SUMMARY
Maternal inheritance of mtDNA is the rule in most animals, but the reasons for this pattern remain unclear. To investigate the consequence of overriding uniparental inheritance, we generated mice containing an admixture (heteroplasmy) of NZB and 129S6 mtDNAs in the presence of a congenic C57BL/6J nuclear background. Analysis of the segregation of the two mtDNAs across subsequent maternal generations revealed that proportion of NZB mtDNA was preferentially reduced. Ultimately, this segregation process produced NZB-129 heteroplasmic mice and their NZB or 129 mtDNA homo-plasmic counterparts. Phenotypic comparison of these three mtDNA lines demonstrated that the NZB-129 heteroplasmic mice, but neither homoplasmic counterpart, had reduced activity, food intake, respiratory exchange ratio; accentuated stress response; and cognitive impairment. Therefore, admixture of two normal but different mouse mtDNAs can be genetically unstable and can produce adverse physiological effects, factors that may explain the advantage of uniparental inheritance of mtDNA.
INTRODUCTION
The maternal inheritance of animal mtDNAs is both virtually universal and highly concerted with specific systems that actively exclude the paternal mitochondria and mtDNAs during fertilization (Al Rawi et al., 2011; DeLuca and O'Farrell, 2012; Sutovsky et al., 2003, 1999; Thompson et al., 2003; Wallace, 2005, 2007). Because the purpose of sexual reproduction is to mix genomes, why is the paternal mtDNA excluded?
The mtDNA encodes the core subunits of the multiple poly-peptide OXPHOS complexes I, III, IV, and V. The sequence of the mtDNA genes is also highly variable within mammalian species (Gómez-Durán et al., 2010; Kazuno et al., 2006; Ruiz-Pesini et al., 2004). Consequently, admixture of two different sets of mtDNA variants for the same OXPHOS polypeptide could be deleterious (Wallace, 2007). This conjecture would predict that if two normal but different mtDNAs were artificially mixed within the same animal, then incompatibility could occur, rendering the heteroplasmic state unstable and adversely affecting the animal's phenotype.
Previous studies in which NZB and Balb/c mtDNAs were mixed in mice by removing a bleb of cytoplasm and a small amount of mtDNA from the oocyte of one strain and transferring it by fusion into the oocyte of the other strain concluded that “The pattern of segregation can be explained by random genetic drift occurring early in oogenesis..”This implies that the genetic differences between NZB and Balb/c mtDNAs are neutral and that heteroplasmy and homoplasmy were functionally indistinguishable (Jenuth et al., 1996; Solignac et al., 1987). Based on this concept, subsequent discussions on mtDNA uniparental inheritance have focused on the concept that heteroplasmy is eliminated by genetic drift due to a “bottleneck”occurring at some point within the female mammalian germline (Birky, 2001; Cao et al., 2007, 2009; Cree et al., 2008; Khrapko, 2008; Wai et al., 2008).
Contrary to the random segregation of mtDNAs via the female germline of NZB-Balb/c heteroplasmic mice, it was found that the Balb/c mtDNAs were selectively lost from liver and kidney whereas NZB mtDNAs were lost from blood and spleen of heteroplasmic animals (Jenuth et al., 1997). The biochemical and molecular basis of this directional segregation remains unknown (Battersby et al., 2003, 2005; Battersby and Shoubridge, 2001), although variation in the mitochondrial outer membrane GTPase, Gimap3, has been linked with the segregation (Jokinen et al., 2010).
In contrast to the proposed random germline segregation of heteroplasmic NZB-Balb/c mtDNAs, studies of the germline segregation of a mouse harboring a heteroplasmic mtDNA frameshift mutation in ND6 revealed rapid and directional loss of the mutant mtDNA (Fan et al., 2008). A similar conclusion was reached for mice rendered heteroplasmic for mtDNA mutations generated by an error prone mitochondrial DNA polymerase (Stewart et al., 2008). Analysis of oocytes from the heteroplasmic ND6 mutant mice led to the conclusion that the directional segregation occurred within the ovary (Fan et al., 2008).
To further investigate the dynamics of germline mtDNA segregation, we prepared mice that are heteroplasmic for 129S6 and NZB mtDNAs, backcrossed onto a C57BL/6J nuclear background. In contrast to previous reports, we observed a directional loss of the NZB mtDNAs from the female germline over successive generations. Furthermore, the heteroplasmic mice were found to be less fit than their homoplasmic counterparts, having reduced physical activity, behavioral abnormalities, and impaired learning. Therefore, our data indicate that the differences between mtDNAs within a mammalian species may not be neutral and that intraspecific heteroplasmy can be sufficiently deleterious as to favor the evolution of uniparental inheritance.
RESULTS
Germline Transmission of NZB-129 mtDNA Heteroplasmy Favors Transmission of 129 mtDNAs
Heteroplasmic mice with roughly equal proportions of 129S6 and NZB mtDNAs were generated by fusion of NZB mtDNA containing cytoplasts to 129S6-derived female embryonic stem cells and transfer of the heteroplasmic stem cells into blastocysts (Sligh et al., 2000). The NZB and 129 mtDNAs come from normal mice but differ by 91 nucleotides including 15 missense mutations, 5 tRNA mutations, 7 rRNA mutations, and 11 control region mutations (Table S1 available online). The resulting female mice were backcrossed for over ten generations with C57BL/6J males from our closed colony to generate a C57BL/6J inbred nuclear background, with offspring from each generation genotyped for the NZB and 129 mtDNAs to maintain the heteroplasmy. Subsequently female lineages were permitted to segregate mixtures of the two mtDNAs, with the proportion of the 129 and NZB mtDNAs quantified at each generation using the polymorphic nucleotide at mtDNA position A4276G. The presence or absence of the A4276G site was interrogated using BamHI restriction digestion, because the 129 mtDNA has a site at this location that is lost in the NZB mtDNA. Following digestion, the relative levels of the fragments was determined by capillary electrophoresis, and the ratio of the two mtDNAs normalized using a standard curve. As an independent validation of this method, in some experiments the proportion of NZB and 129 mtDNAs was also determined using SNaPshot primer extension method with the latter also normalized by comparison with a standard curve (Poole et al., 2010). Supporting SNaPshot data are provided in Figures S1and S2.
The NZB-129 heteroplasmy of animals was determined from a tail tip biopsy taken at day 10 postnatal (P10). In contrast to previous reports (Jenuth et al., 1996), the proportion of NZB mtDNA in progeny tended to be less than that of their heteroplas mic mother. This was evidenced by the frequency and generational rate of production of animals whose mtDNA composition approached homoplasmy for either 129 or NZB mtDNA. During 9 years and 24 generations, the mtDNA admixture in tail tip tissue of 171 mice segregated to undetectable NZB mtDNAs (<3%) within one or two generations. Breeding seven such 129 females revealed that three bred true for homoplasmic for 129 mtDNA. By contrast, more than 10 generations of selective breeding of the highest NZB mtDNA containing females was required to generate animals which approached homoplasmy for NZB mtDNAs. Of the 12 resulting progeny that tested essentially homoplasmic NZB mtDNA (>97%), subsequent breeding of three animals revealed that only one bred true as homoplasmic NZB mtDNA. These results indicate a strong bias favoring progression to homoplasmy for 129S6 mtDNA in the majority of C57BL/6J nuclear DNA animals within one to two generations. In contrast, active selection over prolonged periods was necessary to obtain homoplasmic NZB mtDNA mice on the same nuclear background.
The Progeny of Heteroplasmic Females Are Enriched for 129S6 mtDNA Relative to the Level in Their Mother's mtDNA
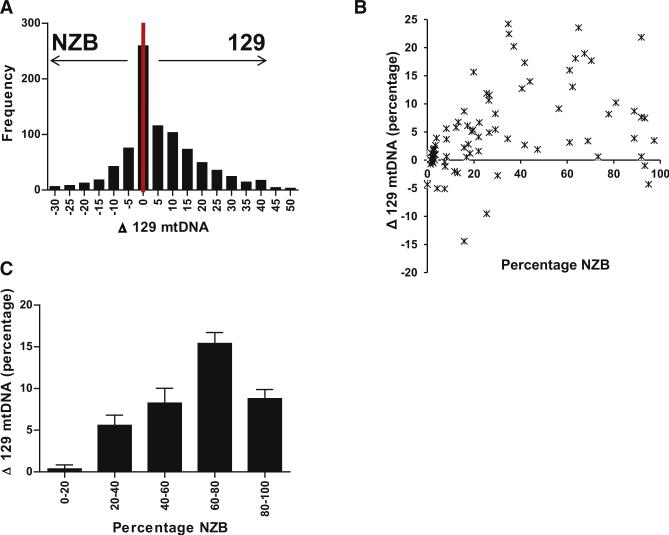
To further quantify the preferential germline transmission of the 129 mtDNAs, we genotyped DNA from tail tips of 864 pups and determined whether the 129-NZB mtDNA heteroplasmy changed relative to their mother. If segregation of different mtDNA types was determined by random drift alone, the mean of the frequency distribution of the progeny's mtDNA genotypes should approximate that of their mother. However, we found that the mean distribution of the progeny's mtDNA heteroplasmy was frequently shifted toward increased 129 mtDNA relative to their mother's (Figure 1A). Of the 864 pups genotyped, 581 (67%) had an increase in the proportion of their 129 mtDNA, whereas 244 (28%) had an increase in NZB mtDNA, and only 39 (5%) had the same mean level of 129-NZB heteroplasmy as their mothers. On average the progeny had a 5.9% (SEM = 0.48, n = 864) increase in the proportion of the 129 mtDNA, which was significantly greater than random (one sample t test p < 0.0001, 95% confidence intervals [CI] 4.92%–6.81%). Therefore, the 129 mtDNA variant was selectively enriched in the mother's offspring.
Figure 1. Change in Progeny 129-NZB mtDNA Heteroplasmy in Offspring from Mothers with Varying Levels of Tail Heteroplasmy.
(A) Frequency histogram of the segregation of mtDNA variants in the germline (n = 864). (B) The average change in 129-NZB mtDNA heteroplasmy of all the progeny of a particular female (n = 78) relative to her tail heteroplasmy. (C) The average change in 129-NZB mtDNA heteroplasmy in pups of females grouped according to their proportions of the different mtDNA variants, 0%–20% group, n = 341; 20%–40%, group n = 140; 40%–60% group, n = 84; 60%–80% group, n = 168; and 80%–100% group, n = 158. The proportion of NZB mtDNA in the tail of a pup was subtracted from the proportion of NZB in the tail of the mother (both at P10). Positive values represent segregation toward 129 mtDNA and negative values toward NZB mtDNA. Error bars represent ± SEM. See also Figure S6 and Table S1.
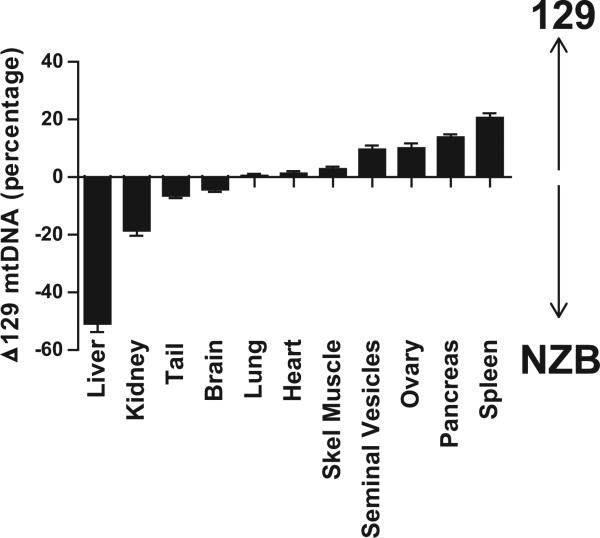
We chose tail tissue at day P10 as a baseline to quantify NZB-129 mtDNA heteroplasmy because tail, brain, lung, heart, and skeletal muscle had similar proportions of 129 and NZB mtDNAs that remained relatively stable into adulthood (Figures 2 and S1). Moreover, the tip of the tail was easily accessible by biopsy early in the mouse's life. The stability of the heteroplasmy of brain, heart, skeletal muscle, and tail was in marked contrast to the loss of 129 mtDNAs observed in liver and kidney or loss of NZB mtDNAs from the spleen and pancreas in adult mice (Figures 2 and S1), a phenomenon reported for NZB-Balb/c heteroplasmic mtDNA mice (Jenuth et al., 1997).
Figure 2. Change in mtDNA Heteroplasmy in Organs of Adult Mice Compared to P10 Tail Tip Heteroplasmy, Determined by Fragment Analysis.
Positive values represent increased 129 mtDNA content negative values increased NZB mtDNA. More than 50 mice were examined for each tissue except for the ovaries (n = 26), and seminal vesicles (n = 21). Error bars represent ± SEM. See also Figure S1.
To determine if the progressive increase in proportion of 129 mtDNA across generations was dependent on the value of maternal 129-NZB heteroplasmy level, we grouped mothers by their tail genotypes in intervals of 20% NZB mtDNAs and analyzed the average change in the percentage of heteroplasmy of the progeny in each group (Figures 1B and 1C). The progeny of mothers with 0%–20% NZB mtDNA had on average 0.36% more of the 129 mtDNA than their mother (SEM = 0.48, n = 341, 95% CI 0.57%–1.3%), progeny of mothers with 20%–40% NZB mtDNA had an average 5.6% more 129 mtDNA (SEM = 1.22, n = 140, 95% CI 3.2%–8.0%), progeny of 40%–60% NZB mothers had 8.2% more 129 mtDNAs (SEM = 1.78, n = 84, 95% CI 4.7%– 11.8%), and progeny of 60%–80% NZB mothers had 15.4% more 129 mtDNA than their mothers (SEM = 1.32, n = 168, 95% CI 12.8%–18.0%). The differences in segregation between the 0%–20% group and the 20%–40% (p = 0.002), 40%–60% (p < 0.0001), 60%–80% (p < 0.0001), and 80%–100% (p < 0.0001) groups were all statistically significant (using a two-tailed unpaired t test with Welch's correction for unequal variances for all comparisons) (Figure 1C). This indicated that as the proportion of the mother's NZB mtDNAs increased, the progeny had a greater tendency to lose NZB mtDNAs.
Once the proportion of NZB mtDNA in the mother exceeded 60%–80%, the rate of NZB mtDNA segregation declined. The progeny of mothers with 80%–100% NZB mtDNAs had progeny with only 8.8% more 129 mtDNA (SEM = 1.10, n = 158, 95% CI 6.63%–11.0%) (Figures 1B and 1C). Grouping these matting by steps of 10%, the progeny of the mothers with 80%–90% NZB mtDNA had a 9.8% bias (n = 64) toward 129 mtDNAs whereas progeny of mothers with 90%–100% NZB mtDNAs had only a 5.5% (n = 47) bias toward 129 mtDNAs, a difference that was also significant (p = 0.0001). Finally, the tendency to lose of the NZB mtDNAs was significantly greater in 60%–80% and 80%– 100% NZB mtDNA females than 20%–40% NZB females (p < 0.0001, and p < 0.0001) (Figure 1C).
Therefore, for mice with approximately equal portions of NZB and 129 mtDNA, there is a strong bias to decrease NZB mtDNA between generations. However, for females with very low levels of NZB or 129 mtDNA heteroplasmy the predilection to lose NZB mtDNAs declines. When the level of the minority mtDNA becomes 10% or less NZB or 129 mtDNA, then the distribution of mtDNAs in the progeny approaches random.
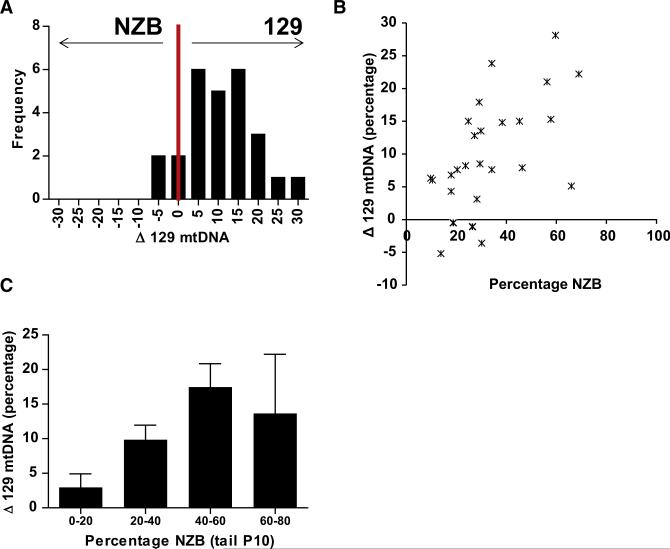
Evidence for Enrichment of 129 mtDNA in the Female Germline
To investigate when the bias in segregation of different mtDNAs occurs, we quantified mtDNA heteroplasmy in ovaries of individual mice and compared this to the value in the tail tissue. The 129 mtDNA was enriched in the ovary relative to the tail in 22 out 26 (84.6%) mice studied, with the average increase being 10.0% (SEM = 1.66 n = 26, 95% CI 6.6%–13.4%) (Figure 3A). As found in tail tissue of progeny compared to their mothers, the increase in proportion of 129 mtDNA in an animal's ovaries increased in proportion with heteroplasmy level of the female (Figures 3B and 3C). Females with 0%–20% NZB mtDNAs in their tails had 3.0% (SEM = 1.96, n = 6) more 129 mtDNA in their ovaries, those with 20%–40% NZB mtDNAs had 9.9% (SEM = 2.11, n = 13), and those with 40%–60% NZB had 17.5% (SEM = 3.38, n = 5) more 129 mtDNA in their ovaries. The differences between 0%–20% group and the 20%–40% and 40%– 60% groups were both statistically significant (two sample t tests p = 0.031 and p = 0.009, respectively). Comparable results were obtained using SNaPshot genotyping (Figure S2). Therefore, the ovary has an increased proportion of 129 mtDNA compared to tail tissue.
Figure 3. Increased 129 mtDNA in the Ovary Compared with Tail at Different Levels of Tail Heteroplasmy Determined by Fragment Analysis.
(A) Frequency histogram of the segregation of mtDNA variants in the ovaries compared to tail tissue of individual mice (n = 26). (B) The change in the mtDNA heteroplasmy of ovaries of female mice relative to their tail tissue at P10. (C) The average change in mtDNA variants in the ovaries of mice within groups with different proportions of mtDNA variants, 20%–40% group, n = 6; 40%–60% group, n = 13; and 60%–80% group, n = 5. Positive values indicate segregation toward 129 mtDNA. Error bars represent ± SEM. See also Figures S2, S3, S4, and S5.
The tendency for the ovaries to have a higher percentage of 129 mtDNA than tail tissue was relatively constant throughout her reproductive lifespan and up to 2 years of age (Figure S3). The apparent tendency for the ovaries to have lower NZB mtDNAs after 2 years may in part be due to the disproportionate survival of mice with low levels of NZB heteroplasmy where the tendency to lose NZB mtDNAs is lowest (the three longest lived animals had tail heteroplasmy levels of 21%, 27.6%, and 19.1% NZB) (Figure S3). Because the same bias was observed in ovaries of young females and old females, this suggests that the predilection of the ovaries to have less 129 mtDNA was present soon after birth.
In contrast to mice heteroplasmic for a frameshift mutation in the mtDNA ND6 gene (Fan et al., 2008), the bias against NZB mtDNA in 129-NZB heteroplasmic progeny did not increase with successive liters from the same female (Figures S4A– S4D). This is also consistent with the bias against the NZB mtDNAs in the germline not being linked with age.
The adult mammalian ovary is composed of primarily somatic cells with a relatively small contribution from germ cells. Hence, the increase in 129 mtDNA observed in ovarian tissue could originate from somatic cells, germ cells, of both cell types. To determine if an increase in the proportion of 129 mtDNA was also observed in mature oocytes, females with various levels of heteroplasmy were super-ovulated, the oocytes separated from the cumulus masses, and the 129-NZB mtDNA heteroplasmy in oocytes quantified (Figure S5). In the limited number of animals analyzed, a significant increase was observed in the proportion of 129 mtDNA in oocytes derived from mothers with 60%–80% heteroplasmy (mean = 4.1%, SEM = 1.47, n = 47) (one sample t test p = 0.008). Therefore, at least a portion of the loss of NZB mtDNA observed in progeny can be attributed to selection for 129 mtDNA occurring in the female germline. These results are consistent with a model where heteroplasmic distribution among progeny appears to be driven by two factors: biased selection against the NZB mtDNA when it is present at over 20% heteroplasmy and a more random segregation when one of the mtDNAs is present at <10%.
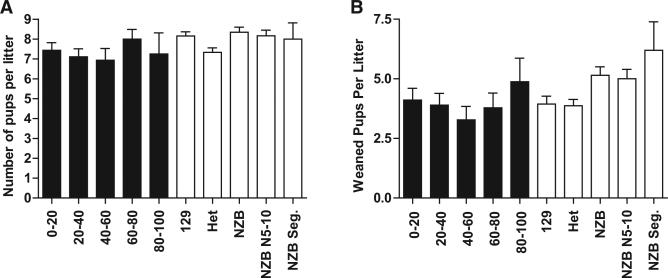
C57BL/6J Nuclear DNA-NZB mtDNA Incompatibility Does Not Cause the Bias Toward 129 mtDNA
One reason that the tendency to lose NZB mtDNAs might increase as the percentage of the NZB mtDNAs rises could be that the NZB mtDNAs are inherently incompatible with the C57BL/6J nucleus. To test this possibility, we compared both fertility and progeny survivorship (i.e., fecundity) of the NZB-129 heteroplasmic mice with that of their homoplasmic NZB or 129 counterparts (Figure 4A). In order to maximize the likelihood that the NZB-129 heteroplasmic and NZB or 129 homoplasmic mtDNA female mice would have essentially the same C57BL/ 6J nuclear DNA background, all females were mated with males from our closed C57BL/6J colony that is maintained by brother-sister mating. Still, small nuclear DNA changes due to de novo mutations cannot be fully excluded (Watkins-Chow and Pavan, 2008).
Figure 4. Effect of mtDNA Genotype on Fertility and Fecundity of Mice.
(A) The average litter size of mice containing different proportions of the 129 and NZB mtDNAs. (B) The average number of pups that were born and survived to weaning. For heteroplasmic mice the mother's percentage of NZB mtDNA is shown, and mice were grouped according to percentage heteroplasmy. For the 0%–20% group 46 litters were examined, 20%–40% group, n = 48; 40%– 60% group, n = 29; 60%–80% group, n = 28; and 80%–100% group, n = 18. 129, segregated to homoplasmic 129 mtDNA (n = 98); Het, mean for all heteroplasmic mice (n = 159); NZB, NZB mice from the backcross (n = 88); NZB N5-10, backcross from generation N5-N10 (n = 67); NZB Seg, mice segregated to homoplasmic NZB from heteroplasmic mice (n = 10). Error bars represent ± SEM.
As an independent control, we generated mice homoplasmic for NZB mtDNAs by crossing an NZB female with a C57BL/6J male and then backcrossing the F1 females to C57BL/6J males at least ten generations. No decrease in fertility was seen for the homoplasmic NZB mice because the number of progeny produced by the NZB mtDNA homoplasmic segregant females was comparable to that of the 129 mtDNA homoplasmic segregant females. Moreover, the number of pups that survived through weaning was even higher for the NZB mtDNA homoplasmic segregant females than for either the 129 homoplasmic segregant females or the NZB-129 heteroplasmic females and was also higher than that of the homoplasmic NZB mtDNA females generated by backcrossing of an NZB female and her daughters with C57BL/6J males (Figure 4B). The increased fecundity of the NZB mtDNA homoplasmic segregant C57BL/ 6J mice, relative to either the heteroplasmic mice or the 129 mtDNA homoplasmic mice, provides independent support that the bias against the NZB mtDNA was not due to an incompatibility between the NZB mtDNAs and the C57BL/6J nucleus.
Because the directional loss of NZB mtDNA from the C57BL/ 6J mouse lineage is unlikely to be due to incompatibility between the NZB mtDNA and the nucleus, it is most likely due to an incompatibility between the NZB and 129 mtDNAs produced by admixture within cells of the heteroplasmic mice. If so, the tendency of the progeny to loss the 129 mtDNA should be the most pronounced when the proportion of the two mtDNAs are relatively equal and should decline when one of the mtDNAs is reduced toward zero, which in fact was observed.
Altered Metabolic Functions of 129-NZB Heteroplasmic mtDNA Mice
If mixture of two mtDNAs in the same cytoplasm can be detrimental, heteroplasmic animals might be less fit than animals with homogenous nuclei but with only one or the other mtDNA. Such phenotypic difference might be most consistently manifest in the brain, heart, lung, and skeletal muscle because the mtDNA heteroplasmy of these organs remains relatively stable throughout the animal's life (Figures 2 and S1). Therefore, we investigated the heteroplasmic mice for alterations in systemic and neurological phenotypes.
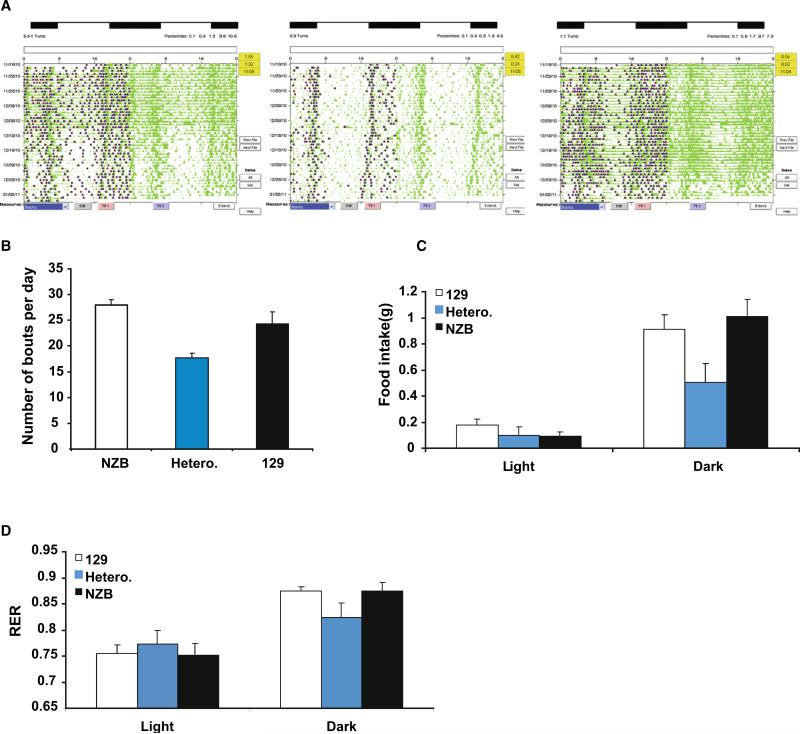
We first examined the effect of NZB-129 heteroplasmy on activity and metabolism in the context of their circadian rhythm. Circadian rhythms are under the combined control of the central nervous system through the light-responsive neurons of the suprachiasmatic nucleus (SCN), which collectively serve as the master oscillator, together with the circadian rhythm of each individual cell in the body. Alterations in mitochondrial function have been linked to SCN-driven time keeping in rodents (Isobe et al., 2011) and individuals with mitochondrial disorders can present with behavioral disorders and alterations in circadian rhythms, appetite, and sleep cycle (Eckel-Mahan and Sassone-Corsi, 2009). Hence mitochondrial dysfunction might perturb both the central and peripheral circadian functions. NZB-129 heteroplasmic and NZB or 129 homoplasmic mice were entrained in a 12 hr light/12 hr dark (LD) cycle for several weeks and then placed into constant darkness (DD; Figure 5A). Mouse activity was monitored by infrared sensors over the course of several weeks in DD. All animals remained rhythmic in LD and DD conditions, and the circadian period (or τ) of the three mtDNA genotype animals did not show striking differences (Figure 5A). However, in both LD and DD conditions, the heteroplasmic mice showed a dramatic reduction in the number of activity bouts relative to the homoplasmic NZB and 129 counterparts (Figures 5A and 5B). When placed in metabolic cages during the dark period the heteroplasmic mice showed a marked decrease in food consumption (energy intake) (Figure 5C). Through indirect calorimetry monitoring of VCO2 and VO2 the heteroplasmic mice were found to also have reduced energy expenditure (respiratory exchange ratio [RER], VCO2/VO2) (Figure 5D) even through the heteroplasmic and homoplasmic mice had similar body weights. Thus, the NZB-129 heteroplasmic mice show a significant reduction in both activity and metabolic rate relative to both 129 and NZB homoplasmic segregant mice.
Figure 5. Behavior and Metabolism of Heteroplasmic Mice and Their Homoplasmic Segregants during the Circadian Cycle.
(A) Quantification of activity bouts in light and dark cycles. Left panel: homoplasmic 129 mtDNA animals. Middle panel: heteroplasmic NZB-129 animals. Right panel: homoplasmic NZB mtDNA animals. (B) Average activity bouts during the continuous dark period of the three mtDNA genotype animals. (C) Average food intake of the mice with the three mtDNA genotypes in the dark and light periods. (D) Average differences in respiratory exchange ratio (RER) during the light and dark period of the heteroplasmic and homoplasmic mice. Error bars represent ± SEM.
Altered Behavior and Cognitive Performance in Heteroplasmic Mice
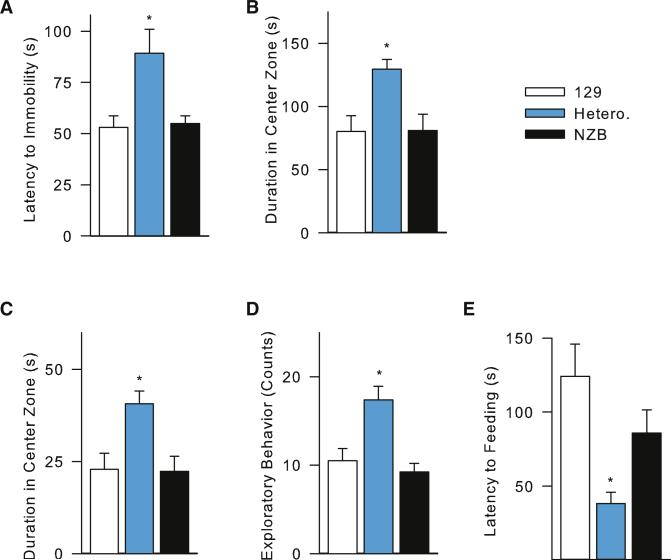
We next analyzed effect of NZB-129 heteroplasmic on behavior and cognition. To investigate the relative response to stress we employed the forced swim (FST) test, yielding the parameter of latency to immobility, representing “behavioral despair”(Alonso et al., 1991). We found that the escape-directed activity of the NZB-129 heteroplasmic mice was significantly greater than either the NZB or 129 segregants, with the latency to immobility being nearly double in the heteroplasmic animals (Figure 6A).
Figure 6. Anxiety-Related Behavior is Elevated in Heteroplasmic Mice in Response to Stress.
(A) Decreased latency to immobility in the forced swim test for 129-NZB heteroplasmic mtDNA versus homoplasmic 129 and homoplasmic NZB mtDNA male mice (n = 9–20; *p % 0.0006). (B) Decreased aversion to anxiogenic center compartment of heteroplasmic mice in the open field (*p % 0.026). (C) Increased time spent in the brightly-lit center compartment in novelty suppression of feeding conflict-based test of heteroplasmic versus homoplasmic mice (*p % 0.006). (D) Increased frequency of NSOF anxiolytic behavior, as determined by body contour (elongation versus contraction) and rearing (*p % 0.001). (E) Decreased latency to feeding in NSOF in heteroplasmic mice (*p % 0.006). Unless otherwise stated, n = 10 and all statistical values were determined by one-way ANOVA, followed by the Student-Newman-Keuls post hoc test. All p values represent significant differences between NZB-129 Hetero. versus homoplasmic 129 and NZB mice. p > 0.05 in all measures for 129 versus NZB. Error bars represent ± SEM.
We next compared the NZB-129 heteroplasmic and 129- and NZB-homoplasmic mice on stress response using the open field (OF) test, which exploits the natural agoraphobia response of mice (Sartori et al., 2012). The 129-NZB heteroplasmic mice spent almost twice as much time in the anxiety-provoking center of the open field than the 129 or NZB homoplasmic animals suggesting decreased adaptive anxiety and fear (Figure 6B).
NZB-129 heteroplasmic and 129- and NZB- homoplasmic mice were also tested for novelty suppression of feeding (Sartori et al., 2012). Again, the heteroplasmic mice spent more time in the brightly lit center of the compartment (Figure 6C), had enhanced exploratory behavior (Figure 6D), and decreased latency to the reward (Figure 6E) than the homoplasmic segregants. Collectively, these results indicate the NZB-129 heteroplasmic mice have abnormal anxiety and fear-associated response to stressful environmental conditions, relative to homoplasmic 129 or NZB homoplasmic mice during light period analysis.
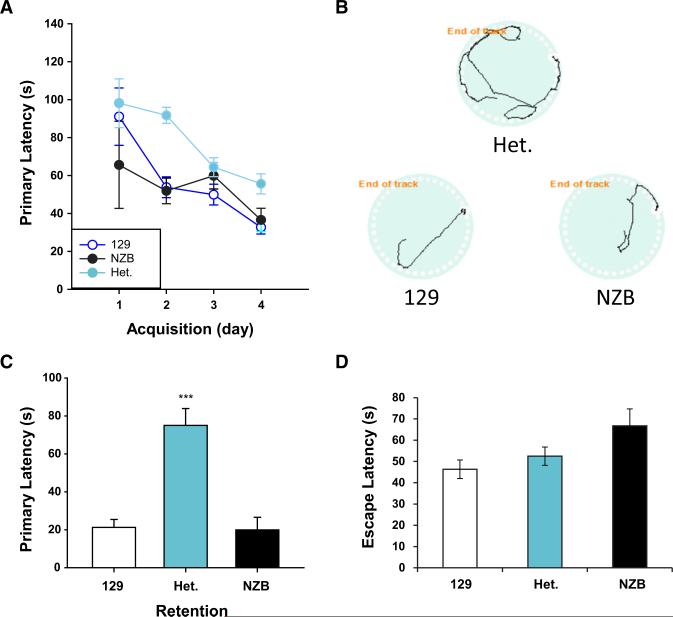
To determine if heteroplasmy affects cognitive performance, we assessed the spatial learning and memory of the NZB-129 heteroplasmic and 129- and NZB- homoplasmic mice using the Barnes maze test (Barnes, 1979). Learning capacity was assessed by the latency to the first nose poke into the escape hole (primary latency) over several days. In all groups, the primary latency, escape latency, and the number of errors gradually decreased to the minimum value on day 4. This progressive improvement in performance over the course of training demonstrate that each group was eventually able to learn the task ([F3,227] = 15.458, p < 0.001). However, the 129-NZB heteroplasmic mice were significantly less efficient in learning the task than the 129 or NZB homoplasmic mtDNA mice ([F2,204] = 13.880, p < 0.001) (Figure 7A). The increased latency in the heteroplasmic mice may be explained by a difference in escape strategy (Sportiche et al., 2010). Indeed, on the final acquisition day, both homoplasmic groups used the more direct, spatial strategy in over 60% of the trials, whereas the heteroplasmic mice employed this method in <30% of the trials (Figure 7B). To assess spatial memory retention, probe trials were suspended for 24-hr after the last training trial and the mice again presented with the task. Remarkably, the heteroplasmic mice took almost four times longer to locate the target hole than either homoplasmic group (p % 0.001, for heteroplasmic mice [Het.] versus 129 and NZB; p = 0.907 for 129 versus NZB) (Figure 7C). After the spatial version of the test, cued trials were conducted to determine if differences were due to sensory, motivational, or motor defects. The heteroplasmic mice located and entered the escape box (escape latency) as efficiently as the 129 and NZB segregants (p = 0.111) (Figure 7D). Taken together, these results suggest that all groups have similar motivation for the goal and eventually learned the task, but consolidation and retention of the spatial cues were more challenging for the heteroplasmic mice. The results indicate that the heteroplasmic mice have impaired spatial memory retention compared to both of their homoplasmic counterparts.
Figure 7. Heteroplasmic Mice Have Impaired Spatial Learning and Memory.
(A) The latency to the first encounter with the target hole for heteroplasmic and homoplasmic mice in the Barnes maze test during the learning period. On the first day the data are from one test trial. On days 2, 3, and 4 each mouse had three trials of 2 min. (B) Representative paths of the mice from day 4 of the acquisition trials. Homoplasmic mice typically used a direct, spatial strategy:. The heteroplasmic mice typically adopted a less direct nonspatial strategy. Het., 129-NZB heteroplasmic mice; 129, 129 homoplasmic mice; NZB, NZB homo-plasmic mice. (C) The effect of heteroplasmy on spatial memory retention. 24 hr after the last acquisition trial the mice were tracked during a 3 min probe trial using the same target hole placement as in the acquisition trials. For all studies, n = 18 for the heteroplasmic mice, n = 17 for the 129 homo-plasmic mice, and n = 9 for the NZB homoplasmic mice. (D) The latency to escape box in cued acquisition trials. Performance of heteroplasmic mice is indistinguishable from both homoplasmic groups in the cued, nonspatial version of the task. For all studies, n = 18 for the 129-NZB heteroplasmic mice (Het.), n = 17 for the 129 homoplasmic mice (129), and n = 9 for the NZB homoplasmic mice (NZB). Error bars represent ± SEM.
Therefore, the 129-NZB heteroplasmic mice show striking behavioral and cognitive differences relative to the 129 and NZB homoplasmic mice. Under routine conditions, the heteroplasmic mice exhibited reduced activity and feeding. However, in response to stress and anxiety the heteroplasmic mice responded more strongly than their homoplasmic counterparts. Yet the increased activity was tempered by a decreased capacity to learn and remember.
DISCUSSION
Our studies demonstrate that admixture of two “normal”but different mtDNAs from the same species within mammalian cells and the female germline can be genetically unstable resulting in the nonrandom segregation of the mtDNA types during tissue aging and germline transmission. Furthermore, mtDNA heteroplasmy within an individual can result in significant physiological, cognitive, and behavioral complications providing an impetus for uniparental inheritance.
The germline segregation of the NZB and 129 mtDNAs over successive generations appears to be driven by two different factors: bias against one of the mtDNAs when the two mtDNAs are present in similar amounts and a more stochastic segregation when the proportion of one of the mtDNAs is ≤10%. The stochastic component of this proposal offers a potential explanation for the conclusion in Balb/c-NZB mtDNA heteroplasmy segregation studies that the mtDNA segregation is random. The Balb/c-NZB studies primarily considered cases in which one of the two mtDNAs was consistently present at low levels (Jenuth et al., 1996).
Our results demonstrate that the segregation of two “normal”mtDNAs of similar proportion does occur in both somatic cells and the germline. We have confirmed the previous report that in Balb/c-NZB heteroplasmic mice the NZB mtDNAs are favored in liver and kidney whereas the Balb/c (similar sequence to 129S6) mtDNAs are favored in spleen (Jenuth et al., 1996). However, we have also demonstrated that biased segregation of mtDNA heteroplasmy also occurs in the female germline.
The mechanism by which heteroplasmic segregation bias is achieved is unknown. Directional loss of a mtDNA ND6 frame-shift mutation was observed in our previous study over successive estrous cycles and generations (Fan et al., 2008), and evidence of bias toward 129S6 mtDNAs was seen in both the ovarian tissue and in oocytes suggesting that this process is also a component of the current bias in 129S6 mtDNA transmission through the germline. However, the physiological mechanism by which one “normal”mtDNA can be distinguished from another remains a mystery. One speculation might be that the two mtDNAs might generate different levels of mitochondrial reactive oxygen species production and that this difference interacts with the common nuclear background in different manners (Hadjivasiliou et al., 2012; Lane, 2011a, 2011b; Moreno-Loshuertos et al., 2006). Another possibility relates to the fact that brain, heart, and muscle have reserve OXPHOS capacity that liver does not (Phillips et al., 2012). Hence, a slight decrease in mitochondrial bioenergetics could be compensated for by the brain, heart, and muscle, but not by the liver. As a result, the liver would be under chronic energetic stress leading to the preferential elimination of the mtDNA that was least compatible with the nuclear DNA-encoded OXPHOS isoforms expressed in that tissue. Behavioral phenotypes could then be manifest because stress and novel learning regimes might require the brain to use its excess capacity, which would, in turn, be limited.
Heteroplasmy of the somatic tissues was found to have deleterious physiological and behavioral consequences. Under normal housing conditions, the heteroplasmic mice had reduced food intake, spontaneous activity levels, and metabolic rates during the dark cycle (Etain et al., 2011). However, under stressful conditions, the heteroplasmic mice were more excitable and resistant to despair (Flaisher-Grinberg et al., 2010; Gindre and Swendsen, 2010; Minassian et al., 2011). The heteroplasmic mice also showed impaired spatial learning and retention. These metabolic and behavioral anomalies are most likely to be the result of the interaction of the NZB and 129 mtDNAs because animals with the same nuclear genome but homoplasmic for one or the other wild-type mtDNAs did not show these behavioral alterations.
The exact molecular mechanism(s) for the incompatibility seen when the NZB and 129 mtDNAs were mixed in the presence of the C57BL/6J nucleus is still unclear. The altered RER implies a partial respiration defect and the 129 and NZB mtDNAs differ in 91 nucleotides including 15 missense mutations (Table S1). Because the mtDNA encodes core polypep-tides of the multiple polypeptide proton-channel containing OXPHOS complexes I, III, IV, and V (Wallace, 2007) and the mitochondria continuously undergo fusion and fission resulting in complementation in trans (Chen et al., 2010; Oliver and Wallace, 1982) the mixing of two different mtDNAs and their protein products could reduce the efficiency of OXPHOS or perturb cellular signaling between the mitochondrion and the nuclear genome (Wallace, 2007). Given the high functional mtDNA sequence variability of humans and other vertebrates (Ji et al., 2012; Mishmar et al., 2003; Ruiz-Pesini et al., 2004; Wallace et al., 2003) most combinations of two different mtDNAs would be functionally deleterious. We propose that the abnormal physiology, behavior, and cognition observed in the heteroplasmic 129-NZB mtDNA mice provides the reason why nature actively prevents mixing of different mtDNAs and thus favors uniparental inheritance.
EXPERIMENTAL PROCEDURES
All experimental procedures involving mice were conducted according to IACUC-approved protocols.
NZB versus 129 mtDNA Sequences
The mtDNAs of the two parental lines were sequenced by the dideoxy-chain termination procedure (Table S1).
Generation of Heteroplasmic Mice
Heteroplasmic mice were generated by fusing synaptosomes from NZB mice to the ρ0 cell line LMEB4 to generate an NZB mtDNA cybrid cell line. The LMEB4(mtNZB) cybrids were enucleated and the cytoplasts fused to rhoda-mine-6G-treated female mouse embryonic stem (ES) cell line CC9.3.1, cells derived from 129/SvEv-Gpic (129S6) mice. The CC9.3.1 (mtNZB) ES cell cybrids were injected into C57BL/6J blastocysts, and female chimeric animals mated with C57BL/6J males to generate the founding agouti female heteroplasmic mouse (Sligh et al., 2000). Heteroplasmic females were backcrossed with C57BL/6J for over ten generations to males from our closed C57BL/6J colony initiated in the early 1990s from Jackson Laboratory C57BL/6J mice (stock number 000664) and maintained by brother-sister mating. By always using males from the same breeding colony as the mate for all backcrosses, the nuclear genotype has been kept as uniform as possible across all mtDNA lineages: NZB-129 heteroplasmic, NZB homoplasmic, and 129 homoplasmic mice. All mouse experimental protocols were conducted with the review and approval for the University of California, Irvine and the Children's Hospital of Philadelphia Institutional Animal Care and Use Committees.
Genotyping of Heteroplasmic Mice
All mice were genotyped at P10 using DNA extracted from tail tip. Total genomic DNA was isolated from tissues using DNeasy kit (QIAGEN, Valencia, CA). To determine the heteroplasmy levels of the mice, fragment analysis and SNaPshot primer extension analysis were used. In the fragment analysis total genomic DNA was PCR amplified using primers (50-/56-FAM/CTGGCCATCG TACTCAACTATA-3′ and 3′-TGTGGGCAATTGATGAATAGGC-5′) flanking the polymorphic A4276G nucleotide that in 129 mtDNA formed part of a BamHI restriction site absent in NZB mtDNA. The PCR fragments were digested with BamH1, mixed with carboxy-X-rhodamine size standard and formamide, and incubated at 95° C for 3 min, followed by immediate cooling on ice, and the relative proportions of the two mtDNAs determined using an ABI Prism 3130×l Genetic Analyzer (Life Technologies, Carlsbad, CA), and Peak Scanner (v1) software (Applied Biosystems) program. To correct for heteroduplex formation, a standard curve was generated by mixing known amounts of cloned 129 and NZB mtDNA (Figure S6A).
In the SNaPshot method, a fragment containing the 4276 polymorphic site was PCR amplified, and unincorporated dNTPs and primers were removed by EXO-Sap-IT treatment (Affymetrix, Santa Clara, CA). The primer extension reaction was performed using the ABI Prism SNaPshot Multiplex Kit (Life Technologies), with the primer (5′-TCTGATTACCAGAAGTAACTCAAGG-3′). The relative proportions of the fluorescent oligonucleotides was determined using a ABI Prism 3130xl Genetic Analyzer, the GeneMapper software, and a standard curved generated using known proportions of the NZB and 129 mtDNA (Figure S6B).
Superovulation
One-month-old female mice housed without exposure to males were super-ovulated (Hogan et al., 1994) by intraperitoneal injection with 5 I.U. pregnant mare serum gonadotropin followed by injection of 5 IU of human chorionic gonadotropin. The oviducts were extracted and placed in hyaluronidase (10 mg/ml in M2 media [Millipore]) at room temperature for 5 min to separate the cumulus cells from the oocytes. Oocytes were then collected for genotyping.
Behavioral Testing
Different cohorts of age-matched male mice were used for each of the behavioral tests in order to minimize any effects that prior experience or stress would have on the phenotypic outcome. All behavioral tests were quantified by Ethovision XT tracking software (Noldus, Netherlands).
Circadian Rhythm Behavioral Monitoring
Animals were entrained in a 12-hr light, 12-hr dark (LD) cycle for 2 weeks prior to recording by infrared sensors. Locomotor activity was detected using passive (pyroelectric) infrared sensors (PU-2201; EK Japan, Japan). Activity data were acquired with a sampling interval of 5 min and using the VitalView Data Acquisition System software (Minimitter, Sunriver, OR). Actograms were created and analyzed by Clocklab software (Actimetrics, Evanston, IL).
Indirect Calorimetry
Whole body metabolic profiling was assessed using a negative-flow CLAMS hardware cage system (Columbus Instruments, Columbus, OH) that measured oxygen consumption, carbon dioxide emission, and food and water intake among other parameters throughout a 25-hr cycle. Animals were given 24-hr to habituate to the cages prior to data accumulation. Substrate partitioning was determined using the RER (VCO2/VO2) calculated from Oxymax software (Columbus Instruments).
Forced Swim Test
Motivational behavior in rodents (Cryan and Holmes, 2005) was assessed by Porsolt's forced swim test (FST) (Porsolt et al., 1977). Transparent cylindrical glass containers (18 cm 3 25 cm) were filled to a depth of 15 ± 1 cm with room temp (23 ± 1° C) tap water. Each mouse was placed in a cylinder and “behavioral despair”was quantified as the latency to immobility by automated tracking software. Immobility was defined as the cessation of intensive, climbing movements with paws against the container wall for 3 s. After 5 min, each mouse was removed from water, dried with a warm towel, and placed in a new cage, alone or with previously tested cage mates.
Open Field Test
To assess response to stress, mice were analyzed in OF tests. Mice were allowed to acclimate to procedure room conditions for at least 1 hour prior to testing. They were then placed in a white-walled, square arena (25×25×35 cm). The effect of stress due to separation from cage mates and agoraphobia within the novel environment was determined by time spent in the center of the area. Exposure to the novel OF environment induces hyponeophagia in rodents (Sartori et al., 2012).
Novelty suppression of feeding was used to determine anxiolytic behavior described by overcoming an adverse stimulus (bright light) to achieve reward (standard chow). Mice were deprived of food for 16 hr prior to placement in the OF. Movement over time (xy coordinates), time spent in the center zone, latency to approach the reward, and exploratory behavior (body contour and rearing) were measured. These measures were quantified by Ethovision XT software within the first 5 min following OF introduction to emphasize anxiety-associated response to novelty.
Barnes Maze Test
Learning and memory were evaluated using the Barnes maze task (Barnes, 1979). Mice were trained to locate a dark escape box hidden underneath a target hole in an elevated platform with 40 holes. The holes were spaced every 9° around the perimeter of the 122 cm circular platform (Noldus), which was evenly illuminated by overhead fluorescent white room lighting (~70 Lux). Mice were given three consecutive, 2 min trials per day to learn the location of the escape box, each from a different starting position. Three large spatial cues were placed on white curtains and walls surrounding the platform. A 1% vinegar solution was used to remove olfactory cues after every trial. Criterion for each group to learn the task was set at a mean search error <4. On day 4 all groups had reached criterion. After 24 hr, the escape box was removed and the mice were tracked in a 3-min probe trial to evaluate memory retention. Three weeks after completion of the spatial test, cued acquisition trials were conducted in the same manner, but with the escape hole on the opposite side of the maze and a proximal cue (a 23 cm tall blue and red flag) was placed directly behind the new target hole.
Statistical Analysis
All analyses were carried out using the Prism 5.0 statistical analysis program (GraphPad, San Diego, CA). To assess the significance of segregation of the mtDNAs Student's t tests (with Welch's correction for unequal variances) were used, where the significance was set at p < 0.05, and to establish 95% CI. For forced swim and open field test analyses, statistical data were determined by one-way ANOVA, followed by the Student-Newman-Keuls post hoc test. For Barnes maze test, differences between groups across acquisition days were determined by two-way ANOVA, followed by Holm-Sidak post hoc test.
Supplementary Material
ACKNOWLEDGMENTS
We express our special appreciation for the technical contributions of Prasanth Potluri, PhD, Marie Lott, Christopher deSolis, Kierstin Keller, Stefanie Navarro, and Paulette Allard. This work was supported by NIH grants HD36437 and HD45913 to G.R.M. and NS21328, NS070298, AG24373, and DK73691, and California Institute of Regenerative Medicine grant RC1-00353 awarded to D.C.W.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2012.09.004.
REFERENCES
- Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–1147. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Castellano MA, Afonso D, Rodriguez M. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol. Behav. 1991;49:69–72. doi: 10.1016/0031-9384(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Battersby BJ, Shoubridge EA. Selection of a mtDNA sequence variant in hepatocytes of heteroplasmic mice is not due to differences in respiratory chain function or efficiency of replication. Hum. Mol. Genet. 2001;10:2469–2479. doi: 10.1093/hmg/10.22.2469. [DOI] [PubMed] [Google Scholar]
- Battersby BJ, Loredo-Osti JC, Shoubridge EA. Nuclear genetic control of mitochondrial DNA segregation. Nat. Genet. 2003;33:183–186. doi: 10.1038/ng1073. [DOI] [PubMed] [Google Scholar]
- Battersby BJ, Redpath ME, Shoubridge EA. Mitochondrial DNA segregation in hematopoietic lineages does not depend on MHC presentation of mitochondrially encoded peptides. Hum. Mol. Genet. 2005;14:2587–2594. doi: 10.1093/hmg/ddi293. [DOI] [PubMed] [Google Scholar]
- Birky CW., Jr. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu. Rev. Genet. 2001;35:125–148. doi: 10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K, Hara T, Hayashi J, Yonekawa H. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat. Genet. 2007;39:386–390. doi: 10.1038/ng1970. [DOI] [PubMed] [Google Scholar]
- Cao L, Shitara H, Sugimoto M, Hayashi J, Abe K, Yonekawa H. New evidence confirms that the mitochondrial bottleneck is generated without reduction of mitochondrial DNA content in early primordial germ cells of mice. PLoS Genet. 2009;5:e1000756. doi: 10.1371/journal.pgen.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree LM, Samuels DC, de Sousa Lopes SC, Rajasimha HK, Wonnapinij P, Mann JR, Dahl HH, Chinnery PF. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet. 2008;40:249–254. doi: 10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- DeLuca SZ, O'Farrell PH. Barriers to male transmission of mitochondrial DNA in sperm development. Dev. Cell. 2012;22:660–668. doi: 10.1016/j.devcel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat. Struct. Mol. Biol. 2009;16:462–467. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etain B, Milhiet V, Bellivier F, Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. Eur. Neuropsychopharmacol. 2011;21(Suppl 4):S676–S682. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Fan W, Waymire KG, Narula N, Li P, Rocher C, Coskun PE, Vannan MA, Narula J, Macgregor GR, Wallace DC. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science. 2008;319:958–962. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Kronfeld-Schor N, Einat H. Models of mania: from facets to domains and from animal models to model animals. J. Psychopharmacol. (Oxford) 2010;24:437–438. doi: 10.1177/0269881108097905. [DOI] [PubMed] [Google Scholar]
- Gindre C, Swendsen J. [Everyday stress, routines and bipolar spectrum]. Encephale. 2010;36(Suppl 2):D92–D96. doi: 10.1016/j.encep.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Gómez-Durán A, Pacheu-Grau D, López-Gallardo E, Díez-Sánchez C, Montoya J, López-Pérez MJ, Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum. Mol. Genet. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- Hadjivasiliou Z, Pomiankowski A, Seymour RM, Lane N. Selection for mitonuclear co-adaptation could favour the evolution of two sexes. Proc. Biol. Sci. 2012;279:1865–1872. doi: 10.1098/rspb.2011.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo, a Laboratory Manual. Second Edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. [Google Scholar]
- Isobe Y, Hida H, Nishino H. Circadian rhythm of metabolic oscillation in suprachiasmatic nucleus depends on the mitochondrial oxidation state, reflected by cytochrome C oxidase and lactate dehydrogenase. J. Neurosci. Res. 2011;89:929–935. doi: 10.1002/jnr.22609. [DOI] [PubMed] [Google Scholar]
- Jenuth JP, Peterson AC, Fu K, Shoubridge EA. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat. Genet. 1996;14:146–151. doi: 10.1038/ng1096-146. [DOI] [PubMed] [Google Scholar]
- Jenuth JP, Peterson AC, Shoubridge EA. Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat. Genet. 1997;16:93–95. doi: 10.1038/ng0597-93. [DOI] [PubMed] [Google Scholar]
- Ji F, Sharpley MS, Derbeneva O, Alves LS, Qian P, Wang Y, Chalkia D, Lvova M, Xu J, Yao W, et al. Mitochondrial DNA variant associated with Leber hereditary optic neuropathy and high-altitude Tibetans. Proc. Natl. Acad. Sci. USA. 2012;109:7391–7396. doi: 10.1073/pnas.1202484109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen R, Marttinen P, Sandell HK, Manninen T, Teerenhovi H, Wai T, Teoli D, Loredo-Osti JC, Shoubridge EA, Battersby BJ. Gimap3 regulates tissue-specific mitochondrial DNA segregation. PLoS Genet. 2010;6:e1001161. doi: 10.1371/journal.pgen.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuno AA, Munakata K, Nagai T, Shimozono S, Tanaka M, Yoneda M, Kato N, Miyawaki A, Kato T. Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet. 2006;2:e128. doi: 10.1371/journal.pgen.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khrapko K. Two ways to make an mtDNA bottleneck. Nat. Genet. 2008;40:134–135. doi: 10.1038/ng0208-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. Evolution. The costs of breathing. Science. 2011a;334:184–185. doi: 10.1126/science.1214012. [DOI] [PubMed] [Google Scholar]
- Lane N. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. Bioessays. 2011b;33:860–869. doi: 10.1002/bies.201100051. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS ONE. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishmar D, Ruiz-Pesini EE, Golik P, Macaulay V, Clark AG, Hosseini S, Brandon M, Easley K, Chen E, Brown MD, et al. Natural selection shaped regional mtDNA variation in humans. Proc. Natl. Acad. Sci. USA. 2003;100:171–176. doi: 10.1073/pnas.0136972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Loshuertos R, Acín-Pérez R, Fernández-Silva P, Movilla N, Pérez-Martos A, Rodriguez de Cordoba S, Gallardo ME, Enríquez JA. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 2006;38:1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- Oliver NA, Wallace DC. Assignment of two mitochondrially synthesized polypeptides to human mitochondrial DNA and their use in the study of intracellular mitochondrial interaction. Mol. Cell. Biol. 1982;2:30–41. doi: 10.1128/mcb.2.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Covian R, Aponte AM, Glancy B, Taylor JF, Chess D, Balaban RS. Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1034–R1048. doi: 10.1152/ajpregu.00596.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JC, Procaccio V, Brandon MC, Merrick G, Wallace DC. Multiplex analysis of mitochondrial DNA pathogenic and polymorphic sequence variants. Biol. Chem. 2010;391:1115–1130. doi: 10.1515/BC.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- Sartori SB, Whittle N, Hetzenauer A, Singewald N. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology. 2012;62:304–312. doi: 10.1016/j.neuropharm.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligh JE, Levy SE, Waymire KG, Allard P, Dillehay DL, Nusinowitz S, Heckenlively JR, MacGregor GR, Wallace DC. Maternal germ-line transmission of mutant mtDNAs from embryonic stem cell-derived chimeric mice. Proc. Natl. Acad. Sci. USA. 2000;97:14461–14466. doi: 10.1073/pnas.250491597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solignac M, Génermont J, Monnerot M, Mounolou JC. Drosophila Mitochondrial Genetics: Evolution of Heteroplasmy through Germ Line Cell Divisions. Genetics. 1987;117:687–696. doi: 10.1093/genetics/117.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportiche N, Suntsova N, Methippara M, Bashir T, Mitrani B, Szymusiak R, McGinty D. Sustained sleep fragmentation results in delayed changes in hippocampal-dependent cognitive function associated with reduced dentate gyrus neurogenesis. Neuroscience. 2010;170:247–258. doi: 10.1016/j.neuroscience.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JB, Freyer C, Elson JL, Wredenberg A, Cansu Z, Trifunovic A, Larsson NG. Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol. 2008;6:e10. doi: 10.1371/journal.pbio.0060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitin tag for sperm mitochondria. Nature. 1999;402:371–372. doi: 10.1038/46466. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, McCauley TC, Sutovsky M, Day BN. Early degradation of paternal mitochondria in domestic pig (Sus scrofa) is prevented by selective proteasomal inhibitors lactacystin and MG132. Biol. Reprod. 2003;68:1793–1800. doi: 10.1095/biolreprod.102.012799. [DOI] [PubMed] [Google Scholar]
- Thompson WE, Ramalho-Santos J, Sutovsky P. Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol. Reprod. 2003;69:254–260. doi: 10.1095/biolreprod.102.010975. [DOI] [PubMed] [Google Scholar]
- Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 2008;40:1484–1488. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 2007;76:781–821. doi: 10.1146/annurev.biochem.76.081205.150955. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Ruiz-Pesini E, Mishmar D. mtDNA variation, climatic adaptation, degenerative diseases, and longevity. Cold Spring Harb. Symp. Quant. Biol. 2003;68:479–486. doi: 10.1101/sqb.2003.68.471. [DOI] [PubMed] [Google Scholar]
- Watkins-Chow DE, Pavan WJ. Genomic copy number and expression variation within the C57BL/6J inbred mouse strain. Genome Res. 2008;18:60–66. doi: 10.1101/gr.6927808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.