of lipid peroxides and in relation to cellular contents of GSH are nonexistent. The hypothesis for the current work was that the combined metal doses deplete more GSH, compared to either metal alone. To test this hypothesis, we treated U937 cells with low doses of 0, 5, 10, and 20 µM (i.e., considering the cumulative, ubiquitous nature and the variable dietary intake in humans of nickel and copper to be averaging approximately 200–300 and 1,000–1,600 µg/day, respectively, (Grandjean, 1984; Georgopoulos et al., 2006) of Cu2+, Ni2+, or Ni2+ + Cu2+ and measured lipid peroxides and GSH levels. U937 is an erythroid leukemia cell line that can be induced to differentiate toward erythrocytes. These cells are easy to culture and have been used in a number of biochemical studies involving signal transduction and gene expression (Adunyah et al., 1997; Subramaniam et al., 1999). The effects of two doses of ethylene diamine tetraacetic acid (EDTA) were also tested to determine whether chelation of extracellular Ni2+ and Cu2+ prevent the loss of intracellular GSH in these cells. The studies are relevant, considering the essential role of GSH in cellular mechanisms and the relative abundance of these metals in the environment and their toxicities, including neurotoxicity, hepatoxicity, and nephrotoxicity (Stohs and Bacchi, 1995).
Methods
Chemicals
NiCl2.6H2O, H2O2, CuCl2.2H2O, and Na-EDTA were purchased from Fisher Scientific (Suwanee, Georgia). All chemicals were of high purity (>99%), according to manufacturer’s instruction, and were used without further purification.
Treatment of U937 cells with metals
NiCl.6H2O, CuCl2.2H2O, and H2O2 solutions were prepared fresh for every treatment using degassed argon and doubly deionized water. U937 cells, obtained from American Type Culture Collection (ATCC; Manassas, Virginia, USA), were maintained at 37°C under a 5% CO2 atmosphere in RPMI 1640 medium containing 10% fetal bovine serum and 50 U/mL each of penicillin and streptomycin (Subramanian et al., 1999). Cells were treated with 0, 5, 10, and 20 µM of Ni2+ and/or Cu2+ and H2O2 (0.01 mM) (Boadi et al., 2005) and incubated for 24 hours at 37°C. Control incubations contained all reagents except the metals.
Sample preparation and analysis of GSH in cells
After incubation, control cells and the treatment groups were pelleted by low-speed centrifugation (2,300 × g). Cells were then washed twice with medium to get rid of any loosely bound metal ions, and the cell pellets were resuspended after the washes in 500 µl of a 5% meta-phosphoric acid (MPA) and then lysed by trituration (30 passages using a 26-gauge syringe). The lysate was centrifuged at 3,000×g for 10 minutes at 4°C. The resultant supernatant was used for the analysis of GSH levels as described by the manufacturer’s instruction for the GSH assay kit from (Cat. No. 354102; EMD/Calbiochem, San Diego, CA), with the following modifications. To an aliquot (100 µl) of the supernatant, the volume was adjusted to 900 µl with a buffer solution [200 mM of potassium phosphate, pH 7.8,25°C, containing 0.2 mM of diethylene triamine pentaacetic acid (DTPA) and 0.025% lubrol]. One hundred microliters of a 12-mM solution of chromogenic reagent in 0.2 N of HCl (reagent 1) was added and mixed, followed by the addition of 100 µl of a 30% NaOH solution (reagent 2). Samples were incubated at 25°C for 30 minutes in the dark, after which absorbance was measured at 400 nm in a spectrophotometer (Spectronic D601). Reduced GSH levels were calculated from a standard curve using GSH freshly prepared in 5% MPA as the standard. Levels of GSH were expressed as nmoles of GSH/106 cells (Rimbach et al., 2001).
Effect of EDTA on GSH in U937 cells
Experiments were also conducted to test the effect of the addition of EDTA (a metal chelator) in the presence of these metal ions on GSH levels in U937 cells. Cells were treated as described above and in the presence of either 50 or 100 µM of EDTA (Li et al., 2009) and incubated for 24 hours at 37°C. Control incubations contained all reagents except the metals. After incubation, levels of GSH in samples were analyzed as previously described.
Analysis of lipid peroxides in U937 cells
Lipid peroxides were analyzed by the thiobarbituric acid (TBA) assay in samples, following the treatments as previously described (Boadi et al., 2005), with some slight modifications described below. Cells in a 1.5 × 106/well concentration from the various treatment groups were washed three times in RPMI 1640 medium, as described above, to remove residual metals before the analysis of lipid peroxides.
Cu2+, Ni2+, Cu2+ + Ni2+, or H2O2 effects on cell growth and viability in U937 cells
Cells were treated in separate experiments to determine whether H2O2(0.01 mM), Ni2+, and/or Cu2+ were affecting cell growth and viability that might compromise lipid peroxide and GSH levels. Cells (2.2 × 106) were incubated with H2O2, Cu2+, Ni2+, or Cu2+ and Ni2+, as described above. Viability (measured by the trypan blue exclusion test) and cell number, determined by cell counting using a Neubauer improved hemocytometer, after the addition of the reagents under investigation. In a separate experiment, effects of the reagents on the levels of GSH were investigated. Cells (1.8 × 106) were incubated as previously described with each of the reagents and in different combinations. Untreated samples (i.e., cells containing none of the above reagents) were also analyzed to determine basal levels of GSH before treatments.
Statistical analysis
Two-way analysis of variance (metal concentration and lipid peroxide or GSH and interaction) was used to compare mean lipid peroxide levels as TBA reactive substances and GSH in U937 cells were subjected to the different treatments. The Student’s t-test was used to determine statistical significance. Difference was designated as significant when P < 0.05. Each value in all figures represents the mean of four different experiments for each dose level of metal tested, which was assayed in triplicates.
Results
Cell growth and viability
Cell number was determined before and after treatments with the reagents. Results indicate that none of the treatments affected cell growth and viability (Table 1) before the GSH and TBA assays. GSH levels in untreated and treatments with the individual regents were not different from each other (Table 2).
Table 1.
Effects of single and combined metal ions and H2O2 on U937 cell number and viability.
| Reagent | Viability (%)a | Cell no. (107well)b |
|---|---|---|
| Ni2+ at (20µM) | 96.4 ± 0.3 | 5.4 ± 0.24 |
| Cu2+ at (20µM) | 97.2 ± 0.4 | 5.7 ± 0.26 |
| Ni2+ + Cu2+ at (5 µM) | 95.4 ± 0.2 | 5.5 ± 0.15 |
| Ni2+ + Cu2+ at (10 µM) | 98.2 ± 0.2 | 5.3 ± 0.15 |
| Ni2+ + Cu2+ at (20 µM) | 97.1 ± 0.5 | 5.7 ± 0.56 |
| H2O2(0.01mM) | 95.6 ± 0.8 | 5.2 ± 0.25 |
U937 cells were incubated at a density of 1.8 × 106 cells/well. Each table in this and other tables in this article represent means for four different experiments for each dose level of metal tested, which was assayed in triplicates.
Viability (measured by trypan blue exclusion) was determined before (96.8 ± 0.25%)
cell number (i.e., growth) after incubation with the above reagents at 37°C for 24 hours.
Table 2.
Levels of GSH in untreated and treated U937 cells with the individual reagents.
| Reagent | GSH level (nmoles/106 cells)a |
|---|---|
| Untreated cells | 10.38 ± 0.42 |
| H2O2(0.01mM) | 9.76 ± 0.21 |
| Ni2+ at (20µM)b | 10.15 ± 0.52 |
| Cu2+ at (20µM)b | 8.30 ± 0.71 |
| Ni2+ + Cu2+ at (5 µM) | 7.99 ± 0.23 |
| Ni2+ + Cu2+ at (10 µM) | 9.21 ± 0.15 |
| Ni2+ + Cu2+ at (20 µM) | 10.20 ± 0.44 |
U937 cells were incubated at a density of 2.1 × 106 cells/well. GSH levels were analyzed after incubation without and with the above individual reagents at 37°C for 24 hours.
GSH levels for the different doses of reagents employed were not different from each other; hence, the values for the high doses were reported for the single metal ions.
Effects of Cu2+, Ni2+, and Cu2+ + Ni2+ on lipid peroxides in U937 cells
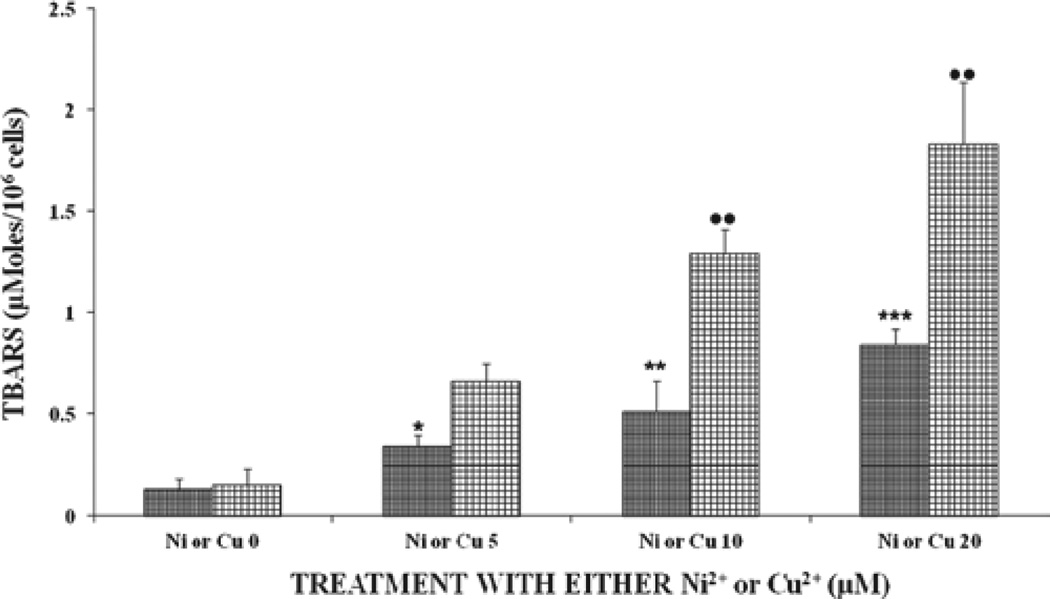
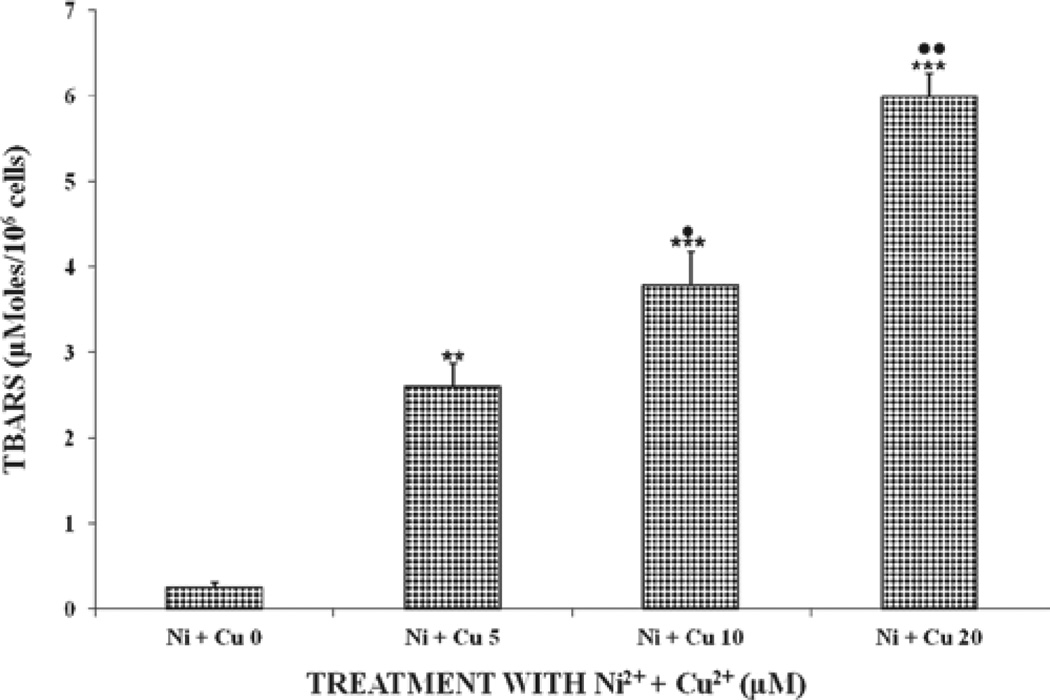
Figure 1 shows the oxidative damage of the two metal ions in U937 cells. Results show that both metal ions increased lipid peroxides in U937 cells in a significant (P < 0.01), dose-dependent manner, with the effect being more pronounced with the Cu2+-treated samples. U937 cells treated Ni2+ + Cu2+ showed increased lipid peroxide levels that were statistically significant (P < 0.001), compared to treatment with either cation alone. Based on the results for Figures 1 and 2, lipid peroxides for the combined treatments increased by ratios of 0.86,2.70,2.25, and 2.60, respectively, for all the tested doses for the combined versus single treatments. The above results indicate that the combined metal ions Ni2+ + Cu2+ were better inducers and showed increased potentiating effects of lipid peroxides levels in cells receiving these metal ions. Therefore, it appears that there is a synergy between Ni2+ + Cu2+ for inducing oxidative damage in U937 cells.
Figure 1.
Effects of different doses of either Ni2+-or Cu2+-induced oxidative damage in U937 cells after incubation at 37°C for 24 hours. Each bar chart ± standard error in this and other figures in this article represent mean for four different experiments for each dose level of metal tested, which was assayed in triplicates. Statistical significances denoted by dot or asterisk symbols are shown as a. comparison between each control subgroup without metal ions (i.e., Ni or Cu) and its treated subgroup for the respective metal ions (i.e., Ni2+ or Cu2+ + 5, Ni2+ or Cu2+ + 10, and Ni or Cu2+ + 20). • or *P < 0.05; •• or **P < 0.01; ••• or ***P < 0.001 in this and other figures. Vertical bars in this and other figures denote standard deviation. The x-axis labels for Figure 1 are defined as follows: Ni2+ or Cu2+ + 0 means control samples were not treated with Ni2+ or Cu2+ ions; Ni2+ or Cu2+ + 5, 10, and 20 means samples were treated with either Ni or Cu2+ ions at 5, 10, and 20 µM, respectively.
Figure 2.
Effects of the combination of Ni and Cuions induced oxidative damage in U937 cells after incubation at 37°C for 24 hours. For statistical significance denoted by dots or asterisks, see legend to Figure 1. The x-axis labels for Figure 2 are defined as follows: Ni2+ + Cu2+ at 0 means control samples were not treated with Ni2+ and Cu2+ ions; Ni2+ + Cu2+ at 5, 10, and 20 means samples were treated with Ni2+ + Cu2+ ions at 5,10, and 20 µM, respectively.
Effects of Cu2+, Ni2+, and Cu2++Ni2+ on GSH in U937 cells
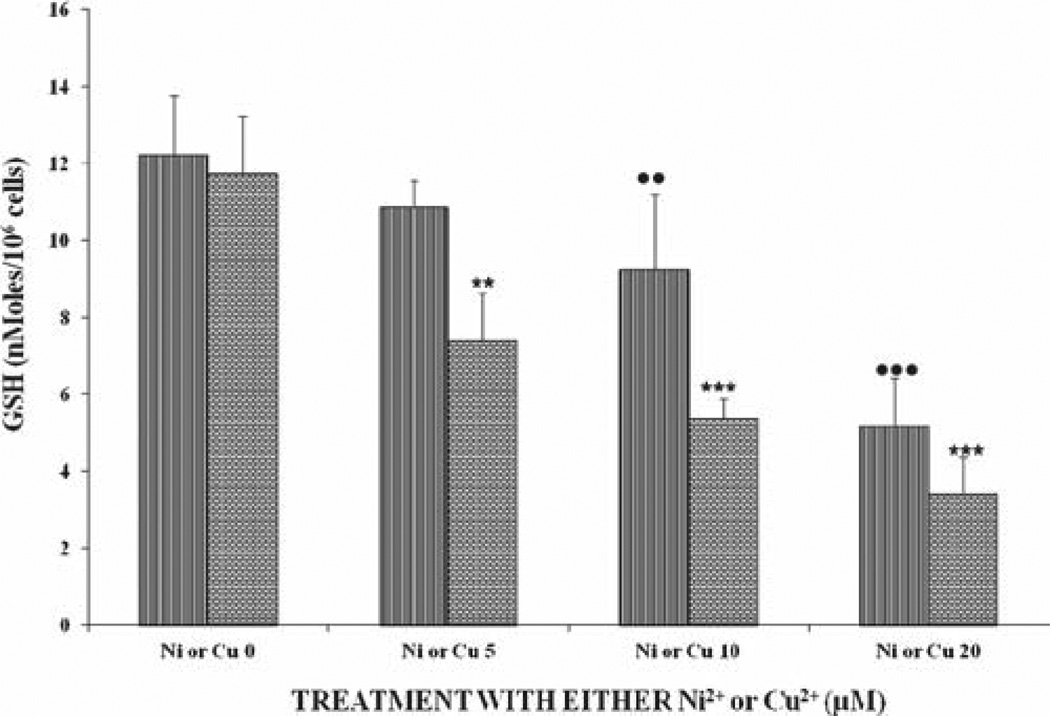
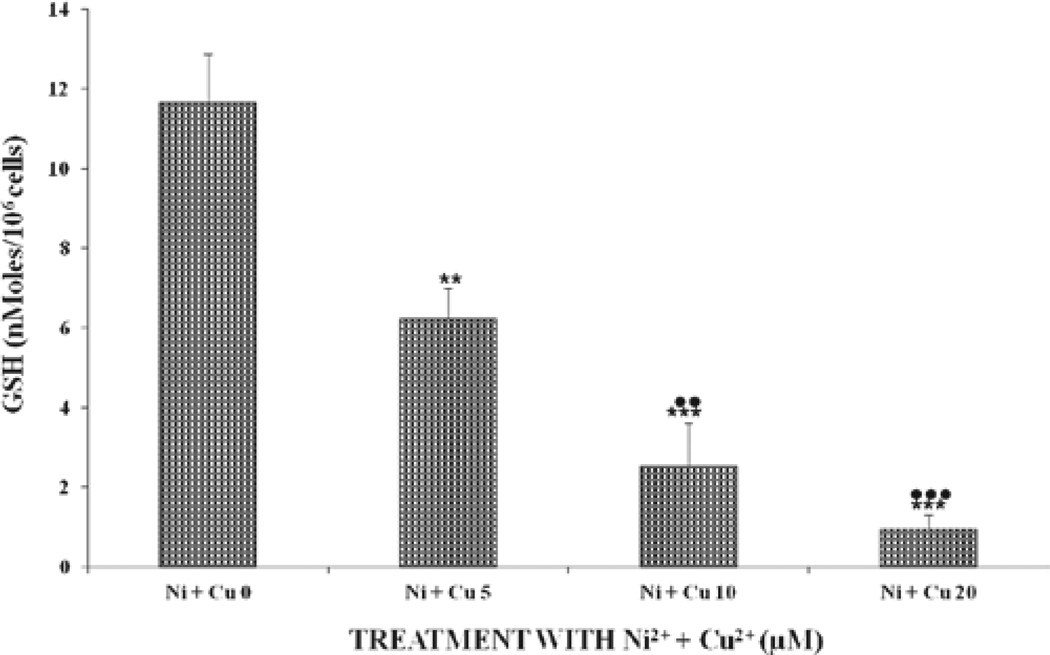
Figure 3 shows the levels of GSH after exposure to either Ni2+ or Cu2+ ions. There was a gradual (for doses of 5 and 10 µM) dose-dependent decrease in GSH levels for the Ni2+- and Cu2+-treated samples. However, the effect of Cu2+ on GSH was statistically significant both at the 5-, 10-, and 20-µM levels within the same group and was also significantly (P < 0.01) different, compared to those of the Ni2+-treated samples. GSH levels decreased in a significant (P < 0.001), dose-dependent manner for cells receiving the combined metal ions (Figure 4). Levels of GSH in cells at the 5-µM dose were reduced by 50%, compared to the control cells (Figure 4). Again, a similar trend in intracellular GSH-level reduction was observed, on comparison between the combined doses and either metal alone. At the combined dose of 20 µM, Ni2+ + Cu2+ cells were almost devoid of GSH (at 0.97 nmoles/106 cells). GSH was reduced by 3.2, 5.3, and 10.3 for the combined doses, compared to the sum of GSH levels for the single treated metal ions.
Figure 3.
Effects of different doses of either Ni2+ or Cu2+ on GSH levels in U937 cells after incubation at 37°C for 24 hours. For statistical significance denoted by dots or asterisks and definitions of the x-axis labels, see legend to Figure 1.
Figure 4.
Effects of the combination of Ni2+ and Cu2+ ions on GSH levels in U937 cells after incubation at 37°C for 24 hours. For statistical significance denoted by dots or asterisks and definitions of the x-axis labels, see legend to Figure 2.
Effects of Cu2+, Ni2+, and Cu2+ + Ni2+ chelation by EDTA on GSH in U937 cells
Table 3 shows the effects of two low doses of EDTA (i.e., 50 and 100 µM, respectively) on its ability to chelate Ni2+ and Cu2+ ions and its consequent effect on GSH levels after either the single or combined metal treatments. There were no significant differences between the levels of GSH for either the Ni2+ or Cu2+ doses tested and in the presence of 50 µM of EDTA. However, EDTA at 100 µM was a better chelator for Ni2+ ions, compared to Cu2+, as evidenced by the decreased and significant differences (P < 0.05) in GSH at 10–20 µM for either metal ion. EDTA at 100 µM resulted in a slight, but not significant, increase in GSH levels, compared to 50 µM for the combined metal doses from 0 to 10 µM. However, there was a significant (P < 0.01) decrease in GSH (3.9 nmole/106 cells) for the 100-µM EDTA, compared to the 50-µM, at 5.2 nmole/106 cells for the combined metal treatments. It is very important and interesting to note that GSH levels generally decreased (P < 0.05) in a dose-dependent manner within each column for either the single or the combined metal treatments for the two doses of EDTA tested.
Table 3.
Effect of EDTA on GSH in U937 cells.
| Ni2+ (µM) | EDTA (50 µM) | EDTA (100 µM) | Cu2+ (µM) | EDTA (50 µM) | EDTA (100 µM) | Ni2+ + Cu2+(µM) | EDTA (50 µM) | EDTA (100 µM) |
|---|---|---|---|---|---|---|---|---|
| 0 | 11.4 ± 0.3 | 10.4 ± 0.3 | 0 | 10.7 ± 0.3 | 11.8 ± 0.3 | 0 | 12.6 ± 0.3 | 11.4 ± 0.3 |
| 5 | 8.2 ± 0.4* | 10.2 ± 0.4 | 5 | 9.2 ± 0.4 | 8.2 ± 0.4 | 5 | 8.2 ± 0.4* | 9.2 ± 0.4 |
| 10 | 6.8 ± 0.2** | 8.4 ± 0.2* | 10 | 7.7 ± 0.2* | 6.4 ± 0.2** | 10 | 6.4 ± 0.2** | 7.4 ± 0.2** |
| 20 | 5.2 ± 0.2** | 6.7 ± 0.2** | 20 | 5.8 ± 0.2** | 5.0 ± 0.2** | 20 | 5.2 ± 0.2** | 3.9 ± 0.2** |
U937 cells were incubated at a density of 1.8 × 106 cells/well. GSH (nmole/106 cells) levels were measured after incubation with the above reagents in the presence of 0.01 mM of H2O2 at 37°C for 24 hours. Statistical significances denoted by asterisk symbols are shown as a comparison between each control subgroup without metal ions (i.e., Ni or Cu) with treatment with EDTA at 50 or 100 µM and its treated subgroup with EDTA for the respective metal ions (i.e., Ni2+ or Cu2+ + 5, Ni2+ or Cu2+ + 10, and Ni2+ or Cu2+ + 20).
P < 0.05;
P < 0.01;
P < 0.001.
Discussion
Many studies have reported metal-induced toxic and carcinogenic effects in humans and animals (Valko et al., 2005). The best evidence supporting the hypothesis of the oxidative nature of metal-induced genotoxic damage is provided by the wide spectrum of nucleobase products formed from the attack of reactive oxygen species (ROS) on DNA in cultured cells and animals exposed to carcinogenic metals (Valko et al., 2005). Further, though other studies (Cartaña et al., 1992; Misra et al., 1990) have reported reduced GSH levels with the concomitant increased lipid peroxides in rat hepatocytes after exposure to Cu+ and Ni+ ions, such studies in transformed cells, such as U937 cells, are nonexistent.
In this article, we present evidence that Cu2+ and Ni2+ ions can also cause increased lipid peroxides and decreased GSH levels in U937 cells. It has been proposed that in the presence of H2O2, these metal ions generate hydroxyl-radical-like species through the Fenton-type reactions, which may result in the degradation of proteins, nucleic acids, or the peroxidative decomposition of polyunsaturated fatty acid (PUFA) (Tamura et al., 1991; Stinson et al., 1992; Minotti, 1993; Kennedy et al., 1997; Valko et al., 2005). The results indicate that neither single nor combinations of reagents at the various concentrations affected cell growth and viability (Table 1). Second, background levels of GSH for the control, as well as the single and combined reagent, treatments were not significantly different from each other (Table 2). Experimental reagents, such as Cu2+, Ni2+, and H2O2, increased the formation of lipid peroxides, as measured by the TBA assay (Duthie et al., 1997).
Cu2+ and Ni2+ are environmentally important metals that appear to play a role in the organization of the nuclear matrix (Kennedy et al., 1997). It has been proposed that in the presence of H2O2, these metal ions generate hydroxyl-radical-like species through Fenton-type reactions (Stinson et al., 1992; Valko et al., 2005):
Ni2+ + H2O2 → Ni3+ + OH− + ·OH
Cu (II)+ H2O2 → Cu (I) + H2O + H+
Cu (I) + H2O2 → Cu (II) + OH− + ˙OH
The resulting hydroxyl-radical–like species may result in the degradation of proteins and nucleic acids or peroxidative decomposition of PUFA (Kennedy et al., 1997).
U937 cells incubated for 24 hours with either Cu2+ or Ni2+ and oxidized through Fenton’s pathway resulted in significant increases in lipid peroxides, confirming the generation of these hydroxyl-like reactive species, causing the increases in the amounts of lipid peroxides as has previously observed by us and others (Boadi et al., 2003, 2005; Valko et al., 2005). It is interesting to note that Cu2+ increased lipid peroxides more, compared to that of Ni2+. Again, the combination of metals at the respective doses increased lipid peroxides, suggesting the effectiveness of the combined metal ions in increasing lipid peroxides over the single treatments, which may exacerbate the oxidative damage in those cells in trying to cope with the oxidative stress. Such a finding is very unique, considering the limited information in the literature on the detrimental effects of combined exposure to these metals and the numerous diseases that have been attributed to oxidative stress (Duthie et al., 1997; Kennedy et al., 1997; Valko et al., 2005). Thus, in the presence of the combined metals, there was a synergistic effect of oxidative stress (Figure 2), which overwhelmed the natural antioxidative capabilities as a result of the increased generation of radicals and the inability of these cells to cope with the damaging effects of these metals. However, the differences in the increased lipid peroxides generated by Cu2+ (Boadi et al., 2003, 2005) over that of Ni2+ may be a result of differences in the abilities of these metals in generating ROS, leading to the formation of lipid peroxides. Bal and Kasprzak (2002) have reported that Ni2+ produces low, but measurable, levels of free radicals in cells. Second, experimental data suggest that oxidative stress may be important in Ni2+-induced carcinogenesis; however, a direct correlation between the ability of Ni2+ to produce oxidative stress and carcinogenicity is not yet fully understood. This observation could explain the observed differences in lipid peroxides between the singly Cu2+- and the Ni2+-treated samples. Thus, in terms of the metal-induced generation of ROS, reports indicate that free radicals have most significantly been evidenced for Cu2+ and iron (Fe2+) (both essential elements) than for Ni2+, Cr2+, and Cd2+, all three well-known carcinogenic metals (Stohs and Bacchi, 1995). We have also shown, in this study, that levels of lipid peroxides increased in a dose-dependent fashion between the combined metals, compared to the single treatments. This could be a result of the ability of the cells to use the free intracellular Cu+ ions, which are not used in the physiological activation of metalloenzymes. Such intracellular free Cu+ and the additive effect of the Ni2+ ions might have caused an increase in the levels of lipid peroxides for the combined metals (Stohs and Bacchi, 1995).
To elucidate the relationship between the formation of lipid peroxides and the antioxidant potential of the U937 cells, we also measured the levels of GSH, a natural antioxidant, in cells. Our results indicate that there was a gradual (for doses of 5 and 10 µM), but dose-dependent, decrease in GSH levels for the Ni+, compared to the Cu+, treated samples. This observation may suggest the low amounts of ROS and/or lipid peroxides as we and others have observed for the Ni+-treated samples (Stohs and Bacchi, 1995). However, the effect of Cu+ on GSH was drastic, both at the 5- and 20-µM levels within the same group, but significantly (P < 0.001) different, compared to those of the Ni+-treated samples. These findings suggest that Cu+ and Ni+ ions act differently and may affect the GSH-redox systems and may also imply that as methods improve, delineation of toxicity may be possible in terms specific molecular interactions, compartmentalization, and the mechanistic details of oxidative stress by the metals (Hansen et al., 2006). Thus, under the conditions prevailing in this study, the generation of ROS might have overwhelmed the natural GSH status in those cells treated with Cu2+ + Ni2+ and therefore contributed to the reduced GSH levels, as observed in our studies, in a dose-dependent manner. GSH levels continued to decrease in a significant (P < 0.001), dose-dependent fashion for cells receiving the combined metal ions (Figure 4), and the effect was more pronounced relative to the cells treated with Cu2+ or Ni2+. GSH levels in cells at the 5-µM dose were reduced to half of its original value, compared to controls. Again, a similar trend in reduced intracellular concentration GSH, and in comparison to each other, was observed for the combined doses of 10 and 20 µM. At the combined dose of 20 µM, cells were almost devoid of GSH (0.97 nmoles/106 cells). Our findings are also in agreement to those of Garcia-Fernandez et al. (2002), who reported a decrease in GSH/GSSG ratios in CHO-K1 and suggested that a homeostatic defense mechanism was activated when cells were exposed to low-metal concentration, but that the ability of the cells to respond by this defense mechanism weakened as the metal dose increased. Alternatively, the presence of these metal-ion cells might have blocked some de novo synthesis of GSH, decreasing the antioxidative status of cells in those groups to better cope with the oxidative stress (Valko et al., 2005).
Although the decreased GSH levels with the concomitant increased lipid peroxides, as we have observed, agrees with what has been reported in previous in vitro and in vivo models (Kilic et al., 2000; Lin et al., 2003), our findings represent the first of such an observation in leukemia cells. This suggests that perhaps both normal and leukemic cells may respond identically to the agents. Nevertheless, it is worth mentioning that metal-treated cells have reduced GSH levels, suggesting a mechanism for toxicity even at very low doses that involves a compromise of cells‘ oxidative status. It is, however, certain that the decreased in GSH content for the various groups will have significant effects on U937-cell metabolism, considering the importance of GSH concentration and metabolic demands of the cells (Choi et al., 2000). The specific contribution of each of the metals to the loss of GSH is not certain. It seems likely that exposure to these exogenous metals may lead to increased ROS that depletes endogenous GSH levels; therefore, subsequent toxicity occurs through a mechanism that simply overwhelms the natural antioxidant defenses. Thus, even if free radical production is most likely the mechanism of cell damage, as previously reported, the loss of intracellular GSH caused by the metal ions observed in the present studies may enhance U937-cell cytotoxicity.
We also investigated the effects of 50 and 100 µM of EDTA on its ability to chelate Ni2+ and Cu2+ ions and assess its effect on GSH levels for either the single or combined metal-ion treatment. We did not observe any significant differences between levels of GSH for all doses of the single metal treatments and in the presence of 50 µM of EDTA. However, EDTA at 100 µM was a better chelator for Ni, compared to Cu, ions, as evidenced by the increased and significant differences (P < 0.05) in GSH levels for the single treatments. This observation may imply that Cu ions were strongly coordinated by DNA bases in the cells, which was likely to bring the radical generation closer to the bases and thus make their oxidation more efficient than in the case of Ni, which is more loosely bound to DNA phosphates (Kasprzak et al., 1986; Sorokin et al., 1996). This may also explain the increased lipid peroxides generated by the cells treated with Cu ions, as observed in this study (Figures 1 and 2). EDTA at 100 µM was better in chelating both metal ions for the combined metals from doses of 0–10 µM. This increase in GSH was moderate, but not statistically significant over that of the 50-µM concentration. It is very important to note that GSH levels were significantly (P < 0.05) decreased for EDTA at 100 µM, compared to the 50-µM levels at the 20-µM combined doses (3.9 versus 5.2 nmole/106 cells, respectively), suggesting the role of GSH in altering Cu+ and Ni+ homeostasis that occurs as a result of membrane damage, leading to downregulation of various metal-dependent systems, including the GSH-enzyme systems (Stohs and Bacchi, 1995). It is important, however, to point out that there seems to be a greater absorption of Cu2+ ions and coordination in the U937 cells and its distribution and effect on antioxidant defense mechanisms may all contribute to the production and tissue-damaging effects of ROS, as observed for Cu2+ (Boadi et al., 2005), in comparison to Ni2+ in the present studies. GSH levels generally decreased (P < 0.05) in a dose-dependent manner within each column for either the single or the combined metal treatments for the two doses of EDTA tested, suggesting that increased metal accumulation may have a permanent and deleterious effect by decreasing GSH levels and/or preventing the free-radical-scavenging properties of GSH (Winterbourn, 1993; Valko et al., 2005).
In conclusion, the metals assayed in the present study (i.e., Cu2+ and Ni2+) caused damage to U937 cells, with the effect being severe at the combined doses. The increased formation of free radicals and other reactive species by the combined metal treatments may account for the increased lipid peroxides and a concomitant reduction in GSH levels. Reversal of GSH by low amounts of EDTA was minimal, suggesting the role of Cu2+ and Ni2+ ions in biological systems, such as U937 cells, indicating that enhanced formation of free radicals and other reactive species can be regarded as a common factor in determining metal-induced toxicity and carcinogenicity. Nevertheless, studies are currently ongoing in our laboratory to evaluate the effects of the above metals on other GSH-redox systems, such as GSSG levels, GSH peroxidase, GSH reductase, glucose-6-phosphate dehydrogene, and superoxide dismutase activities, in U937 cells.
Conclusions
Multiple exposure of U937 cells to the combination metal ions Cu2+ + Ni2+ can cause an increased generation of lipid peroxides as well as decreased levels in GSH levels, compared to the single treatments of either metal ion. EDTA minimally reduced the intracellular loss of GSH for the single treatment of either metal ion, compared to the combined treatments. Thus, the loss of intracellular GSH in U937 cells caused by the metal ions may enhance the oxidative damage in these cells.
Acknowledgment
The authors thank Dr. Gary McCollum of Vanderbilt University for reading and offering his constructive criticisms and suggestions. This study did not involve the use of humans or experimental animals.
Declaration of interest
This study was supported with grants from Tennessee State University through the “EARDA” program, NIAMS Grant no. RO3AR46384–02 to Samuel E. Adunyah of Meharry Medical College, MBRS SCORE Grant no. SO6GM08037–29, an NCI supplementary grant to Meharry Medical College through VICC, and NIH-NCRR RCMI Grant no. 2G12RRO3032–16 to Meharry Medical College, and the financial support of the Samuel P. Massey Chair of Excellence for Environmental studies chaired by Dr. Lonnie Sharpe at Tennessee State University. Dr. Samuel E. Adunyah is also supported by 5 U54 RR026140-03 (NCRR) / 8 U54 MD007593-03 (NIMHD) and NCI 1 U54 CA163069-01 grants.
References
- Adunyah SE, Wheeler BJ, Cooper RS. Evidence for the involvement of LCK and MAP kinase (ERK-1) in the signal transduction mechanism of interleukin-15. Biochem Biophys Res Commun. 1997;232:754–758. doi: 10.1006/bbrc.1997.6367. [DOI] [PubMed] [Google Scholar]
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Bal W, Kasprzak KS. Induction of oxidative DNA damage by carcinogenic metals. Toxicol Lett. 2002;127:55–62. doi: 10.1016/s0378-4274(01)00483-0. [DOI] [PubMed] [Google Scholar]
- Boadi WY, Iyere PA, Adunyah SE. Effect of quercetin and genistein on copper- and iron-induced lipid peroxidation in methyl linolenate. J Appl Toxicol. 2003;23:363–369. doi: 10.1002/jat.933. [DOI] [PubMed] [Google Scholar]
- Boadi WY, Iyere PA, Adunyah SE. In vitro exposure to quercetin and genistein alters lipid peroxides and prevents the loss of glutathione in human progenitor mononuclear (U937) cells. I Appl Toxicol. 2005;25:82–88. doi: 10.1002/jat.1049. [DOI] [PubMed] [Google Scholar]
- Cartana J, Romeu A, Arola L. Effects of copper, cadmium and nickel on liver and kidney glutathione redox cycle of rats (Rattus sp.) Comp Biochem Physiol C, Comp Pharmacol Toxicol. 1992;101:209–213. doi: 10.1016/0742-8413(92)90262-6. [DOI] [PubMed] [Google Scholar]
- Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J Biol Chem. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Collins AR, Duthie GG, Dobson VL. Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidised pyrimidines) in human lymphocytes. Mutat Res. 1997;393:223–231. doi: 10.1016/s1383-5718(97)00107-1. [DOI] [PubMed] [Google Scholar]
- García-Fernández AJ, Bayoumi AE, Pérez-Pertejo Y, Motas M, Reguera RM, Ordóñez C, Balaña-Fouce R, Ordóñez D. Alterations of the glutathione-redox balance induced by metals in CHO-K1 cells. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132:365–373. doi: 10.1016/s1532-0456(02)00079-0. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Georgopoulos PG, Wang SW, Georgopoulos IG, Yonone-Lioy MJ, Lioy PJ. Assessment of human exposure to copper: a case study using the NHEXAS database. J Expo Sci Environ Epidemiol. 2006;16:397–409. doi: 10.1038/sj.jea.7500462. [DOI] [PubMed] [Google Scholar]
- Grandjean P. Human exposure to nickel. IARC Sci Publ. 1984;53:469–485. [PubMed] [Google Scholar]
- Kasprzak KS, Waalkes MP, Poirier LA. Antagonism by essential divalent metals and amino acids of nickel(II)-DNA binding in vitro . Toxicol Appl Pharmacol. 1986;82:336–343. doi: 10.1016/0041-008x(86)90210-3. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, Moore K, Jr, Caulfield JL, Tannenbaum SR, Dedon PC. Quantitation of8-oxoguanine and strand breaks produced by four oxidizing agents. Chem Res Toxicol. 1997;10:386–392. doi: 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- Kilic D, Sayan H, Gönül B, Egehan I. The effect of granulocyte macrophage-colony stimulating factor on glutathione and lipid peroxidation in a rat model. Eur J Surg Oncol. 2000;26:701–704. doi: 10.1053/ejso.2000.0984. [DOI] [PubMed] [Google Scholar]
- Lakritz J, Plopper CG, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal Biochem. 1997;247:63–68. doi: 10.1006/abio.1997.2032. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu J, Liu Y, Li X. Effects of EDTA on mechanism of lead accumulation in Typha orientalis Presl. Bull Environ Contam Toxicol. 2009;83:553–557. doi: 10.1007/s00128-009-9787-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Li W, Zhao Y, Chou IN. Alterations in cytoskeletal protein sulfhydryls and cellular glutathione in cultured cells exposed to cadmium and nickel ions. Toxicology. 1993;77:65–79. doi: 10.1016/0300-483x(93)90138-i. [DOI] [PubMed] [Google Scholar]
- Lin W-C, Liao Y-C, Liau M-C. Effect of CGA-II on cell viability, lipid peroxidation, glutathione concentration, and its related enzyme activities in primary rat hepatocytes. Am J Chinese Med. 2003;31:415–423. doi: 10.1142/S0192415X0300103X. [DOI] [PubMed] [Google Scholar]
- Minotti G. Sources and role of iron in lipid peroxidation. Chem Res Toxicol. 1993;6:134–146. doi: 10.1021/tx00032a001. [DOI] [PubMed] [Google Scholar]
- Misra M, Rodriguez RE, Kasprzak KS. Nickel induced lipid peroxidation in the rat: correlation with nickel effect on antioxidant defense systems. Toxicology. 1990;64:1–17. doi: 10.1016/0300-483x(90)90095-x. [DOI] [PubMed] [Google Scholar]
- Rimbach G, Gohil K, Matsugo S, Moini H, Saliou C, Virgili F, Weber SU, Packer L. Induction of glutathione synthesis in human keratinocytes by Ginkgo biloba extract (EGb761) Biofactors. 2001;15:39–52. doi: 10.1002/biof.5520150104. [DOI] [PubMed] [Google Scholar]
- Sorokin VA, Valeev VA, Gladchenko GO, Sysa IV, Blagoi YP, Volchok IV. Interaction of bivalent copper, nickel, manganese ions with native DNA and its monomers. J Inorg Biochem. 1996;63:79–98. doi: 10.1016/0162-0134(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Stinson TJ, law S, Jeffery EH, Plewa MJ. The relationship between nickel chloride-induced peroxidation and DNA strand breakage in rat liver. Toxicol Appl Pharmacol. 1992;117:98–103. doi: 10.1016/0041-008x(92)90222-e. [DOI] [PubMed] [Google Scholar]
- stohs JS, Bacchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Subramaniam SV, Cooper RS, Adunyah SE. Interleukin-17 induces rapid tyrosine phosphorylation and activation of raf-1 kinase in human monocytic progenitor cell line U937. Biochem Biophys Res Commun. 1999;262:14–19. doi: 10.1006/bbrc.1999.0746. [DOI] [PubMed] [Google Scholar]
- Tamura H, Kitta K, Shibamoto T. Formation of reactive aldehydes from fatty acids in a Fe2+ /H202 oxidation system. J Agric Food Chem. 1991;39:439–442. [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Wataha JC, Lewis JB, Lockwood PE, Rakich DR. Effect of dental metal ions on glutathione levels in THP-1 human monocytes. J Oral Rehabil. 2000;27:508–516. doi: 10.1046/j.1365-2842.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- White AR, Cappai R. Neurotoxicity from glutathione depletion is dependent on extracellular trace copper. J Neurosci Res. 2003;15:889–897. doi: 10.1002/jnr.10537. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Superoxide as an intracellular radical sink. Free Radic Biol Med. 1993;14:85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]